Abstract
Obestatin and ghrelin are two peptides derived from the same prohormone. It is well established that ghrelin is produced by endocrine cells in the gastric mucosa. However, the distribution of human obestatin immunoreactive cells is not thoroughly characterized. A polyclonal antibody that specifically recognizes human obestatin was produced. Using this antibody and a commercial antibody vs ghrelin, the distribution of obestatin and ghrelin immunoreactive cells was determined in a panel of human tissues using immunohistochemistry. The two peptides were detected in the mucosa of the gastrointestinal tract, from cardia to ileum, and in the pancreatic islets. Interestingly, epithelial cells in the ducts of mammary glands showed distinct immunoreactivity for both ghrelin and obestatin. By double immunofluorescence microscopy, it was shown that all detected cells were immunoreactive for both peptides. Furthermore, the subcellular localization of obestatin and ghrelin was essentially identical, indicating that obestatin and ghrelin are stored in the same secretory vesicles. (J Histochem Cytochem 56:793–801, 2008)
Keywords: chromogranin A, ghrelin, gut, immunofluorescence, immunohistochemistry, intestine, mammary glands, obestatin, pancreas
Ghrelin is a 28 amino acid peptide that originally was isolated from the stomach. It is generated by processing of a 117 amino acid peptide, preproghrelin, by specific proteases and is stored in secretory vesicles of endocrine cells. The peptide has been shown to be further processed by addition of an octanoyl group to a serine residue, and this acylation is important for the endocrine/biological activity of this peptide (Kojima et al. 1999). Ghrelin is a multifunctional molecule, involved in many biological processes ranging from appetite regulation (Asakawa et al. 2001; Inui 2001) and growth hormone release (Kojima et al. 1999; Arvat et al. 2000) to gut motility (Tack et al. 2006) and cell proliferation (Jeffery et al. 2002,2005).
Ghrelin is produced in the oxyntic glands of the gastric mucosa, which is the main source of circulating ghrelin (Ariyasu et al. 2001). Previous reports have described the identification of ghrelin-immunoreactive (IR) cells in human tissue including pancreas, pituitary, hypothalamus, immune cells, lung, placenta, ovary, and testis (Gualillo et al. 2001; Hattori et al. 2001; Korbonits et al. 2001; Date et al. 2002; Volante et al. 2002; Gaytan et al. 2003,2004; Raghay et al. 2006). Furthermore, ghrelin has been identified in various tumors (Korbonits et al. 2001; Papotti et al. 2001; Iwakura et al. 2002; Volante et al. 2003; Tsolakis et al. 2004; Ekeblad et al. 2007).
Obestatin, an amidated 23 amino acid peptide, has been isolated from rat stomach (Zhang et al. 2005) and is derived from the carboxy-terminal part of proghrelin, whereas ghrelin is derived from the N-terminal part of the same precursor. It has been reported that obestatin has inhibitory effects on feeding and digestive motility and thus antagonizes the stimulatory effect of ghrelin through interaction with the orphan GPR39 receptor (Zhang et al. 2005; Lagaud et al. 2007). These findings have lately been questioned (Gourcerol et al. 2006; Lauwers et al. 2006; Bassil et al. 2007), and further studies are needed to determine the physiological function of obestatin. In a recent publication, the distribution of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats was characterized (Zhao et al. 2007). However, the allocation of obestatin in human tissues remains largely unknown. In this study, we characterized the presence of obestatin-IR cells and ghrelin-IR cells in a large panel of human tissues.
Materials and Methods
Antibody Production
A peptide, CFNAPFDVGIKLSGVQYQQHSQAL-amide, corresponding to human obestatin with an additional N-terminal cysteine residue, was synthesized. The peptide was coupled through the cysteine residue to maleimide-activated keyhole limpet hemocyanin. Free peptide was removed using dialysis. A rabbit was immunized with the peptide-carrier complex using a standard immunization protocol. The antiserum was used without further purification.
