Abstract
Bromelain, a mixture of proteases derived from pineapple stem, has been reported to have therapeutic benefits in a variety of inflammatory diseases, including murine inflammatory bowel disease. The purpose of this work was to understand potential mechanisms for this anti-inflammatory activity. Exposure to bromelain in vitro has been shown to remove a number of cell surface molecules that are vital to leukocyte trafficking, including CD128a/CXCR1 and CD128b/CXCR2 that serve as receptors for the neutrophil chemoattractant IL-8 and its murine homologues. We hypothesized that specific proteolytic removal of CD128 molecules by bromelain would inhibit neutrophil migration to IL-8 and thus decrease acute responses to inflammatory stimuli. Using an in vitro chemotaxis assay, we demonstrated a 40% reduction in migration of bromelain- vs. sham-treated human neutrophils in response to rhIL-8. Migration to the bacterial peptide analog fMLP was unaffected, indicating that bromelain does not induce a global defect in leukocyte migration. In vivo bromelain treatment generated a 50 – 85% reduction in neutrophil migration in 3 different murine models of leukocyte migration into the inflamed peritoneal cavity. Intravital microscopy demonstrated that although in vivo bromelain treatment transiently decreased leukocyte rolling, its primary long-term effect was abrogation of firm adhesion of leukocytes to blood vessels at the site of inflammation. These changes in adhesion were correlated with rapid re-expression of the bromelain-sensitive CD62L/L-selectin molecules that mediate rolling following in vivo bromelain treatment and minimal re-expression of CD128 over the time period studied. Taken together, these studies demonstrate that bromelain can effectively decrease neutrophil migration to sites of acute inflammation and support the specific removal of the CD128 chemokine receptor as a potential mechanism of action.
Keywords: proteinase, intravital microscopy, leukocyte adhesion, CD128, CD62L, neutrophil
Background
Bromelain is a natural mixture of proteolytic enzymes that is derived from pineapple stems and has been proposed be useful for therapy of immune-mediated diseases (1, 2). Bromelain has been used either alone or in a multi-enzyme preparation, most commonly combined with trypsin and rutin, in multiple clinical trials in both humans and animals. The level of proof, method of bromelain administration and dose, and quality of the studies vary, but beneficial effects were suggested or proven in a variety of inflammatory diseases and models of inflammation. These include the experimental allergic encephalomyelitis (EAE) model of the human autoimmune disease multiple sclerosis (3), carrageenan-induced pleurisy in the rat (4 – 6), immunologically mediated arteriosclerosis in rat aortic allografts (7), rheumatologic diseases in mice and humans (8 – 13), and allergic asthma (14) and rhinitis (15). Some studies demonstrated that bromelain had efficacy similar to standard anti-inflammatory drugs such as dexamethasone (5, 6) or non-steroidal anti-inflammatory agents (NSAIDs) (8, 10, 11, 16). Our previous studies showed that oral administration of 5 mg bromelain/day markedly decreased the development and severity of inflammatory bowel disease in IL-10−/− mice (17). Bromelain was also anecdotally reported to induce remission in 2 patients with refractory ulcerative colitis (18).
Despite the promising results of bromelain treatment in animal models and human clinical trials, the mechanisms that are primarily responsible for its anti-inflammatory effects are still unclear. However, proteolytic activity is required for the anti-inflammatory effect of bromelain on T cell activation and cytokine secretion in vitro (19 – 21) and in murine models of inflammatory bowel disease in vivo (17). We previously showed that in vitro bromelain treatment proteolytically removed at least 14 cell surface molecules that have been associated with leukocyte adhesion and/or activation (19, 22). Among the bromelain-sensitive molecules identified were CD62L (L-selectin), CD128a (CXCR1) and CD128b (CXCR2). The latter two molecules make up the receptor for IL-8, a chemokine that regulates neutrophil activation and chemotaxis to sites of acute inflammation (23). Leukocyte migration to sites of inflammation is a complex process (reviewed in 24) that first requires decreasing the velocity of leukocyte flow in the bloodstream through selectin-mediated rolling followed by chemokine-activated changes in integrin affinity that allow firm adhesion to the blood vessel wall. The binding of IL-8 to its receptor on neutrophils is thought to regulate the integrin affinity changes that result in firm adhesion, thus allowing the neutrophils to firmly adhere and then to transmigrate through the endothelium toward the source of chemoattractant. For example, IL-8 is increased in rectal dialysate from patients with ulcerative colitis, leading to the attraction and activation of neutrophils that drive the colonic inflammation that is characteristic of that disease (25). Use of anti-IL-8 antibodies to decrease adhesion and migration of neutrophils has been shown to decrease the severity of the resulting inflammation in several different in vivo models (reviewed in 26). The purpose of these studies was to determine the effects of bromelain on the migration of leukocytes, particularly neutrophils, and to further elucidate the mechanisms underlying these effects.
Materials and Methods
Reagents and Approvals
Bromelain was purchased from Sigma-Aldrich (catalog #B-4882; St. Louis, MO) or obtained as a gift from Hong Mao Biochemicals Company, Ltd. (Nikompattana, Thailand). Antibodies were obtained from R & D Systems (Minneapolis, MN), BD PharMingen (San Diego, CA), or Caltag Laboratories (Burlingame, CA). Unless specified, all other reagents were obtained from Sigma-Aldrich (St. Louis, MO), VWR (Atlanta, GA), or Invitrogen (Carlsbad, CA). All aspects of this study that involved participation of humans in research, including the consent form, were approved by the Institutional Review Board of Duke University Medical Center. All animal studies were approved by the Institutional Animal Care and Use Committee of Duke University.
