Abstract
Intrafusal fibers within muscle spindles make up a small subpopulation of muscle fibers. These proprioceptive fibers differ from most extrafusal fibers because, even in maturity, their diameters remain small, and they retain expression of developmental myosins. Although both extrafusal and intrafusal fibers contain satellite cells (SCs), comparatively little is known about intrafusal SCs. Analyzing chicken fast-phasic posterior (PLD) and slow-tonic anterior (ALD) latissimus dorsi muscles, we show that SCs of both intrafusal and extrafusal fibers express Pax7. We further test the hypotheses that intrafusal fibers display parameters reflective of extrafusal immaturity. These hypotheses are that intrafusal fibers contain (a) higher SC frequencies (number of SC nuclei/all nuclei within basal lamina) and concentrations (closer together) and (b) smaller myonuclear domains than do adjacent extrafusal fibers. IHC techniques were applied to PLD and ALD muscles excised at 30 and 138 days posthatch. The hypotheses were validated, suggesting that intrafusal fibers have greater capacities for growth, regeneration, and repair than do adjacent extrafusal fibers. During maturation, extrafusal and intrafusal fibers show similar trends of decreasing SC frequencies and concentrations and increases in myonuclear domains. Thus, extrafusal and intrafusal fibers alike should exhibit reduced capacities for growth, regeneration, and repair during maturation. (J Histochem Cytochem 56:831–840, 2008)
Keywords: muscle spindles, intrafusal, extrafusal, Pax7, satellite cells, myonuclear domain, anterior latissimus dorsi, posterior latissimus dorsi
Muscle spindles are minute mechanoreceptors located in variable numbers within the bellies of skeletal muscles (Maier 1992). Spindles relay static and dynamic orientation information about a muscle through afferent nerves to the central nervous system (Barker et al. 1974; MacIntosh et al. 2006). They also receive motor neuron input to control contractility of the spindle (Taylor et al. 2000). Each spindle consists of a fusiform-shaped connective tissue capsule containing comparatively tiny intrafusal muscle fibers, situated parallel to the long axis of the larger extrafusal fibers (De Anda and Rebollo 1967; Maier 1992). It has been suggested that intrafusal fibers persist in a comparatively immature state because, unlike extrafusal fibers, they reach their maximum size shortly after birth (Kozeka and Ontell 1981) and maintain expression of developmental myosin heavy chain isoforms (Maier 1991; Walro and Kucera 1999) and the myogenic regulatory factor Myf5 (Zammit et al. 2004).
Satellite cells (SCs), mononuclear stem cells located between the plasmalemma and basal lamina of skeletal muscle fibers (Mauro 1961; Anderson 2006), have the ability to proliferate and differentiate into new myonuclei during the growth, regeneration, and repair of muscle fibers (Charge and Rudnicki 2004; Collins et al. 2005; Zammit et al. 2006). SC numbers and frequency (no. SC nuclei/all nuclei within basal lamina) within extrafusal muscle fibers decrease with postnatal maturation (Halevy et al. 2004; Shefer et al. 2006). SC concentration (surface area of plasmalemma/SC), a variable that is independent of myonuclear number, also decreases (more plasmalemma/SC) with maturation (Allouh et al. 2008). SCs are now routinely identified by immunolabeling methods that localize the expression of paired box transcription factor 7 (Pax7) (Zammit et al. 2006; Day et al. 2007). Whereas Pax7 is expressed by quiescent SCs and their proliferating progeny, Pax7 is not expressed by normal myonuclei (Seale et al. 2000; Halevy et al. 2004; Shefer et al. 2006). Although SCs are present within intrafusal fibers (Katz 1961; Maier 1992), very little is known of the biology of this SC population.
Myonuclear domain is the calculated volume of cytoplasm per myonucleus (Hall and Ralston 1989; Allen et al. 1999). Each myonucleus is thought to be responsible for protein synthesis within its own domain (Hall and Ralston 1989; Ono et al. 1994). Myonuclear domains in extrafusal fibers increase in size with normal growth and aging (Winchester and Gonyea 1992; Mozdziak et al. 1994; Ohira et al. 2001). Myonuclear domains are normally smaller in the more frequently activated, slower contracting types of extrafusal fibers (Roy et al. 2005; Aravamudan et al. 2006). Nothing is known of the size of myonuclear domains within intrafusal fibers.
The tapered ends of extrafusal fibers also maintain a comparatively immature state. The expression of a developmental myosin is retained within the fiber ends of mature chicken pectoralis muscle (Rosser et al. 2000). Throughout posthatch development, myonuclear domains within these tapered fiber ends are comparable in size to those of the pectoralis of week-old chicks and do not increase in size during subsequent growth as do the domains along rest of fiber lengths (Rosser et al. 2002). High SCs frequencies and greater SC concentrations (less surface area of plasmalemma/SC), similar to what one observes in week-old chicks, are found within the tapered fiber ends of the chicken pectoralis throughout posthatch development (Allouh et al. 2008).
Because both intrafusal fibers and the tapered ends of extrafusal fibers persist in comparatively immature states, we postulate that they might share similarities in characteristics related to fiber growth and maturation. Thus, this study tests the hypotheses that intrafusal fibers will contain (a) higher SC frequencies and concentrations and (b) smaller myonuclear domains than the adjacent extrafusals. IHC techniques were used to quantify SC and myonuclear numbers of both intrafusal and extrafusal fibers of the anterior latissimus dorsi (ALD) and posterior latissimus dorsi (PLD) muscles of chickens 30 and 138 days posthatch. These muscles are, respectively, well-characterized synergistic slow and fast contracting muscles in which intrafusal fiber distribution has been mapped (Ovalle et al. 1999). Here we show that, independent of muscle type or age, higher SC numbers and smaller myonuclear domains do distinguish intrafusal fibers. However, intrafusal fibers mirror muscle and age dependent trends observed in extrafusal fibers.
