Abstract
Aims and Objectives
Suck development is a challenging hurdle for preterm infants who endure an extensive oxygen history due to respiratory distress syndrome (RDS). The fine structure of the non-nutritive suck (NNS) was studied in preterm infants according to RDS severity.
Design and Methods
Recordings of NNS were completed cribside in the neonatal intensive care unit (NICU) in 55 preterm infants distributed among one healthy control group and two RDS infant groups. NNS pressure amplitude (cmH20) and within-burst suck cycle period (ms) were the dependent measures extracted from digitized records of pacifier nipple compression pressure.
Results and Conclusions
RDS preterm infants demonstrated significant differences in NNS suck pressure amplitude compared to healthy preterm infants. Periods of oxygen supplementation restrict orofacial movement and limit orosensory experiences necessary for suck development and neural maturation. RDS infants may be excellent candidates for patterned oral stimulation programs designed to advance the maturation of sucking skills.
Keywords: non-nutritive suck (NNS), respiratory distress syndrome (RDS), pressure amplitude, suck cycle period, oromotor, suck central pattern generator (sCPG)
Introduction
Oral feeding difficulties represent one of the most frequently encountered problems in preterm infants (Comrie & Helm, 1997; Lau & Hurst, 1999). The non-nutritive suck (NNS) may be dysfunctional and lack organization, especially for those preterm infants who have an extensive history of intubation, continuous positive airway pressure, and nasal cannulation due to respiratory distress syndrome (RDS) (Barlow & Finan, 2007; Barlow & Estep, 2006). Lengthy oxygen supplementation procedures cost the preterm infant precious sensory and motor experiences during a critical period of brain development when the central patterning of suck and feeding skills are being refined. Interruption of these experiences may impair fragile syntheses of how the brain maps these functions (Bosma, 1970). These preterm infants often lack a functional suck and manifest oromotor dyscoordination which may persist well into early childhood and lead to significant delays in the emergence of other oromotor behaviors, including, babbling, speech-language production, and feeding (Adams-Chapman, 2006; Ballantyne et al., 2006). Early oral feeding difficulties and extended hospitalization underscores the need to facilitate the development of normal oral motor skills (Fucile, Gisel, & Lau, 2002).
Suck is a precocious motor skill in term infants that can be differentiated by nutritive and non-nutritive modes. The non-nutritive suck is defined as any repetitive mouthing activity on a blind nipple or pacifier, which does not deliver a liquid stimulus (Goldson, 1987; Wolff, 1968). As shown in Figure 1, the NNS is characterized by periods of suck bursts with rest segments or “pauses” between individual suck bursts. The maturation and coordination of the NNS precedes the suck-swallow-breathe pattern associated with the nutritive suck (Medoff-Cooper, 2005; Gewolb, Vice, Schweitzer-Kenney, Taciak, & Bosma, 2001; Lau & Schanler, 1996; 1999). The NNS has been observed in utero as early as 14 weeks gestational age (GA), and continues to develop until it is functionally incorporated into the preterm motor repertoire by 32 weeks GA (Mizuno & Ueda, 2005). Both suck modes are thought to be controlled by a neuronal network known as the suck central pattern generator (sCPG) (Barlow & Estep, 2006; Barlow, Finan, Park, 2004; Iriki et al., 1988; Tanaka et al, 1999). This rhythm-generating circuit is located in the reticular formation of the brainstem and is centrally modulated by multiple inputs, including descending pathways from sensorimotor cortex, and primary trigeminal mechanoreceptive afferents originating in the oral sensorium.
Figure 1.
