Abstract
Highly active antiretroviral therapy (HAART) has dramatically altered the spectrum of morbidity and mortality in HIV-infected patients. This has been attributed to improvements in the lung microenvironment leading to enhanced pulmonary immunity, either by preventing the progressive loss of immune function or by actually promoting immune restoration. However, these changes have been accompanied by the recognition of new pulmonary complications in HIV-infected subjects, especially those associated with immune reconstitution. In this review we will describe how HIV infection alters the normal pulmonary environment, highlight the effect of HAART on these perturbations, and discuss potential complications of HAART in the lung, focusing on the pulmonary immune reconstitution inflammatory syndrome.
Introduction
The World Health Organization estimates that 34-46 million people worldwide are infected with HIV [1]. Pulmonary disease is a major source of morbidity and mortality in these patients. In the pre-HAART era over 60% of patients presented to a physician at some point during their illness with associated lung disease [2, 3]. Infections appear to occur in an orderly fashion, with increased susceptibility to common pathogens occurring earlier than susceptibility to opportunistic pathogens, which generally reflects substantial impairment of the host immune response. However, the development of highly active antiretroviral therapy (HAART) has greatly influenced the morbidity and mortality of HIV infection. This has generally been attributed to improvements in immunologic function, either by preventing the progressive loss of immunity in HIV infection or by actually promoting immune reconstitution. While the effects of HAART are well described in the vascular compartment, their effects at the tissue level, including the lung, are just beginning to be understood. In this review we will describe how HIV infection alters the normal pulmonary environment, highlight the effect of HAART on these perturbations, and discuss potential complications of HAART in the lung, focusing on the pulmonary immune reconstitution inflammatory syndrome.
Effect of HIV on Pulmonary Immune Responses
The respiratory tract from the oropharynx to the alveoli serves as an interface between the host and the environment. Thus pulmonary immune responses are felt to represent a form of mucosal immunity. Pulmonary immunity can be divided into innate and acquired responses. Most pathogens gaining access to the respiratory tract are phagocytosed by alveolar macrophages (AM), the principal arm of innate immunity. Importantly, AM phagocytosis of most foreign material gaining access to the alveolar space does not result in an inflammatory response due to the general immunosuppressive properties of alveolar macrophages [4]. This results in a generalized paucity of lung inflammation under normal conditions, allowing gas exchange to occur unimpeded.
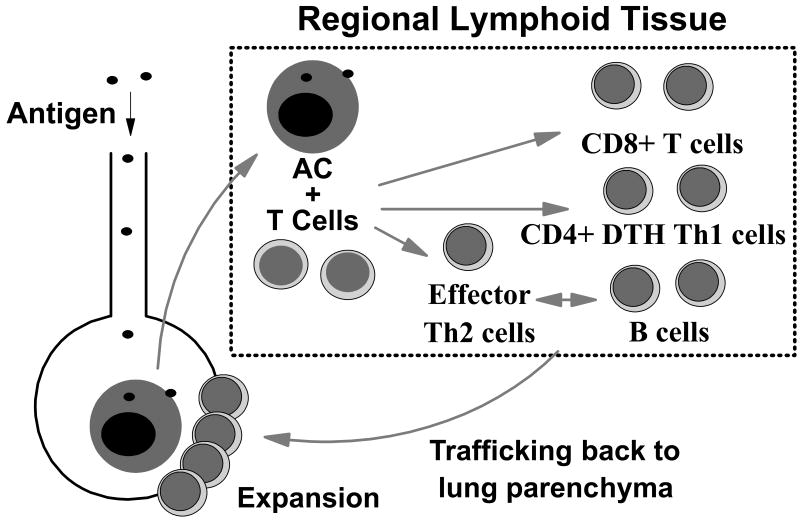
Failure of innate host defenses leads to persistence of antigen in the respiratory tract and initiation of a specific acquired immune response. This process involves an intricate network of positive and negative feedback loops between antigen presenting cells (i.e. alveolar macrophages, dendritic cells), B lymphocytes, and T lymphocytes. As in other lymphoid tissues, the primary immune response does not occur at the site of initial challenge. Rather, new antigen is taken up, processed, and transported by accessory cells to regional lymphoid tissue [5] (Figure 1). There antigen is presented to naïve CD4 T lymphocytes to form specific effector Th2 cells that are important in the generation of antigen-specific B lymphocytes (humoral immune response), Th1 T cells involved in delayed type hypersensitivity (DTH) reactions, and CD8 cytotoxic T cell (CTL) responses. These cells then must traffic back to the site of initial challenge, in this case the alveolar space, under the control of local chemokine production in the lung [6]. During the initial antigenic response, memory B and T cells are also created which allow the host to respond more rapidly upon re-exposure to the same antigen. Importantly, memory cells make up the predominant resident lymphocyte population in the normal lung [7].
