Abstract
Maintaining good glycemic control is a daily challenge for people with type 1 diabetes. Insulin requirements are changing constantly due to many factors, such as levels of stress and physical activity. The basal insulin requirement also has a circadian rhythm, adding another level of complexity. Automating the adjustment of insulin dosing would result in improved glycemic control, as well as an improved quality of life by significantly reducing the burden on the patient. Building on our previous success of using run-to-run control for prandial insulin dosing (a strategy adapted from the chemical process industry), we show how this same framework can be used to adjust basal infusion profiles. We present a mathematical model of insulin-glucose dynamics which we augment in order to capture the circadian variation in insulin requirements. Using this model, we show that the run-to-run framework can also be successfully applied to adjust basal insulin dosing.
Keywords: type 1 diabetes, run-to-run control, basal infusion, circadian variation
1 Introduction
Type 1 diabetes mellitus is a metabolic disease characterized by hyperglycemia (high blood glucose concentration), which is caused by an absolute deficiency of insulin secretion. There are an estimated 18 million people worldwide with the disease [1], [2], and with a clear rising trend in its incidence [3]. People with type 1 diabetes fully depend on exogenous insulin, and managing their disease is a daily challenge.
Insulin dosing is divided into two regimens: basal and bolus insulin. The basal insulin covers the requirements in the absence of meals. The insulin bolus is associated with the carbohydrate content of meals (the insulin-to-carbohydrate ratio), and is calculated to offset the appearance of glucose from a meal. Both of these require adjustment over time.
The two main insulin delivery methods are continuous subcutaneous insulin infusion (CSII) pumps and multiple daily injections. CSII pumps are becoming the preferred method to deliver insulin, as CSII therapy significantly improves glycemic control [4]. Pumps have the advantage that basal insulin delivery can be set at different rates for distinct segments of the day (e.g., the Paradigm® pump, Medtronic MiniMed, Inc., Northridge, CA supports up to 48 segments). These segments are selected to accommodate changing insulin requirements due to the sleep-awake cycle and activity levels; a range of one to seven segments are common. The CSII pump is also used to deliver the meal-related insulin boluses.
Since the Diabetes Control and Complications Trial [5], the first large, prospective clinical trial, the accumulated evidence is strong that blood glucose levels must be normalized in order to avoid the complications associated with diabetes [6]. Tight glucose control (i.e., as close to normal as possible) should be maintained for life in order to accrue the full benefits. Many factors influence the insulin dose requirements, including weight, physical condition, and stress levels. Due to the constantly changing insulin requirement, frequent blood glucose monitoring is mandated. Based on these glucose levels the insulin dosage must be modified, dietary changes implemented (such as alteration in the timing, frequency and content of the meals), and activity patterns changed.
Automating insulin delivery has, therefore, been an active field of research. There have been multiple algorithms proposed for closed-loop control of glycemia, including MPC [7]–[10], PD [11]–[13], PID [14]–[17], and H∞ [18], [19] — for a recent review of the literature in this area see [20]. All of these controllers are based on the assumption that a continuous glucose sensor is available, and although sensor technology has improved significantly over the last few years, it is still short of the reliability and accuracy required for commercial implementation of such a closed-loop system [20].
Even before continuous glucose monitoring, there have been substantial efforts to develop algorithms that use sparse blood glucose measurements. Most of these have evolved from within the medical community, and consist of heuristics based on clinical experience [21]–[29]. None of these algorithms use the newer monomeric insulin formulations, and most are aimed at multiple insulin injection therapy, rather than the use of CSII pumps.
We have previously tested a run-to-run control strategy to adjust the insulin-to-carbohydrate ratio based on post-meal blood glucose measurements [30]–[34], with the clinical results of the latest version of the algorithm being very encouraging [35]. The basal insulin infusion rate in these studies has been manually adjusted by the physician using retrospective continuous glucose monitoring [36].
Adjusting the basal infusion rates is not straightforward, as the effect of glucose appearance from a meal and the related insulin bolus make it difficult to distinguish if blood glucose changes are due to the basal or bolus dose. A common situation for many individuals is to have their basal rates set too low, and to compensate for the difference in the prandial bolus, which is not the best strategy. CSII pump manufacturers are introducing features to help subjects adjust their basal rates, but none provides rate adjustment recommendations.
In this paper we present the use of run-to-run control to adjust basal infusion rates using sparse blood glucose measurements. The performance measure used in our algorithm is derived, in part, from our clinical heuristic. The paper is organized as follows: we first introduce the clinical heuristic; we follow that with an overview of the run-to-run algorithm, and the derivation of the performance measure. We present simulation results of our control strategy, for which we introduce modifications to one of the previously published models of glucose kinetics in order to capture the circadian variation in insulin sensitivity and changes in physical activity. We conclude the paper with a discussion of the results.
2 Clinical Adjustment of Basal Infusion Rates
When starting a subject on CSII therapy, there are a number of steps the physician will follow [37]. The first one is to estimate the total insulin requirement, which depends on the subject's weight and other factors such as physical condition and stress. For a nominal subject under typical stress levels, this is calculated as
| (1) |
where W is the subject's weight. Basal insulin requirements are roughly 50% of the total daily dose
| (2) |
The change in insulin sensitivity over the course of the day is driven by the circadian variation in hormone levels. Basal insulin needs are highest in the early morning, stable during the day, and lowest in the middle of the night, when hormones are at their nadir [37]. For this reason, a day is divided into four segments: midnight to 4:00, 4:00 to 10:00, 10:00 to 18:00 and 18:00 to midnight. For each segment the basal infusion rate is set to
| (3) |
as the initial estimate. From this starting point, it is necessary to fine-tune the infusion rates based on the subject's response. Initially, the same basal rate is used for the third and fourth segments. This is to provide a transition point from the outset at a time of the day when some people may have a drastic change in physical activity levels. Based on the person's actual patterns the physician can choose different ways of segmenting the day, but in most cases these four suffice.
