Abstract
Tumor invasion into blood and/or lymphatic channels is an important component of cancer staging and prognosis. Standard pathological methods do not provide sufficient contrast to discriminate between invasion into each type of vessel and are complicated by tissue retraction artifacts. We evaluated the ability of a triple-stain immunohistochemical method, combining cytokeratin, CD34, and podoplanin stains in a single section, to distinguish blood from lymphatic vascular invasion in oral squamous cell carcinoma and confirmed its results using multispectral analysis. The triple-stain method was significantly more sensitive in detecting invasive events than the standard hematoxylin and eosin staining method and easily discriminated between blood and lymphatic vessel invasion. Invasive events were present in blood and/or lymphatic vessels in the majority of patients with and without presentation of lymph node metastasis, indicating that vessel invasion in this cancer model is common and is not a rate-limiting step for lymph node metastasis. (J Histochem Cytochem 56:803–810, 2008)
Keywords: vessel invasion, metastasis, oral squamous cell carcinoma, immunohistochemistry
The ability of a primary tumor to invade blood or lymphatic vessels is used in staging and prognosis of many human cancers (Greene et al. 2002). Evaluation of this ability in standard hematoxylin/eosin (H and E)–stained tumor sections is difficult, owing to the lack of contrast between individual tumor cells and the small vessels they invade, the tendency of vessel spaces to collapse because of loss of pressure after tissue excision, and the phenomenon of retraction, in which tumor tissue shrinks at a higher rate than the surrounding tissue, leaving tumor nests surrounded by vessel-like pseudospaces. In addition, many blood and lymphatic vessels are structurally similar and cannot be differentiated using standard H and E, although the consequences of blood and lymphatic metastasis are not equivalent. An improved method for the pathologist to better identify vessel invasion and to differentiate between invasion of the blood and lymphatic systems may improve cancer diagnosis by identifying tumors with more severe or metastatic phenotypes.
Metastasis is the leading cause of death among cancer patients, but the process by which cancer cells establish colonies in new organs is not well understood (Ries et al. 2005). Invasion into the vasculature is one of the first physical steps required for metastasis, and recent work suggests that an increased number of vessels may encourage lymphatic metastasis by providing increased access to the circulation, but current staining methods are inadequate for direct observation of tumor cell infiltration into the vessels (Hanahan and Weinberg 2000; Alitalo et al. 2005; Cao 2005).
Immunohistochemical (IHC) stains are superior to H and E stains for providing contrast, but are limited by the difficulty of staining a single slide with multiple specific antibodies and the lack of sufficient visual contrast to evaluate overlapping stains. Multispectral imaging provides a means to definitively analyze a single slide for multiple colors.
We developed a new triple-staining method that simultaneously labels tumor antigen and blood and lymphatic vessel markers, and tested it on a group of primary oral squamous cell carcinomas (OSCCs). OSCCs are useful for the study of human lymphatic metastasis because of their high frequency of lymphatic metastasis and correlation between metastasis and decreased prognosis. We then evaluated the effectiveness of the triple-stain method in identifying invasion into lymphatic (podoplanin+, CD34+/−) and blood (podoplanin−, CD34+) vessels using a multispectral camera and analysis system.
Materials and Methods
Study Population
Paraffin-embedded slides from 48 patients with newly diagnosed squamous cell carcinoma of the oral cavity were selected from the archives of the Hospital of the University of Pennsylvania upon the basis of pathological lymph node status, absence of distant metastasis, and availability of material. Clinical factors and survival data were obtained from chart review and the Social Security Death Index and subsequently deidentified at the Hospital of the University of Pennsylvania under Institutional Review Board approval. Tumors were pathologically staged according to American Joint Committee on Cancer guidelines. All patient resections were performed at the Hospital of the University of Pennsylvania from 1998 to 2003. Thirty (62.5%) patients were alive at the time of statistical analysis; for these patients, the median follow-up for survival was 4.6 years, with a range of 1.8–7.1 years.