Materials and Methods
Experimental Model
White Leghorn chickens (Gallus gallus; Hy-Line W-36, Clark Hy-Line, Brandon, Canada) were hatched and raised under identical conditions at the University of Saskatchewan, Department of Animal and Poultry Science, as described in our previous studies (Rosser et al. 2000,2002). Following the Canadian Council on Animal Care Guidelines, and with the approval of the University of Saskatchewan Committee on Animal Care and Supply, five birds were killed by cervical dislocation at 30 and 138 days posthatch.
The ALD and PLD are more formally termed latissimus dorsi pars cranialis and latissimus dorsi pars caudalis (Vanden Berge and Zweers 1993), respectively. They are the most superficial muscles on the dorsal surface of the thorax and are synergists that act to adduct the humerus (George and Berger 1966; Raikow 1985). Whereas the ALD is a slow (tonic) contracting muscle originating on a variable number of cervical and thoracic vertebrae and inserting on the humerus, the PLD is a fast (phasic) muscle originating on thoracic vertebrae and inserting immediately caudad to the ALD (George and Berger 1966; Harvey and Marshal 1986). The ALD is one of a few key slow contracting muscles used to fix the folded avian wing against the body (Raikow 1985; Meyers 1992).
Tissue Preparation and Sectioning
The ALD and PLD were excised bilaterally from each 30-day bird and from the left side of each 138-day bird and then weighed. Samples were removed from each muscle, such that the long axis of each sample was parallel to the direction of the muscle fibers. Each sample, ∼0.5–1.0 cm × 2–4 cm × thickness of muscle, was coated with Tissue-Tek OCT Compound (Sakura Finetek; Torrance, CA) and immediately frozen in isopentane cooled by liquid nitrogen (Sewry and Dubowitz 2001). Samples were stored at −80C. Serial cross-sections of ALD and PLD samples from each chicken were cut at 12 μm thickness using a cryostat at −20C. Tissue sections were placed on ProbeOn Plus charged microscope slides (Fisher Scientific; Nepean, Canada) and stored at −20C.
IHC
IHC labeling was performed using slides from several locations along the length of the each muscle. At each location, several consecutive slides were labeled. The blocking solution consisted of 5% goat serum, 1% BSA, and 5 mM EDTA in PBS (0.02 M sodium phosphate buffer, 0.15 M sodium chloride, pH 7.2) and was applied to each slide for 15–20 min at room temperature. The primary antibody anti-Pax7 (Developmental Studies Hybridoma Bank; Iowa City, IA), a mouse monoclonal was used to label SCs. Anti-laminin (L9393; Sigma Chemical, St. Louis, MO), a rabbit polyclonal labeled basal laminae. Anti-myosin (NA4; gift from Dr. E. Bandman, University of California, Davis, CA; now available at Developmental Studies Hybridoma Bank) labeled all myosin heavy chains. The primary labeling solution was applied either for 90 min at room temperature or overnight at 4C. The primary solution consisted of either (A) 1:200 anti-laminin and 1:15 anti-Pax7 in block solution or (B) 1:200 anti-laminin and 1:5000 anti-myosin in block solution. Primary solutions A and B were alternated with each serial slide.
Alexa Fluor 488 goat anti-mouse IgG (A-11001; Invitrogen, Carlsbad, CA) was used to label anti-Pax7 and NA4 green and Alexa Fluor 546 goat anti-rabbit IgG (A-11010; Invitrogen) to label anti-laminin red when viewed with epifluorescent microscopy. The secondary labeling solution, containing both secondary antibodies each diluted 1:200 in PBS at pH 7.2, was applied to each slide for 30 min at room temperature in the dark. Hoechst 33258 (bisbenzimide; Sigma Chemical) was applied to each slide for 5 min at a dilution of 1:1,500,000 in PBS to label the DNA in nuclei blue under epifluorescent microscopy. Finally, 4% formaldehyde in PBS was applied to each slide for 3 min. Slides were mounted in Geltol (Thermo Scientific; Pittsburgh, PA) and stored at 4C in the dark.
Imaging and Analyses
Images of cross-sections were captured using a Sony S70 digital still camera (Sony; Tokyo, Japan) attached to a Zeiss Axioskop 20 microscope (Carl Zeiss; Oberkochen, Germany) equipped for epifluorescence. Spindles from each muscle studied were located and photographed, such that at least 30 intrafusal fibers were analyzed from each muscle. The different colored fluorescent images from each field of view were compiled using Adobe Photoshop (Adobe System; San Jose, CA). The final images consisted of all nuclei in blue, SC nuclei and myosin in green, and basal lamina in red. These images were used to identify intrafusal fibers and count the number of SCs and myonuclei per muscle fiber. All intrafusal fibers and 200 adjacent extrafusal fibers from each sample were analyzed in this way. The ellipse minor axis of intrafusal and extrafusal fibers was determined using Scion Image 1.63 (developed by the U.S. National Institutes of Health and available on the internet by anonymous FTP from Zippy.nimh.nih.gov) and used to calculate the cross-sectional area of each fiber. Ellipse minor axis of Scion Image is identical to lesser fiber diameter (Rosser et al. 2000). Lesser fiber diameter, which is defined as the maximum aspect across the lesser aspect of a fiber, is routinely used to overcome distortion that may result when a muscle fiber is cut obliquely rather than transversely (Dubowitz 1985).