Figure 1.
Normal pulmonary immune responses. Antigen reaching the lower respiratory tract which is not cleared by phagocytosis is taken up, processed, and transported by accessory cells to regional lymphoid tissue. There antigen is presented to naïve CD4 T lymphocytes to form CD4 Th2 T cells for B cell help, CD4 Th1 delayed type hypersensitivity T cells, and CD8 cytotoxic T cells. These effector cells then traffic back to the site of initial challenge in the lung where they can undergo further expansion in situ.
HIV infection impacts all components of the pulmonary immune response. The end result is a generalized state of cellular activation and accumulation of immune cells and pro-inflammatory mediators in the alveolar space. Interestingly, this does not appear to be due to defective alveolar macrophage phagocytic function as alveolar macrophages from HIV-infected subjects are not defective in their ability to ingest pneumococcus opsonized with pooled IgG [8], mycobacteria [9], or Cryptococcus [10]. This could reflect chronic macrophage activation in HIV-infected subjects due to persistent levels of interferon-gamma (IFN-γ) in the alveolar space [9, 11, 12]. In fact, virtually every macrophage and lymphocyte cytokine studied to date is found in increased concentrations in bronchoalveolar lavage [13], further supporting the presence of chronic immune activation and inflammation in the lungs of these subjects.
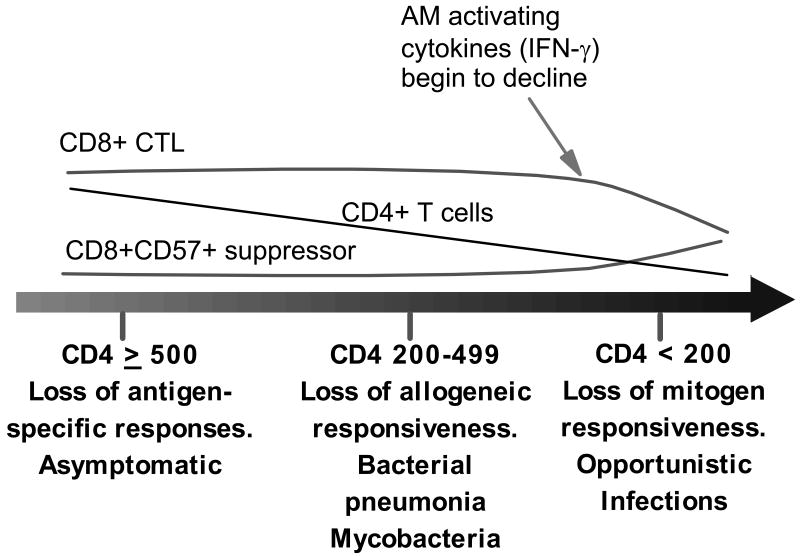
While one might hypothesize that chronic immune activation should protect against pulmonary infections, the opposite is true. In contrast to innate phagocytic function, acquired pulmonary immune defects are clearly present in HIV infection and appear to progress in an orderly fashion (Figure 2). Early studies demonstrated that the rapid decline in the percentage of peripheral blood CD4 cells in HIV infection is mirrored in the alveolar space [14]. Chronic macrophage activation may actually contribute to the loss of CD4 cells in the lung by promoting HIV infection in T cells through several mechanisms including direct infection of T cells during AM-T cell interactions and through secondary activation of T cells, which by itself renders them more susceptible to HIV infection [15]. Since memory CD4 T cells are preferentially infected with HIV [16], antigen-specific responses are lost early in HIV infection, with losses of less stringent allogeneic and mitogenic T cell responses occurring later as disease progresses. This could explain why susceptibility to common bacterial pathogens occurs earlier than opportunistic infections, which require more profound immunosuppression. Concomitant with a decrease in CD4 T cells, there is an increase in the number and percentage of CD8+ cells. The predominant CD8+ cell in the alveolar space during the course of HIV infection is the cytotoxic T lymphocyte, mostly antigen-specific CTL against HIV infected cells [17]. We have speculated this represents an appropriate in situ immune response to HIV antigen in the lung. Importantly, CTL are an abundant source of the macrophage activating cytokine IFN-γ [12, 18]. However, with HIV disease progression there is a gradual loss of the CTL population, which is replaced by CD8+ CD57+ cells with immune suppressive properties [19]. Since IFN-γ secretion by CD8+ CTL makes up for the decline in CD4+ IFN-γ secreting memory cells, this cytokine remains elevated in the alveolar space until late stage HIV infection. Only then, as the number of HIV-specific CTL decline, does the concentration of this important macrophage activating cytokine also decline. Thus late stage HIV infection is characterized by a loss of CD4+ cells in the lung, an accumulation of immune suppressive CD8+ cells in its place, and a loss of IFN-γ in the alveolar space. All of these defects contribute to decreased phagocytic function and defects in acquired immunity in late stage disease.