Triple-stain Immunohistochemistry
Slides were deparaffinized and rehydrated, then incubated in 0.75% trypsin (Abcam Inc.; Cambridge, MA) in a humidified chamber at 37C for 10 min, washed in PBS, and incubated for 10 min in 10 mM sodium citrate (pH 6.0) at 95C. Slides were allowed to cool for 20 min, then blocked against endogenous peroxidase by incubation in 0.6% H2O2 in PBS at room temperature for 20 min. Slides were washed in PBS and incubated with pan-cytokeratin antibody (Vector Laboratories; Burlingame, CA) at 1:500 for 1 hr at 37C. After washing, the slides were incubated with biotinylated secondary antibody (Vector Laboratories) for 30 min at room temperature, washed again, and incubated with ABC complex (Dako; Carpinteria, CA) for 30 min at room temperature. Slides were developed with DAB (Vector Laboratories) for 30 sec, rinsed, and stored overnight in PBS at 4C. Slides were warmed through an incubation in room-temperature PBS, blocked against the peroxidase used during the previous stain for 20 min in 1.2% H2O2 in PBS, and blocked for 1 hr at 37C using the Mouse-On-Mouse kit (MOM; Vector Laboratories). The slides were briefly washed, incubated for 5 min in MOM diluent, and incubated with CD34 antibody (Qbend 10; Dako) at 1:250 for 1 hr. The slides were washed, incubated with biotinylated secondary antibody and ABC complex, and developed with Vector SG (Vector Laboratories) for 5 min. Slides were washed and the steps repeated for anti-podoplanin (AngioBio Co.; Del Mar, CA) at 1:50, which was developed with Vector VIP (Vector Laboratories) for 1 min. Slides were counterstained in hematoxylin (Dako), washed and dehydrated, then permanently mounted with Clarion (Sigma-Aldrich Corp.; St. Louis, MO).
Multispectral Visualization and Analysis
Entire slides were visually scanned at 10× by a pathologist. Three representative pictures of intratumoral and peritumoral hot spots were acquired at 10× using a Leica DMRA2 microscope (Leica Microsystems, Inc.; Bannockburn, IL). All occurrences of vessel invasion were captured in more detail at 20×. Pictures were captured and analyzed using the Nuance Multispectral Imaging System (Cambridge Research and Instrumentation, Inc.; Woburn, MA). Nonspecific background staining was individually subtracted from each image. Individual spectra were defined for the Nuance system by using known structures: artery for CD34, basal epithelium for podoplanin, epithelium for pan-cytokeratin, and cell nuclei for hematoxylin. Images were unmixed using Nuance software. Vessels in hot spots were classified as blood vessels if the vessel contained only CD34, as lymphatic vessels if the vessel contained only podoplanin or podoplanin and CD34, and were counted using the UTHSCSA ImageTool program (developed at the University of Texas Health Science Center at San Antonio, TX, and freely available from the Internet). Unmixed images of vessel invasion were compared with the original identifications to determine the accuracy of the original classifications.
Statistical Analysis
Description of variables included simple statistics such as mean, median, standard deviation, and range for continuous variables, and frequencies and percentages for categorical variables. The strength of agreement between two methods with respect to binary variables (e.g., positive/negative) was measured by κ statistic (Fleiss 1981). The κ values can range from 0 to 1, with values of 0.61 or greater indicating substantial or stronger agreement (Landis and Koch 1977). The null hypothesis that all agreement between staining methods is due to random chance (κ = 0) was tested by χ2 test (Fleiss 1981). The strength of agreement between two methods with respect to continuous variables was determined by paired t-test. The analysis of case classification used standard definitions of sensitivity (% correctly classified as disease positive), specificity (% correctly classified as disease negative), and overall accuracy (% correctly classified for positive or negative disease status). For analysis purposes, the pathological N stage was assumed as “truth” with respect to disease status, to which staining methods (e.g., single-slide H and E or triple-stain) were compared. Survival was estimated by the method of Kaplan and Meier (Kaplan and Meier 1958), and differences in survival between groups were assessed by the log rank test (Mantel 1966). All reported p values are two-sided. Significance was defined as p<0.05. Statistical analyses were performed with SPSS V12.0 (SPSS, Inc.; Chicago, IL).