In vitro Leukocyte Migration Assays
Healthy adults were recruited as blood donors after they gave informed consent for participation. Neutrophils (>90% purity) were obtained from their peripheral blood by density gradient centrifugation using Histopaque-1077 and -1119 (Sigma-Aldrich). The cells obtained were labeled with 1 µg/ml Calcein AM in PBS for 30 min at 37°C, then treated with RPMI1640 media or 100 µg/ml bromelain at 37°C for 30 min. Cells were washed, then 2 × 104 cells were added to the upper well of each chemotaxis chamber (ChemoTx microplates, Neuro Probe Inc, Gaithersburg, MD). The chemoattractants rhIL-8/CXCL8 (20 ng/ml) (Biosource, Camarillo, CA) or formyl-methionyl-leucyl-proline (fMLP, 10 nM) were placed in the lower chamber. Migration was allowed to occur for 3 hrs; cells present in the lower chamber were documented by flow cytometry. Results were expressed as a Migration Index, defined as the number of cells migrated in response to the stimulus divided by the number of cells migrated in response to media alone for each treatment condition. In some experiments, leukocyte migration was also confirmed by examining filters under fluorescence microscopy.
In vivo Leukocyte Migration Assays
Saline or 200 µg bromelain (10 mg/kg) in saline was injected intravenously (i.v.) into the tail vein of 5–8 wk old female Balb/C mice (Jackson Laboratories, Bar Harbor, ME). Thirty minutes later, 1 ml of 3% (w/v) thioglycollate, 300 ng/ml rhIL-8, or 1 µM fMLP was injected intraperitoneally (i.p.) to provide an inflammatory stimulus. Mice were euthanized 6 (thioglycollate) or 4 (IL-8 or fMLP) hrs later, and peritoneal lavage was performed with 10 ml ice-cold media containing RPMI1640, 5% fetal bovine serum, 5 mM EDTA, 10 mM HEPES, and 10 U/ml heparin. A total cell count was obtained using a hemocytometer and cells were further characterized by light microscopy and flow cytometry. Cytocentrifuge preparations were stained with a modified Wright-Giemsa stain (Hema 3 stain set, Fisher Scientific, Kalamazoo, MI) and a differential was performed. Flow cytometric analysis of peritoneal lavage cells included antibodies against the following antigens: Gr-1/Ly-6C/G (neutrophils; clone RB6-8C5), CD3ε (T cells; clone145-2C11), F4/80 (macrophages; clone BM8), and CD117/c-kit (mast cells; clone 2B8). Cells with the phenotype of Gr-1+ F4/80− were identified as neutrophils. The cytokines and chemokines present in peritoneal lavage fluid were quantitated using a Luminex bead-based fluorescent multiplex immunoassay (BioRad, Hercules, CA).
Intravital Microscopy
Peritoneal inflammation was elicited by injection of 1 ml of 3% thioglycollate i.p. 30 minutes after i.v. injection of 200 µg bromelain (10 mg/kg) in 0.1 ml saline or saline alone, as for the in vivo leukocyte migration assays. Six hours later, mice were anesthetized using Nembutal (80 mg/kg i.p) and the tail vein was cannulated using a 30 1/2 gauge needle with PE10 tubing. A 1 – 2 cm incision was made in the abdomen, the animal was placed in the lateral recumbent position on the stage of a fluorescence microscope, and a segment of intestine was exteriorized to allow visualization of mesenteric vessels. Rhodamine 6G dye (1.25 mg/kg in 100 µl saline) was injected i.v. to visualize leukocytes. Body core temperature was maintained at 37°C throughout the study using a temperature-controlled heating blanket (Homeothermic System, Harvard Apparatus) placed on the microscope stage. The temperature of the intestine/mesentery preparation was thermostatically controlled at 37°C using a circulating water bath. Leukocyte trafficking was visualized under fluorescence microscopy using Carl Zeiss MPS (Carl Zeiss, Hanover, MD). Fluorescence epi-illumination was provided with a 100 W mercury-arc lamp (AttoArc HBO, Carl Zeiss, Inc) with a rhodamine filter set (excitation 545 nm and emission 610 nm). Images were captured with a SIT camera (Hamamatsu, C2400-08) connected to an image processor (Hamamatsu Image Processor Argus, Hamamatsu Photonics) and a videocassette recorder (JVC BR-S378U). After observations were made, mice that were initially pre-treated with either bromelain or saline received an i.v. dose of 200 µg bromelain to assess its immediate effects on leukocyte migration. Bolus injection of FITC-dextran (50 µl of 5 mg/ml; 150 kDa MW) was used to quantitate blood flow in the vessels observed. At the conclusion of recording, mice were euthanized by barbiturate overdose and a total blood leukocyte count was obtained. Three mice were studied under each treatment condition, with two or more vessels examined in the majority of the mice studied.
Analysis of Bromelain Effect on Cell Surface Molecules
To assess the effects of bromelain on leukocyte expression of cell surface molecules in vivo, mice were treated with 200 µg bromelain i.v. then euthanized immediately or 30 minutes later. Peripheral blood was obtained from the inferior vena cava and anticoagulated using 3 mg EDTA/ml. Leukocyte expression of bromelain-sensitive molecules was determined by flow cytometry using antibodies against CD62L (clone MEL-14), CD128a/CXCR1 (MAB330/clone 42705), and CD128b/CXCR2 (MAB331/clone 48311.211 or 6C6).
Statistical Analysis
Student’s t test or analysis of variance (ANOVA) was used to compare differences between groups. A p value of ≤ 0.05 was considered to be statistically significant.