Figure 2.
Changes in the alveolar space with HIV progression. Over time there is a progressive loss of CD4 T cells (especially memory T cells leading to early loss of antigen-specific responses), loss of CD8+CD57- cytotoxic T lymphocyte (CTL), and an increase in CD8+ CD57+ cells with immune suppressive properties. As CD4 T cells and CD8 T cells decrease in late stage infection IFN-γ declines resulting in decreased phagocytic function and more advanced defects in acquired immunity.
Despite the emphasis on cellular immune defects in HIV infection, it was soon recognized that these subjects also have significant perturbations in humoral immunity. HIV infection results in increased numbers of activated circulating B cells producing non-specific IgG [20] likely driven by an altered cytokine milieu [21]. High immunoglobulin concentrations are found in the lung of HIV-infected subjects [22, 23]. However, the antibody that is produced appears to have defective opsonic function, including activity against pneumococcus [24, 25]. Thus these patients are also susceptible to pathogens controlled by antibody production, especially bacterial infections.
Effects of HAART on Pulmonary Immunity
HAART is effective in reducing plasma HIV viral loads and returning many of the cellular abnormalities in blood to normal [26]. The effect of HAART on tissue viral loads is less well defined, primarily because different tissue compartments behave differently in response to HIV infection as well as in response to therapy. In lymph nodes HAART induces early rapid decline in detectable HIV RNA followed by a slower decay [27], kinetics similar to that found in blood. The virologic response is associated with decreased cellular proliferation and downregulation of inflammatory genes [28]. The GI tract appears to be very sensitive to HIV infection, with rapid loss of CD4+ T cells in gut-associated lymphoid tissue (GALT) [29]. Furthermore, HAART-induced suppression of viral replication in GALT in patients with chronic HIV infection is incomplete and associated with poor CD4+ T cell reconstitution in the GI tract [29]. In contrast, we have recently shown that HAART induces a rapid and significant decrease in the viral load in the alveolar space within six months, both in the acellular and cellular fractions [30].
If, as speculated above, the presence of HIV antigens in the lung drives a CTL response and induces generalized cellular activation in the pulmonary microenvironment, then control of the pulmonary viral load should return alveolar constituents towards a normal state. Indeed, HAART is associated with a delayed but significant decrease in the absolute number and percentage of alveolar lymphocytes [30]. This decline is due exclusively to a decrease in the absolute number of CD8+ lymphocytes in the alveolar space. As a result, the number of HIV-infected subjects with lymphocytic alveolitis declines on HAART. Even more striking, HAART induces a dramatic decline in BAL concentrations of pro-inflammatory cytokines and chemokines [31], which may have significant implications for recruitment of inflammatory cells to the lung. Interestingly, while cytokines and chemokine concentrations decrease, both IFN-γ and the IFN-γ inducible chemokines [i.e. interferon-inducible protein 10 (IP-10) and monokine induced by interferon-γ (MIG)] remain easily detectable in the alveolar space both in HIV-infected subjects and normal volunteers. These chemokines contribute to the recruitment of memory cells to the lung [32]. Furthermore, there is a very strong correlation between the number of lymphocytes in the alveolar space and the concentration of IP-10 and MIG, but not other chemokines such as IL-8, RANTES, or monocyte chemoattractant protein (MCP). This correlation is weak in untreated subjects, but strengthens markedly with time on HAART.