In order to optimize the basal rates, the subject wears a continuous glucose monitoring system for a period of three days. Such a system records the blood glucose concentration every five minutes, thus providing a full history of the glucose changes. During this time they skip a meal each day, with the purpose of obviating the effects of the prandial insulin bolus and the glucose appearing from the corresponding meal. Basal infusion rates are adjusted to target a fasting blood glucose of 90 mg/dL. The process is repeated as necessary until fasting levels, in the absence of a meal, are at the target level [36].
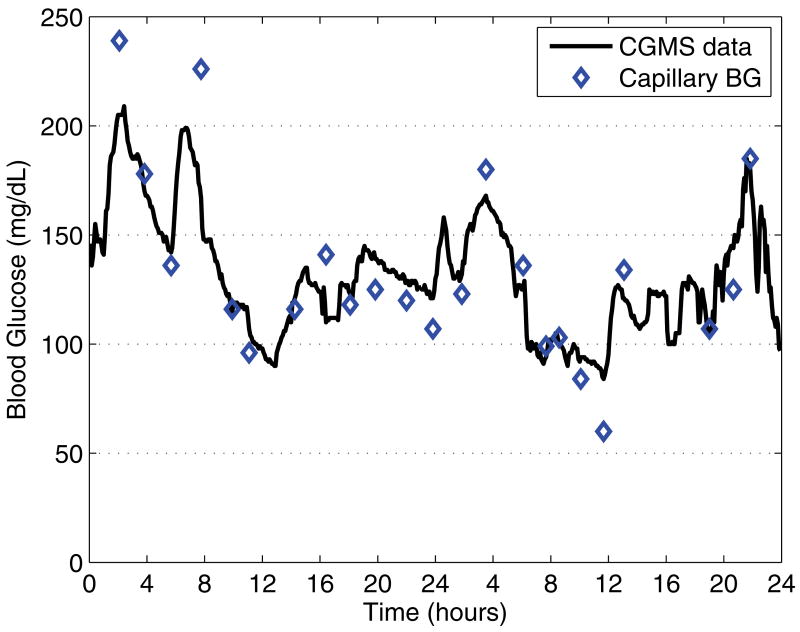
Figure 1 shows a two-day sequence before the basal rate is adjusted, with the subject skipping breakfast on the second day. The hyperglycemic excursion during the course of the night indicates that the overnight basal infusion rate is too low (midnight to 4:00), while the morning segment (4:00 to 10:00) is within the clinically acceptable range. Figure 2, first panel, shows the overnight segment, and again no breakfast in the morning. This figure also shows the effects of dinner and the three meals on the following day, highlighting why manual adjustment requires meals to be skipped.
Fig. 1.
Two-day sequence (starting at midnight) of CGMS blood glucose data before the adjustment of basal rates; the subject skipped breakfast on the second day. The hyperglycemic excursion during the night indicates that the basal infusion rate for this period is too low, while the morning segment of the second day is correct. Diamonds indicate the capillary blood glucose calibration measurements.
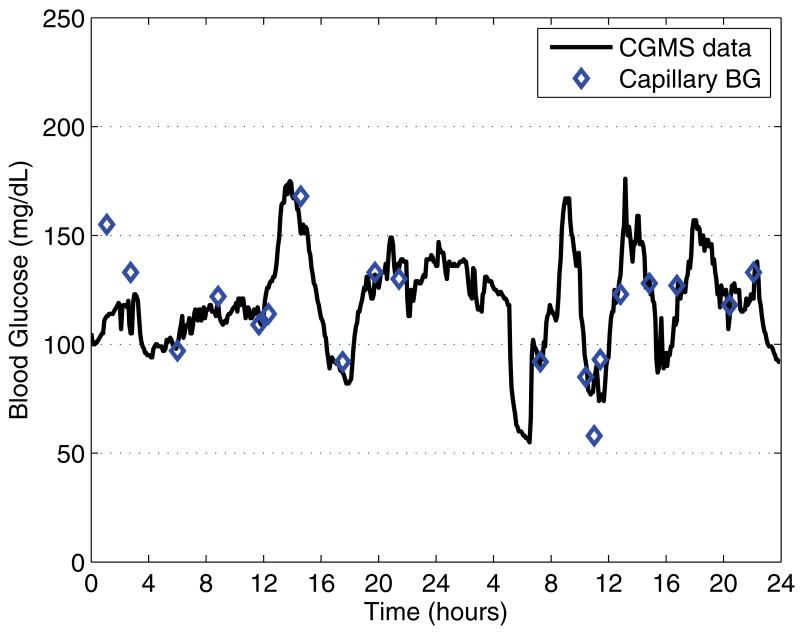
Fig. 2.
Two-day sequence (starting at midnight) of CGMS blood glucose data after the adjustment of basal rates; the subject skipped breakfast on first day. Nighttime and morning basal rates are correctly set. Diamonds indicate the capillary blood glucose calibration measurements.