The mean lengths of myonuclei and SCs were determined by sectioning tissue longitudinally at 12 μm thickness with a −20C cryostat. Three chickens at each age were used to measure the lengths of 50 myonuclei and 50 SC nuclei per muscle studied. Longitudinal sections were labeled according to the preceding protocol, omitting primary solution B. Scion Image 1.63 was used to measure the lengths of myonuclei and SC nuclei.
Calculations and Statistics
Mean values for each of the following parameters were obtained from both intrafusal and extrafusal fibers of each muscle studied. The frequency of SCs was calculated using the average number of satellite cell nuclei (SCN) and myonuclei (MN) per fiber and the formula SCN frequency = (SCN/([SCN + MN]) × 100% (Schmalbruch and Hellhammer 1977). The mean number of SCN and MN per unit length was determined using the formula Z = n × L/(d + l), where Z = number of SCN or MN per unit length of fiber, n = number of nuclear profiles (SCN or MN) per fiber profile, L = unit length of fiber (1mm), d = section thickness, and l = average SCN or MN length (Schmalbruch and Hellhammer 1977). The mean concentration or surface area of plasmalemma per SC was calculated using the formula S = πEL/ZSC, where S = surface area of plasmalemma per SC, E = mean ellipse minor axis, L = unit length of fiber (1 mm), and ZSC = number of SC per unit length of fiber. The mean cross-sectional area was quantified according to the formula C = π(E/2)2, where C = mean cross-sectional area of fiber and E = mean ellipse minor axis of fiber. The mean volume of cytoplasm per myonucleus (myonuclear domain) was calculated using the formula V = CL/ZMN, where V = volume of cytoplasm per myonuclei, C = mean cross-sectional area of fiber, L = unit length of fiber (1 mm), and ZMN = number of MN per unit length of fiber (Rosser et al. 2002).
ALD and PLD muscles from five animals at each posthatch age of 30 and 138 days were studied. Data throughout this report are presented as means and SEs. The mean SC frequency, mean surface area of plasmalemma per SC, and mean myonuclear domain were each analyzed for differences between type of fiber (intrafusal vs extrafusal fibers), muscle type (ALD vs PLD), and age (30 vs 138 days posthatch). Comparisons were performed using a one-way ANOVA test at 5% level of significance (p≤0.05). In cases where the homogeneity of variance assumption was violated (p≤0.05), the analogous non-parametric (Mann-Whitney) test was performed instead (Rosner 2006). These statistical tests were performed using SPSS 15.0 for Windows, evaluation version (SPSS; Chicago, IL). Differences between posthatch ages in myonuclear length or satellite cell length in ALD or PLD were assessed through two-sample t-tests performed using Microsoft Excel (Microsoft; Redmond, WA).
Results
The ALD and PLD each underwent an ∼6- to 7-fold increase in wet weight from 30 to 138 days posthatch (Table 1). The mean lengths of myonuclei and satellite cell nuclei did not change with age in either the ALD or PLD (Table 1). Thus, for the calculations outlined in the Materials and Methods section, data were pooled such that the average lengths of ALD myonuclei and SC nuclei were 10.89 ± 0.02 and 9.84 ± 0.04 μm, respectively. PLD myonuclei and SC nuclei lengths used were 11.57 ± 0.09 and 10.34 ± 0.06 μm, respectively.
Table 1.
Comparison of muscle weights and nuclear lengths
| Anterior latissimus dorsi muscle
|
Posterior latissimus dorsi muscle
|
|||
|---|---|---|---|---|
| 30 day | 138 day | 30 day | 138 day | |
| Mean muscle weight (g)a | 0.07 ± 0.01 | 0.40 ± 0.04 | 0.13 ± 0.01 | 0.89 ± 0.05 |
| Myonuclei mean length (μm)b | 10.88 ± 0.04 | 10.90 ± 0.03 | 11.65 ± 0.12 | 11.48 ± 0.14 |
| Satellite cell nuclei mean length (μm)b | 9.79 ± 0.08 | 9.88 ± 0.04 | 10.32 ± 0.10 | 10.36 ± 0.09 |
Significant difference (p≤0.05) between the 30- and 138-day posthatch muscle.
No significant difference (p>0.05) between the 30- and 138-day posthatch muscle.
In comparing mean muscle weights, mean nuclear lengths, and mean satellite cell nuclear lengths, within either anterior latissimus dorsi or posterior latissimus dorsi muscles, only muscle weight was significantly different between ages. Each value represents mean ± SE.