Results
Bromelain Treatment Decreases rhIL-8-Induced Migration of Human Neutrophils in vitro
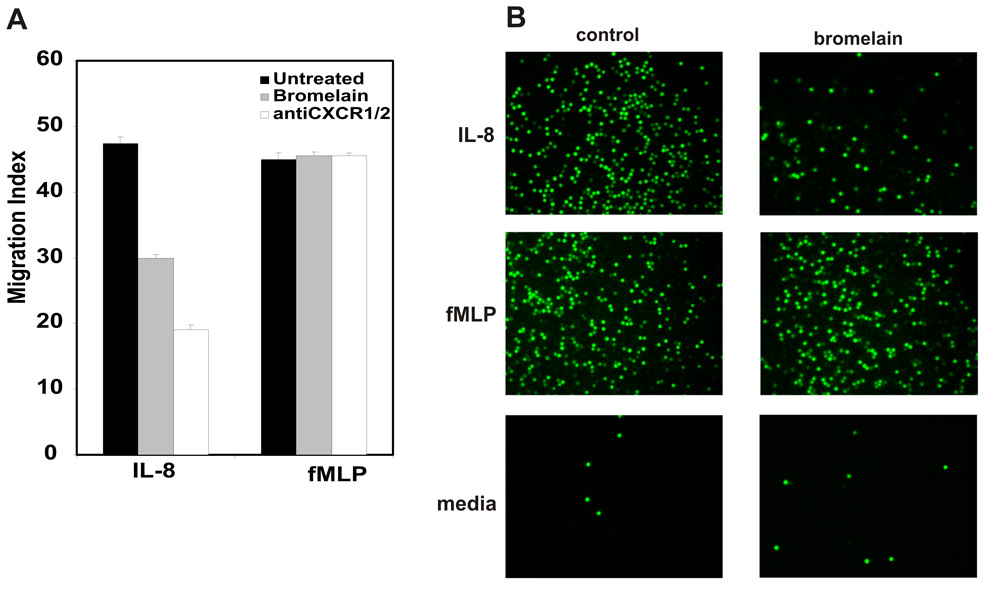
Bromelain treatment has previously been shown to remove the CD128a/CXCR1 and CD128b/CXCR2 receptors for IL-8, a chemokine that stimulates neutrophil chemotaxis and activation, from human neutrophils (22). Therefore, we determined the effect of bromelain treatment on IL-8-stimulated migration of human neutrophils using an in vitro chemotaxis assay. Flow cytometry demonstrated that the bromelain treatment conditions used reduced neutrophil CXCR1 to ~15% and CXCR2 to ~40% of that observed in sham (media)-treated neutrophils (data not shown). As shown in Figure 1, sham-treated human neutrophils migrated robustly in response to IL-8. In contrast, bromelain-treated neutrophils demonstrated a 40% reduction in IL-8-induced migration. A combination of CXCR1 and CXCR2 antibodies inhibited neutrophil migration by ~60%. This somewhat stronger inhibition by the CXCR1/CXCR2 antibody combination is consistent with the incomplete removal of CD128a/CXCR1 and CD128b/CXCR2 by the bromelain treatment conditions used in these studies. Importantly, neutrophil migration stimulated by the bacterial peptide analog fMLP was unaffected by bromelain treatment, indicating that bromelain does not induce a global defect in leukocyte migration.
Figure 1. Bromelain treatment inhibits IL-8-stimulated migration of human neutrophils in vitro.
A. Calcein-labeled human neutrophils that migrated through filters in response to IL-8 or fMLP as described in the Methods were quantitated by flow cytometry. Data shown is mean of triplicates wells from a single experiment, representative of 5 such experiments performed. Untreated and bromelain-treated neutrophils were tested as well as neutrophils exposed to saturating amounts of anti-CXCR1 and -CXCR2 antibodies. * indicates p≤ 0.01 vs. untreated cells. B. Fluorescence microscopy of the wells in the chemotaxis chamber at the conclusion of the migration period visually illustrates the reduction observed when bromelain-treated neutrophils migrate in response to IL-8, but not to fMLP.
Bromelain Treatment Inhibits Thioglycollate-Induced Migration of Murine Neutrophils in vivo
Since bromelain treatment specifically inhibited IL-8-mediated neutrophil migration in vitro, we wished to determine whether similar effects were also observed in vivo. We chose to use the well-established murine model of thioglycollate-induced peritonitis, since this normally results in robust neutrophil migration into the peritoneal cavity by 6 hrs after thioglycollate injection. We found that mice treated with 10 mg/kg bromelain i.v. before i.p. thioglycollate injection had a ~70% reduction in absolute neutrophil migration into the peritoneal cavity compared with saline-treated mice (Figure 2; p = 0.01). Wright-Giemsa-stained cytocentrifuge preparations from these mice visually confirmed the magnitude of the reduction in neutrophil migration into the peritoneal cavity in bromelain-treated, thioglycollate-stimulated mice (Figure 2).
Figure 2. Bromelain treatment decreases thioglycollate-stimulated neutrophil migration in vivo.
A. Data shown represent the mean ± SEM of the total (GR-1+ F4/80−) neutrophils obtained by peritoneal lavage 6 hrs after injection of thioglycollate (for saline and bromelain-pretreated mice; n = 5) or saline alone (for unstimulated mice; n = 4). Mice pretreated with bromelain had a ~70% reduction in neutrophil migration into the peritoneal cavity when stimulated with thioglycollate compared with saline-treated mice (* indicates p = 0.01). B. Flow cytometry shows 41%, 11%, and 0.4% neutrophils in peritoneal lavage specimens from representative saline- and bromelain-treated, thioglycollate-stimulated and unstimulated mice, respectively. C. Wright-Giemsa-stained cytocentrifuge preparations from these mice illustrate the reduction in neutrophil migration into the peritoneal cavity in bromelain-treated, thioglycollate-stimulated mice, consistent with the flow cytometric data.