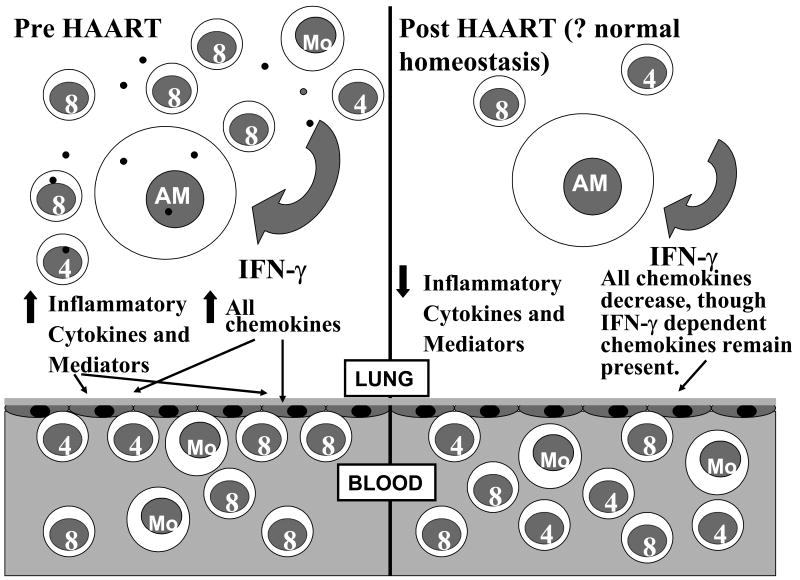
Based on the above findings we can propose the following model concerning HIV in the lung and the effect of HAART on these changes (Figure 3). In untreated patients there is persistent HIV antigen in the lung leading to generalized cellular activation and augmented cytokine and chemokine secretion in response to pathogens and other particulate antigens, including those that would not induce an inflammatory response under normal circumstances. This not only leads to further cellular activation, but also promotes influx of inflammatory cells to the alveolar space in a nonspecific manner, including B cells which are producing non-specific antibody. HIV-specific CTL are also present in the alveolar space and are a rich source of IFN-γ, further leading to even higher concentrations of IFN-γ. The large amount of non-specific cytokine and chemokine secretion lead to a relatively poor correlation between lung lymphocyte numbers and chemokine concentrations. With HAART the pulmonary viral load decreases, reducing the antigenic load driving the nonspecific inflammatory pulmonary response. Less cellular activation is seen and nonspecific cytokine secretion resolves. What remains is persistent low level IFN-γ production from resident memory cells that inhabit the lung [7], which in turn maintains the BAL concentration of IFN-γ inducible chemokines leading to the normal trafficking of these cells into the alveolar space rather than a massive influx of non-specific inflammatory cells. This results in a much tighter relationship between chemokine concentrations in the lung and lung lymphocyte numbers. In summary, HAART therapy is able to return the pulmonary environment towards normal, with normal cellular composition, reduced cellular activation, and normal inflammatory mediator concentrations.
Figure 3.
Effect of HAART on the alveolar environment in HIV infection. In untreated patients there is persistent HIV antigen in the lung leading to generalized cellular activation and augmented cytokine and chemokine secretion in response to pathogens and other particulate antigens. This leads to further cellular activation and promotes influx of inflammatory cells to the alveolar space in a nonspecific manner, including B cells which are producing non-specific antibody. With HAART the pulmonary viral load decreases, reducing the antigenic load driving the nonspecific inflammatory pulmonary response. Less cellular activation is seen and nonspecific cytokine secretion resolves. Low level IFN-γ and IFN-γ inducible chemokine production continues leading to the normal trafficking of memory cells into the alveolar space rather than a massive influx of non-specific inflammatory cells.
Clinical Implications of HAART on pulmonary disease in HIV infection
While HAART is clearly associated with changes in the alveolar environment, it is not yet clear whether this translates into immunologic recovery. One would expect these immunologic changes to result in an enhanced ability to respond appropriately to infectious challenges. In fact, there are some early data suggesting this is true. In a review, Johnson and Gerber cited data demonstrating that HAART (a) can improve the ability to handle opportunistic infections, (b) allow discontinuation of treatment for opportunistic infections in some patients, (c) allow discontinuation of prophylaxis against some opportunistic infections, and (d) enhance responsiveness to vaccines [26]. In the lung HAART has been associated decreased opportunistic infections [33], decreased mycobacterial infections [34], and decreased incidence of bacterial pneumonia [35]. This improved immunologic milieu has led to the development of guidelines on when primary and secondary prophylaxis against opportunistic pathogens can be discontinued [36].