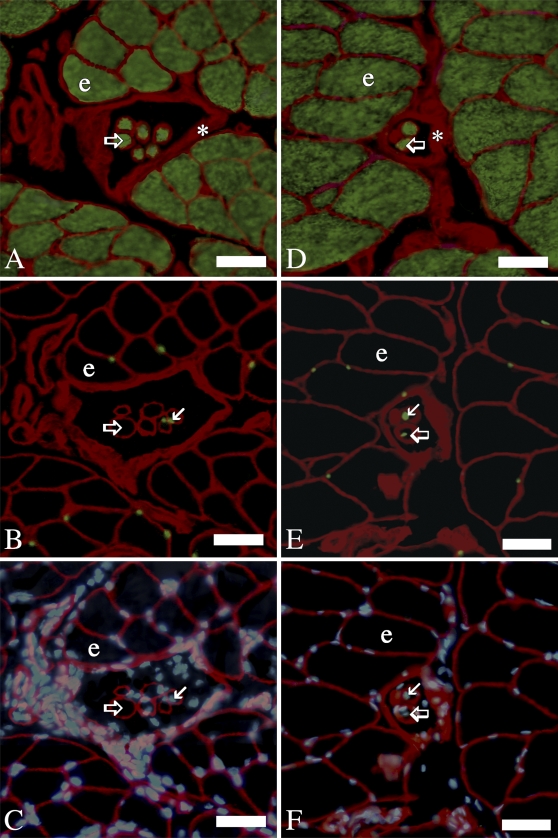
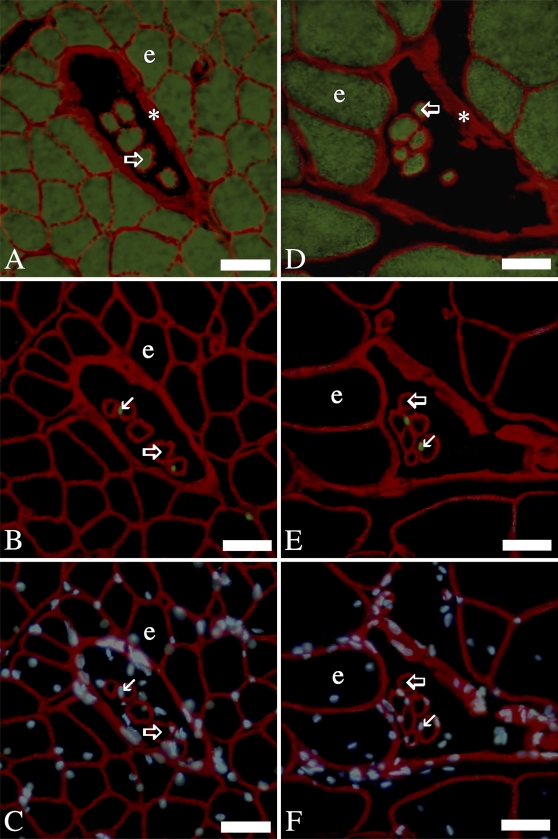
Laminin is a major constituent of the basement membrane in a variety of cell types including endothelium, nerve, and muscle (Tzu and Marinkovich 2008). To distinguish muscle fibers from other cell types and structures that are surrounded by basement membrane, muscle fibers were identified by their skeletal muscle myosin heavy chain content in ALD (Figures 1A and 1D) and PLD (Figures 2A and 2D). Extrafusal and intrafusal fibers were differentiated by the location of intrafusal fibers within the capsules of the muscle spindles (Figures 1A, 1D, 2A, and 2D), which were identified by their rich laminin content (Maier and Mayne 1995). SCs in extrafusal and intrafusal fibers were recognized by their characteristic position beneath basal laminae and Pax7 labeling, in both the ALD (Figures 1B and 1E) and PLD (Figures 2B and 2E). Myonuclei were distinguished by their position beneath muscle fiber basal laminae in ALD (Figures 1C and 1F) and PLD (Figures 2C and 2F) and lack of Pax7 labeling Figures 1B, 1E, 2B, and 2E). Although no SCs are shown in the extrafusal fibers of the micrograph of Pax7 labeling of 138-day PLD (Figure 2E), SCs were in fact present in low numbers (Figures 3A and 3B). In our previous study (Allouh et al. 2008) of chicken pectoralis, throughout posthatch development, 2–3% of the nuclei expressing Pax7 were located in interstitial spaces outside of the basal laminae. In this study, a comparatively small number of the Pax7-labeled nuclei were also located in the interstitial spaces of both ALD and PLD. However, because these nuclei were pertinent to neither our current calculations nor hypotheses, no attempt was made to quantify their numbers.
Figure 1.
IHC labeling of serial cross-sections of anterior latissimus dorsi (ALD) muscles: 30- (A–C), and 138-day (D–F). In each of the preceding, the first image (A,D) is from a section cut serial to the subsequent two images that are both from a second section. In all images, laminin is colored red. (A,D) Myosin is green. (B,E) Pax7 is green. (C,F) Nuclei are blue. Several intrafusal fibers clustered within a spindle capsule are near the center of each image and are considerably smaller in diameter than the larger extrafusal fibers situated outside of the capsule. Open/large arrows, intrafusal fibers; line/small arrows, satellite cells; asterisk, capsule of muscle spindle; e, extrafusal fiber. Bar = 30 μm.
Figure 2.
IHC labeling of serial cross-sections of posterior latissimus dorsi (PLD) muscles: 30- (A–C) and 138-day (D–F). In all images, laminin is red. (A,D) Myosin is green. (B,E) Pax7 is green. (C,F) Nuclei are blue. Open/large arrows, intrafusal fibers; line/small arrows, satellite cells; asterisk, capsule of muscle spindle; e, extrafusal fiber. Bar = 30 μm.
Figure 3.