Bromelain Treatment Does Not Reduce Peritoneal Production of Pro-Inflammatory Cytokines and Chemokines
Bromelain treatment has previously been shown to decrease production of certain pro-inflammatory cytokines and chemokines in vitro (20, 21). Decreased production of such mediators might decrease leukocyte migration to a site of inflammation by reducing pro-inflammatory or chemotactic stimuli. To address this possibility, the concentrations of a panel of 10 cytokines and chemokines (IFN-γ, TNF, IL-4, IL-6, IL-10, IL-12 p40 and p70, KC, MCP-1, MIP-1α) were measured in peritoneal lavage fluid from unstimulated and thioglycollate-stimulated mice using a multiplex fluorescence immunoassay. As shown in Figure 3, the concentration of each of these cytokines/chemokines was low to non-detectable in peritoneal lavage fluid from unstimulated mice. Thioglycollate stimulation markedly increased the concentrations of IL-6, KC, MCP-1, and MIP-1α in peritoneal lavage fluid, with lesser but statistically significant increases in IL-4, IL-10, IL-12 p40, IL-12 p70, and TNF. Bromelain treatment significantly increased levels of KC (the murine homologue of IL-8) that was present in lavage fluid (p = 0.006), but had no effect on levels of the other cytokines/chemokines tested (Figure 3). These data indicate that the stimulus for thioglycollate-elicited neutrophil emigration into the peritoneal cavity via the CD128a/CXCR1 and CD128b/CXCR2 receptors is not decreased in bromelain- vs. saline-treated mice over the time period examined.
Figure 3. Bromelain treatment in vivo does not decrease peritoneal expression of pro-inflammatory cytokines and chemokines.
Data shown is the mean ± SEM of the cytokine/chemokine concentration, reported as pg/ml peritoneal lavage fluid, for unstimulated (n = 2) and saline- (n = 5) or bromelain-pretreated (n = 6) thioglycollate-stimulated mice. Levels of most of the cytokines/chemokines tested increased in lavage fluid from thioglycollate-stimulated vs. unstimulated mice, but were not affected by bromelain treatment. The exception was KC (the murine homologue of IL-8), which was significantly increased (* denotes p = 0.006) in bromelain-treated mice.
Bromelain Treatment Inhibits IL-8 Induced Migration of Murine Neutrophils in vivo
Thioglycollate is a non-specific peritoneal inflammatory stimulus. To begin to determine the mechanisms by which bromelain decreased leukocyte migration in vivo, we measured leukocyte migration into the peritoneal cavity following i.p. injection of either rhIL-8 (which is recognized by murine CD128a/CXCR1 and CD128b/CXCR2 receptors) or fMLP, used as a non-specific stimulus of neutrophil chemotaxis. As seen for thioglycollate stimulation, bromelain treatment decreased the neutrophils present in the IL-8-stimulated peritoneal cavity by ~75% (Figure 3, left; p = 0.0001). However, in contrast to what we observed with human neutrophils in vitro, we found that i.v. bromelain treatment also decreased leukocyte migration by ~85% when fMLP was injected into the peritoneal cavity in vivo (Figure 3, right; p = 0.004). It is important to note that, although fMLP has direct chemotactic activity toward neutrophils that we exploited in our in vitro assays, in vivo fMLP injection also stimulates leukocyte migration by inducing production of the IL-8 homologue KC within the peritoneal cavity (27). Thus, taken together, these data are consistent with the hypothesis that bromelain may inhibit leukocyte migration via an IL-8-dependent mechanism.
Bromelain Treatment Primarily Affects Firm Adhesion of Leukocytes to Blood Vessels in vivo
Leukocyte adhesion to blood vessels and their subsequent extravasation into inflamed tissues is a complex process involving interactions with multiple adhesion molecules. We previously showed that in vitro bromelain treatment removed human CD62L, a cell surface molecule that mediates leukocyte rolling, as well as components of the human IL-8 receptor that stimulate firm adhesion to blood vessels at the site of inflammation (19, 22). Murine CD62L, CD128a/CXCR1, and CD128b/CXCR2 are also susceptible to proteolytic removal by bromelain. Mice deficient in CD62L/L-selection molecules that mediate leukocyte rolling have markedly impaired migration into the inflamed peritoneal cavity (28). Removal of CD128a/CXCR1, and CD128b/CXCR2 molecules might also be predicted to decrease leukocyte migration, by preventing IL-8-induced changes in integrin affinity that allow firm adhesion. To further elucidate the mechanisms by which bromelain affected leukocyte trafficking, mice were treated in vivo with either bromelain or saline, then injected i.p. with thioglycollate to generate a stimulus for leukocyte migration into the peritoneal cavity. The effect of bromelain treatment on leukocyte rolling and firm adhesion (“sticking”) to mesenteric blood vessels was then directly observed using intravital fluorescence microscopy (see supplemental videos).
Saline-treated, thioglycollate-injected mice had a high density of leukocytes adherent to their mesenteric blood vessels, with frequent rolling leukocytes (Table 1). In contrast, the mesenteric vessels in mice treated with bromelain prior to thioglycollate injection showed only rare adherent leukocytes (Table 1). The frequency of rolling leukocytes did not differ between saline- and bromelain-treated mice (Table 1). Thus, the primary effect of bromelain treatment given 6 hrs prior to observation was to decrease firm adhesion of leukocytes to blood vessels in the inflamed peritoneal cavity, with minimal to no effect on leukocyte rolling.
Table 1.