Once these basal infusion rates are set, they require periodic re-adjustment. Sometimes a person will realize that their blood glucose levels have not been under control, and at that point will seek assistance in adjusting their dosing. In the meantime, they have gone for several days with less than optimal glycemic control.
3 Run-to-Run Control
Taking the clinical heuristic as a starting point, we have adapted a run-to-run control algorithm to adjust basal insulin infusion rates using only sparse blood glucose measurements. The strategy is adapted from the chemical process industry [38], in the same spirit as the insulin-to-carbohydrate ratio adjustment algorithms previously developed [30]–[35].
The standard run-to-run control algorithm [38] consists of the following steps:
-
Parameterize the input profile for run k, uk(t), as U(t, vk). Also consider a sampled version, ψk, of the output yk(t), such that it has the same dimension as the manipulated variable vector vk. Thus we have
Choose an initial guess for vk (when k = 1).
Complete the run using the input uk(t) corresponding to vk. Determine ψk from the measurements yk(t).
-
Update the input parameters as
where K is an appropriate gain matrix and ψr represents the reference values to be attained. Increment k for the next run, and repeat steps 3–4 until convergence.
The algorithm allows for individualizing the number of segments and the corresponding timing. Using the run-to-run strategy, each day is considered a run. For the current day, a performance measure is calculated for each segment and then used to automatically adjust the amount of insulin for each corresponding segment on the following day. Because the algorithm adjusts based on the glucose measurements at the end of a segment, the number of segments dictates the number of glucose measurements required. These measurements drive the algorithm to maintain, increase or decrease the amount of insulin infused for the corresponding segment on the following day. This dose correction process is designed to be independent of physician or subject intervention.
In the absence of meals or other events affecting blood glucose levels (e.g., exercise), the basal insulin infusion rate must keep the subject's blood glucose in the normal range of 70–110 mg/dl. If the amount of insulin infused over the time segment is too low, the blood glucose level will rise above the desired range. If the basal dose is too large, it will tend to lower blood glucose into the hypoglycemic range.
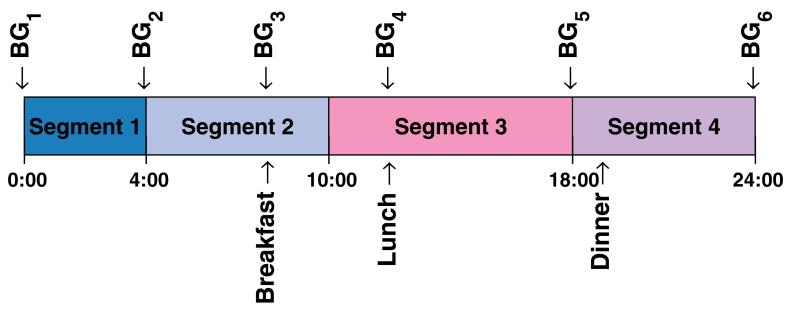
Given that the number of blood glucose measurements has to be minimized for the strategy to be practical, these have to be taken at strategic times in relation to the segments chosen. Figure 3 illustrates this. For segment one, adjustment is made using measurements at start and end of the segment (BG1 & BG2). Segment two is adjusted with the measurement right before breakfast (BG3), in order to avoid the prandial glucose excursion, and the measure at the start of the segment (BG2). In the case of segment three, the first measurement (BG4) is delayed to the start of lunch to avoid effects from breakfast; this also minimizes number of fingersticks needed. Finally, for segment four, the first measurement is not delayed, as BG5 is also used for the end of segment three; the ending measurement (BG6) is also (BG1) for the following day.
Fig. 3.
Nominal basal segments for a day, indicating meal and blood glucose measurement times.
Regardless of the blood glucose level coming into a segment, the perfect basal insulin dose should keep this level constant during the segment. The deviation
| (4) |
where G is the corresponding blood glucose measurement, is the performance measure that is used as the controlled variable (ψ). The controlled variable vector (assuming four segments) is then
| (5) |
where k is the current day.
Using the above formulation, there exist multiple “perfect” basal doses, which will keep blood glucose at a different target over the segment. Because of this, a second controlled variable vector is set to
| (6) |
where
| (7) |
such that the desired fasting blood glucose can be targeted.
The input parameters (the basal infusion rate for each segment) are then updated using
| (8) |
where K1 and K2 are gain matrices, and ψr = 0 mg/dl and ϕr = 80 mg/dl are the reference target values. The new doses are implemented the following day, and the procedure repeated.
In cases when there are too many complicating events to capture the effect of the basal rate, the adjustment for that day can be omitted. As the initial guess for vk at k = 0, the same settings as in the manual adjustment procedure (3) can be used.
Given that the insulin requirement to maintain blood glucose constant for a given segment is independent of the other segments, it is appropriate for the gain matrix K1 to be designed as a diagonal matrix. For the same reasons, the gain matrix K2 is also designed as a diagonal matrix. The tuning for each gain matrix can then be done independently for each segment, allowing for the possibility that different levels of aggressiveness in the adjustments be allowed for different times of day.
The algorithm (8) is guaranteed to yield a single optimum solution. Because the start of a segment coincides with the end of the previous segment, this provides a sufficient constraint to guarantee a unique solution at steady-state, which is a blood glucose at the desired reference value (see appendix for proof).
4 Simulation Model
Currently, there are no published models of insulin-glucose dynamics that incorporate the circadian variation of insulin sensitivity, nor the changing insulin requirements due to changing levels of physical activity. Since the run-to-run control strategy proposed is meant to compensate for precisely these factors, one of the current models was modified to incorporate an approximation of such effects.