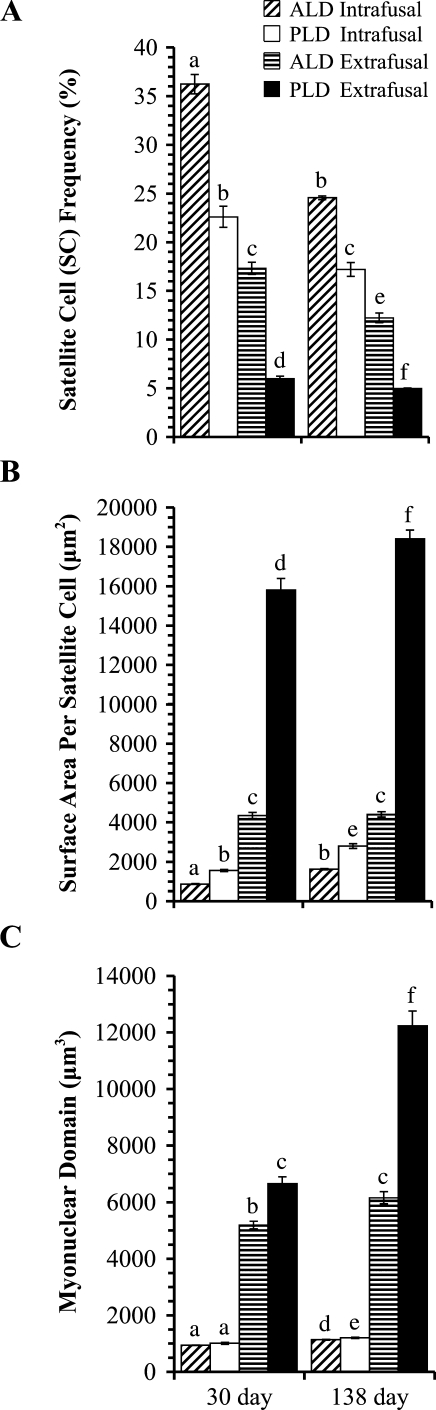
ALD and PLD intrafusal and extrafusal fiber data. Satellite cell frequency (A) decreases in each muscle and fiber type with age but is always greater in the intrafusal than extrafusal fibers of the same muscle. Surface area per satellite cell (B) usually increases with age, but always less in the intrafusal than extrafusal fibers of the same muscle. Myonuclear domain (C) increases in each muscle and fiber type with age and is always smaller in intrafusal than extrafusal fibers of the same muscle. Within each graph (A–C), bars beneath the same lowercase letters are not significantly (p>0.05) different from one another. Bars beneath different lowercase letters are significantly (p≤0.05) different from one another. Each value is expressed as mean ± SE (n=5).
The mean frequency of SCs (Figure 3A) was significantly higher in intrafusal than extrafusal fibers in all muscles examined; in ALD at 30 (p<0.001) and 138 days (p<0.001) and in PLD at 30 (p=0.008) and 138 days (p=0.008). In 30-day ALD, mean SC frequency in intrafusal and extrafusal fibers ranged, respectively, from 32.9% to 38.7% and from 15.6% to 19.4%. In 138-day ALD, it ranged from 24.1% to 25.0% in intrafusal and from 11.1% to 13.9% in extrafusal fibers. In 30-day PLD, it extended from 20.0% to 25.0% in intrafusal and from 5.0% to 6.6% in extrafusal fibers. In 138-day PLD, values in intrafusal and extrafusal fibers ranged, respectively, from 15.4% to 19.2% and from 4.8% to 5.1%. Mean SC frequency decreased significantly from 30 to 138 days posthatch in ALD intrafusal (p=0.008) and extrafusal fibers (p<0.001) and in PLD intrafusal (p=0.003) and extrafusal fibers (p=0.032). In comparing the two muscles, mean SC frequency was always significantly greater in the ALD than PLD; in intrafusal fibers at 30 (p<0.001) and 138 days (p=0.008) and in extrafusal fibers at 30 (p<0.001) and 138 days (p=0.008).
Mean surface area per SC (Figure 3B) was significantly lower in intrafusal than extrafusal fibers in all muscles studied: in ALD at 30 (p=0.008) and 138 days (p=0.008) and in PLD at 30 (p=0.008) and 138 days (p=0.008). In 30-day ALD, mean surface area per SC in intrafusal and extrafusal fibers ranged, respectively, from 806 to 961 and 3900 to 4716 μm2. Within 138-day ALD, it ranged from 1554 to 1694 μm2 in intrafusal fibers and from 4004 to 4790 μm2 in extrafusal fibers. In 30-day PLD, it extended from 1435 to 1686 μm2 in intrafusal fibers and from 14,264 to 17,045 μm2 in extrafusal fibers. The 138-day PLD ranged from 2560 to 3151 μm2 in intrafusal fibers and from 17,396 to 19,547 μm2 in extrafusal fibers. Mean surface area per SC increased significantly from 30 to 138 days posthatch in ALD intrafusal (p<0.001), PLD intrafusal (p=0.008), and PLD extrafusal (p=0.008) fibers. However, the apparent numeric increase in surface area per SC with age in ALD extrafusal fibers was not significant (p=0.770). Contrasting the two muscles, mean surface area per SC was consistently lower in the ALD than PLD: in intrafusal fibers at 30 (p≤0.001) and 138 days (p=0.008) and in extrafusal fibers at 30 (p=0.008) and 138 days (p=0.008).