Intravital Microscopy Reveals Differential Effects of Bromelain on Leukocyte Rolling and Firm Adhesion*
| Adherent cells/field | Rolling cells/sec | |
|---|---|---|
| Saline pre-treatment | ||
| Initial | 96 ± 69 | 3.8 ± 0.6 |
| After bromelain injection | 115 ± 81 | 0.2 ± 0.1** |
| Bromelain pre-treatment | ||
| Initial | 8 ± 8 | 4.5 ± 1.5 |
| After bromelain injection | 62 ± 52 | 0.6 ± 0.4** |
Data shown is the mean ± SEM for vessels examined in 3 mice per treatment group. Numbers of adherent and rolling cells were normalized to reflect the area of the vessel imaged for each mouse studied. Units are arbitrary, based on the size of the microscopic field imaged.
indicates p < 0.03 (Student’s t test) vs. initial value
Injection of an additional (10 mg/kg) dose of bromelain into thioglycollate-injected mice essentially abrogated leukocyte rolling in mesenteric vessels within 10 sec of its injection into the tail vein, in mice initially treated with saline or with bromelain (Table 1). This additional dose of bromelain induced arrest of rolling leukocytes but did not cause detachment of the leukocytes that were already firmly adherent to the blood vessel walls. The arrested cells are scored as adherent, but the numbers of adherent cells/field initially and after bromelain injection are not statistically different in saline- or bromelain-treated mice.
Effects of Bromelain on Leukocyte Cell Adhesion Molecules in vivo
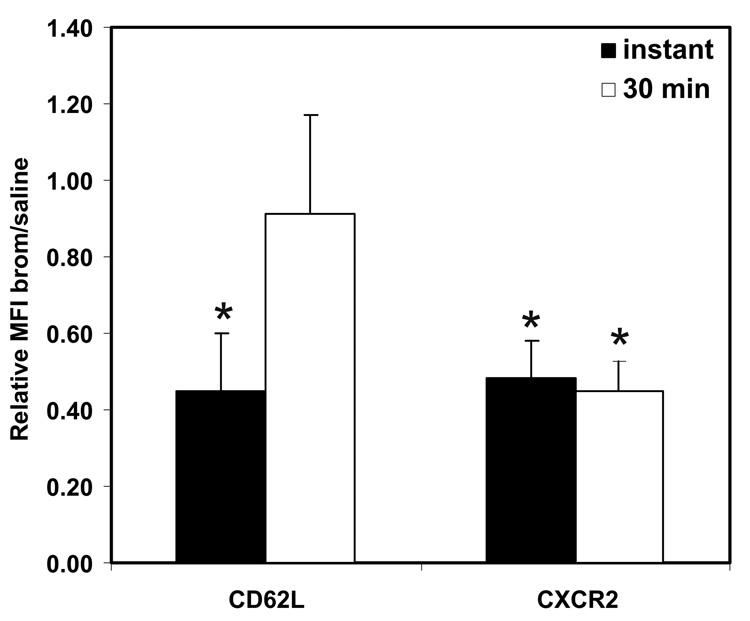
Although bromelain abrogated leukocyte rolling immediately after its i.v. injection, the intravital microscopy studies clearly showed that the primary long-term effect of in vivo bromelain treatment was on firm adhesion. To address the mechanisms underlying these observations, the expression of the bromelain-sensitive cell surface molecules CD62L and CD128b/CXCR2 on murine peripheral blood leukocytes in vivo was determined by flow cytometry either immediately or 30 min after i.v. injection of 10 mg/kg bromelain (Figure 5). We found that CD128b was reduced to ~50% normal immediately after bromelain injection (p = 0.05) and this level of expression was maintained over the next 30 minutes. In contrast, although leukocyte CD62L levels also initially reduced to ~45% of normal levels by in vivo bromelain treatment, CD62L expression returned to that seen in saline-treated mice within 30 min of bromelain injection. Based upon these observations, CD62L-mediated leukocyte rolling would be expected to be decreased only transiently (as observed), with more long-term suppression of the firm adhesion that is regulated by CD128 molecules, as was observed 6 hrs after bromelain injection.
Figure 5. Effect of bromelain treatment on cell adhesion molecules in vivo.
The expression of the bromelain-sensitive cell surface molecules CD62L and CD128b/CXCR2 on murine peripheral blood leukocytes was determined by flow cytometry either immediately or 30 min after i.v. injection of 10 mg/kg bromelain. Data shown represents the mean ± SEM of the ratio of mean fluorescence intensity (MFI) observed for bromelain-treated mice to that observed for saline-treated mice (n = 3/group). CD128b/CXCR2 was reduced to ~50% normal immediately after as well as 30 minutes following bromelain injection. In contrast, although leukocyte CD62L levels were initially reduced to ~45% by in vivo bromelain treatment, the reduction was transient and CD62L expression had returned to that seen in saline-treated mice by 30 min after bromelain injection. * indicates significant difference (p<0.05) compared with saline-treated mice.
Discussion
The purpose of this work was to investigate potential mechanisms for the anti-inflammatory activity of bromelain, particularly with respect to effects on the leukocyte trafficking that is characteristic of acute inflammation. The reported studies demonstrate significant reductions in the IL-8-mediated migration of bromelain- vs. sham-treated human neutrophils in vitro and in thioglycollate-, IL-8-, and fMLP-stimulated neutrophil migration into the inflamed peritoneal cavity of bromelain-treated mice. Although in vivo bromelain treatment transiently decreased leukocyte rolling, its primary long-term effect was abrogation of firm adhesion of leukocytes to blood vessels at the site of inflammation. These changes in adhesion were correlated with transient effects on expression of the bromelain-sensitive CD62L/L-selectin that mediate leukocyte rolling and longer lasting reduction of the expression of the CD128 molecules that regulate firm adhesion.