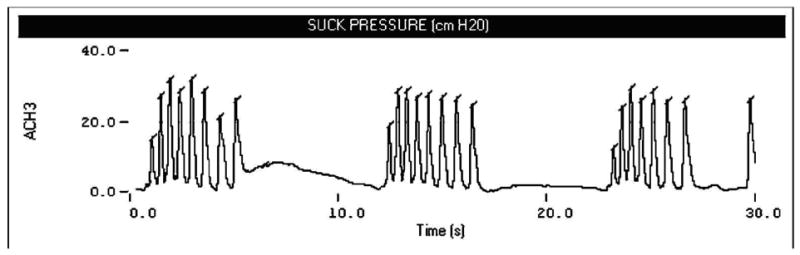
Waveform display of the classic ‘burst-pause’ suck CPG patterning as demonstrated by a preterm control neonate. Tick marks indicate peak-picking algorithm tags for NNS pressure peaks during burst production. These tick marks are used for pressure amplitude (cmH20) and within-burst suck cycle (ms) period measurements.
The utility of the NNS has been shown to benefit growth, maturation, gastric motility, while decreasing stress (Lau & Hurst, 1999; Lau & Schanler, 1996; DiPetro et. al, 1993; Pickler et. al, 1992; Field, 1993) and enhancing oral feeds (McCain, 1995). The NNS accelerates the transition from tube to independent oral feeding and is presumed to enhance the maturation of neural systems responsible for ororhythmic activity (Bernbaum et al., 1983; Field, et al, 1982; Measel & Anderson, 1979). Recent evidence suggests that the sensory consequences associated with the production of NNS have beneficial effects on oral feeding performance and the development of specific sucking skills (Fucile et al., 2002, 2005).
In general, oromotor control measures sampled in the neonatal intensive care unit (NICU) are limited when considering the ‘fine structure’ of the NNS among preterm infants with a history of respiratory disease and related complications. Lau and Schanler (1996) differentiated between coarse and fine structures of the NNS. The fine structure analysis includes measurement of select within-NNS burst characteristics, including individual peak amplitudes and cycle periods of suck (see Figure 1). The goal of the current study was to examine the ‘fine’ structure of the NNS burst pattern while accounting for birth weight, GA at birth, and postmenstrual age at two consecutive visits (designated PMA1 and PMA2) among three preterm infant groups, including healthy controls, mild RDS, and moderate-severe RDS classified according to oxygen history. It was hypothesized that preterm infants with a history of RDS, would manifest significant differences in the fine structure of the NNS in terms of nipple compression pressure amplitude and NNS burst cycle period when compared to healthy preterm control neonates.
Patients and methods
Patients
The Human Subjects Committees at the University of Kansas Medical Center and Stormont-Vail Regional Health Center approved the research protocol for this study and informed consent was obtained from the parents prior to the participants' enrollment into the study. A total of 55 preterm infants (23 female, 32 male), distributed among three groups, participated in the study. The mean GA at birth was 30.06 weeks (S.D. = 2.2) and the mean birth weight was 1339.7 g (S.D. = 386.2). General inclusion criteria for the study population were: head circumference within the 10-90th percentile of mean for PMA, neurological examination showing no anomalies for PMA: response to light, sound, and spontaneous movements of all extremities, and with stable vital signs (heart rate, blood pressure, age appropriate respiratory rate, and oxygen saturation >92 SpO2) to allow for NNS. All infants were extubated for >5 days at the time of testing. Exclusion criteria included intracranial hemorrhage, periventricular leukomalacia, neonatal seizures and culture positive sepsis or meningitis at time of testing, chromosomal anomalies or craniofacial malformation.
The three operationally defined test groups included healthy preterm controls [CONTROL], mild RDS [RDS1] and moderate-severe RDS [RDS2]. The healthy preterm CONTROL group included 17 infants (9 female, 8 male) with an average birth GA of 31.5 weeks (S.D. = 1.4), and a mean birth weight of 1518.7 g (S.D. = 318.6). These infants were not intubated but had an average of 1.4 days of nasal oxygen supplementation (range 0 to 4 days). The RDS1 group included 11 infants (4 female, 7 male) with an average birth GA of 30.5 weeks (S.D. = 2.1), and a mean birth weight of 1442.4 g, (S.D. = 275.1). RDS1 infants required intubation and/or 5-7 days of oxygen therapy (mean = 5.1 days). The RDS2 group included 27 infants (10 female, 17 male) with an average birth GA of 29.0 weeks (S.D. = 2.2), and a mean birth weight of 1185.3 g (S.D. = 264.33). RDS2 infants required intubation and received a minimum of seven days of oxygen therapy (mean = 37.4 days) for inclusion. The clinical characteristics of the three preterm infant test groups are summarized in Table 1.