Myonuclear domain (volume of cytoplasm per myonucleus; Figure 3C) was always significantly smaller in intrafusal than extrafusal fibers: in 30-day ALD (p=0.008), 138-day ALD (p=0.008), 30-day PLD (p≤0.001), and 138-day PLD (p=0.008). In 30-day ALD, myonuclear domain ranged from 926 to 972 μm3 in intrafusal fibers and from 4777 to 5477 μm3 in extrafusal fibers. Within 138-day ALD, it ranged from 1099 to 1188 μm3 in intrafusal fibers and from 5674 to 6708 μm3 in extrafusal fibers. The range of myonuclear domains in the 30-day PLD extended from 902 to 1103 μm3 in intrafusal fibers and from 6228 to 7569 μm3 in extrafusal fibers. In 138-day PLD, it ranged from 1143 to 1270 μm3 in intrafusal fibers and from 10,650 to 13,875 μm3 in extrafusal fibers. Mean myonuclear domain increased significantly from 30 to 138 days posthatch within ALD intrafusal (p=0.008), ALD extrafusal (p=0.005), PLD intrafusal (p=0.002), and PLD extrafusal fibers (p≤0.001). In comparing the two muscles, mean myonuclear domains were significantly smaller within the ALD than PLD in 138-day intrafusal (p=0.045), 30-day extrafusal (p≤0.001), and 138-day extrafusal (p≤0.001) fibers. In 30-day intrafusal fibers, however, the difference in myonuclear domain between the ALD and PLD was not significant (p=0.151).
Discussion
It has long been known that intrafusal fibers contain SCs, but very little progress has been made in their characterization. We found that Pax7 is expressed within SC nuclei of both intrafusal and extrafusal muscle fibers. As outlined in our studies of chicken pectoralis extrafusal fibers (Halevy et al. 2004; Allouh et al. 2008), SCs were identified by their expression of Pax7 and their location beneath the basal laminae of the fibers. A previous study of human muscle also reported on the expression of Pax7 by satellite cells in intrafusal fibers, but apart from an IHC image, neither quantitative data nor references to muscle groups were provided (Reimann et al. 2004). Regardless, the similar expression of Pax7 across SCs of different fiber types and species provides further support of the importance of Pax7 expression to SC function. Indeed, studies of mice lacking Pax7 showed elimination of SCs and impaired muscle growth after the initial postnatal phase (Kuang et al. 2006; Relaix et al. 2006).
This study provided new observations and insights about muscle spindles. Regardless of muscle or age studied, intrafusal fibers had higher frequencies and concentrations of SCs and smaller myonuclear domains than the surrounding extrafusal fibers. The values obtained for each of these three parameters quantified are indicative of greater capacities for growth, regeneration, and repair (Rosser et al. 2002; Allouh et al. 2008). Thus, intrafusal fibers should have greater capacities for growth, regeneration, and repair than do extrafusal fibers. As outlined in the following text, these observations can be related to both the comparatively immature state of intrafusal fibers and/or their higher frequencies of contraction. In addition, during maturation, intrafusal fibers show the same trends of decreasing SC frequency and concentration and increasing myonuclear domain size as the associated extrafusal fibers. This suggests that, like extrafusal fibers, intrafusal fibers will show a reduced capacity for growth, regeneration, and repair with aging.
The comparatively immature state of intrafusal fibers may certainly be related to their relatively higher frequencies and concentrations of SCs and smaller myonuclear domains. Immature fibers are smaller in diameter than mature fibers (Rosser et al. 2000). Invariably, intrafusal fibers are tiny compared with the adjacent extrafusal fibers (Kozeka and Ontell 1981; Maier 1992). SC frequency is greater in the younger, smaller diameter fibers within extrafusal populations (Campion 1984; Shefer et al. 2006), and SC frequency has been inversely correlated with fiber diameter (Allouh et al. 2008). Similarly, myonuclear domains are smaller in younger, smaller, diameter fibers (Rosser et al. 2002) and increase in size with normal growth and aging in extrafusal fibers (Winchester and Gonyea 1992; Mozdziak et al. 1994; Ohira et al. 2001). Myonuclear domain size has also been inversely correlated with fiber size (Hikida 2007). Therefore, small diameters associated with the relative immaturity of intrafusal fibers can explain their relatively higher SC frequency and concentration and smaller myonuclear domains.
The higher activity levels of intrafusal fibers can also be correlated with higher SC numbers and smaller myonuclear domains. In general, greater contractile activity of a muscle corresponds with higher SC frequencies (Gibson and Schultz 1982; Gibson and Schultz 1983). Also, myonuclear domains are normally smaller in those fiber types that are more frequently activated (Roy et al. 2005; Aravamudan et al. 2006). Unlike extrafusal fibers, intrafusal fibers constantly send and receive information to keep the body continually aware of its position in space (Barker et al. 1974; Maier 1992). Even within a muscle at rest, intrafusal fibers send to the CNS afferent signals that are linearly related to the muscle's length (Dorward 1970; Vallbo 1974). Whereas extrafusal fibers of birds and mammals are supplied by alpha motor nerves (Kernell 2006), intrafusal fibers are supplied by smaller γ motor nerves (Moschovakis et al. 1991; Maier 1992; Taylor et al. 2000). It has been suggested that the evolution of γ motor neurons in higher vertebrates must have conferred an adaptive advantage related to activity patterns not closely coupled to those of alpha-motoneurons (Windhorst 2007).