Western Blotting
The specificity of the obestatin and ghrelin antibodies was evaluated by Western blot analysis. Obestatin (2.0 μg) and ghrelin (2.0 μg) (cat. no. 031-80; Phoenix Pharmaceuticals, Burlingame, CA) peptides were used. Peptides were subjected to SDS-PAGE 16.5% tris-tricine gel (BioRad; Hercules, CA) and transferred to polyvinylidene difluoride membrane (Amersham Biosciences; Buckinghamshire, UK). The membrane was blocked in PBS, pH 7.4, with 5% BSA (Sigma-Aldrich; Steinheim, Germany) and 0.5% Tween-20 (Sigma-Aldrich) for 1 hr at room temperature. The membrane was incubated with rabbit anti-obestatin antibody (1:300) in PBS with 1% BSA and 0.1% Tween-20 overnight at 4C and rinsed in PBS with 0.5% Tween-20 three times, followed by incubation with horseradish peroxidase–conjugated anti-rabbit secondary antibody (1:10,000; Amersham Biosciences) in PBS with 5% BSA and 0.5% Tween-20 for 1 hr. After washing the membrane three times in PBS with 0.5% Tween-20, bound antibodies were visualized using Lumi-Light reagent (Roche; Basel, Switzerland) and detected with the ChemiDoc XRS imaging system (BioRad). The membrane was thereafter stripped with 0.2 M NaOH and 0.5 M NaCl, blocked, and incubated with the rabbit anti-ghrelin antibody (1:2000, cat. no. H-031-30; Phoenix Pharmaceuticals) in PBS with 1% BSA and 0.1% Tween-20 overnight at 4C. After appropriate washing steps and incubation with horseradish peroxidase–conjugated anti-rabbit secondary antibody (1:10,000; Amersham Biosciences) in PBS with 5% BSA and 0.5% Tween-20, bound antibodies were visualized and detected as described above.
Human Tissues
Human tissues were obtained from surgically removed material, except for pituitary tissue, which was obtained at autopsy, and third trimester placenta and umbilical cord, which were obtained at partus. To be able to detect rare IR cells in the tissues, only large specimens of high histological quality were used in the study. The panel of collected human tissues is listed in Tables 1 and 2. Tissue samples originated from macroscopically and microscopically normal tissue and were examined by an experienced pathologist. Mammary tissue was represented by women of fertile age and postmenopausal women. Tissues were fixed in 3.7% formaldehyde in PBS and embedded in paraffin. The paraffin blocks were cut into 4-μm sections and attached to positively charged glass slides (Superfrost Plus; Menzel Gläser, Braunschweig, Germany).
Table 1.
Distribution of obestatin immunoreactive cells and ghrelin immunoreactive cells in human tissues
| Tissue | Cases | Obestatin | Ghrelin |
|---|---|---|---|
| Digestive tract | |||
| Cardia | 1 | ++ | ++ |
| Fundus | 5 | ++ | ++ |
| Corpus | 7 | ++ | ++ |
| Antrum | 3 | ++ | ++ |
| Duodenum | 3 | ++ | ++ |
| Jejunum | 3 | ++ | ++ |
| Ileum | 4 | ++ | ++ |
| Pancreas | 3 | ++ | ++ |
| Mammary glands | 3 | + | + |
Immunoreactivity was detected in all cases. ++, immunoreactivity clearly detected at an antibody dilution of 1:16,000; +, immunoreactivity clearly detected at an antibody dilution of 1:2000.
Table 2.
Human tissues non-immunoreactive for obestatin and ghrelin
| Tissue | Cases | Tissue | Cases |
|---|---|---|---|
| Digestive tract | Reproductive | ||
| Esophagus | 1 | Ovaries | 3 |
| Colona | 6 | Tuba | 1 |
| Rectum | 7 | Uterus | 3 |
| Anus | 3 | Epididymis | 2 |
| Liver | 2 | Testis | 5 |
| Gallbladder | 2 | Prostate | 2 |
| Ductus deferens | 3 | ||
| Lymphatic | |||
| Tonsil | 3 | Respiratory | |
| Thymus | 4 | Trachea | 1 |
| Appendix | 3 | Lungb | 3 |
| Endocrine | Other | ||
| Pituitary | 5 | Skin | 3 |
| Parathyroid | 5 | Spleen | 3 |
| Thyroid | 4 | Placenta | 6 |
| Adrenal gland | 1 | Umbilical cord | 3 |
Samples represented by cecum, ascendens, transversum, descendens, and sigmoideum colon.
Samples represented by bronchi, bronchioli, and alveoli.
No immunoreactivity was detected in any of the cases.