The model used is that of Hovorka et al. [7], replacing the subcutaneous insulin infusion model with that presented in [39]. The core of the model consists of two compartments for glucose (plasma and tissue) and one compartment for plasma insulin. The model divides the action of insulin on glucose kinetics into three additional components (x1 for the effects of insulin on glucose distribution/transport, x2 for glucose disposal, and x3 for endogenous glucose production), which are described by
| (9a) |
| (9b) |
| (9c) |
where the parameters kb1, kb2, and kb3 correspond to the insulin sensitivity of each mechanism of action of insulin, and I(t) is the plasma insulin concentration.
The circadian change in insulin sensitivity has been modeled by introducing a modulating gain which affects all sensitivities equally. This is introduced by replacing I(t) in equation 9 with I′(t) as given by
| (10) |
where the modulating gain km(t) is given, in the Lapalce domain, by
| (11) |
with the input kp(t) being a step profile corresponding to the desired gain for each segment. Given that the model is now time-varying with respect to time of day, t = 0 is set to correspond to midnight. The dynamics are set according to expected behavior based on what is known of the underlying physiology. The delay introduced in the input (τd) is relative to when the infusion profile changes, as the change in sensitivity is known to start earlier. This parameter is optimized such that the desired infusion profile maintains the blood glucose concentration as close to 80 mg/dl as possible, in the absence of any simulated meals.
A set of cases was created from actual subject pump infusion profiles. We obtained 13 manually optimized basal infusion profiles from a group of subjects [36]. Table 1 shows the multipliers for each segment assuming that the basal insulin dose requirement has been calculated according to the clinical guidelines (equation 2). In all cases the timing for the segments is the same as the nominal (i.e., midnight to 4:00, 4:00 to 10:00, 10:00 to 18:00 and 18:00 to midnight). The delay τd was optimized independently for each case. The median delay was 11 min, with a range of 10.4–16.7 min.
Table 1.
For the standard segments of the day, 13 cases were created based on subject data that had their basal rates manually optimized in a previous study [36].
| Case | Segment 1 | Segment 2 | Segment 3 | Segment 4 |
|---|---|---|---|---|
|
| ||||
| 1 | 0.91 | 1.52 | 1.52 | 1.60 |
| 2 | 0.73 | 1.02 | 0.73 | 0.87 |
| 3 | 0.69 | 1.37 | 0.91 | 1.26 |
| 4 | 0.72 | 1.44 | 1.04 | 0.96 |
| 5 | 0.29 | 0.87 | 0.87 | 0.87 |
| 6 | 0.79 | 1.35 | 0.79 | 1.13 |
| 7 | 0.99 | 1.48 | 1.38 | 1.58 |
| 8 | 1.08 | 2.27 | 1.95 | 2.38 |
| 9 | 0.89 | 0.98 | 0.71 | 0.53 |
| 10 | 0.29 | 0.91 | 0.29 | 0.34 |
| 11 | 0.60 | 1.42 | 1.20 | 1.05 |
| 12 | 0.53 | 1.60 | 1.00 | 0.71 |
| 13 | 0.87 | 1.03 | 0.87 | 0.87 |
One of the limitations of the Hovorka et al. [7] model is the component that describes the absorption of glucose from the consumption of a mixed meal. In daily living conditions, with the correct bolus dose of insulin to cover a meal, the blood glucose concentration is expected to return to basal levels within two to three hours after the start of the meal [31], [40]. The dynamics of the model for this case are much slower than this, resulting in extremely long settling times that are not physiologically correct. For this reason, the time constants for the absorption part of the model have been modified such that the settling time is more consistent with the subject data. This results in an initial rate of change that is too aggressive, but this will not affect the results of the simulations presented, as no blood glucose measurements are used within the first two hours of any postprandial period. The parameter in the Hovorka et al. [7] model that was changed is t2max,G, from a value of 40 min to a value of 5 min.