The extrafusal fibers of the ALD have a greater frequency and concentration of SCs and smaller myonuclear domains than the extrafusal fibers of the PLD. Slower contracting extrafusal fibers are typically activated more frequently than fast contracting fibers (Anapol and Herring 2000). Our study of chicken SCs is in agreement with the findings of studies of mammalian SCs that concluded that extrafusal fibers of slow contracting muscles, associated with the maintenance of posture or sustained locomotion, have more SCs and a greater capacity for growth, regeneration, and repair compared with muscles activated in short, sporadic bursts (Schmalbruch and Hellhammer 1977; Gibson and Schultz 1983; Shefer et al. 2006). Myonuclear domains of slow fibers have also been found to be smaller than those of fast contracting fibers (Roy et al. 2005; Aravamudan et al. 2006). The smaller myonuclear domain of slow fibers is correlated with a greater need for protein synthesis associated with sustained contraction (Edgerton and Roy 1991; Tseng et al. 1994; Rosser et al. 2002).
Similarities between intrafusal and extrafusal fibers in the trends of posthatch SC numbers and myonuclear domain size can be used to formulate additional theories. The decrease in SC numbers associated with maturation of extrafusal fibers has been related to a diminished capacity for growth, regeneration, and repair (Shefer et al. 2006; Brack and Rando 2007). It can, therefore, be inferred that this should also hold true for intrafusal fibers as they mature. Similarly, the larger myonuclear domains that we observed with maturation of extrafusal fibers in the avian pectoralis (Rosser et al. 2002), ALD, and PLD would be associated with a lessened ability for protein synthesis and growth (see preceding discussion). The same conclusion can be applied to the increase of myonuclear domain sizes observed during maturation of intrafusal fibers.
In summary, Pax7 can be used to label SCs in both extrafusal and intrafusal fibers of the chicken. Greater SC frequencies and concentrations and smaller myonuclear domain sizes within intrafusal fibers suggest that they have a greater capacity for growth, regeneration, and repair than the adjacent extrafusal fibers. These results also imply that intrafusal fibers are less developmentally mature and/or more physiologically active than extrafusal fibers. Similarities in developmental trends between intrafusal and extrafusal fibers in decreasing SC numbers and increasing myonuclear domain sizes indicate that, as they mature, intrafusal fibers will, like extrafusal fibers, show a diminished capacity for growth, regeneration, and repair. Studies further exploring these concepts could involve experimental paradigms that stress muscles to stimulate SC division, works labeling markers of SC differentiation and proliferation, and physiologic models comparing the contractile activities of intrafusal and extrafusal fibers within the same muscle preparations.
Acknowledgments
A Discovery Grant awarded to B.W.C.R. from the Natural Sciences and Engineering Research Council (NSERC) of Canada provided funds for this study. Two Undergraduate Student Research Awards and an Alexander Graham Bell Canada Graduate Scholarship from NSERC funded L.J.K. A Summer Student Research Project from the College of Medicine, University of Saskatchewan, funded C.N.N. The Jordan University of Science and Technology and the College of Medicine, University of Saskatchewan, provided funds for M.Z.A. Z.Y.R. is supported by the National Institute on Aging (AG021566 and AG013798) and the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (NRI, 2003-35206-12843).
Lesley A. McLeod of the Department of Community Health and Epidemiology, University of Saskatchewan, provided assistance with statistical analyses. Dr. Everett Bandman at the University of California, Department of Food Sciences and Technology, generously provided the anti-myosin antibody. The antibody against Pax7 developed by A. Kawakami was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA.
References
- Allen DL, Roy RR, Edgerton VR (1999) Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22:1350–1360 [DOI] [PubMed] [Google Scholar]
- Allouh MZ, Yablonka-Reuveni Z, Rosser BWC (2008) Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 56:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anapol F, Herring SW (2000) Ontogeny of histochemical fiber types and muscle function in the masseter muscle of miniature swine. Am J Phys Anthropol 112:595–613 [DOI] [PubMed] [Google Scholar]
- Anderson JE (2006) The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J Exp Biol 209:2276–2292 [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC (2006) Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol 100:1617–1622 [DOI] [PubMed] [Google Scholar]
- Barker D, Hunt CC, McIntyre AK (1974) Muscle receptors. In: Autrum H, Jung R, Loewenstein WR, MacKay DM, Teuber HL, eds. Handbook of Sensory Physiology, vol. 3, no 2. Berlin, Springer-Verlag, 1–310
- Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3:226–237 [DOI] [PubMed] [Google Scholar]
- Campion DR (1984) The muscle satellite cell: A review. Int Rev Cytol 87:225–251 [DOI] [PubMed] [Google Scholar]
- Charge SBP, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122:289–301 [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z (2007) Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Anda G, Rebollo MA (1967) The neuromuscular spindles in the adult chicken. I. Morphology. Acta Anat (Basel) 67:437–451 [DOI] [PubMed] [Google Scholar]
- Dorward PK (1970) Response characteristics of muscle afferents in the domestic duck. J Physiol 211:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V (1985) Muscle Biopsy: A Practical Approach. Philadelphia, Balliere-Tindall
- Edgerton VR, Roy RR (1991) Regulation of skeletal muscle fiber size, shape and function. J Biomech 24:123–133 [DOI] [PubMed] [Google Scholar]
- George JC, Berger AJ (1966) Avian Myology. New York, Academic Press
- Gibson MC, Schultz E (1982) The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202:329–337 [DOI] [PubMed] [Google Scholar]