With the decrease in pulmonary infections in HIV-infected subjects on HAART, other pulmonary complications have become more frequently recognized, leading to a dramatic change in the spectrum of pulmonary disease in HIV infection. These complications include pulmonary hypertension [37], emphysema [38, 39], a variety of malignancies [40], and immune reconstitution inflammatory syndrome, or IRIS [41]. HIV-related chronic obstructive pulmonary disease in particular is becoming more frequently recognized. The fall in pulmonary viral load as well as the number of alveolar CD8+ lymphocytes could significantly decrease the incidence of HIV-associated emphysema, a condition postulated to be mediated both directly by the virus as well as by lung cytotoxic T cells [38, 42].
Immune reconstitution syndrome is perhaps the most intriguing complication in patients on HAART because it is likely being mediated by the very immunologic improvements we seek when treating patients. IRIS is best defined as “a paradoxical deterioration in clinical status attributable to the recovery of the immune status during HAART” [41]. This definition addresses two important points: 1) it correctly excludes toxicities of the individual drugs used and 2) it implicates immune recovery rather than residual defects in cell-mediated immunity as responsible for IRIS. When applying this definition, it is estimated that between 10% and 25% of patients who begin HAART will experience IRIS of varying intensity and severity [43].
IRIS has been broadly categorized as either infectious or non-infectious. Infectious IRIS has been described for many different pathogens with a wide array of symptoms depending on the organ systems involved and pathogens identified [40, 44]. It occurs when subclinical or a partially treated infection persists prior to initiation of HAART and provides the antigenic substrate necessary for an immunopathologic inflammatory response. Infectious IRIS can occur very rapidly (i.e. days after starting HAART) and usually occurs within the first 3 months after initiating therapy [45]. Pulmonary IRIS is most often due to an ongoing or partially treated tuberculosis [46, 47] or atypical mycobacterial (ie: Mycobacterium avium) infection. The inflammatory response to these mycobacterial antigens can present as infiltrates or mediastinal lymphadenopathy and are responsible for about a third of cases of IRIS reported [48]. In the lung, early infectious IRIS is occurring at a time when antigen-specific memory cells are making their way back into the alveolar space. One can picture a scenario where antigen-specific memory cells against mycobacteria are released from lymph nodes after initiating HAART. These cells traffic back to the lung where they encounter pre-existing mycobacterial antigens, leading to the inflammatory response characteristic of IRIS.
Non-infectious IRIS is increasingly described and frequently occurs after 6 months of successful therapy. Late pulmonary IRIS may manifest as sarcoidosis (up to 3 years) after HAART initiation [49]. In the few studies that examined cell phenotype during sarcoid IRIS, a CD4 alveolitis was seen and in one study a significant percentage of CD25 cells were noted [50, 51]. Both HIV and sarcoidosis exhibit a state of peripheral lymphopenia, setting the stage for memory cell expansion by homeostatic proliferation [52]. The role of the thymus and naïve cells in IRIS of any type is not defined.
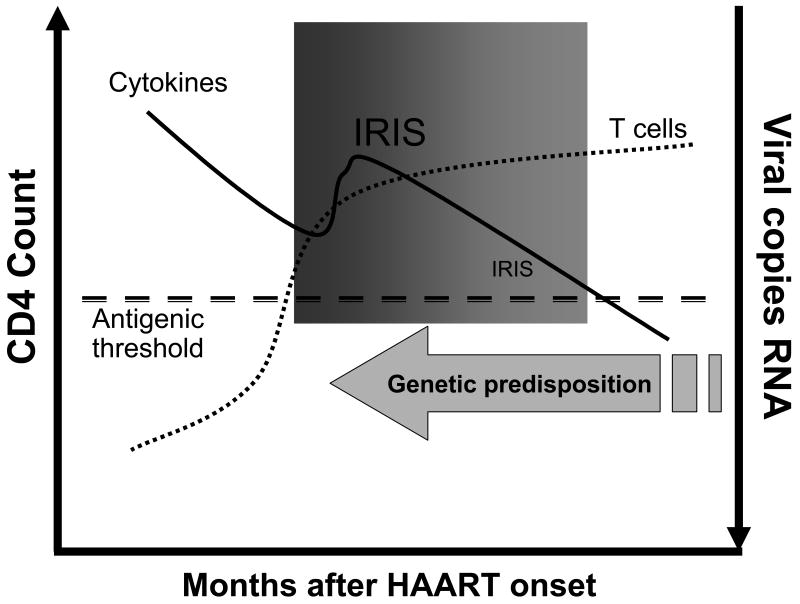
Predictors of IRIS are few, but it appears that a low CD4 nadir, CD4 T cell increase after one month of therapy, rate of decrease in HIV RNA levels, CD8 T cell numbers and previous opportunistic infections all may play a role [46, 53-55]. Although the antigen driving IRIS is speculated to be mycobacterial or fungal, HIV or other ubiquitous viral antigens have not entirely been excluded as a cause of IRIS. However, the very rapid reduction in HIV RNA seen post HAART initiation argues somewhat against HIV antigens. Human herpesvirus-8, implicated in multicentric Castleman's disease, some lymphomas, and Kaposi sarcoma, can cause IRIS and may co-exist in an immunosuppressed patient [56, 57]. The timing of early IRIS implicates existing memory cells in the pathogenesis. However, this theory remains unproven. As such, modeling IRIS is difficult and must take into account individual genetic predisposition, antigenic stimuli, cytokine fluctuations, T cell redistribution, and HIV viral kinetics (Figure 4).