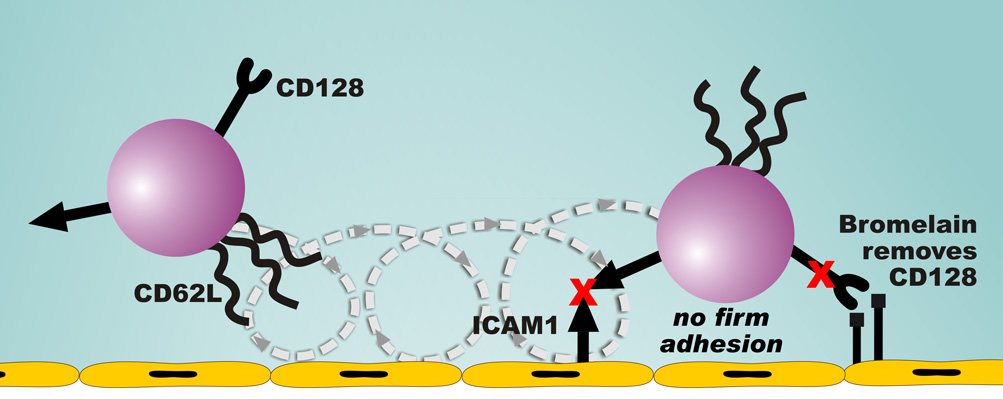
These studies presented here demonstrate that bromelain can effectively decrease neutrophil migration to sites of acute inflammation and support the specific removal of the CD128 chemokine receptor as a potential mechanism for this effect (Figure 6). Human neutrophils express CD128a/CXCR1 and a lesser number of CD128b/CXCR2 receptors. Although these receptors are often referred to as IL-8 receptors, they also bind other CXC chemokines. In mice, the IL-8 homologue KC serves as the primary neutrophil chemoattractant. However, the marked and significant decreases in neutrophil migration that we observed in vivo in bromelain-treated mice stimulated with rhIL-8 highlight the importance of murine CD128a/CXCR1 and CD128b/CXCR2 in the acute inflammatory response. Bromelain treatment of human neutrophils in vitro did not affect their migration in response to fMLP (Figure 1). However we did observe a decrease in fMLP-stimulated migration of neutrophils into the peritoneal cavity of bromelain-treated mice in vivo (Figure 4A). The human studies were done in vitro using purified neutrophils, whereas a variety of cell types may also have been affected by i.p. fMLP treatment in the murine in vivo studies. We feel that the decrease in neutrophil migration observed in our bromelain-treated mice that were stimulated with fMLP can be explained by the significant contribution of in vivo induction of the IL-8 homologue KC to the neutrophil migration that was previously reported for this model (27).
Figure 6. Mechanism for bromelain effects on leukocyte migration.
Although bromelain treatment in vivo removes CD62L, these molecules are rapidly re-expressed and the effect of bromelain on leukocyte rolling is transient. The predominant effect of bromelain on leukocytes is to reduce firm adhesion. We propose that longer lasting proteolytic removal of the CD128a/CXCR1 and CD128b/CXCR2 receptors by bromelain impairs chemokine-mediated changes in integrin affinity, resulting in the observed decrease in firm adhesion.
Figure 4. Bromelain treatment decreases IL-8 and fMLP-stimulated neutrophil migration in vivo.
A. Data shown is the mean ± SEM total (GR-1+ F4/80−) neutrophils present in peritoneal lavage fluid 4 hrs after injection of rhIL-8 (n = 14 for saline-treated; n = 14 for bromelain-treated mice) or the bacterial peptide analog fMLP (n = 13 for saline-treated; n = 10 for bromelain-treated mice). Mice treated with bromelain had significantly reduced neutrophil migration in response to both rhIL-8 (* denotes p = 0.0001) and fMLP (p = 0.004). B. Wright-Giemsa-stained cytocentrifuge preparations from these mice illustrate the reduction in neutrophil migration into the peritoneal cavity in bromelain-treated mice when stimulated with either rhIL-8 or fMLP.
Although the present studies support proteolytic removal of CD128 chemokine receptors as a mechanism for its effects on leukocyte migration, studies from our laboratory and others suggest that multiple mechanisms likely contribute to the observed anti-inflammatory effects of bromelain. We previously showed that at least 14 leukocyte cell surface molecules are proteolytically removed by bromelain treatment (19, 22). Each of these bromelain-sensitive molecules has been individually shown to play a role in leukocyte adhesion and/or activation. However, the net effect of bromelain treatment may depend on the activity of specific proteinases within the bromelain preparations used (29) as well as on the complement of bromelain-sensitive cell surface molecules expressed by the treated cells. Published studies have shown mixed effects of bromelain on activation and cytokine secretion by leukocytes (3, 19, 20, 30, 31) and colon epithelial cells (21). We found no significant decreases in the levels of pro-inflammatory cytokines in the peritoneal lavage fluid of bromelain-treated mice. In fact, levels of the IL-8 homologue KC were increased in peritoneal fluid, suggesting that the chemokine stimulus to neutrophil migration is likely higher in bromelain- vs. saline-treated mice despite the decreased neutrophil migration observed following bromelain treatment. The peritoneal fluid of bromelain-treated mice did not exhibit the marked decreases in the production of pro-inflammatory cytokines that have been previously reported in bromelain-treated lymphocytes (20) and colon biopsies (32). This difference may reflect both our earlier time point for cytokine measurement (6 hrs rather than 16 or 24 hrs) as well as biologic differences in activation pathways of lymphocytes vs. other leukocytes and the paucity of lymphocytes in the peritoneal cavity at the time of our analysis. The majority of inflammatory cells present in the peritoneal cavity at the time of our analysis were neutrophils and resident macrophages. Others have also reported activation of macrophage cytokine secretion by bromelain (30). We hypothesize that the net effect of bromelain in any given biologic situation depends on the cell types present, since each cell type may express different relative numbers of bromelain-sensitive pro-inflammatory vs. anti-inflammatory surface molecules and thus have differing net biologic responses to their proteolytic removal. Interestingly, the enhanced secretion of the IL-8 homologue KC into the peritoneal fluid that we observed in bromelain-treated mice is consistent with a previous report that IL-8 secretion was enhanced in Salmonella-infected human colon epithelial cells following bromelain treatment (21).