Results
Triple-stain Immunohistochemistry
Three chromagens (DAB, Vector SG, and Vector VIP) were chosen on the basis of limited spectral interference with each other and with the hematoxylin counterstain. At high intensity levels, chromagens may scatter light rather than acting as pure absorbers. To limit light scattering, each chromagen was titrated for intensity vs development time, and a development time was chosen that fell within the linear portion of the intensity curve. Each antibody was tested for efficacy on control skin and tonsil tissue. Pan-cytokeratin antibodies stained epithelial cells, CD34 antibodies stained arteries and small vessels, and podoplanin antibodies stained basal epithelium, small vessels, and a thin reticular network within germinal centers. Tests with tumor tissue showed that pan-cytokeratin consistently stained tumor cytoplasm and that podoplanin often stained tumor cytoplasm, particularly at the basal layer of tumor nests.
Triple stains against cytokeratin, CD34, and podoplanin (Figure 1) were performed on slides from a series of 48 patients with oral squamous cell carcinoma with a wide range of pathological T and N stages (Table 1). To compare the results of the triple-stain protocol, which was performed on a single slide per patient, with the standard pathological H and E method, we also examined a single slide from the same block stained by H and E. Invasive events were identified by a combination of cytokeratin staining and malignant cytology. The triple-stain method identified angio/lymphatic vessel invasion (Figure 2) in 33 patients, compared with 9 for the H and E method (Table 2). The triple-stain method also identified almost 10 times as many occurrences of vascular invasion (102 invasive events) as the H and E method (13 invasive events), with the most profound increase in the identification of intratumoral invasive events. The invasive events involved both blood and lymphatic vessels and were both intratumoral and peritumoral. Although many instances of blood vessel invasion were identified (23 invasive events), the majority of invasive events were in lymphatic vessels (79 invasive events), and the majority of those events involved a single malignant cell (Table 3). Multispectral analysis confirmed 88/102 invasive events (86%) and changed the identity of the vessel from CD34+podoplanin− blood to CD34+podoplanin+ lymphatic vessel in five instances (4.9%). Multispectral analysis was shown to be highly concordant with the triple-stain method alone (κ = 0.91, p<0.001), and we conclude that the triple-stain method alone is sufficient to significantly improve identification of invasive events compared with the standard H and E method.
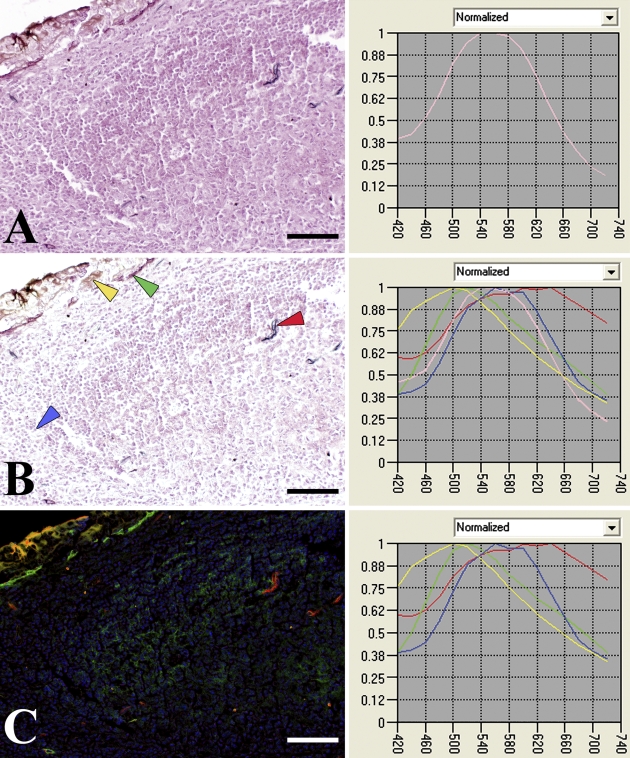
Figure 1.