Table I. Clinical Characteristics of Study Infants*.
| VARIABLE | CONTROL
(n=17) |
RDS1
(n=11) |
RDS2
(n=27) |
|
|---|---|---|---|---|
| Gender (males : females) | 8 : 9 | 7 : 4 | 17 : 10 | |
| Birth GA (weeks) | 31.5 (1.4) | 30.5 (2.1) | 29.0 (2.2) | |
| Birth Weight (grams) | 1518.7 (318.6) | 1442.4 (275.1) | 1185.3 (409.9) | |
| PMA @ Session (weeks) | Session 1 | 33.6 (1.7) | 33.4 (1.3) | 34.1 (2.2) |
| Session 2 | 34.7 (1.6) | 34.5 (1.1) | 34.9 (2.1) | |
| Mean | 34.2 (1.7) | 34.0 (1.2) | 34.5 (2.2) | |
| % Oral Feeding | Session 1 | 13.2 (4.0) | 9.4 (4.4) | 3.4 (3.2) |
| Session 2 | 35.0 (9.5) | 40.6 (10.5) | 10.4 (7.5) | |
| Mean | 24.1 (6.8) | 25.0 (7.5) | 6.9 (5.4) | |
| Oxygen Therapy History (days) | Ventilator | 0.0 (0.0) | 1.3 (1.3) | 6.4 (11.0) |
| CPAP | 0.7 (1.1) | 2.3 (2.2) | 9.6 (10.2) | |
| Cannula | 0.7 (1.3) | 1.6 (1.6) | 21.9 (15.2) | |
| Total | 1.4 (1.7) | 5.2 (1.8) | 37.9 (26.0) | |
Expressed as mean (sd)
Methods
The fine structure of the NNS burst pattern was digitally sampled during two consecutive visits occurring at approximately 33.7 and 34.7 weeks PMA, designated as PMA1 and PMA2, respectively. Testing occurred at the University of Kansas Medical Center (Kansas City, KS) and Stormont-Vail Regional Health Center (Topeka, KS) neonatal intensive care units.
A mobile oromotor physiology recording station, known as the Actifier (Finan & Barlow, 1996) was positioned cribside within the NICU approximately 15 minutes before a scheduled feed. This device utilizes a custom machined receiver and a silicone Soothie™ pacifier. A 2-point scale calibration of pacifier intraluminal air pressure was completed using water manometry and registered prior to digitization. Following a brief examination of physiologic state, the infant was cradled by the tester in a supportive inclined posture and swaddled with the neonate's hands at midline. Sampling of NNS behavior was not initiated until the infant was in an optimal behavioral state, i.e., drowsy to quiet alert (stages 3 or 4 of the Preterm Infants Behavioral Scale, Newborn Individualized Developmental Care and Assessment Program; NIDCAP (Als, 1995). The infant remained connected to the physiological monitors at all times (respiration, EKG, and SpO2). With the pacifier pressure-recording receiver outside the mouth, a specially designed real time non-nutritive suck data acquisition and analysis program developed in our laboratory, known as Neosuck RT, was triggered to initiate sampling. This maneuver provided a ‘no-contact’ pacifier pressure baseline condition that was used by the Neosuck RT analysis software to automatically adjust (demean) the pressure signal offset to zero volts output. The examiner presented the Soothie™ pacifier to the infant's mouth to establish an adequate latch. The infant's position was maintained while the NNS pressure waveform was sampled.
A 2-minute contiguous block of NNS behavior, objectively based on the infant's greatest number of suck cycles with nipple compression pressures greater than 1 cmH2O, was subjected to computerized measurement of NNS amplitudes and NNS cycle periods within burst, using a specially designed software NNS waveform discrimination algorithm. The first derivative of the suck pressure signal was used to index peak values NNS pressures at each derivative zero crossing. A hysteresis function prevented small deviations (reversals) in the digitized NNS pressure trajectory from being indexed as a valid pressure peak if the time interval between any two consecutive peaks was less than the preset peak period threshold value of 1 cm H20. This algorithm permitted objective identification of NNS cycle amplitude peaks and derivation of NNS cycle periods during the infant's most productive 2-minute suck record epoch.