In summary, the studies presented here demonstrate that bromelain can effectively decrease IL-8-induced neutrophil migration both in vitro and in vivo and support proteolytic removal of CD128 chemokine receptors as a potential mechanism for this effect. Further studies will be necessary to determine the contribution of these effects on neutrophil influx on the ultimate development and severity of both acute and chronic inflammatory responses.
Supplementary Material
This video clip shows representative images of leukocytes within mesenteric vessels of a living anesthetized mouse that was initially treated with saline i.v., then given thioglycollate i.p. to induce peritoneal inflammation and imaged as described in the Methods. At the start of the video, leukocytes (bright spots) can be seen adherent to the vessel wall in the center of the field. These adherent leukocytes do not move with blood flow. Rolling leukocytes can be visualized as they percolate around these adherent cells. Cells in the bulk flow travel too fast to be visualized under these conditions. The transient increase in blood pressure associated with i.v. injection of bromelain (00:12.35 on the digital time stamp) moves the blood vessel out of the plane of focus, causing blurring of the image. When the image clears, rolling leukocytes are no longer evident but adherent cells remain. These images demonstrate that bromelain treatment very rapidly decreases leukocyte rolling, but does not cause detachment of leukocytes that have already become firmly adherent.
This video clip shows representative images of leukocytes within mesenteric vessels of a living anesthetized mouse that was initially treated with bromelain i.v., then given thioglycollate i.p. to induce peritoneal inflammation and imaged as described in the Methods. In contrast to what is seen with saline-treated mice (Supplemental video 1), few leukocytes are adherent to the vessel wall in bromelain-treated mice approximately 6 hrs after the initial dose of bromelain. The majority of leukocytes seen are visualized as they roll along the vessels walls. The transient increase in blood pressure associated with i.v. injection of an additional bolus of bromelain (00:10.53 on the digital time stamp) moves the blood vessel out of the plane of focus, causing blurring of the image. When the image clears, rolling leukocytes are no longer evident. The vessel now appears empty, since leukocytes moving in the bulk flow pass through too quickly to be clearly seen. Bromelain treatment acutely results in rapid cessation of leukocyte rolling, however this effect is transient. The longer term effect of i.v. bromelain treatment is to decrease firm adhesion of leukocytes to vessels at the site of inflammation.
Acknowledgement
The authors would like to thank Chau T. Trinh and Paula K. Greer for expert technical assistance and Dr. Miriam Wahl for loan of the thermostatically controlled microscope stage and circulating water bath used for intravital microscopy. This work was supported by grant number AT002288-01 from the National Institutes of Health, National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell & Molec Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol. 1988;22:191–203. doi: 10.1016/0378-8741(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 3.Targoni OS, Tary-Lehmann M, Lehmann PV. Prevention of murine EAE by oral hydrolytic enzyme treatment. J Autoimmunity. 1999;12:191–198. doi: 10.1006/jaut.1999.0271. [DOI] [PubMed] [Google Scholar]
- 4.Majima M, Kawashima N, Hiroshi I, Katori M. Effects of an orally active non-peptide bradykinin B2 receptor antagonist, FR173657, on plasma exudation in rat carrageenin-induced pleurisy. Brit J Pharmacol. 1997;121:723–730. doi: 10.1038/sj.bjp.0701194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majima M, Nishiyama K, Iguchi Y, Yao K, Ogino M, Ohno T, Sunahara N, Katoh K, Tatemichi N, Takei Y, Katori M. Determination of bradykinin-(1–5) in inflammatory exudate by a new ELISA as a reliable indicator of bradykinin generation. Inflamm Res. 1996;45:416–423. doi: 10.1007/BF02252938. [DOI] [PubMed] [Google Scholar]
- 6.Ogino M, Majima M, Kawamura M, Hatanaka K, Saito M, Harada Y, Katori M. Increased migration of neutrophils to granulocyte-colony stimulating factor in rat carrageenin-induced pleurisy: roles of complement, bradykinin, and inducible cyclooxygenase-2. Inflamm Res. 1996;45:335–346. doi: 10.1007/BF02252946. [DOI] [PubMed] [Google Scholar]
- 7.Gaciong Z, Paczek L, Bojakowski K, Socha K, Wisniewski M, Heidland A. Beneficial effect of proteases on allograft arteriosclerosis in a rat aortic model. Nephrol Dialysis, Transplant. 1996;11:987–989. [PubMed] [Google Scholar]
- 8.Wittenborg A, Bock PR, Hanisch J, Saller R, Schneider B. Comparative epidemiological study in patients with rheumatic diseases illustrated in a example of a treatment with non-steroidal anti- inflammatory drugs versus an oral enzyme combination preparation. Arzneimittel-Forschung. 2000;50:728–738. doi: 10.1055/s-0031-1300280. [DOI] [PubMed] [Google Scholar]
- 9.Brown AC. Lupus erythematosus and nutrition: a review of the literature. J Renal Nutrit. 2000;10:170–183. doi: 10.1053/jren.2000.16323. [DOI] [PubMed] [Google Scholar]
- 10.Masson M. Bromelain in blunt injuries of the locomotor system. A study of observed applications in general practice. Fortschritte der Medizin. 1995;113:303–306. [PubMed] [Google Scholar]
- 11.Akhtar NM, Naseer R, Farooqi AZ, Aziz W, Nazir M. Oral enzyme combination versus diclofenac in the treatment of osteoarthritis of the knee – a double-blind prospective randomized study. Clin Rheumatol. 2004;23:410–415. doi: 10.1007/s10067-004-0902-y. [DOI] [PubMed] [Google Scholar]
- 12.Rovenska E, Svik K, Stancikova M, Rovensky J. Enzyme and combination therapy with cyclosporin A in the rat developing adjuvant arthritis. Inter J Tiss React. 1999;21:105–111. [PubMed] [Google Scholar]