Image translation using multispectral Nuance system. (A) A representative region of background was selected as the pink spectrum and subtracted from the entire image, a control region. (B) Spectra were then selected from representative areas from regions of positive controls (CD34, artery, red arrow; podoplanin, basal epithelium, green arrow; pan-cytokeratin, epithelium, yellow arrow; hematoxylin, nuclei, blue arrow). (C) Unmixing was performed using the four selected spectra. Bar = 0.1 mm.
Table 1.
Characteristics of 48 oral squamous cell carcinoma patients
| All patients | No./ 48 | %/100 |
|---|---|---|
| Age | ||
| ≤60 | 26 | 54.2 |
| >60 | 22 | 45.8 |
| Gender | ||
| Male | 36 | 75.0 |
| Female | 12 | 25.0 |
| Site of origin | ||
| Tongue | 26 | 54.2 |
| Floor of mouth | 15 | 31.3 |
| Mandible | 4 | 8.3 |
| Buccal/retromolar | 3 | 6.3 |
| Grade | ||
| 1 | 1 | 2.1 |
| 2 | 32 | 66.7 |
| 3 | 15 | 31.3 |
| Pathological T stage | ||
| 1 | 11 | 22.9 |
| 2 | 14 | 29.2 |
| 3 | 6 | 12.5 |
| 4 | 17 | 35.4 |
| Pathological N stage | ||
| N0 | 21 | 43.8 |
| N1 | 4 | 8.3 |
| N2b | 18 | 37.5 |
| N2c | 5 | 10.4 |
| Pathological report for angio/lymphatic vessel invasion | ||
| Positive | 7 | 14.6 |
| Negative | 41 | 85.4 |
| Local recurrence | ||
| Positive | 5 | 10.4 |
| Negative | 43 | 89.6 |
| Prior surgery | 0 | 0 |
| Prior chemotherapy/radiation | 1 | 2.1 |
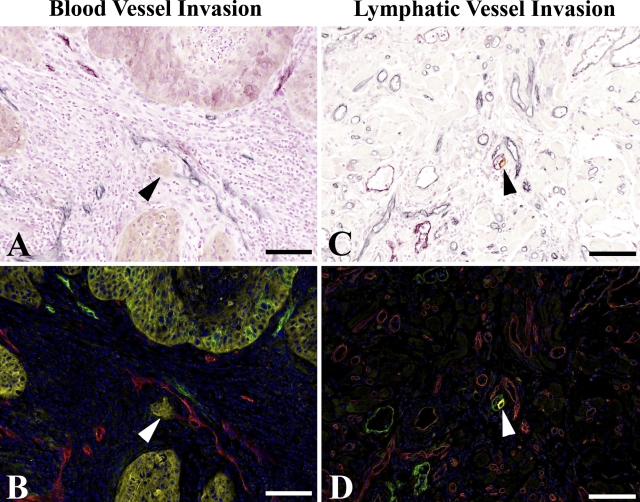
Figure 2.
Triple-stain immunohistochemistry and multispectral imaging identify occurrences of vessel invasion. Intratumoral invasion of tumor cells into blood vessel (A,B), arrowheads. Peritumoral invasion of tumor cell into lymphatic vessel (C,D), arrowheads. Triple-stain method under light microscope shows blood vessels as blue and lymphatic vessels as purple (A,C); unmixing by multispectral analysis shows blood vessels as red and lymphatic vessels as green (B,D). Blood and lymphatic vessels are clearly distinguished. Bar = 0.1 mm.
Table 2.