5 Results
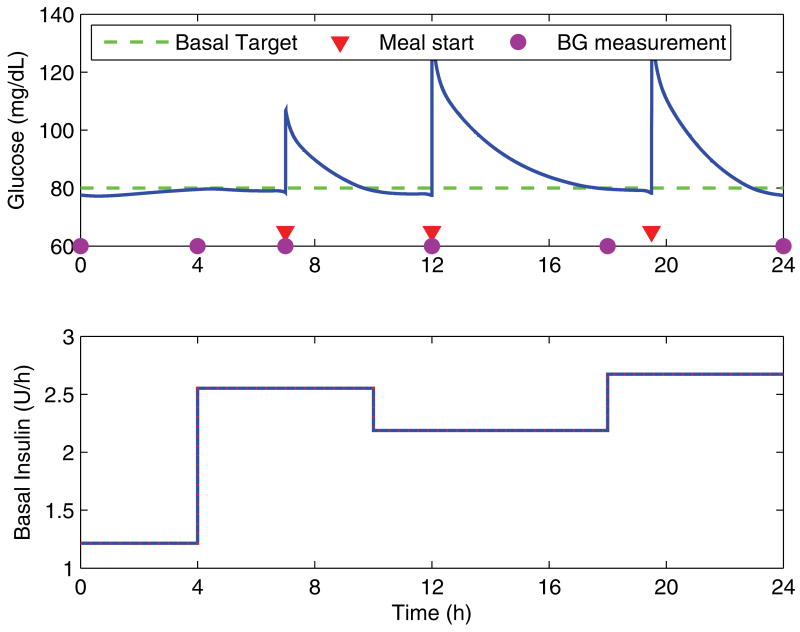
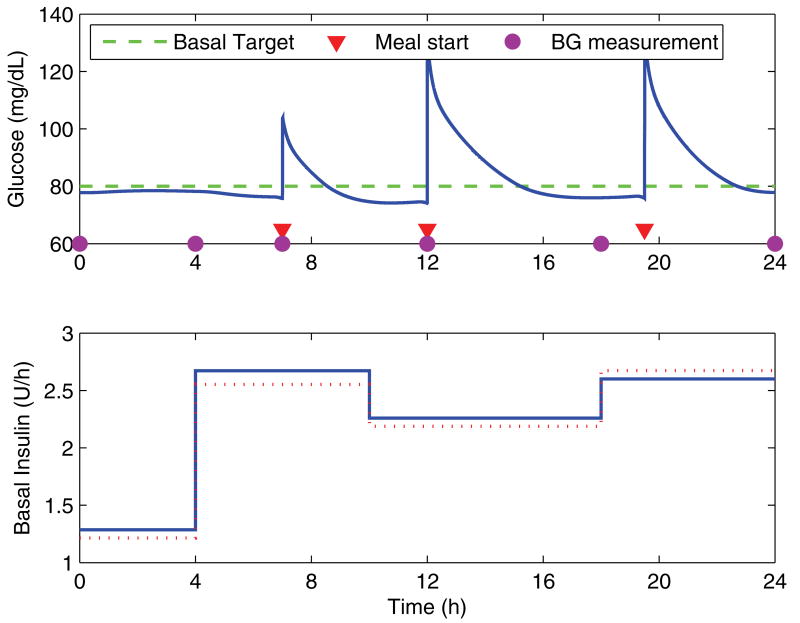
Figure 4 shows the simulation over a day using the correct basal and prandial dosing, which illustrates the adjusted timing of the blood glucose measurements in order to minimize the effects of the meals. For all cases, the algorithm is initialized using the clinical heuristics (equation 3), with the total basal dose calculated from the actual requirements according to the model (rounded down to the nearest whole unit of insulin). Meals are assumed to be the same from day to day, and the dose of the prandial insulin bolus to be correctly matched. There are constraints on the rate of change of the dosing for any given segment from one day to the next, with a 20% maximum increase and a 30% maximum decrease.
Fig. 4.
Blood glucose response over a day with the corresponding basal infusion profile. The circles indicate the timepoints at which blood glucose measurements are taken for use by the algorithm, and the triangles indicating the starting time of meals.
Clinically, it is desirable to have the basal infusion rates converge within a time frame of seven days. Therefore, the gains of the run-to-run controller were manually tuned to meet this specification. For the simulations, the gain matrices were set to be Kj = kjI, j = 1, 2, where kj is a scalar, and I the identity matrix, which sets the same tuning for all segments. The gains were set to k1 = 0.01 and k2 = 0.0067. Setting k2 to be smaller than k1 is desirable, as converging to the desired blood glucose level is a secondary objective to maintaining blood glucose steady throughout the corresponding segment.
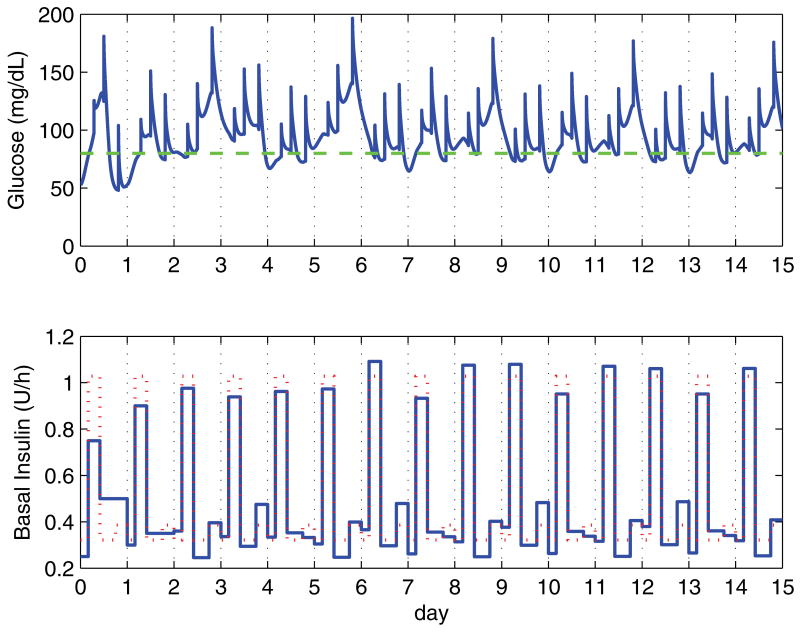
For the 13 cases, the algorithm was considered to have converged once the basal profile was within 10% of the actual profile for all four segments. On average, the algorithm converged in 6.3 days, with a standard deviation of 2.1 days. There is one case (number ten) which does not converge to within 10%, but does so within 15%. With the 15% metric, all cases converge, with an average of 5.1 days and a standard deviation of 2.3 days. Case ten is a particularly challenging scenario, as the subject has a very high insulin sensitivity, except during the 4:00 to 10:00 segment, in which the insulin requirement is 2.7 to 3.1 times higher than for the other segments. Over all the cases, the constraints are rarely active, with the exception of case ten. Overall, the median for the number of times a constraint is active over 15 days of simulation is one time, with a range of 0 to 14.