Statistical Analysis
Given the hierarchically nested design of the data, in which the NNS Amplitude and within-burst NNS Cycle Period responses (level-1) were nested within two weekly visits (level-2) and visits were nested within 55 preterm infants (level-3), three-level models were estimated using MULTILEV in LISREL 8.7 (Jöreskog & Sörbom, 2004). Models were tested examining the effects of preterm group (CONTROL, RDS1, RDS2) as well as covariates including gestational age at birth, birth weight, and postmenstrual age on the NNS Amplitude and NNS Cycle Period response variables.
The multilevel regression analysis for each dependent variable was conducted estimating a series of three-level models. First, an intercept-only model (i.e., null model) was estimated in order to determine the number of random variance components. Second, grouping variables and covariates were introduced into the model with their significant random effects. Finally, the best fitting model was found by comparing deviance statistics (-2lnL) of each potential model.
Results
NNS Amplitude
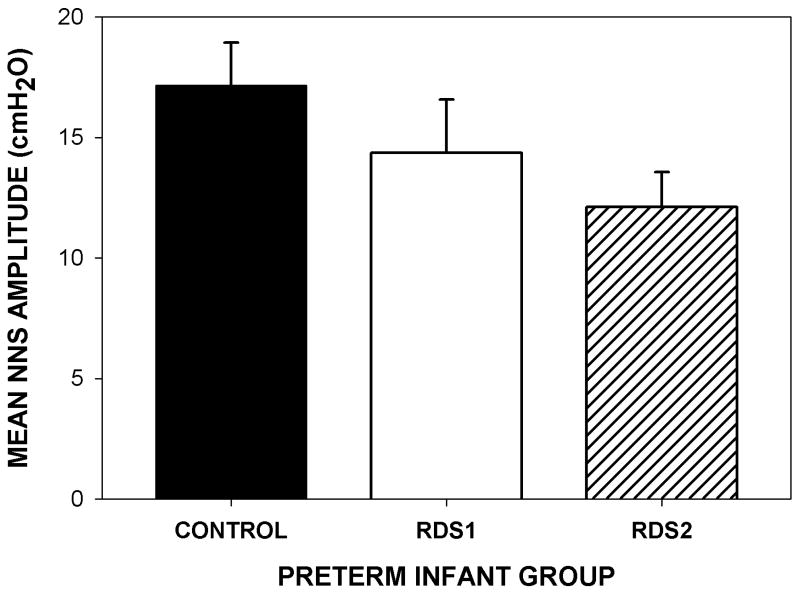
The mean NNS amplitude decreased with RDS severity as shown in Figure 2. The healthy control group yielded the highest mean of NNS amplitude pressure (M = 17.15 cm H20, SE = 1.79), followed by those in the RDSl group (M = 14.35 cm H20, SE = 2.22) and the RDS2 group (M = 12.12 cm H20, SE = 1.43).
Figure 2.
Bar graph depicting the estimated marginal means and standard error of NNS amplitude by group collapsed across both visits.
Although the covariates (birth weight, GA at birth, PMA measured at each session) had large correlations with RDS severity level, they demonstrated much smaller correlations with NNS amplitude suck pressure. As a result, only the grouping variable (RDS preterm infant group) was included in the model as a predictor. The fixed effects of the predictor (RDS group) variable indicate the expected amount to which the estimated mean NNS amplitude suck pressure score decreases as a function of preterm infant group. The random effects of the predictor variable were not included because the likelihood ratio test suggested that they were not tenable.