Increased sensitivity of triple-stain method over hematoxylin and eosin (H and E) in identifying occurrences of vessel invasion by tumor cells
| Paired differenceb
|
|||||
|---|---|---|---|---|---|
| Triple stain | H and E | Mean ± SE | 95% CI for mean | p | |
| Patients presenting with angio/lymphatic vessel invasiona | 33 | 9 | 0.88 | ||
| Number of invasive eventsc | 102 | 13 | 1.85 ± 0.38 | 1.09 to 2.62 | <0.001 |
| Intratumoral events | 48 | 4 | 0.92 ± 0.26 | 0.40 to 1.44 | 0.001 |
| Peritumoral events | 54 | 9 | 0.94 ± 0.26 | 0.42 to 1.46 | 0.001 |
Single-slide method. χ2 test of the null hypothesis that agreement between methods is no greater than expected by random chance.
Number of invasive events by triple-stain minus number of invasive events by H and E, per patient.
Paired t-test of the null hypothesis that paired difference means = 0.
Table 3.
Characteristics of tumor cell invasion into vessels
| Blood vessel invasion | Lymphatic vessel invasion | |
|---|---|---|
| Number of patients positive | 14 | 26 |
| Total number of invasive events | 23 | 79 |
| Intratumoral invasive events | 12 | 36 |
| Peritumoral invasive events | 11 | 43 |
| Total number of cells | 208 | 568 |
| Cells per event (mean) | 9.0 | 7.2 |
| Cells per event (median) | 2.0 | 1.0 |
| Percent single-cell events | 43.5 | 53.2 |
Clinical Evaluation
Because vascular invasion is an early step in metastasis, we examined the abilities of the different methods to classify patients as N0 or N+ (Table 4). In N+ patients, the H and E method identified very few cases with angio/lymphatic invasion, and as a result, had very low sensitivity (25.9% and 22.2% for the standard pathological method using multiple blocks and the single-block H and E, respectively). However, because these methods identified few cases with angio/lymphatic invasion overall, specificity was high (100% and 85.7% for pathological method and single-block H and E, respectively). In N+ patients, the triple-stain method identified invasion in many more cases, and as a result had a much higher sensitivity (81.5%) than the H and E method. But this method also identified invasion in over half of the N0 patients, resulting in a low specificity (47.6%). Even with the misclassification of some N0 patients, the triple-stain method still resulted in the correct classification of the highest number of patients, giving it the highest overall accuracy of the three methods (66.7%).
Table 4.
Sensitivity and specificity of tested methods to predict presence of metastasis in the lymph nodes by the presence of angio/lymphatic vessel invasion in the primary tumor. Standard pathological method using H and E, H and E on a single slide, and triple-stain immunohistochemistry on a single slide
| Angio/lymphatic vessel invasion
|
||||
|---|---|---|---|---|
| Positive | Negative | Total | Case classificationa | |
| Standard H and E | ||||
| N+ | 7 | 20 | 27 | Sensitivity 7/27 (25.9%) |
| N0 | 0 | 21 | 21 | Specificity 21/21 (100%) |
| Total | 7 | 41 | 48 | Accuracy 28/48 (58.3%) |
| Single-slide H and E | ||||
| N+ | 6 | 21 | 27 | Sensitivity 6/27 (22.2%) |
| N0 | 3 | 18 | 21 | Specificity 18/21 (85.7%) |
| Total | 9 | 39 | 48 | Accuracy 24/48 (50.0%) |
| Single-slide triple-stain | ||||
| N+ | 22 | 5 | 27 | Sensitivity 22/27 (81.5%) |
| N0 | 11 | 10 | 21 | Specificity 10/21 (47.6%) |
| Total | 33 | 15 | 48 | Accuracy 32/48 (66.7%) |
Case classification assumes pathological N stage is “truth” to which these methods are compared.
Using the triple-stain method, the percentage of patients with invasive events was higher in the N+ group than in the N0 group (81.5% vs 52.4%). There was a significant difference between the N+ and N0 groups in the average number of total colonies per patient (2.8 vs 1.2, p=0.04) using the triple-stain method, but not using the H and E method.