Figure 4.
Necessary requirements to develop immune reconstitution inflammatory syndrome. IRIS occurs when the CD4 count is increasing and viral RNA is decreasing, suggesting an adequate and appropriate response to HAART is a prerequisite. Other factors such as cytokine milieu and T cell subset redistribution are likely contributing factors. Most clinical cases of IRIS (bolded) occur early, within a few months of HAART initiation. However, there is clearly a window (shaded box) in which IRIS can occur “late” provided that an antigenic threshold is maintained (unrecognized opportunistic pathogen burden or unmasked autoantigens related to injury and T cell rebirth). Finally, if an individual has a genetic predisposition to a vigorous proinflammatory response to antigen after HAART initiation, IRIS may be early and fulminant.
Although much is known regarding T cell subset redistribution in the blood during HAART, very little is known regarding the role of different T cell subsets in IRIS or local lung T cells during an IRIS episode [55]. Immunologically, both CD8 and CD4 T cells have been implicated in IRIS and vary depending on the site of IRIS. For example, immune reconstitution uveitis is CD8 dependent. However, in early pulmonary immune reconstitution to Mycobacterium tuberculosis, CD4 T cells are important contributors to the immune response [44]. Furthermore, early IRIS occurs during a time of rapid expansion of the memory cell population in the vascular compartment whereas late IRIS is temporally associated with a second phase of CD4 improvement when expansion of naïve T cells predominates. Thus it is still unclear if regulatory, naïve, or memory cells are responsible for pulmonary IRIS. Further study is needed to delineate predictors of IRIS and biomarkers of disease, which will provide an immunologic rationale for future treatment strategies.
Conclusion
HIV infection causes profound changes in the lung compartment characterized by macrophage and lymphocyte activation, secretion of pro-inflammatory cytokines and chemokines, and accumulation of CD8 T cells in the alveolar space leading to a lymphocytic alveolitis. All of these changes impact pulmonary immunity, leading to a progressive loss in the ability to respond to pathogenic organisms, starting with a decreased ability to respond to common bacteria and mycobacteria and progressing to susceptibility to opportunistic pathogens once profound immunologic defects occur. Most of the changes seen in the lung can be attributed to a direct effect of HIV on immune cells. As such, therapy to reduce the HIV burden should have significant beneficial effects. Indeed, HAART rapidly reduces the viral burden in the lung, reduces the number of CD8 T cells in the alveolar space, and reduces the amount of pro-inflammatory cytokines and chemokines in BAL, thus returning the alveolar microenvironment towards normal. While this clearly has had the expected clinical benefit of reducing susceptibility to pulmonary infections, new complications have been discovered. The immune reconstitution inflammatory syndrome in particular is likely a direct effect of improved immunologic responsiveness, occurring when a recovering pulmonary immune system encounters residual pathogenic antigens still present in the lung. Thus, while the clinical outlook for HIV-infected subjects has greatly improved in the HAART era, one still has to be vigilant for potential pulmonary complications in these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS/WHO. The joint United Nations Programme on HIV/AIDS and the World Health Organization AIDS epidemic update. 2003. [Google Scholar]
- 2.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis. 1990;141:1582–98. doi: 10.1164/ajrccm/141.6.1582. [DOI] [PubMed] [Google Scholar]
- 3.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990;141:1356–72. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- 4.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaltreider HB, Curtis JL, Arraj SM. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after immunization with sheep erythrocytes intratracheally. II. Dose-dependence and kinetics of appearance of antibody-forming cells in hilar lymph nodes and lungs of unprimed and primed mice. Am Rev Respir Dis. 1987;135:87–92. doi: 10.1164/arrd.1987.135.1.87. [DOI] [PubMed] [Google Scholar]