Based on the null model (intercept-only model), the estimated mean NNS amplitude across all measurements, two sessions, and all babies is 14.13 cmH2O. The estimated variances of level-1, level-2, and level-3 residual errors are 37.71, 49.98, and 34.30, respectively. The intra-class correlation (ICC) derived from the estimated variances revealed that most of the variability in the NNS amplitude scores occurs between sessions (41%), followed by among measurements (31%), and finally among babies (28%), respectively. The substantial ICCs shows that there is some clustering of the scores within sessions and within babies, suggesting that multilevel analysis is more suitable for these data than the ordinal least square analysis.
The Full Model includes two grouping (dummy) variables and three covariates (birth weight, GA@birth, and PMA@test) and is given by:
1. Fixed Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | p | χ2 | p |
| Intercept | 10.17 | 22.68 | .45 | .20 | .65 | .20 | .65 |
| D1 | -2.55 | 2.92 | -.87 | .76 | .38 | .75 | .39 |
| D2 | -4.29 | 2.82 | -1.52 | 2.32 | .13 | 2.28 | .13 |
| BW | .00 | .00 | .11 | .01 | .91 | .01 | .91 |
| GA@BIRTH | .21 | .99 | .22 | .05 | .83 | .05 | .83 |
| PMA@test | -.02 | .60 | -.03 | .00 | .98 | .00 | .98 |
Intercept = estimated NNS AMP mean for ‘Healthy Control’ group
Intercept + D1 = estimated NNS AMP mean for ‘RDS1’ group
Intercept + D2 = estimated NNS AMP mean for ‘RDS2’ group
2. Random Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | p | χ2 | p |
| 29.28 | 11.39 | 2.57 | 6.61 | < .05 | 7.61 | < .01 | |
| 48.99 | 9.52 | 5.15 | 26.48 | < .01 | 1389.06 | < .01 | |
| 37.71 | .60 | 63.13 | 3985.05 | < .01 | 263642 | < .01 | |
The likelihood-ratio test shows that neither group membership nor other covariates significantly predict the NNS amplitude scores. The residual ICC show that 28% of total residual variance occurs among babies, and the squared multiple correlation indicate that approximately 20% of level-3 AMP variance is accounted for by the group membership and other covariates. The small z-scores for the covariates (BW, GA@birth, and PMA@test) confirm these are not significant predictors of NNS amplitude among the 55 infants tested. The full model does not provide significant improvement in fit over the null model, .
The Semi-Final Model includes the same grouping variables as the null model but no covariates and is given by:
Fixed Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | p | χ2 | p |
| Intercept | 17.15 | 1.79 | 9.60 | 92.08 | < .01 | 54.04 | < .01 |
| D1 | -2.80 | 2.85 | -.98 | .97 | .33 | .96 | .33 |
| D2 | -5.03 | 2.29 | -2.20 | 4.84 | < .05 | 4.64 | < .05 |
Intercept = estimated NNS AMP mean for ‘Control’ group
Intercept + D1 = estimated NNS AMP mean for ‘RDS1’ group
Intercept + D2 = estimated NNS AMP mean for ‘RDS2’ group
Random Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | p | χ2 | p |
| 29.57 | 11.44 | 2.58 | 6.68 | < .05 | 7.72 | < .05 | |
| 48.98 | 9.52 | 5.15 | 26.48 | < .01 | 1392.26 | < .01 | |
| 37.71 | .60 | 63.13 | 3985.05 | < .01 | 263642 | < .01 | |
Babies in the RDS2 group (M = 12.12, SE = 1.43) have a significantly lower estimated NNS AMP mean than those in the healthy Control group (M = 17.15, SE = 1.79). However, the estimated NNS AMP means do not differ between babies in the Control group and those in the RDS1 group (M = 14.35, SE = 2.22). The residual ICC shows that 25% of total residual variance occurs among babies, and the squared multiple correlations indicate that approximately 14% of level-3 NNS AMP variance is accounted for by group membership. This model does not provide significant improvement in fit over the null model, .