Immunohistochemistry
Immunohistochemistry was performed using the EnVision Plus-HRP Detection Kit (Dako; Glostrup, Denmark). All immunostainings were performed using the following protocol. Paraffin-embedded sections were deparaffinized. For antigen retrieval, the sections were subjected to pretreatment (microwave heating for 10 min at 750 W followed by 15 min at 380 W using Tris-HCl buffered saline, pH 8.0). The tissue sections were incubated 5 min in a 0.03% hydrogen peroxide solution to block endogenous peroxidase activity. The sections were incubated overnight at 4C with the obestatin or ghrelin antibody in PBS with 1% BSA, followed by incubation with secondary antibody (conjugated to horseradish peroxidase–labeled polymer) in PBS and 1% BSA. Bound antibodies were visualized by 5-min incubation with liquid 3,3′-diaminobenzidine substrate chromogen. The sections were finally counterstained in Mayer's hematoxylin, mounted, and evaluated under light microscope. Appropriate washing with PBS was performed between each step, and all incubations were performed in a moist chamber at room temperature.
Dilution series (1:500–1:32,000) were performed to determine the optimal dilution of the obestatin and ghrelin antibodies in gastric mucosa and pancreas. All tissues were screened using the obestatin and ghrelin antibodies diluted 1:8000 and 1:2000. An additional screen was performed on sections from the gastrointestinal tract and pancreas using antibody dilutions of 1:16,000. To determine the relative abundance of obestatin-IR cells in the gastrointestinal tract, IR cells of all cases were counted in 10 randomly chosen unit areas of the mucosa.
The specificity tests performed for the obestatin and ghrelin antibodies included the following: (a) negative controls, where the antibodies were replaced by normal rabbit serum (Vector Laboratories; Burlingame, CA) at the respective dilution; (b) neutralization tests, conducted by preabsorption (2 hr at 4C) of the antibody with the homologous antigen (ghrelin, 10 μM, cat. no. 031-80; Phoenix Pharmaceuticals, or obestatin, 10 μM) before application to the sections; (c) cross-reaction tests, performed by incubation of the ghrelin antibody with obestatin peptide (2 hr at 4C) and vice versa.
Immunofluorescence Microscopy
Paraffin-embedded sections were deparaffinized and subjected to pretreatment for antigen retrieval (microwave heating for 10 min at 700 W in Tris-HCl–buffered saline, pH 8.0). Sections were incubated 30 min with blocking solution (donkey serum, diluted 1:5 in PBS; Jackson ImmunoResearch, Newmarket, UK).
For triple immunofluorescence staining, the sections were incubated for 1 hr in blocking solution containing rabbit anti-obestatin (1:400), mouse anti-chromogranin A (1:2000, clone LK2H10; Chemicon, Temecula, CA), and chicken anti-ghrelin (1:400, cat. no. Y-031-44; Phoenix Pharmaceuticals). Sections were washed in PBS and incubated for 1 hr with a cocktail of secondary antibodies including donkey anti-mouse conjugated to tetramethyl rhodamine isothiocyanate (TRITC, 1:100; Jackson ImmunoResearch), donkey anti-chicken conjugated to aminomethylcoumarin acetic acid (AMCA, 1:50; Jackson ImmunoResearch), and donkey anti-rabbit conjugated to FITC (1:100; Dako Cytomation, Glostrup, Denmark) diluted in blocking solution. Appropriate washing in PBS was performed between each step, and incubation was performed in a dark moist chamber at room temperature. The sections were mounted with Vectashield (Vector Laboratories) and evaluated. Tissues were photographed by an Axiocam HRm camera using Axiovision imaging software, a ×63 Plan-Apochromat objective, and a Zeiss Axioplan 2 microscope (Carl Zeiss; Göttingen, Germany). Triple immunofluorescence stainings were performed on all IR tissues.
For double immunofluorescence staining of mammary tissue, the sections were prepared as described above and incubated overnight at 4C in blocking solution containing rabbit anti-obestatin (1:100) and chicken anti-ghrelin (1:100, cat. no. Y-031-44; Phoenix Pharmaceuticals). Sections were washed in PBS with 0.05% Tween-20 and incubated 1 hr at room temperature with secondary antibodies: donkey anti-chicken conjugated to TRITC (1:50; Jackson ImmunoResearch) and donkey anti-rabbit conjugated to FITC (1:50; Dako Cytomation) diluted in blocking solution. Appropriate washing in PBS with 0.05% Tween-20 was performed between each step, and incubation was performed in a dark moist chamber. Sections were mounted and visualized as previously described.