It is possible that patients with invasive events have a less favorable form of disease. Although the numbers of patients in this study were not sufficient to confirm this hypothesis, we did find that the 11 N0 patients presenting with invasive events showed a poorer prognosis (5-year survival rate + SE; 11.1% + 10.5%) than the 10 N0 patients with no identified invasive events (5-year survival rate + SE; 37.5% + 17.1%, p=0.07), and all 5 patients that presented with a local recurrence also presented with invasive events.
Vessel Quantitation
Hot spots were defined as areas with a high density of vessels observed under low power (10×). Counting the number of vessels in hot spots revealed a substantial variability in vessel numbers, both intratumoral and peritumoral (Table 5). Tumors from 43 of 47 patients (91.5%) contained CD34+podoplanin+ intratumoral lymphatic vessels, and 43 of 44 (97.8%) contained CD34+podoplanin+ peritumoral lymphatic vessels, but the number of double-stained vessels averaged under 30% of the total number of lymphatic vessels present in both areas. Numbers of intratumoral and peritumoral lymphatic vessels were similar, but the number of peritumoral blood vessels was higher. Both intratumoral and peritumoral blood vessels also tended to be present in much higher numbers than lymphatic vessels.
Table 5.
Characteristics of blood and lymphatic vessels
| Intratumoral (n=47) | Peritumoral (n=44) | |
|---|---|---|
| Lymphatic vessels present (podoplanin+, CD34+) | 43 (91.5%) | 43 (97.8%) |
| Vessel countsa | ||
| Lymphatic vessels (podoplanin+, CD34+) | 3.6 ± 3.6 (0.0–16.3) | 5.5 ± 4.9 (0.0–18.7) |
| Lymphatic vessels (podoplanin+, CD34−) | 15.1 ± 10.1 (3.0–52.0) | 14.8 ± 9.3 (1.7–42.3) |
| Blood vessels (podoplanin−, CD34+) | 101.5 ± 38.1 (25.3–189.3) | 243.5 ± 85.0 (93.7–520.7) |
Mean ± SD (Range).
Discussion
Tumor infiltration into vessels is an important prognostic factor for many cancers (Greene et al. 2002). However, tumor cell infiltration is difficult to evaluate and the type of vessel cannot be determined by standard H and E staining. Vessel invasion can be successfully investigated by IHC staining of consecutive slides, but the analysis is time-consuming (Van den Eynden et al. 2006). This data shows that triple staining can be achieved on archival paraffinized tissue to highlight the presence of tumor in vessels and to distinguish between blood and lymphatic vessels. Triple staining is difficult because of cross-reaction between antibodies and color overlap; in this protocol, cross-reaction among the stains was minimized by the dual use of an increased concentration of H2O2 block (4× normal) and MOM block between the stains. The low level of cross-reaction was confirmed by eye using controls in which only one primary antibody was used but the rest of the staining protocol was unchanged, and also by the unmixing calculations of the Nuance multispectral system, which separates the spectral signatures of the chromagens and quantifies them individually. This spectral separation allowed us to confirm the presence of CD34 on some podoplanin+ lymphatic vessels, although the numbers of double-stained vessels varied widely among patients. The high degree of concordance between the triple-stain method and the multispectral analysis indicates that the color overlap using this method is minimal, and therefore the immunohistochemistry method may reliably be used alone, giving improved results with little loss of speed.
Many multicolor staining systems use immunofluorescent reagents (Uchihara et al. 1995; Wahlby et al. 2002; Van Vlierberghe et al. 2005) but have not yet been accepted into wide clinical use, partially because reading a three-color stain in dark-field can be quite difficult and time-consuming. In addition, fluorescent reagents are more expensive than standard reagents, extremely sensitive to light and time, may suffer from the decreased clarity of morphology in a frozen-section system, and are often difficult to optimize to a paraffinized system. This multicolor protocol allows for triple-color staining that can be performed on archived paraffinized tissue, quickly and easily read in bright-field, and stored for a longer period of time. This technique also has the potential to be automatable with standard, relatively inexpensive reagents, and may therefore have a cost/benefit ratio that is clinically relevant. The system does not require fluorescence filters or the overlay of multiple pictures, making it potentially less expensive and time-consuming than immunofluorescent protocols.