- 13.Rovenska E, Svik K, Stancikova M, Rovensky J. Inhibitory effect of enzyme therapy and combination therapy with cyclosporin A on collagen-induced arthritis. Clin ExpRheumatol. 2001;19:303–309. [PubMed] [Google Scholar]
- 14.Secor ER, Jr, Carson WF, IV, Cloutier MM, Guernsey LA, Schramm CM, Wu CA, Thrall RS. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Clin Immunol. 2005;237:68–75. doi: 10.1016/j.cellimm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornhill SM, Kelly AM. Natural treatment of perennial allergic rhinitis. Alternative Med Rev. 2000;5:448–454. [PubMed] [Google Scholar]
- 16.Inoue K, Motonaga A, Dainaka J, Nishimura T, Hashii H, Yamate K, Ueda F, Kimura K. Effect of etodolac on prostaglandin E2 biosynthesis, active oxygen generation and bradykinin formation. Prostaglandins Leukotrienes & Essential Fatty Acids. 1994;51:457–462. doi: 10.1016/0952-3278(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 17.Hale LP, Greer PK, Trinh CT, Gottfried MR. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol. 2005;116:135–142. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kane S, Goldberg MJ. Use of bromelain for mild ulcerative colitis. Ann Int Med. 2000;132:680. doi: 10.7326/0003-4819-132-8-200004180-00026. [DOI] [PubMed] [Google Scholar]
- 19.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J Immunol. 1992;149:3809–3816. [PubMed] [Google Scholar]
- 20.Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163:2568–2575. [PubMed] [Google Scholar]
- 21.Mynott TL, Crossett B, Prathalingam SR. Proteolytic inhibition of Salmonella enterica Serovar Typhimurium -induced activation of the MAP kinases ERK and JNK in cultured human intestinal cells. Infect. Immun. 2002;70:86–95. doi: 10.1128/IAI.70.1.86-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin Immunol. 2002;104:183–190. doi: 10.1006/clim.2002.5254. [DOI] [PubMed] [Google Scholar]
- 23.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34:311–318. [PubMed] [Google Scholar]
- 24.Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Research. 2000;22:299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- 25.Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, Macdermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704–712. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- 26.Lindley IJD. Interleukin 8. In: Mire-Sluis A, Thorpe R, editors. Cytokines. San Diego: Academic Press; 1998. pp. 125–140. [Google Scholar]
- 27.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine regulates neutrophil recruitment. Amer J Pathol. 2001;158:715–721. doi: 10.1016/S0002-9440(10)64014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hale LP, Greer PK, Trinh CT, James CL. Proteinase activity and stability of natural bromelain preparations. Intl Immunopharmacol. 2005;5:783–793. doi: 10.1016/j.intimp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Engwerda CR, Andrew D, Murphy M, Mynott TL. Bromelain activates murine macrophages and natural killer cells in vitro. Cellular Immunol. 2001;210:5–10. doi: 10.1006/cimm.2001.1793. [DOI] [PubMed] [Google Scholar]
- 31.Enwerda CR, Andrew D, Ladhams A, Mynott TL. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cellular Immunol. 2001;210:66–75. doi: 10.1006/cimm.2001.1807. [DOI] [PubMed] [Google Scholar]
- 32.Onken JE, Greer PK, Calingaert B, Hale LP. Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin Immunol. 2008;126:345–352. doi: 10.1016/j.clim.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video clip shows representative images of leukocytes within mesenteric vessels of a living anesthetized mouse that was initially treated with saline i.v., then given thioglycollate i.p. to induce peritoneal inflammation and imaged as described in the Methods. At the start of the video, leukocytes (bright spots) can be seen adherent to the vessel wall in the center of the field. These adherent leukocytes do not move with blood flow. Rolling leukocytes can be visualized as they percolate around these adherent cells. Cells in the bulk flow travel too fast to be visualized under these conditions. The transient increase in blood pressure associated with i.v. injection of bromelain (00:12.35 on the digital time stamp) moves the blood vessel out of the plane of focus, causing blurring of the image. When the image clears, rolling leukocytes are no longer evident but adherent cells remain. These images demonstrate that bromelain treatment very rapidly decreases leukocyte rolling, but does not cause detachment of leukocytes that have already become firmly adherent.
This video clip shows representative images of leukocytes within mesenteric vessels of a living anesthetized mouse that was initially treated with bromelain i.v., then given thioglycollate i.p. to induce peritoneal inflammation and imaged as described in the Methods. In contrast to what is seen with saline-treated mice (Supplemental video 1), few leukocytes are adherent to the vessel wall in bromelain-treated mice approximately 6 hrs after the initial dose of bromelain. The majority of leukocytes seen are visualized as they roll along the vessels walls. The transient increase in blood pressure associated with i.v. injection of an additional bolus of bromelain (00:10.53 on the digital time stamp) moves the blood vessel out of the plane of focus, causing blurring of the image. When the image clears, rolling leukocytes are no longer evident. The vessel now appears empty, since leukocytes moving in the bulk flow pass through too quickly to be clearly seen. Bromelain treatment acutely results in rapid cessation of leukocyte rolling, however this effect is transient. The longer term effect of i.v. bromelain treatment is to decrease firm adhesion of leukocytes to vessels at the site of inflammation.