Specificity tests for the obestatin and ghrelin antibodies included neutralization and cross-reaction tests as described above. For chromogranin A, the test for specificity was performed by replacement of the primary antibody by non-immune mouse serum (Dako Cytomation).
Ethical Approval
This study was reviewed and approved by the local ethics committee.
Results
Western Blot Analysis
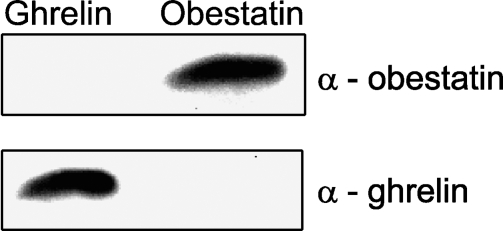
The specificity of the obestatin and ghrelin antibodies was confirmed by Western blot (Figure 1). The obestatin antibody detected obestatin with an estimated molecular size of ∼2.6 kDa. After stripping the membrane and incubating with the ghrelin antibody, a strong band corresponding to the ghrelin peptide was shown at the expected molecular mass of 2.7 kDa. Thus, there was no cross-reactivity between the ghrelin and obestatin antibodies.
Figure 1.
Western blot analysis with obestatin and ghrelin peptides. (Top panel) Obestatin peptide clearly recognized by the anti-obestatin antibody (dilution 1:300). (Bottom panel) Same membrane after incubation with anti-ghrelin antibody (dilution 1:2000) detects a band corresponding to the ghrelin peptide.
Distribution of Obestatin-IR Cells and Ghrelin-IR Cells
The distribution of obestatin and ghrelin immunoreactivity in human tissues is summarized in Tables 1 and 2. In previous reports, ghrelin-IR cells were located in the gastric mucosa and pancreas. Therefore, appropriate dilutions for immunohistochemistry of the obestatin and ghrelin antibodies were determined using these tissues. In gastric mucosa and pancreas, both the obestatin and ghrelin antibodies gave strong and distinct immunostaining without an interfering background at a dilution of 1:8000. Both peptides showed a similar immunostaining pattern in the gastric mucosa that was confined to the cytoplasm.
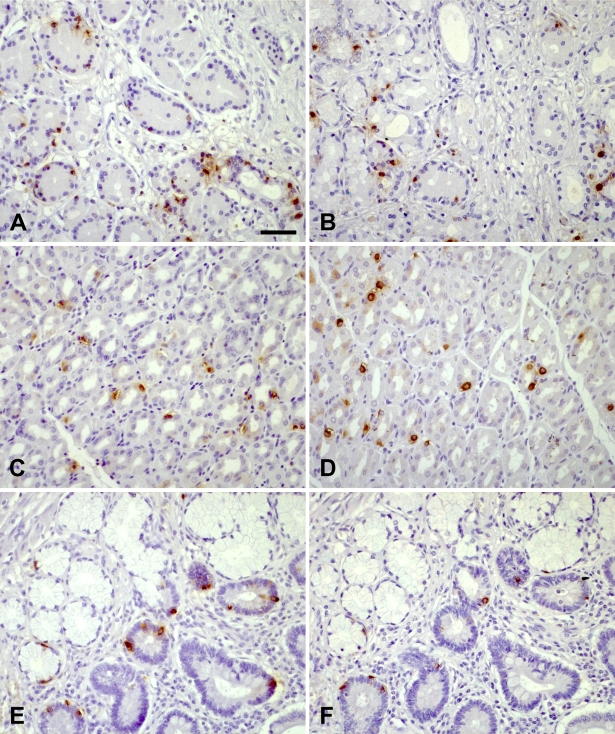
Obestatin-IR cells and ghrelin-IR cells were found throughout the gastrointestinal tract. IR cells were seen in all parts of the stomach including cardia (Figures 2A and 2B), fundus, corpus (Figures 2C and 2D), and antrum. The IR cells were most numerous in the stomach region, less abundant in the duodenum, occasional in jejunum, and rare in ileum. No IR cells were detected in the colonic mucosa or caudally. A substantial number of obestatin-IR cells and ghrelin-IR cells were found in the crypts of Lieberkühn, whereas only a few scattered IR cells were seen in the Brunner's glands (Figures 2E and 2F). The vast majority of the obestatin-IR cells and ghrelin-IR cells were located in the deeper third of the glands, whereas few cells were found in superficial mucosa regions of the cardia, fundus, duodenum (Figure 3), and ileum. A limited number of both obestatin-IR and ghrelin-IR cells were found in the periphery of pancreatic islets. A few scattered IR cells were detected in connection to the exocrine pancreatic ducts. No IR cells were seen in the other organs and tissues examined in this screen (1:8000 antibody dilution).