The two RDS preterm groups are combined in the Final Model as given by:
Fixed Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | p | χ2 | p |
| Intercept | 17.15 | 1.80 | 9.54 | 90.94 | < .01 | 53.60 | < .01 |
| D3 | -4.38 | 2.17 | -2.02 | 4.09 | < .05 | 3.95 | < .05 |
Intercept = estimated NNS AMP mean for ‘Control’ group
Intercept + D3 = estimated NNS AMP mean for ‘RDS1’ and ‘RDS2&RDS+’ groups combined
Random Component.
| Wald Test | LR Test | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | z | χ2 | P | χ2 | p |
| 30.26 | 11.56 | 2.62 | 2.62 | < .05 | 7.97 | < .01 | |
| 48.98 | 9.52 | 5.15 | 26.48 | < .01 | 1392.26 | < .01 | |
| 37.71 | .60 | 63.13 | 3985.05 | < .01 | 263642 | < .01 | |
Babies in the healthy Control group (M = 17.15, SE = 1.80) have a significantly higher estimated NNS AMP mean than those in other two RDS groups combined (M = 12.77, SE = 1.21). The residual ICC shows that 26% of total residual variance occurs among babies, and the squared multiple correlations indicate that approximately 12% of level-3 NNS AMP variance is accounted for by group membership. This reduced model provides significant improvement in fit over the null model, .
NNS Burst Cycle Period
NNS cycle periods occurring within a burst failed to differentiate as a function of RDS preterm infant group or the covariates.
Discussion
Wolff (1968) suggested that detailed analysis of sucking rhythms might be useful for correlating abnormal brain function with observable behavior in the young infant. Disruption of the suck CPG in premature infants may be caused, in part, by missed or degraded critical experiences in utero that support its development. Preterm infants with the added complication of lung disease, requiring intubation, are also at risk for sensory deprivation and motor restriction. The results of this study demonstrate quantitative differences in the ‘fine’ structure of the NNS pressure amplitude among three premature infant test groups over two consecutive visits, while adjusting for gestational age at birth, birth weight, and post menstrual maturational effects.
The hallmark of the central pattern generation of the suck in healthy preterm infants appears to revolve around stability and distinguishes this group from preterm infants with a history of RDS. As a group, the healthy control infants manifest consistent NNS pressure amplitudes over the two test sessions, PMA1 and PMA2, sampled in the present study. Examination of the clinical discharge summaries revealed that healthy control infants transitioned smoothly to oral feeds and were discharged from the NICU, often by the 35th week PMA. In contrast, RDS infants manifested significant reduction in NNS amplitude and typically required 2 to 3 additional weeks in the NICU in order to successfully complete the transition-to-oral feeds. In our view, preterm neonates with a history of RDS represent a model of sensory deprivation and oromotor restriction, given the extended periods of oxygen supplementation and intubation that restrict the neonate from engaging in suck activity for weeks, and in some cases, months. Extended periods of orofacial restriction deprive the preterm infant of critical orosensory experiences that are hypothesized to play an important role in establishing early sensorimotor behaviors such as feeding, babbling, and speech.
The invasiveness of oxygen supplementation is presumed to cost the preterm infant precious sensory and motor experiences during a critical period of brain development when the beneficial and multiple effects of NNS could otherwise be utilized to facilitate neural maturation, reduce stress, establish the central patterning of suck, and enhance the transition to oral feeding. Trussing the lower face with tubes and tape also restricts the range and type of oral movements. Support for this hypothesis is derived from studies of brain development in animal models. For example, the combination of sensory deprivation and motor restriction in rat has been shown to disrupt development of key brain structures involved in sensorimotor control, including motor cortex and cerebellum (Pascual et al., 1998, 1993; Pascual & Figueroa, 1996). This is consistent with the notion of a critical period during early postnatal life, when manipulations in trigeminal sensory systems may result in drastic effects in the structure and function of the developing brain. Bosma (1973) suggested that “appropriate oral experiences may be critical in the final weeks of gestation, and that their interruption may impair fragile syntheses of central neural representations of these functions (p. 7).” Thus, we further speculate that the NNS profile exhibited by infants with RDS, particularly those with moderate-severe RDS, may benefit directly from patterned oral somatosensory stimulation protocols designed to advance the maturation of specific sucking skills through synchronous stimulation of the suck CPG circuitry (Barlow & Finan, 2007; Barlow & Estep, 2006; Fucile et al., 2002, 2005).