- 6.D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–75. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 7.Saltini C, Kirby M, Trapnell BC, Tamura N, Crystal RG. Biased accumulation of T lymphocytes with “memory”-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990;171:1123–40. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon SB, Molyneux ME, Boeree MJ, et al. Opsonic phagocytosis of Streptococcus pneumoniae by alveolar macrophages is not impaired in human immunodeficiency virus-infected Malawian adults. J Infect Dis. 2001;184:1345–9. doi: 10.1086/324080. [DOI] [PubMed] [Google Scholar]
- 9.Day RB, Wang Y, Knox KK, Pasula R, Martin WJ, Twigg HL. Alveolar Macrophages from HIV Infected Subjects are Resistant to Mycobacterium tuberculosis In Vitro. Am J Respir Cell Mol Biol. 2003 doi: 10.1165/rcmb.2003-0059OC. [DOI] [PubMed] [Google Scholar]
- 10.Cameron ML, Granger DL, Matthews TJ, Weinberg JB. Human immunodeficiency virus (HIV)-infected human blood monocytes and peritoneal macrophages have reduced anticryptococcal activity whereas HIV-infected alveolar macrophages retain normal activity. J Infect Dis. 1994;170:60–7. doi: 10.1093/infdis/170.1.60. [DOI] [PubMed] [Google Scholar]
- 11.Buhl R, Jaffe HA, Holroyd KJ, et al. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993;150:1019–28. [PubMed] [Google Scholar]
- 12.Twigg HL, 3rd, Spain BA, Soliman DM, et al. Production of interferon-gamma by lung lymphocytes in HIV-infected individuals. Am J Physiol. 1999;276:L256–62. doi: 10.1152/ajplung.1999.276.2.L256. [DOI] [PubMed] [Google Scholar]
- 13.Twigg HL., 3rd Bronchoalveolar lavage fluid in HIV-infected patients. “Cytokine soup”. Chest. 1993;104:659–61. doi: 10.1378/chest.104.3.659. [DOI] [PubMed] [Google Scholar]
- 14.Agostini C, Poletti V, Zambello R, et al. Phenotypical and functional analysis of bronchoalveolar lavage lymphocytes in patients with HIV infection. Am Rev Respir Dis. 1988;138:1609–15. doi: 10.1164/ajrccm/138.6.1609. [DOI] [PubMed] [Google Scholar]
- 15.Twigg HL, 3rd, Lipscomb MF, Yoffe B, Barbaro DJ, Weissler JC. Enhanced accessory cell function by alveolar macrophages from patients infected with the human immunodeficiency virus: potential role for depletion of CD4+ cells in the lung. Am J Respir Cell Mol Biol. 1989;1:391–400. doi: 10.1165/ajrcmb/1.5.391. [DOI] [PubMed] [Google Scholar]
- 16.Tardif MR, Tremblay MJ. LFA-1 is a key determinant for preferential infection of memory CD4+ T cells by human immunodeficiency virus type 1. J Virol. 2005;79:13714–24. doi: 10.1128/JVI.79.21.13714-13724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plata F, Autran B, Martins LP, et al. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987;328:348–51. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- 18.Jassoy C, Harrer T, Rosenthal T, et al. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNF-alpha), and TNF-beta when they encounter their target antigens. J Virol. 1993;67:2844–52. doi: 10.1128/jvi.67.5.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadat-Sowti B, Parrot A, Quint L, Mayaud C, Debre P, Autran B. Alveolar CD8+CD57+ lymphocytes in human immunodeficiency virus infection produce an inhibitor of cytotoxic functions. Am J Respir Crit Care Med. 1994;149:972–80. doi: 10.1164/ajrccm.149.4.7511468. [DOI] [PubMed] [Google Scholar]
- 20.Zamarchi R, Barelli A, Borri A, et al. B cell activation in peripheral blood and lymph nodes during HIV infection. Aids. 2002;16:1217–26. doi: 10.1097/00002030-200206140-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bergamini A, Bolacchi F, Bongiovanni B, et al. Human immunodeficiency virus type 1 infection modulates the interleukin (IL)-1beta and IL-6 responses of human macrophages to CD40 ligand stimulation. J Infect Dis. 2000;182:776–84. doi: 10.1086/315803. [DOI] [PubMed] [Google Scholar]
- 22.Fahy RJ, Diaz PT, Hart J, Wewers MD. BAL and serum IgG levels in healthy asymptomatic HIV-infected patients. Chest. 2001;119:196–203. doi: 10.1378/chest.119.1.196. [DOI] [PubMed] [Google Scholar]
- 23.Gordon SB, Miller DE, Day RB, et al. Pulmonary immunoglobulin responses to Streptococcus pneumoniae are altered but not reduced in human immunodeficiency virus-infected Malawian adults. J Infect Dis. 2003;188:666–70. doi: 10.1086/377480. [DOI] [PubMed] [Google Scholar]
- 24.Eagan R, Twigg HL, 3rd, French N, et al. Lung fluid immunoglobulin from HIV-infected subjects has impaired opsonic function against pneumococci. Clin Infect Dis. 2007;44:1632–8. doi: 10.1086/518133. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Oishi K, Yoshimine H, et al. Decreased serum opsonic activity against Streptococcus pneumoniae in human immunodeficiency virus-infected Ugandan adults. Clin Infect Dis. 2003;37:1534–40. doi: 10.1086/379511. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SC, Gerber JG. Advances in HIV/AIDS therapy. Adv Intern Med. 2000;45:1–40. [PubMed] [Google Scholar]