Figure 2.
Immunohistochemical stainings of human tissue. Sections immunostained for ghrelin (A,C,E) and obestatin (B,D,F). Cardia (A,B): cells immunoreactive for obestatin and ghrelin are located in the deeper regions of the mucosa including the cardiac glands. Corpus (C,D): obestatin and ghrelin immunoreactive cells are scattered throughout the whole mucosa. Duodenum (E,F): scattered obestatin and ghrelin immunoreactive cells are seen in the crypts of Lieberkühn, some also in connection to Brunner's glands. Bar = 50 μm.
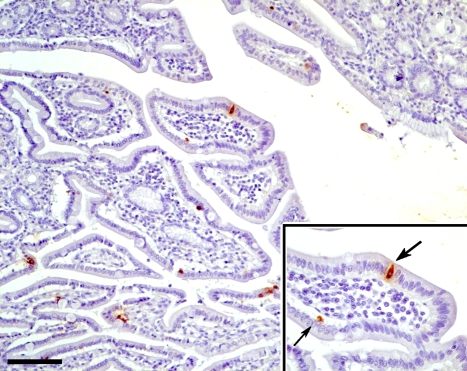
Figure 3.
Section of duodenum showing ghrelin immunoreactive cells, some superficially located. Some cells are of the open type reaching the lumen with long processes (thick arrow); others are of the closed type (thin arrow). (Inset) Higher magnification of a part of the mucosa. Bar = 100 μm.
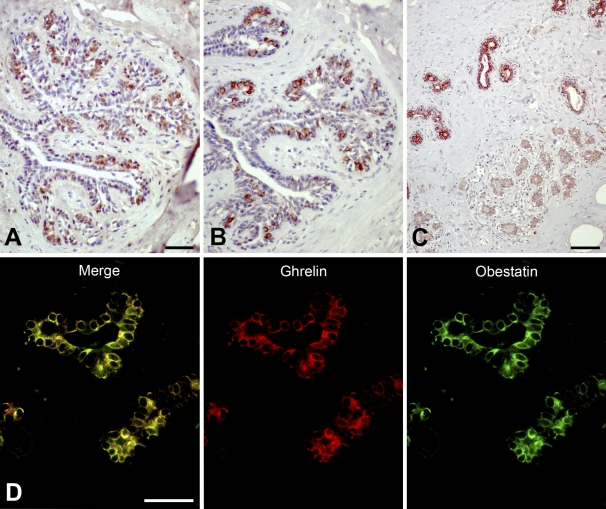
The screening of the tissue panel with a dilution of 1:2000 (for both obestatin and ghrelin antibodies) was performed to detect cells with weaker immunoreactivity. At this dilution, obestatin-IR cells and ghrelin-IR cells were found in the ducts of the mammary glands (Figure 4). Both antibodies gave similar staining patterns. Cytoplasmic immunoreactivity was seen in ductal epithelial cells, whereas the myoepithelial cells were non-IR. Immunostaining was most apparent in the ducts, whereas the lobuli showed occasional weak immunostaining or were non-IR. In some ducts, the vast majority of the epithelial cells were IR; other ducts showed a more scattered staining pattern. The staining pattern was similar in all specimens examined.
Figure 4.
Immunostaining for obestatin and ghrelin peptides in human mammary glands. Ghrelin (A) and obestatin (B) immunoreactivity is detected in inner cells of the ducts. Occasional weaker staining is found in the lobuli. (C) Section taken from inner part of the glands showing obestatin immunoreactivity in the ductal epithelium, whereas occasional immunoreactive cells are found in the lobuli. (D) Immunofluorescence staining of ducts in mammary tissue. Ghrelin [tetramethyl rhodamine isothiocyanate (TRITC)] is visualized as red and obestatin (FITC) as green. Yellow color in the merged image indicates co-localization of obestatin and ghrelin. Bars: A,B = 50 μm; C = 100 μm; D = 20 μm.