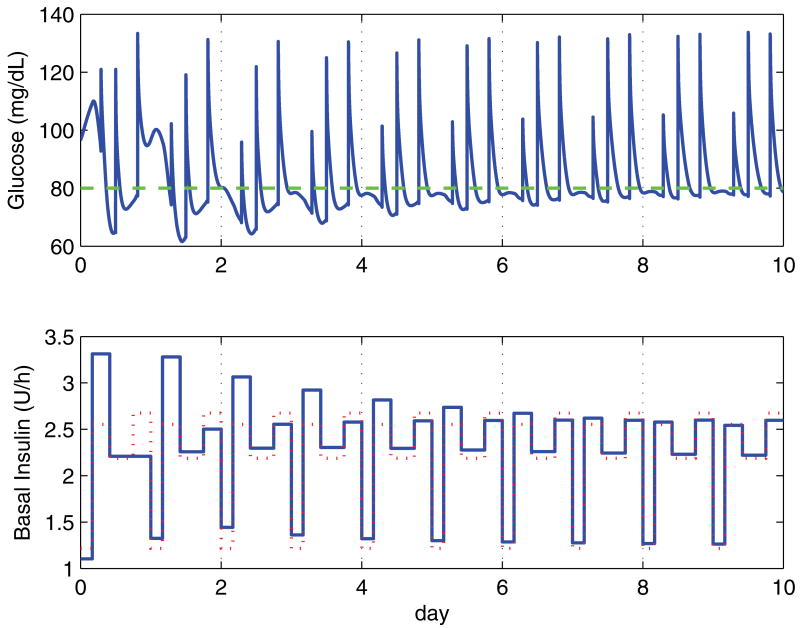
Figure 5 shows the simulation for a representative case (number eight) over 10 days. This case takes five days to converge, and has no active constraints. Figure 6 shows day seven of this run; this is the same profile as the perfect day example shown in figure 4. The reason the profile does not converge perfectly to the underlying profile is due in large measure to the remaining effects of the meals, which are not completely eliminated even after several hours.
Fig. 5.
Blood glucose response over 10 days with the corresponding basal infusion profile for case eight. The dotted line in the insulin plot indicates the optimal basal infusion profile. Initial basal rates are set according to the clinical heuristics, with segment one slightly underdosed, segment two is overdosed, segment three is very close to target, and segment four is underdosed. These settings result in periods of hyper- and hypoglycemia, with levels converging to clinically acceptable levels by the fifth day.
Fig. 6.
Blood glucose response for day seven of the simulation run for case eight. The dotted line in the insulin plot indicates the optimal basal infusion profile. The blood glucose profile shows good glycemic control over the day, with the basal infusion rates being very close to the optimal rates.
Case ten is shown in figure 7 for a simulation run of 15 days. Even small changes in the basal profile from one day to the next, compounded with the deviations introduced by the meals, introduce large changes in the blood glucose response. In this case, an unconstrained design would drive the subject to frequent hypoglycemia. De-tuning the controller would alleviate this issue, but at the expense of a very sluggish response for the remaining 12 cases.
Fig. 7.
Blood glucose response over 15 days for the worst case (number ten). The initial setting based on the clinical heuristics are significantly off, with the subject showing high insulin sensitivity in all but the morning segment, which has significantly higher insulin requirements. The dotted line in the insulin plot indicates the optimal basal infusion profile.
6 Conclusions
Adjusting basal and prandial insulin dosing is a daily chore for people with type 1 diabetes. The success in developing an algorithm to adjust prandial insulin dosing based on medical expertise [31], [35], [41] has further motivated the development of a similar strategy for basal insulin dosing.
A major hurdle has been the lack of a mathematical model that incorporates the dynamics associated with the circadian changes in basal insulin requirements. We presented a first approximation of such a model by using the manually optimized profiles of a group of subjects. Although not based on the underlying physiology, it serves the purpose of challenging the algorithm appropriately.
Using only five, properly timed, blood glucose measurements, we have shown that the proposed run-to-run algorithm can successfully adjust basal infusion rates for the nominal four segments. Different daily segments can be easily accommodated by the algorithm, requiring only the proper timing for the blood glucose measurements. Being able to make dose adjustment decisions even in the presence of meals is a significant improvement over current clinical strategies. Even with the error introduced by the tail end of the meal response, basal rates converge in less than a week on average.
In the clinical testing of the algorithm the additional challenge will be that the meal related insulin dose might not be correct either. To avoid this potential confounding factor, initial testing can be done by skipping meals as in the manual adjustment protocol. Eventually, a strategy that combines the adjustment of basal and bolus insulin dosing will be required. Appropriate heuristics will need to be incorporated, as the interplay between these two components of insulin therapy are not negligible.
The benefits of tight glycemic control are significant, and a run-to-run framework for the adjustment of basal and bolus insulin dosing can be an important tool in accomplishing this goal.
Acknowledgments
This work was supported by the National Institutes of Health, grants R01-DK068706 and R01-DK068663.
A Appendix
Without loss of generality, we can assume the four standard segments, with the initial and ending blood glucose measurements coinciding for the end of a segment and the beginning of the next one. Thus segment one uses G1k and G2k, segment two uses G2k, and G3k and so on.
To simplify the notation, we use deviation variable form, thus
| (A.1) |
and the update law in deviation variable form, with the references being zero, is
| (A.2) |
and without loss of generality, we can assume Kj = kjI, j = 1, 2, where kj is a scalar, and I the identity matrix.
