Abstract
Regulation of intracellular cAMP by multiple pathways enables differential function of this ubiquitous second messenger in a context-dependent manner. Modulation of Gs-stimulated intracellular cAMP has long been known to be modulated by the Gi and Gq/Ca2+ pathways. Recently, the G13 pathway was also shown to facilitate cAMP responses in murine macrophage cells. We report here that this synergistic regulation of cAMP synthesis by the Gs and G13 pathways is mediated by a specific isoform of adenylyl cyclase, AC7. Furthermore, this signaling paradigm exists in several hematopoietic lineages and can be recapitulated by exogenous expression of AC7 in HEK 293 cells. Mechanistic characterization of this synergistic interaction indicates that it occurs downstream of receptor activation and it can be mediated by the α subunit of either G12 or G13. Our results demonstrate that AC7 is a specific downstream effector of the G12/13 pathway.
The second messenger, cAMP, is ubiquitously utilized by many cell types to regulate a variety of fundamental physiological functions (1-6). This precise control of various cellular functions and subsequent physiological processes requires that regulation of intracellular cAMP concentration occur in a cell type-dependent manner (7, 8). Specificity is achieved at multiple levels including synthesis, degradation, and selective response to extracellular stimuli (7-11). Key mechanisms for differential regulation in the synthetic pathway occur at the level of adenylyl cyclase (AC)3 enzymes.
Synthesis of cAMP by membrane-bound AC is primarily regulated by the heterotrimeric Gs protein and its modulation by ligand binding to G-protein-coupled receptors (12, 13). There are nine membrane-bound mammalian ACs that can be divided into four subgroups based on their sequence similarities and regulatory properties (11, 14, 15). AC1, AC3, and AC8 can be stimulated by Ca2+/calmodulin upon elevation of intracellular free Ca2+ through voltage-gated Ca2+ channels or capacitative entry. The activities of AC2, AC4, and AC7 isoforms are stimulated by the βγ subunits of G proteins. In this case, regulation requires coordinate stimulation with Gs and Gβγ acts synergistically to enhance the catalytic activities of this class of ACs. AC5 and AC6 are generally inhibited by a variety of pathways, including Ca2+ and Gαi. The AC9 isotype is unique among the membrane ACs in that it is insensitive to activation by the diterpene, forskolin.
The complex regulation of AC activities makes them ideal for translating inputs from multiple pathways into integrated cAMP responses. Regulation of AC activities by the Gi and Gq/Ca2+ pathways has been well studied both in vitro and in vivo (11, 14, 15). Recently, regulation of cAMP responses by the G13 pathway was also reported (16), thereby establishing that cAMP can be regulated by all major groups of heterotrimeric G proteins. In the latter study, a genetically engineered BRET sensor for cAMP, CAMYEL, was used to measure transient changes in intracellular cAMP in real time and in greater detail in living cells. It was shown that activation of the G13 pathway greatly enhanced Gs-stimulated cAMP responses in murine macrophage cells and that this synergistic interaction was transient and occurred at the level of cAMP synthesis, not its degradation.
Here we report the identification of a specific AC isoform, AC7, as the key integrator that mediates the regulation of cAMP responses from the G13 pathway. The extension of this observation to both human erythroleukemia cells (HEL) and primary macrophages derived from bone marrow demonstrate that this regulation is a conserved signaling motif utilized by several hematopoietic lineages. We further show that regulation of AC7 activity is mediated by the α subunit of G13 and both Gα12 and Gα13 are capable of mediating the synergistic effect on AC7 activity. However, the constitutively active proteins, Gα13QL or Gα12QL, act as dominant negative proteins with respect to cAMP regulation. These results suggest that AC7 may be a direct or indirect downstream effector of the G12/13 pathway.
EXPERIMENTAL PROCEDURES
Reagents—Isoproterenol, prostaglandin E1, complement C5a, uridine 5′-diphosphate, thrombin, and forskolin were obtained from Sigma. Sphingosine 1-phosphate (Avanti Polar Lipids), doxycyclin (Clontech), pertussis toxin and cholera toxin (List Biological) were purchased from the sources indicated.
Cell Culture and Generation of Stable Cell Lines via Retroviral Transfection—Protocols for culturing RAW 264.7 cells and for retroviral infection were described previously (16). The HEL cell line was obtained from ATCC and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and 20 mm NaHEPES, pH 7.4. HEK 293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and 20 mm NaHEPES, pH 7.4. Transfection with DNA was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Retrovirus was made with the Phoenix Amphotropic packaging cell line (Orbigen). Infection of cells was initiated by application of virus-containing supernatant harvested from the packaging cell line and 6 μg/ml Polybrene on top of the targeted cells and centrifuged at 1,200 × g for 2 h at 32 °C. Cells were then cultured with the viral supernatant for 1 day at 32 °C. The viral supernatant was then removed and cells were cultured with fresh medium containing 100-500 μg/ml hygromycin (depending on cell line) at 37 °C for selection, expansion, and maintenance. The transduction efficiency of this method ranges from 50 to 80% based on the number of cells surviving selection.