Neutralization tests for obestatin and ghrelin antibodies in the gastrointestinal tract and the mammary glands resulted in lack of immunoreactivity. The same finding was seen when substituting the primary antibody with normal rabbit serum. When performing cross-reactivity experiments, the immunoreactivity remained unchanged.
In the high antibody concentration screen (1:2000), pituitary, prostate, testis, placenta, ovaries, thyroid, and parathyroid tissues showed weak immunoreactivity. The immunoreactivity in these tissues was assessed as background because it could not be blocked with the respective peptides.
Co-localization Studies
The immunohistochemical screening indicated that obestatin and ghrelin have similar distribution patterns in all tissues examined. Immunofluorescence microscopy, conducted on a majority of the cases of IR tissues, showed that all detected obestatin-IR cells were also IR for ghrelin and vice versa (Figures 4 and 5). The subcellular distribution of both peptides showed an intracellular granular pattern, typical for large secretory granules in endocrine cells. Furthermore, in all IR tissues, the subcellular localization of obestatin and ghrelin was found to be essentially identical.
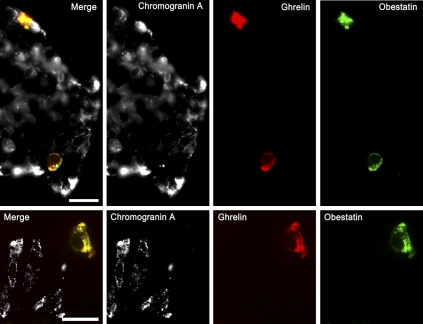
Figure 5.
Triple immunofluorescence for the localization of ghrelin, obestatin, and chromogranin A in human tissue. Chromogranin A (TRITC) is visualized as white; ghrelin (aminomethylcoumarin acetic acid) as red, and obestatin (FITC) as green. The left image in each set is a merged image generated from the white, red, and green channels. Yellow color indicates co-localization of obestatin and ghrelin. (Top panels) Pancreas. (Bottom panels) Gastric mucosa. Bar = 20 μm.
To further characterize the obestatin and ghrelin cells, sections were also immunostained with the endocrine marker chromogranin A. Very weak immunoreactivity for chromogranin A was found in a few obestatin/ghrelin-IR cells of the gastrointestinal tract and pancreas. However, chromogranin A was undetectable in the vast majority of obestatin/ghrelin-IR cells in all tissues examined. In mammary tissue, no chromogranin A immunoreactivity was detected (data not shown).
All control immunostainings resulted in lack of immunoreactivity. When performing cross-reactivity experiments, the immunoreactivity remained unchanged.
Discussion
In this study, obestatin-IR cells and ghrelin-IR cells were found throughout the gastrointestinal tract and in the pancreas. Interestingly, obestatin-IR cells and ghrelin-IR cells were also detected in the ductal epithelium of the mammary glands. Ghrelin-IR cells have been identified in human breast cancer and cell lines (Jeffery et al. 2005). However, the presence of obestatin-IR cells and ghrelin-IR cells in normal mammary glands has not previously been described. Although the immunoreactivity detected in the mammary glands is specific, the amount of obestatin and ghrelin in the cells in the mammary tissue seems to be less than in the gastrointestinal tract. This assumption is based on the fact that the antibodies have to be less diluted to detect the IR cells in the mammary glands. Because both obestatin and ghrelin are detected in the same cells, and the immunostaining can be completely blocked by the corresponding peptide, it is unlikely that the immunoreactivity we describe is caused by cross-reactivity with any unrelated protein. Currently, the functions of obestatin and ghrelin in mammary glands are unknown. The abundance of IR cells in the mammary glands indicates that these cells might influence circulating obestatin and ghrelin levels. It is also possible that these peptides act in a paracrine way or are secreted into the ducts and have an exocrine function.
Others have identified ghrelin-IR cells in human tissues that in this report are classified as non-IR for obestatin and ghrelin. These tissues include pituitary, lung, placenta, ovaries, testis, thyroid, and parathyroid (Gualillo et al. 2001; Korbonits et al. 2001; Volante et al. 2002; Gaytan et al. 2003,2004; Raghay et al. 2006). The lung samples, represented by sections of bronchi, bronchioli, and alveoli, do not show any obestatin or ghrelin immunoreactivity. In adult lung tissues, the amount of ghrelin-IR cells is reported to be very low (Volante et al. 2002), and it is possible that obestatin/ghrelin cells are so rare in our specimens that we are unable to detect any of these cells.