Our work confirms that of Kurtz et al. (2005), which showed that double staining oral carcinomas for CD31 (vessels) and cytokeratin (tumor) increased the number of vascular-positive patients to 42% (17/40) from 30% (12/40) in the original pathological reports based on H and E staining alone. Our addition of the podoplanin stain to simultaneously identify tumor invasion in both blood and lymphatic vessels in a three-color method increased the percentage of patients with identified vascular invasion from 15% (7/48) to 69% (33/48), a dramatic increase in sensitivity on both the CD31/cytokeratin method (Kurtz et al. 2005) and the podoplanin alone method of Kyzas et al. (2005) in head and neck squamous cell carcinomas (23/81, 29.4%).
Our work also confirms the results of Van den Eynden et al. (2006), which found that ∼70% of invasive events in breast cancer were missed by H and E evaluation when compared with immunohistochemical staining through a laborious process of comparing CD34 and podoplanin stains on consecutive sections (Van den Eynden et al. 2006).
The high numbers of invasive events and the display of vessel invasion by the majority of N0 patients indicates that vessel invasion is a common instead of a rare occurrence and is not a rate-limiting step in metastasis, consistent with the high numbers of tumor cells frequently present in the bloodstream and lymphatics that are not correlated with the presence of metastasis (Hewitt and Blake 1975; Fidler et al. 1978; Tarin et al. 1984; Racila et al. 1998; Sleeman et al. 2001). The relatively high numbers of blood vessels containing tumor cells (the “seed” of Paget's hypothesis) in patients without distant metastasis indicate that the patterning of metastasis in this disease is extremely dependent on the targeted organ (the “soil”) (Paget 1889). The ability of this method to distinguish between the non-equivalent paths of blood and lymphatic vessel invasion will enable more specific study of the process of metastasis, improving upon the standard method that combines these two different types of invasion into the single category of “lymphovascular invasion.”
The clinical significance of vessel invasion is not clear at this time, owing to the large number of N0 patients that displayed vessel invasion, although vessel invasion may possibly indicate a more dangerous phenotype, identifying patients who could benefit from further treatment. The number of patients, when subdivided, was insufficient to draw conclusions in this study. Future studies will determine the clinical significance of vessel invasion, which may potentially differ across types of disease.
Despite the growing literature correlating lymphangiogenesis, usually assessed by the quantitation of vessels in hot spots, with metastasis, the presence of vessel invasion in these patients was not linked with the number of lymphatic vessels, nor with any distinction between podoplanin+CD34− and podoplanin+CD34+ vessels (Beasley et al. 2002; Maula et al. 2003; Franchi et al. 2004; Cao 2005; Kyzas et al. 2005). Double-stained vessels were present in nearly all tumors, but their numbers varied widely. These results are a middle ground between Fiedler et al. (2006), which found CD34 positivity in the majority of tumor lymphatic vessels, and Xuan et al. (2005), which found CD34 expression in lymphatic vessels to be very rare, and suggest that CD34 positivity in lymphatic vessels may depend upon other unknown factors. Reports indicating that VEGF-C assists invasion have raised the possibility that enlarged vessels are easier to invade, but we found instead that the invaded lymphatic vessels were small (data not shown) (Alitalo et al. 2005).
By easily identifying a much larger group of invasive events, this method should allow the field to study the process of vessel invasion in more detail. A better understanding of the phenotype of the vessels being invaded may resolve the question of whether the correlation between lymphangiogenesis and metastasis is because a newly formed vessel is easier to invade, or because a tumor that is able to invade also spurs lymphangiogenesis. Improved information about a primary tumor's true capabilities must be beneficial to patients in the long term.
Acknowledgments
This work was supported by University of Pennsylvania Cancer Center Support Grant CA-016520 (to RM).
The authors thank Wendy Snyder, Clinical Research Coordinator of the Department of Otorhinolaryngology at the Hospital of the University of Pennsylvania, for her assistance in collecting patient data, and Danielle Murphy of the University of Pennsylvania for helpful advice about multicolor staining.