We know that once steady-state is reached, there will be no further corrections, thus vk+1 = vk. For now drop the k subscript as we refer to the same day. Looking at just the first segment, we have
| (A.3) |
| (A.4) |
and let
| (A.5) |
then G2d = fG1d. Similarly, we have
| (A.6) |
| (A.7) |
| (A.8) |
Since G5d = G5 − ϕr and G1d = G1 − ϕr, we have
| (A.9) |
| (A.10) |
| (A.11) |
but G5 is the same as G1 on the following day, thus
| (A.12) |
and if we are indeed in steady state, then G1k+1 = G1k, thus
| (A.13) |
| (A.14) |
thus we have only one solution, which holds for the other segment endpoints as well. It is straightforward to show that this still holds true when the measurements do not coincide, as illustrated in the case shown in figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cesar C. Palerm, Email: palerm@ieee.org.
Howard Zisser, Email: hzisser@sansum.org.
Lois Jovanovič, Email: ljovanovic@sansum.org.
Francis J. Doyle, III, Email: frank.doyle@icb.uscb.edu.
References
- 1.Eiselein L, Schwartz HJ, Rutledge JC. The challenge of type 1 diabetes mellitus. ILAR J. 2004;45(3):231–236. doi: 10.1093/ilar.45.3.231. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Diabetes (fact sheet no. 312) 2006 September; Retrieved on 11 January 2007 from WHO Web site: http://www.who.int/mediacentre/factsheets/fs312/en/
- 3.Gale EAM. Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia. 2005;48(12):2445–2450. doi: 10.1007/s00125-005-0028-z. [DOI] [PubMed] [Google Scholar]
- 4.Weissberg–Benchell J, Antisdel–Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26:1079–1087. doi: 10.2337/diacare.26.4.1079. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT. Glycemic control and cardiovascular disease—should we reassess clinical goals? N Engl J Med. 2005;353(25):2707–2709. doi: 10.1056/NEJMe058282. [DOI] [PubMed] [Google Scholar]
- 7.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–20. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 8.Lynch SM, Bequette BW. Model predictive control of blood glucose in type I diabetics using subcutaneous glucose measurements. Proceedings of the American Control Conference; 2002. pp. 4039–4043. [Google Scholar]
- 9.Parker RS, Doyle FJ, III, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 10.Schaller HC, Schaupp L, Bodenlenz M, Wilinska ME, Chassin LJ, Wach P, Vering T, Hovorka R, Pieber TR. On-line adaptive algorithm with glucose prediction capacity for subcutaneous closed loop control of glucose: evaluation under fasting conditions in patients with type 1 diabetes. Diabet Med. 2006;23(1):90–93. doi: 10.1111/j.1464-5491.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo Y, Shimoda S, Sakakida M, Nishida K, Sekigami T, Ichimori S, Ichinose K, Shichiri M, Araki E. Strict glycemic control in diabetic dogs with closed-loop intraperitoneal insulin infusion algorithm designed for an artificial endocrine pancreas. J Artif Organs. 2003;6(1):55–63. doi: 10.1007/s100470300009. [DOI] [PubMed] [Google Scholar]
- 12.Sekigami T, Shimoda S, Nishida K, Matsuo Y, Ichimori S, Ichinose K, Shichiri M, Sakakida M, Araki E. Comparison between closed-loop portal and peripheral venous insulin delivery systems for an artificial endocrine pancreas. J Artif Organs. 2004;7(2):91–100. doi: 10.1007/s10047-004-0251-2. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda S, Nishida K, Sakakida M, Konno Y, Ichinose K, Uehara M, Nowak T, Shichiri M. Closed-loop subcutaneous insulin infusion algorithm with a short-acting insulin analog for long-term clinical application of a wearable artificial endocrine pancreas. Front Med Biol Eng. 1997;8(3):197–211. [PubMed] [Google Scholar]
- 14.Marchetti G, Barolo M, Jovanovic L, Zisser H, Seborg D. An improved pid switching control strategy for type 1 diabetes. Engineering in Medicine and Biology Society, 2006. EMBS '06. 28th Annual International Conference of the IEEE; 2006. pp. 5041–5044. [DOI] [PubMed] [Google Scholar]
- 15.Panteleon AE, Loutseiko M, Steil GM, Rebrin K. Evaluation of the effect of gain on the meal response of an automated closed-loop insulin delivery system. Diabetes. 2006;55(7):1995–2000. doi: 10.2337/db05-1346. [DOI] [PubMed] [Google Scholar]
- 16.Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery-the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125–44. doi: 10.1016/j.addr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Steil GM, Saad MF. Automated insulin delivery for type 1 diabetes. Curr Opin Endocrinol Diabetes. 2006;13(2):205–211. [Google Scholar]
- 18.Parker RS, Doyle FJ, III, Ward JH, Peppas NA. Robust H∞ glucose control in diabetes using a physiological model. AIChE Journal. 2000;46(12):2537–2549. [Google Scholar]
- 19.Ruiz-Velzquez E, Femat R, Campos-Delgado DU. Blood glucose control for type I diabetes mellitus: A robust tracking H∞ problem. Control Eng Pract. 2004;12(9):1179–1195. [Google Scholar]