Using a high concentration of obestatin and ghrelin antibody, respectively (1:2000 dilution), placenta, ovaries, testis, pituitary, thyroid, and parathyroid generates weak stainings for ghrelin and/or obestatin. However, these immunostainings are assessed to be nonspecific, because they cannot be blocked by immunogenic peptides. Thus, we cannot confirm the results of these previous reports. As indicated in Table 2, the number of cases for some tissues is low. Regarding these tissues, it cannot be totally excluded that obestatin/ghrelin cells are present in some human individuals.
This study showed that obestatin-IR cells and ghrelin-IR cells are identified throughout the human gastrointestinal tract, from the cardia through fundus, corpus, antrum, duodenum, jejunum, to the ileum, as well as in the pancreas. The highest number of obestatin-IR cells and ghrelin-IR cells was detected in the cardia specimen. We could only obtain one sample of cardia, and because ghrelin or obestatin IR cells have not previously been described in this part of the stomach, it is important to further study the function of these peptides at this location. It should be noted that other tissue samples from this case, including fundus, antrum, and duodenum, appear as normal regarding the distribution of obestatin and ghrelin immunoreactivity. The immunoreactivity in the gastrointestinal tract is very strong because it can be clearly detected even at antibody dilutions of 1:16,000. A recent study focusing on the digestive tract of Sprague-Dawley rats showed that obestatin is present in the stomach, duodenum, jejunum, colon, and pancreas (Zhao et al. 2007). In the digestive tract of rats and humans, the distribution of obestatin-IR cells and ghrelin-IR cells is similar. However, no obestatin or ghrelin immunoreactivity was detected in the human colon. Moreover, in rats, only ∼60% of the obestatin-IR cells in the oxyntic mucosa were IR for ghrelin. In humans, obestatin seems to completely co-localize with ghrelin. It is possible that this discrepancy is related to differences in sensitivity of detection and/or species differences. Furthermore, it is reported that most obestatin-IR cells in the rat stomach are of the closed type, whereas in the intestine, most cells are of the open type. In humans, most of the IR cells are of the closed type, with occasional open-type cells in the duodenal mucosa.
It has been reported that ghrelin-IR cells (Dornonville de la Cour et al. 2001) and obestatin-IR cells (Zhao et al. 2007) present in the rat gastric mucosa contain chromogranin A. In this study, the vast majority of obestatin/ghrelin cells detected in human tissues were non-IR for this endocrine marker; a very weak staining was identified in a minor subset of obestatin/ghrelin cells. Thus, the distribution and the protein characteristics of obestatin/ghrelin cells in the digestive tract of humans and rats are similar but not identical. The function of these cells might be, in part, species specific.
Because chromogranin A is a main constituent of secretory vesicles of most endocrine cells, it is often used as an endocrine marker. This protein is co-stored with peptide hormones and is often processed in a cell type–specific pattern (Portela-Gomes and Stridsberg 2001). Because the monoclonal chromogranin A antibody we used is widely used as a marker of neuroendocrine tumors, the finding that obestatin-IR cells and ghrelin-IR cells in human tissues seem to be non-IR for this antibody is of clinical importance. Thus, lack of chromogranin A immunoreactivity does not rule out the presence of obestatin/ghrelin cells. These peptides have to be stained for separately in pathological conditions affecting the mucosa of the gastrointestinal tract, pancreas, and mammary glands.
In conclusion, we identified obestatin-IR cells and ghrelin-IR cells in the gastrointestinal tract, pancreas, and mammary glands. Our results indicate that it is important to further characterize obestatin/ghrelin cells concerning the presence of endocrine markers. In this aspect, it is also of importance to compare the obestatin/ghrelin cells in mammary glands with those in the gastrointestinal tract. Such studies could give us information that is valuable for showing the physiological function of obestatin and ghrelin in mammary glands.
Acknowledgments
This work was supported by the Swedish Cancer Society, the Selanders Foundation, and the Lions Foundation for Cancer Research at the Uppsala University Hospital.
The authors thank Professor Lars Grimelius for pathological expertise and Åsa Forsberg for excellent technical assistance. We gratefully acknowledge Dr Rose-Marie Amini for interpreting breast histology sections.