- Gibson MC, Schultz E (1983) Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6:574–580 [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BWC, Rinkevich Y, Reshef R, Rozenboim I, et al. (2004) Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231:489–502 [DOI] [PubMed] [Google Scholar]
- Hall ZW, Ralston E (1989) Nuclear domains in muscle cells. Cell 59:771–772 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Marshal IG (1986) Muscle. In: Sturkie PD, ed. Avian Physiology. 4th ed. New York, Springer-Verlag, 74–86
- Hikida RS (2007) Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1104–1105 [DOI] [PubMed] [Google Scholar]
- Katz B (1961) The terminations of the afferent nerve fibre in the muscle spindle of the frog. Philos Trans R Soc Lond B Biol Sci 243:221–240 [Google Scholar]
- Kernell D (2006) The Motoneurone and Its Muscle Fibers. Oxford, Oxford University Press
- Kozeka K, Ontell M (1981) The 3-dimensional cytoachitecture of developing murine muscle spindles. Dev Biol 87:133–147 [DOI] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA (2006) Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh BR, Gardier PF, McComas AJ (2006) Skeletal Muscle: Form and Function. 2nd ed. Windsor, Human Kinetics
- Maier A (1991) Axon contacts and acetylcholinesterase activity on chicken intrafusal muscle fiber types identified by their myosin heavy chain composition. Anat Embryol (Berl) 184:497–505 [DOI] [PubMed] [Google Scholar]
- Maier A (1992) The avian muscle spindle. Anat Embryol (Berl) 186:1–25 [DOI] [PubMed] [Google Scholar]
- Maier A, Mayne R (1995) Basal lamina development in chicken muscle spindles. Dev Dyn 202:284–293 [DOI] [PubMed] [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA (1992) The morphological basis of folded-wing posture in the American kestrel (Falco sparverius). Anat Rec 232:493–494 [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Burke RE, Fyffe RE (1991) The size and dendritic structure of HRP-labeled gamma motoneurons in the cat spinal cord. J Comp Neurol 311:531–545 [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Schultz E, Cassens RG (1994) Satellite cell mitotic-activity in posthatch turkey skeletal-muscle growth. Poult Sci 73:547–555 [DOI] [PubMed] [Google Scholar]
- Ohira Y, Tanaka T, Yoshinaga T, Kawano F, Nomura T, Nonaka I, Allen DL, et al. (2001) Onotogenetic, gravity-dependent development of rat soleus muscle. Am J Physiol 280:C1008–1016 [DOI] [PubMed] [Google Scholar]
- Ono T, Ono K, Mizukawa K, Ohta T, Tsuchiya T, Tsuda M (1994) Limited diffusibility of gene products directed by a single nucleus in the cytoplasm of multinucleated myofibers. FEBS Lett 337:18–22 [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Dow PR, Nahirney PC (1999) Structure, distribution and innervation of muscle spindles in avian fast and slow skeletal muscle. J Anat 194:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikow RJ (1985) Locomotor systems. In: King AS, McLelland J, eds. Form and Functions of Birds, vol 3. New York, Academic Press, 57–146
- Reimann J, Brimah K, Schroder T, Wernig A, Beauchamp JR, Partridge TA (2004) Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res 315:233–242 [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhish S, Mansouri A, et al. (2006) Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B (2006) Fundamentals of Biostatistics. Belmont, Thomson-Brooks/Cole
- Rosser BWC, Dean MS, Bandman E (2002) Myonuclear domain size varies along the lengths of maturing skeletal muscle fibers. Int J Dev Biol 46:747–754 [PubMed] [Google Scholar]
- Rosser BWC, Farrar CM, Crellin NK, Andersen LB, Bandman E (2000) Repression of myosin isoforms in developing and denervated skeletal muscle fibers originates near motor endplates. Dev Dyn 217:50–61 [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Siengthai B, Edgerton VR (2005) Activity-dependent influences are greater for fibers in rat medial gastrocnemius than tibialis anterior muscle. Muscle Nerve 32:473–482 [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U (1977) The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec 189:169–175 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786 [DOI] [PubMed] [Google Scholar]
- Sewry CA, Dubowitz V (2001) Histochemistry and immunocytochemistry of muscle in health and disease. In: Karpati G, Hilton-Jones D, Griggs RC, eds. Disorders of Voluntary Muscle. 7th ed. Cambridge, Cambridge University Press, 251–282
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294:50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R, Rawlinson S (2000) Distinctive patterns of static and dynamic gamma motor activity during locomotion in the decerebrate cat. J Physiol 529:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BS, Kasper CE, Edgerton VR (1994) Cytoplasm-to-myonucleus ratio and succinate dehydrogenase activities in adult rat slow and fast muscle fibers. Cell Tissue Res 275:39–49 [DOI] [PubMed] [Google Scholar]
- Tzu J, Marinkovich MP (2008) Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 40:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB (1974) Afferent discharge from human muscle spindles in non-contracting muscles: steady state impulse frequency as a function of joint angle. Acta Physiol Scand 90:303–318 [DOI] [PubMed] [Google Scholar]
- Vanden Berge JC, Zweers GA (1993) Myologia. In: Baumel JJ, King AS, Breazille JE, Evan HE, Vanden Berge JC, eds. Handbook of Avian Anatomy: Nomina Anatomica Avium. Cambridge, MA, Nuttall Ornithological Club, 189–247
- Walro JM, Kucera J (1999) Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci 22:180–184 [DOI] [PubMed] [Google Scholar]
- Winchester PK, Gonyea WJ (1992) A quantitative study of satellite cells and myonuclei in stretched avian slow tonic muscle. Anat Rec 232:369–377 [DOI] [PubMed] [Google Scholar]
- Windhorst U (2007) Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73:155–202 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, et al. (2004) Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol 273:454–465 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191 [DOI] [PubMed] [Google Scholar]