Abstract
Polycyclic aromatic hydrocarbons (PAHs) are contaminants increasing in the environment largely due to burning of fossil fuels. Our previous work identified a synergistic toxicity interaction in zebrafish embryos occurring when PAHs that are agonists for the aryl hydrocarbon receptor (AHR) co-occur with PAHs that are CYP1A inhibitors. This toxicity is mediated by the AHR2, and morpholino knockdown of CYP1A exacerbated toxicity. This study tested two hypotheses: 1) in the absence of functional CYP1A, metabolism of PAHs is shunted towards CYP1B1, which has been shown in mammals to produce more reactive metabolites of PAHs; alternatively 2) CYP1B1 serves a protective role similar to CYP1A. We used a morpholino approach to knockdown CYP1B1 alone and in co-knockdown with CYP1A to determine whether we could alter deformities caused by synergistic toxicity of PAHs. CYP1B1 knockdown was not different from non-injected controls; nor were CYP1B1+CYP1A co-knockdown deformities different from CYP1A knockdown alone. These data suggest that CYP1B1 is not a significant factor in causing synergistic toxicity of PAHs, nor, in contrast to CYP1A, in providing protection.
Keywords: Polycyclic aromatic hydrocarbons, Zebrafish, CYP1B1, CYP1A
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic contaminants, currently increasing in the environment largely due to burning of fossil fuels (Van Metre and Mahler, 2005). Certain combinations of PAH (AHR agonist + CYP1A inhibitor) can result in synergistic embryotoxicity; this interaction has been shown to be regulated by the AHR2 in zebrafish, and CYP1A plays a protective role in this toxicity (Billiard et al., 2006). CYP1B1 overlaps in function with CYP1A, shares 39% protein similarity (Godard et al., 2005), and is thought to have a greater tendency to metabolize substrates to more toxic products than CYP1A. For example, CYP1B1 can metabolize the PAH benzo(a)pyrene (BaP) to the genotoxic metabolite trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene and in the presence of epoxide hydrolase, is ten times more active than CYP1A in doing so (Shimada et al., 1999). CYP1B1 null mice are protected from BaP induced bone marrow toxicity; BaP was only able to cause bone marrow toxicity in a low affinity AHR mouse unable to produce sufficient levels of CYP1A to detoxify BaP (Galvan et al., 2003). The authors showed that liver CYP1A serves to metabolize and prevent BaP from reaching the bone marrow where CYP1B1 can form the more toxic dihydrodiol epoxide PAH metabolite, leading to apoptosis of progenitor B-lmphocytes. This situation resembles the PAH synergistic developmental toxicity in our zebrafish studies, in which low levels of the PAH-type AHR agonist β-naphthoflavone (BNF) become toxic when CYP1A is unable to metabolize it (Billiard et al., 2006). Thus, one hypothesis is that a metabolic shift toward CYP1B1 is responsible, at least partly, for the synergistic toxicity in developing zebrafish. Alternatively, given the similarity of protein identity and function between CYP1A and CYP1B1, we also tested the hypothesis that CYP1B1 serves a protective function similar to CYP1A.
We used a morpholino approach to transiently knockdown expression of CYP1B1 in developing zebrafish embryos. Experiments were conducted using the zebrafish AB* strain following confirmation that the cDNA region surrounding the CYP1B1 ATG start sight was not different from the published Tubigen strain sequence NM_001013267 (data not shown). Morpholinos against CYP1B1 (5’-TCTCAGAGCCAGCAGGACATCCATC-3’), CYP1A (5′-TGGATACTTTCCAGTTCTCAGCTCT-3′), and a standard control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′) were obtained from GeneTools (Philomath, OR, USA). Embryos were injected with 3–5nl of 0.15mM morpholino and dosed with 21 µg/L 7-ethoxyresorufin, as well 1 µg/L BNF, 50 µg/L or 100 µg/L of the CYP1A inhibitor α-naphthoflavone (ANF) alone and in combination with BNF, from 24hpf to 96hpf (Billiard et al., 2006). At 96hpf, embryos were imaged for EROD activity and deformities, and results were quantified using IP Lab software (Scanalytics Inc., Fairfax, VA, USA) and expressed as % control as previously described (Billiard et al., 2006). Data presented as mean ± SEM (n= 3 pools of 5 embryos). While the large numbers of embryos required for these studies prevented inclusion of a control morpholino in every experiment, we have previously shown this control morpholino is not different from non-injected controls using these same chemical exposures (Billiard et al., 2006).