References
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, et al. (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758 [DOI] [PubMed] [Google Scholar]
- Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, et al. (2000) Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest 23:493–495 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, et al. (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345 [DOI] [PubMed] [Google Scholar]
- Bassil AK, Haglund Y, Brown J, Rudholm T, Hellstrom PM, Naslund E, Lee K, et al. (2007) Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br J Pharmacol 150:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, et al. (2002) Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 51:124–129 [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Bjorkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Hakanson R (2001) A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 99:141–150 [DOI] [PubMed] [Google Scholar]
- Ekeblad S, Lejonklou MH, Grimfjard P, Johansson T, Eriksson B, Grimelius L, Stridsberg M, et al. (2007) Co-expression of ghrelin and its receptor in pancreatic endocrine tumours. Clin Endocrinol (Oxf) 66:115–122 [DOI] [PubMed] [Google Scholar]
- Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C, Pinilla L, et al. (2004) Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab 89:400–409 [DOI] [PubMed] [Google Scholar]
- Gaytan F, Barreiro ML, Chopin LK, Herington AC, Morales C, Pinilla L, Casanueva FF, et al. (2003) Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J Clin Endocrinol Metab 88:879–887 [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St-Pierre DH, et al. (2006) Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides 27:2811–2819 [DOI] [PubMed] [Google Scholar]
- Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, Kojima M, Kangawa K, Dieguez C, et al. (2001) Ghrelin, a novel placental-derived hormone. Endocrinology 142:788–794 [DOI] [PubMed] [Google Scholar]
- Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C (2001) GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 86:4284–4291 [DOI] [PubMed] [Google Scholar]
- Inui A (2001) Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci 2:551–560 [DOI] [PubMed] [Google Scholar]
- Iwakura H, Hosoda K, Doi R, Komoto I, Nishimura H, Son C, Fujikura J, et al. (2002) Ghrelin expression in islet cell tumors: augmented expression of ghrelin in a case of glucagonoma with multiple endocrine neoplasm type I. J Clin Endocrinol Metab 87:4885–4888 [DOI] [PubMed] [Google Scholar]
- Jeffery PL, Herington AC, Chopin LK (2002) Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J Endocrinol 172:R7–11 [DOI] [PubMed] [Google Scholar]
- Jeffery PL, Murray RE, Yeh AH, McNamara JF, Duncan RP, Francis GD, Herington AC, et al. (2005) Expression and function of the ghrelin axis, including a novel preproghrelin isoform, in human breast cancer tissues and cell lines. Endocr Relat Cancer 12:839–850 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, et al. (2001) The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 86:881–887 [DOI] [PubMed] [Google Scholar]
- Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP (2007) Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun 357:264–269 [DOI] [PubMed] [Google Scholar]
- Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W (2006) Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun 351:21–25 [DOI] [PubMed] [Google Scholar]
- Papotti M, Cassoni P, Volante M, Deghenghi R, Muccioli G, Ghigo E (2001) Ghrelin-producing endocrine tumors of the stomach and intestine. J Clin Endocrinol Metab 86:5052–5059 [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M (2001) Selective processing of chromogranin A in the different islet cells in human pancreas. J Histochem Cytochem 49:483–490 [DOI] [PubMed] [Google Scholar]
- Raghay K, Garcia-Caballero T, Nogueiras R, Morel G, Beiras A, Dieguez C, Gallego R (2006) Ghrelin localization in rat and human thyroid and parathyroid glands and tumours. Histochem Cell Biol 125:239–246 [DOI] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, et al. (2006) Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 55:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolakis AV, Portela-Gomes GM, Stridsberg M, Grimelius L, Sundin A, Eriksson BK, Oberg KE, et al. (2004) Malignant gastric ghrelinoma with hyperghrelinemia. J Clin Endocrinol Metab 89:3739–3744 [DOI] [PubMed] [Google Scholar]
- Volante M, Allia E, Fulcheri E, Cassoni P, Ghigo E, Muccioli G, Papotti M (2003) Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol 162:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volante M, Fulcheri E, Allia E, Cerrato M, Pucci A, Papotti M (2002) Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem 50:1013–1021 [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 310:996–999 [DOI] [PubMed] [Google Scholar]
- Zhao CM, Furnes MW, Stenstrom B, Kulseng B, Chen D (2007) Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell Tissue Res 331:575–587 [DOI] [PubMed] [Google Scholar]