Acknowledgments
This study was supported by grants NIH R01 DC03311-06 (SM Barlow), NIH P30 HD02528, and NIH P30 DC005803. The authors would like to thank the parents who allowed their children to participate in this study and the NICU medical teams at the University of Kansas Medical Center and Stormont-Vail Regional Health Center for their support. Additionally, the authors express their sincere gratitude to Lana Seibel MA, Mimi Urish MA, Monique Fees BA, Meredith Poore BGS, and Shinying Chu BA for data collection, Jose Gierbolini, MD, Medical Director Newborn Services at Stormont-Vail Regional Health Center, and to Rajesh Vantipalli, MSCS, Research Engineer of the Communication Neuroscience Laboratories for software design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susan Stumm, Graduate Research Associate, Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas USA.
Steven M. Barlow, Professor, SPLH, Programs in Neuroscience and Human Biology, Director Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas USA.
Meredith Estep, Graduate Research Associate, Communication Neuroscience Laboratories, Program in Neuroscience, University of Kansas, Lawrence, Kansas USA.
Jaehoon Lee, Statistician, Advanced Statistical Methods Core, NIH Center for Biobehavioral Neurosciences in Communication Disorders, University of Kansas, Lawrence, Kansas USA.
Susan Cannon, Research Associate, Communication Neuroscience Laboratories, Kansas University Medical Center, Kansas City, Kansas USA.
Joy Carlson, Stormont-Vail Regional Medical Center, Topeka, Kansas USA.
Donald Finan, Assistant Professor, Department of Speech-Language-Hearing Science, Center for Neuroscience, University of Colorado, Boulder, Colorado USA.
References
- Adams-Chapman I. Speech and language outcome at 30 months adjusted age among a cohort of ELBW infants. Pediatric Academic Society. 2006;5532:177. [Google Scholar]
- Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the premature infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York: 1995. pp. 77–85. [Google Scholar]
- Ballantyne M, Frisk V, Green P. Language impairment in extremely-low-birth-weight infants. Pediatric Academic Society. 2006;5532:178. [Google Scholar]
- Barlow SM, Finan DS. Patterns for the premature brain: driving the suck central pattern generator in premature infants with RDS. Pediatric Academic Society. 2007;6430:5. [Google Scholar]
- Barlow SM, Estep M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Communicative Disorders. 2006;39:366–380. doi: 10.1016/j.jcomdis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Park SY. Central pattern generation and sensorimotor entrainment of respiratory and orofacial systems. In: Maassen B, Hulstijn W, Kent R, Peters HFM, van Lieshout PHMM, editors. Speech Motor Control in Normal and Disordered Speech. Oxford University Press; 2004. pp. 211–224. [Google Scholar]
- Bernbaum JC, Pereira GR, Watkins JB, Peckham GJ. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics. 1983;71(1):41–45. [PubMed] [Google Scholar]
- Bosma JF. Summarizing and perspective comments: Part V. Form and function in the infant's mouth and pharynx. In: Bosma JF, editor. Second Symposium on Oral Sensation and Perception. Springfield, Illinois: Charles C. Thomas Publisher; 1970. pp. 550–555. [Google Scholar]
- Comrie JD, Helm JM. Common feeding problems in the intensive care nurseries, maturation, organization, evaluation, and management strategies. Semin Speech Lang. 1997;18:239–261. doi: 10.1055/s-2008-1064075. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Cusson RM, Caughy MO, et al. Behavioral and physiologic effects of nonnutritive sucking during gavage feeding in preterm infants. Pediatric Research. 1994;36:207–214. doi: 10.1203/00006450-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Field T. Sucking for stress reduction, growth and development during infancy. Pediatric Basics. 1993;64:13–16. [Google Scholar]