EROD activity was increased by exposure to BNF in the non-injected controls (NI) and CYP1B1-knockdown larvae, and not in the CYP1A-knockdown or CYP1A+1B1-double knockdown larvae. BNF-induced EROD activity was inhibited by co-exposure with ANF in all groups (data not shown). No significant deformity rescue or exacerbation was observed between CYP1B1-knockdown and NI controls, nor between CYP1B1+CYP1A double knockdown compared to CYP1A-knockdown alone (Fig. 1).
Fig. 1.

Quantification at 96 hours post fertilization of pericardial edema (a), jaw-eye gap length (b), and representative images (c) of non-injected (NI; i.e. no morpholino), CYP1B1-MO, CYP1A-MO, and CYP1A+1B1-MOs exposed to the AHR agonist BNF, CYP1A inhibitor ANF, alone and in combination, and DMSO vehicle control. A two-factor ANOVA for injection and treatment was significant for both factors and interaction term (p≤0.008). Fisher’s PLSD was used as a posthoc test. Both deformity measures show that CYP1B1-MO is indistinguishable from NI (edema p=0.2, jaw p=0.9) and that CYP1A+1B1-MOs are not different from CYP1A alone (edema p=0.7, jaw p= 0.6). Knockdown of CYP1A with and without CYP1B1-MO exacerbates toxicity at the coexposure dose of 1µg/L BNF+ 50µg/L ANF, a dose not toxic to NI or CYP1B1-MO larvae (p≤ 0.01). All groups showed similar toxicity levels at the dose of 1µg/L BNF+ 100µg/L ANF, which were significantly elevated above single treatments and vehicle controls (p< 0.0001). Images of deformed larvae are outlined in black.
To confirm knockdown of CYP1B1, we dosed control morpholino and CYP1B1-morpholino injected embryos with a more potent AHR ligand, PCB126 at a non-toxic dose of 1 µg/L, and used whole mount immunolocalization with CYP1B1 polyclonal antibodies (a generous gift from D. Buhler) to visualize protein knockdown at 72hpf. Immunohistochemistry was conducted as described previously for zfCYP1A (Andreasen, et al., 2002). CYP1B1 antibody was used at a concentration of 18 µg/mL, and Alexa Fluor® 594 goat anti-rabbit IgG at a dilution of 1:1500 (Molecular Probes, Eugene, OR) to impart fluorescence.
Localization of CYP1B1 at 72hpf was found in epithelial cells, and was inducible by PCB126 (Fig. 2). This induction was lessened in CYP1B1 knockdown embryos, although not completely. As the CYP1B1 antibody was made prior to the discovery of CYP1C1, it is possible that this polyclonal antibody recognizes CYP1C1 as well as CYP1B1. Regardless, we were able to obtain some knock-down with the CYP1B1 morpholino.
Fig. 2.