References
- Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438:946–953 [DOI] [PubMed] [Google Scholar]
- Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, et al. (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62:1315–1320 [PubMed] [Google Scholar]
- Cao Y (2005) Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer 5:735–743 [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Gersten DM, Hart IR (1978) The biology of cancer invasion and metastasis. Adv Cancer Res 28:149–250 [DOI] [PubMed] [Google Scholar]
- Fiedler U, Christian S, Koidl S, Bates DO, Christofori G, Augustin H (2006) The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol 168:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss J (1981) Statistical Methods for Rates and Proportions. 2nd ed. New York, Wiley
- Franchi A, Gallo O, Massi D, Baroni G, Santucci M (2004) Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer 101:973–978 [DOI] [PubMed] [Google Scholar]
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, eds (2002) AJCC Cancer Staging Manual. New York, Springer-Verlag
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Hewitt H, Blake E (1975) Quantitative studies of translymphnodal passage of tumor cells naturally disseminated from a non-immunogenic murine squamous carcinoma. Br J Cancer 31:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [Google Scholar]
- Kurtz KA, Hoffman HT, Zimmerman B, Robinson RA (2005) Perineural and vascular invasion in oral cavity squamous carcinoma. Arch Pathol Lab Med 129:354–359 [DOI] [PubMed] [Google Scholar]
- Kyzas P, Geleff S, Batistatou A, Agnantis NJ, Stefanou D (2005) Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol 206:170–177 [DOI] [PubMed] [Google Scholar]
- Landis J, Koch C (1977) The measurement of interrater agreement for categorical data. Biometrics 33:159–174 [PubMed] [Google Scholar]
- Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170 [PubMed] [Google Scholar]
- Maula SM, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R (2003) Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 63:1920–1926 [PubMed] [Google Scholar]
- Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 1:99–101 [PubMed] [Google Scholar]
- Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW (1998) Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA 95:4589–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries L, Harkins D, Krapcho M, Mariotto A, Miller B, Feuer E, Clegg L, et al., eds (2005) SEER Cancer Statistics Review, 1975–2003. Bethesda, MD, National Cancer Institute. http://seer.cancer.gov/csr/1975_2003/ (based on November 2005 SEER data submission)
- Sleeman JP, Krishnan J, Kirkin V, Baumann P (2001) Markers for the lymphatic endothelium: in search of the Holy Grail? Microsc Res Tech 55:61–69 [DOI] [PubMed] [Google Scholar]
- Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B (1984) Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 44:3584–3592 [PubMed] [Google Scholar]
- Uchihara T, Kondo H, Akiyama H, Ikeda K (1995) Single-laser three-color immunolabeling of a histological section by laser scanning microscopy: application to senile plaque-related structures in post-mortem human brain tissue. J Histochem Cytochem 43:103–106 [DOI] [PubMed] [Google Scholar]
- Van den Eynden G, Van der Auwera I, Van Laere S, Colpaert C, van Dam P, Dirix L, Vermeulen P, et al. (2006) Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer 94:1643–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vlierberghe RL, Sandel MH, Prins FA, van Iersel LB, van de Velde CJ, Tollenaar RA, Kuppen PJ (2005) Four-color staining combining fluorescence and brightfield microscopy for simultaneous immune cell phenotyping and localization in tumor tissue sections. Microsc Res Tech 67:15–21 [DOI] [PubMed] [Google Scholar]
- Wahlby C, Erlandsson F, Bengtsson E, Zetterberg A (2002) Sequential immunofluorescence staining and image analysis for detection of large numbers of antigens in individual cell nuclei. Cytometry 47:32–41 [PubMed] [Google Scholar]
- Xuan M, Fang Y-R, Wato M, Hata S, Tanaka A (2005) Immunohistochemical co-localization of lymphatics and blood vessels in oral squamous cell carcinomas. J Oral Pathol Med 34:334–339 [DOI] [PubMed] [Google Scholar]