- 20.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 21.Beyer J, Schrezenmeir J, Schulz G, Strack T, Kstner E, Schulz G. The influence of different generations of computer algorithms on diabetes control. Comput Methods Programs Biomed. 1990;32(3–4):225–232. doi: 10.1016/0169-2607(90)90104-h. [DOI] [PubMed] [Google Scholar]
- 22.Chanoch LH, Jovanovic L, Peterson CM. The evaluation of a pocket computer as an aid to insulin dose determination by patients. Diabetes Care. 1985;8(2):172–176. doi: 10.2337/diacare.8.2.172. [DOI] [PubMed] [Google Scholar]
- 23.Chiarelli F, Tumini S, Morgese G, Albisser AM. Controlled study in diabetic children comparing insulin-dosage adjustment by manual and computer algorithms. Diabetes Care. 1990;13(10):1080–1084. doi: 10.2337/diacare.13.10.1080. [DOI] [PubMed] [Google Scholar]
- 24.Jovanovic L, Peterson CM. Home blood glucose monitoring. Compr Ther. 1982;8(1):10–20. [PubMed] [Google Scholar]
- 25.Peters A, Rübsamen M, Jacob U, Look D, Scriba PC. Clinical evaluation of decision support system for insulin-dose adjustment in IDDM. Diabetes Care. 1991;14(10):875–880. doi: 10.2337/diacare.14.10.875. [DOI] [PubMed] [Google Scholar]
- 26.Peterson CM, Jovanovic L, Chanoch LH. Randomized trial of computer-assisted insulin delivery in patients with type I diabetes beginning pump therapy. Am J Med. 1986;81(1):69–72. doi: 10.1016/0002-9343(86)90184-1. [DOI] [PubMed] [Google Scholar]
- 27.Schiffrin A, Mihic M, Leibel BS, Albisser AM. Computer-assisted insulin dosage adjustment. Diabetes Care. 1985;8(6):545–552. doi: 10.2337/diacare.8.6.545. [DOI] [PubMed] [Google Scholar]
- 28.Schrezenmeir J, Dirting K, Papazov P. Controlled multicenter study on the effect of computer assistance in intensive insulin therapy of type 1 diabetics. Comput Methods Programs Biomed. 2002;69(2):97–114. doi: 10.1016/s0169-2607(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 29.Skyler JS, Skyler DL, Seigler DE, O'Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311–318. doi: 10.2337/diacare.4.2.311. [DOI] [PubMed] [Google Scholar]
- 30.Owens CL, Zisser H, Jovanovic L, Srinivasan B, Bonvin D, Doyle FJ., III Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans Biomed Eng. 2006;53(6):996–1005. doi: 10.1109/TBME.2006.872818. [DOI] [PubMed] [Google Scholar]
- 31.Palerm CC, Zisser H, Bevier WC, Jovanovič L, Doyle FJ., III Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30(5):1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 32.Palerm CC, Zisser H, Jovanovic L, Doyle FJ., III Flexible run-to-run strategy for insulin dosing in type 1 diabetic subjects. Proceedings of the International Symposium on Advanced Control of Chemical Processes; Gramado, Brazil. 2006. pp. 521–526. [Google Scholar]
- 33.Palerm CC, Zisser H, Jovanovič L, Doyle FJ., III A run-to-run framework for prandial insulin dosing: handling real-life uncertainty. Int J Robust Nonlin. 2007;17(13):1194–1213. [Google Scholar]
- 34.Zisser H, Jovanovic L, Doyle F, III, Ospina P, Owens C. Run-to-run control of meal-related insulin dosing. Diabetes Technol Ther. 2005;7(1):48–57. doi: 10.1089/dia.2005.7.48. [DOI] [PubMed] [Google Scholar]
- 35.Palerm CC, Zisser H, Bevier W, Doyle FJ, III, Jovanovič L. Improved clinical outcome using sparse measurements and run-to-run control for prandial insulin dosing. Diabetes Technology Meeting; Atlanta, GA, USA. 2006. p. A127. [Google Scholar]
- 36.Zisser H, Bevier WC, Jovanovič L. Restoring euglycemia in the basal state using continuous glucose monitoring in subjects with type 1 diabetes mellitus. Diabetes Technol Ther. doi: 10.1089/dia.2007.0220. submitted. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic L. Optimizing insulin therapy in patients with diabetes, CME Activity jointly sponsored by Washington Hospital Center and MedStar Research Institute. 2002. Insulin therapy and algorithms for treating type 1 diabetes mellitus; pp. 13–19. [Google Scholar]
- 38.Srinivasan B, Primus CJ, Bonvin D, Ricker NL. Run-to-run optimization via control of generalized constraints. Control Eng Pract. 2001;9(8):911–919. [Google Scholar]
- 39.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005;52(1):3–12. doi: 10.1109/TBME.2004.839639. [DOI] [PubMed] [Google Scholar]
- 40.Bevier WC, Zisser H, Palerm CC, Finan DA, Seborg DE, Doyle FJ, III, Okada Wollitzer A, Jovanovič L. Calculating the insulin to carbohydrate ratio using the hyperinsulinemic euglycemic clamp — a novel use for a proven technique. Diabetes Metab Res Rev. doi: 10.1002/dmrr.727. in press. [DOI] [PubMed] [Google Scholar]
- 41.Palerm CC, Zisser H, Bevier WC, Jovanovič L, Doyle FJ., III . Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. In: Peppas NA, Hoffman AS, Kanamori T, Tojo K, editors. Advances in Medical Engineering, Drug Delivery Systems and Therapeutic Systems. AIChE; New York, NY, USA: 2006. pp. 115–119. [Google Scholar]