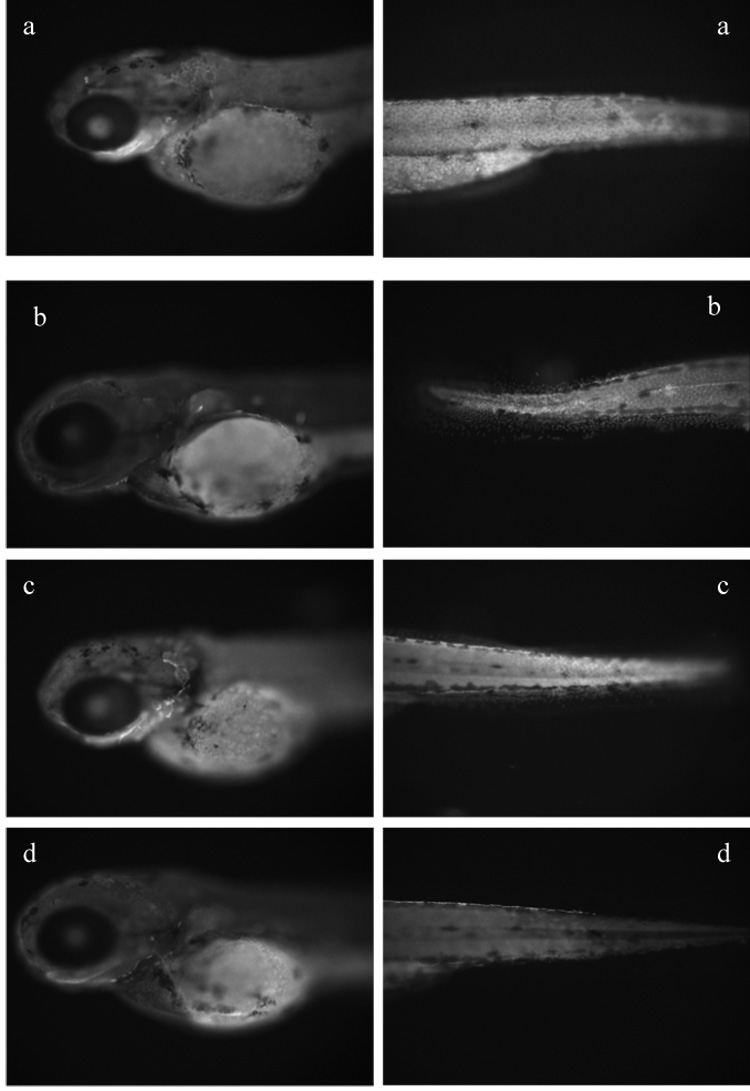
Immunolocalization of CYP1B1 (530 nm excitation and 586 nm emission filter; 100X, f500 microscopy). (A) Control-morpholino embryo exposed to PCB 126 showing induction of CYP1B1 indicated by strong fluorescence in epithelial cells. (B) Control-morpholino embryo exposed to DMSO showing less CYP1B1 induction, as indicated by limited fluorescence compared to (A). (C) CYP1B1 morpholino embryo exposed to DMSO and (D) CYP1B1 morpholino embryo exposed to PCB 126 showing less CYP1B1 induction as indicated by limited fluorescence compared to (A).
In light of recent studies identifying CYP1C1 as a fish specific closely related CYP1 family member, it has been suggested that the piscine CYP1B1 and CYP1C1 enzymes, which have 48% protein similarity, together serve the function of the singular CYP1B1 enzyme in mammals (Godard et al., 2005). It would thus be interesting to see in future studies whether knockdown of CYP1C1 alters PAH toxicity.
In conclusion, we were not able to observe any rescue or exacerbation of deformities by CYP1B1 knockdown by itself or in combination with CYP1A knockdown. Still, these data do not support a significant role for CYP1B1 in synergistic developmental toxicity of PAHs.
Acknowledgements
Elwood Linney, Dawoon Jung, Seth Kullman, NIEHS-supported Duke University Superfund Basic Research Center (P42 ES10356), Integrated Toxicology & Environmental Health Program (T32 ES07031) and EPA STAR fellowship (AT-L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Toxicological Sciences. 2002;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. Toxicological Sciences. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Galvan N, Jaskula-Sztul R, Macwilliams PS, Czuprynski CJ, Jefcoate CR. Toxicology and Applied Pharmacology. 2003;193:84–96. doi: 10.1016/s0041-008x(03)00338-7. [DOI] [PubMed] [Google Scholar]
- Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. Biochemical and Biophysical Research Communications. 2005;331:1016–1024. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, et al. Chemical Research in Toxicology. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Environmental Science and Technology. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Environmental Health Perspectives. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]