- Field T, Ignatoff E, Stringer S, Brennan J, Greenberg R, Widmayer S, et al. Nonnutritive sucking during tube feedings: effects on preterm neonates in an intensive care unit. Pediatrics. 1982;70(3):381–384. [PubMed] [Google Scholar]
- Finan DS, Barlow SM. The Actifier: a device for neurophysiological studies of orofacial control in human infants. Journal of Speech and Hearing Research. 1996;39:833–838. [PubMed] [Google Scholar]
- Fucile S, Gisel E, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Developmental Medicine and Child Neurology. 2005;47(3):158–162. doi: 10.1017/s0012162205000290. [DOI] [PubMed] [Google Scholar]
- Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. The Journal of Pediatrics. 2002;141(2):230–236. doi: 10.1067/mpd.2002.125731. [DOI] [PubMed] [Google Scholar]
- Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suckle and swallow in preterm infants. Dev Med Child Neurology. 2001;43:22–27. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- Goldfield EC, Wolff PH, Schmidt RC. Dynamics of oral-respiratory coordination in full-term and preterm infants: II. Continuing effects at 3 months post term. Developmental Science. 1999;2(3):374–384. [Google Scholar]
- Goldson E. Nonnutritive sucking in the sick infant. Journal of Perinatology. 1987;7(1):30–34. [PubMed] [Google Scholar]
- Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Dev Brain Research. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8.7 for Windows (computer software) Lincolnwood, Illinois: Scientific Software International, Inc.; 2004. [Google Scholar]
- Lau C, Hurst N. Oral Feeding in Infants. Current Problems in Pediatrics. 1999:105–124. doi: 10.1016/s0045-9380(99)80052-8. [DOI] [PubMed] [Google Scholar]
- Lau C, Schanler RJ. Oral motor function in the neonate. Clinics in Perinatology. 1996;23(2):161–178. [PubMed] [Google Scholar]
- McCain GC. Promotion of preterm infant nipple feeding with nonnutritive sucking. J Pediatric Nursing. 1995;10:3–8. doi: 10.1016/S0882-5963(05)80093-4. [DOI] [PubMed] [Google Scholar]
- Measel CP, Anderson GC. Nonnutritive sucking during tube feedings: effect upon clinical course in preterm infants. J Obsteric Gynecol Neonatal Nursing. 1979;8:265–271. doi: 10.1111/j.1552-6909.1979.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Developmental Medicine and Child Neurology. 2005;47(5):299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B. Nutritive sucking research: from clinical questions to research answers. J Perinat Neonat Nurs. 2005;19:265–272. doi: 10.1097/00005237-200507000-00013. [DOI] [PubMed] [Google Scholar]
- Pascual R, Fernandez V, Ruiz S, Kuljis RO. Environmental deprivation delays the maturation of motor pyramids during the early postnatal period. Early Hum Dev. 1993;33:145–155. doi: 10.1016/0378-3782(93)90209-d. [DOI] [PubMed] [Google Scholar]
- Pascual R, Figueroa H. Effects of preweaning sensorimotor stimulation on behavioral and neuronal development in motor and visual cortex of the rat. Biol Neonate. 1996;69:399–404. doi: 10.1159/000244337. [DOI] [PubMed] [Google Scholar]
- Pascual R, Hervias MC, Toha ME, Valero A, Figueroa HR. Purkinje cell impairment induced by early movement restriction. Biol Neonate. 1998;73:47–51. doi: 10.1159/000013959. [DOI] [PubMed] [Google Scholar]
- Pickler RH, Higgins KE, Crummette BD. The effect of nonnutritive sucking on bottle-feeding stress in preterm infants. J Obstretic, Gynecologic and Neonatal Nursing. 1992;22(3):230–234. doi: 10.1111/j.1552-6909.1993.tb01804.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kogo M, Chandler SH, Matsuya T. Localization of oral-motor rhythmogenic circuits in the isolated rat brainstem preparation. Brain Research. 1999;821:190–199. doi: 10.1016/s0006-8993(99)01117-8. [DOI] [PubMed] [Google Scholar]
- Wolff PH. The serial organization of sucking in the young infant. Pediatrics. 1968;42(6):943–956. [PubMed] [Google Scholar]