- 27.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–4. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189:572–82. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 29.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–47. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twigg HL, III, W M, Valentine F, Schnizlein-Bick CT, Basset R, Zheng L, Wheat J, Day RB, Rominger H, Collman R, Fox L, Coombs R, Bucy RP for the Adult AIDS Clinical Trials Group Protocol 723 Team. Effect of highly active antiretroviral therapy on viral burden in the lungs of HIV-infected subjects. Journal of Infectious Diseases. 2007 doi: 10.1086/523766. [DOI] [PubMed] [Google Scholar]
- 31.Twigg HI, Day RB, Smith PA, Knox KS. Highly Active Antiretroviral Therapy (HAART) Markedly Decreases Bronchoalveolar Lavage (BAL) Chemokine Concentrations. Am J Respir Cell Mol Biol. 2007;175:248A. [Google Scholar]
- 32.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 33.Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531–2. doi: 10.1056/nejm199705223362118. [DOI] [PubMed] [Google Scholar]
- 34.Kirk O, Gatell JM, Mocroft A, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–72. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JH, Moore RD, Keruly JC, Chaisson RE. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med. 2000;162:64–7. doi: 10.1164/ajrccm.162.1.9904101. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–29. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 37.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–41. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 38.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–72. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 39.Crothers K, Griffith TA, McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–5. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. Aids. 2006;20:1095–107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 41.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–27. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol. 2005;14:48–52. doi: 10.1097/01.pas.0000142168.72253.11. [DOI] [PubMed] [Google Scholar]
- 43.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–15. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 44.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. Aids. 2004;18:1615–27. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 46.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. Aids. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 47.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882–92. doi: 10.1086/313809. [DOI] [PubMed] [Google Scholar]
- 49.Morris DG, Jasmer RM, Huang L, Gotway MB, Nishimura S, King TE., Jr Sarcoidosis following HIV infection: evidence for CD4+ lymphocyte dependence. Chest. 2003;124:929–35. doi: 10.1378/chest.124.3.929. [DOI] [PubMed] [Google Scholar]
- 50.Foulon G, Wislez M, Naccache JM, et al. Sarcoidosis in HIV-infected patients in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:418–25. doi: 10.1086/381094. [DOI] [PubMed] [Google Scholar]
- 51.Naccache JM, Antoine M, Wislez M, et al. Sarcoid-like pulmonary disorder in human immunodeficiency virus-infected patients receiving antiretroviral therapy. Am J Respir Crit Care Med. 1999;159:2009–13. doi: 10.1164/ajrccm.159.6.9807152. [DOI] [PubMed] [Google Scholar]
- 52.Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–40. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breton G, Duval X, Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–12. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 54.Robertson J, Meier M, Wall J, Ying J, Fichtenbaum CJ. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639–46. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]
- 55.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57:167–70. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 56.Aaron L, Lidove O, Yousry C, Roudiere L, Dupont B, Viard JP. Human herpesvirus 8-positive Castleman disease in human immunodeficiency virus-infected patients: the impact of highly active antiretroviral therapy. Clin Infect Dis. 2002;35:880–2. doi: 10.1086/342696. [DOI] [PubMed] [Google Scholar]
- 57.Weir A, Wansbrough-Jones M. Mucosal Kaposi's sarcoma following protease inhibitor therapy in an HIV-infected patient. Aids. 1997;11:1895–6. doi: 10.1097/00002030-199715000-00022. [DOI] [PubMed] [Google Scholar]