Isolation of BMDM and Retroviral Infection—AC7 knock-out mice were purchased from the Jackson Laboratory and bred by intercrossing heterozygous males and females. All experimental procedures involving animals in this study were reviewed and approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center. Bone marrow-derived primary macrophages were isolated from mouse femurs and cultured as described (17). Briefly, 6-8-week-old mice were sacrificed and femurs were isolated. Bone marrow cells were flushed out of the femur bones and red blood cells were lysed. The remaining cells were cultured on non-TC-coated dishes in Dulbecco's modified Eagle's medium supplemented with macrophage colony-stimulating factor provided by addition of 10% conditioned medium from CMG14-12 cells (kindly provided by Dr. T. Roach), 55 μm β-mercaptoethanol, 10% fetal bovine serum, 2 mm l-glutamine, 20 mm NaHEPES, pH 7.4, 100 units/ml penicillin, and 100 μg/ml streptomycin. PlatE cells (kindly provided by Dr. T. Roach) were transfected with retrovirus constructs using FuGENE HD (Roche) to produce retrovirus for infection of BMDM. A 1:1 mixture of viral supernatant and BMDM growth medium supplemented with 8 μg/ml Polybrene was added on top of BMDM cells on the 2nd day after BMDM isolation. After culturing the cells at 32 °C for 1 day the media was supplemented with fresh viral supernatant for another day. Infected BMDMs were expanded for 4 days and briefly selected with 50 μg/ml hygromycin before being used for BRET assays. The transduction efficiency of this method is about 20-40% based on the number of BMDMs that survived selection.
Assay of BRET in Live Cells—Adherent cells were plated in 96-well solid-white tissue culture plates (Greiner) at a density of 40,000-60,000 cells per well the day before assays. Suspension cells were plated on the day of assay at 100,000 cells per well. Cells were serum starved in Hanks' balanced salt solution, pH 7.4, for 1 h before treatments. The BRET assay was carried out with a POLARstar Optima plate reader from BMG LabTech. Emission signals from Renilla luciferase and yellow fluorescent protein were measured simultaneously using a BRET filter set (475-30/535-30). Cells in each well were assayed in 80 μl of Hanks' balanced salt solution with 2 μm coelenterazine-h and stimulations were initiated by injection of 20 μl of ×5 ligand. Calculation of cAMP concentration was done as described before (16).
Knockdown of Protein Expression by RNAi—siRNA oligomers targeting mRNA for mouse AC7, mouse AC9, human AC7, and human Gα13 were SMARTPool products purchased from Dharmacon. They were used either as a pool of 4 different oligos (designated as -P) or as individual oligos (letter designation other than -P). Cells were transfected with 200 nm siRNA using HiPerFect reagent (Qiagen). Cells were plated into 96-well tissue culture plates at 24 h post-transfection and assayed at 48 h post-transfection. Mouse AC2 and AC3 were knocked down using stable expression of shRNA in the pFBneo vector (Stratagene) (18). The sequences for the AC2-D and AC3-A oligomers are 5′-CCG GAT CAA GCT GGA ATT TGA A-3′ and 5′-TCC GGG TCA TCA CCA AGA TCAA-3′, respectively.
DNA Constructs and Inducible Expression of Proteins—Mouse Gα13QL cDNA was a kind gift from Dr. William Singer. Human Gα12 and Gα12QL cDNAs were obtained from the Missouri S & T cDNA Resource Center. All cDNAs were cloned into the pSLIKneo construct where gene expression is driven by an inducible TRE promoter (19). Lentivirus was produced by co-transfection of 4 plasmids into 293T cells and concentrated viral supernatant was used to infect RAW cells as described (19). Stable RAW cell lines were established using medium containing 500 μg/ml G418. Expression was induced by culturing the cells with medium containing 0.5 μg/ml doxycyclin for 1 day.
Quantification of mRNA and Protein Expression—Samples were taken for analysis by Western blot or qRT-PCR to assess the expression level of protein or mRNA, respectively. Antibodies used in the experiments include anti-Gα13 (B-860) (20), anti-Gα12 (Santa Cruz Biotechnology), anti-FLAG (Sigma), anti-V5 (Invitrogen), anti-His (R&D systems), and anti-myc (Cell Signaling Technology). Primers used for qRT-PCR are: mAC2 (F) 5′-TAC TGC CAA AAA CGT CCA TCC-3′ and (R) 5′-GCA TTG CAA AAG CTG TCC AG-3′; mAC3 (F) 5′-CAA TGG CAC TGA CAG CAT GC-3′ and (R) 5′-CGT GGC GCG AGA AGT AGT AAA-3′; mAC7 (F) 5′-GCC TTC GAC TGC TGA ATG AGA-3′ and (R) 5′-GCC ATG TAG GTG CTG CCA AT-3′; mAC9 (F) 5′-GCT CAG CTC CTG GAT GAG GT-3′ and (R) 5′-ACA TCA CAA TGG AAC TGT CCT GAC-3′; hAC7 (F) 5′-CGC GAG CAG CAA GAC AAG A-3′ and (R) 5′-AGG CTC ATC CTC AGG AAG ACC-3′. The qRT-PCR were done with an ABI 7500 Real Time PCR system from Applied Biosystems. Primers used for genotyping wild type and knock-out alleles of wild type, 5′-GTT CTC ACC ATG TGG GGT TAG TAT G-3′, knock-out, 5′-GGG TGG GAT TAG ATA AAT GCC TGC TCT-3′, and 5′-ACA AGC TGG GGC ATA TAG CAG TTAG-3′.
Enzyme-linked Immunoassay (EIA) for cAMP—Cells were plated on 96-well tissue culture plates and cultured for 1 day. Prior to treatments, cells were cultured in serum-free medium for 1 h. Ligands (×10) were then added to stimulate the cells. After the addition of ligands, reactions were stopped at the indicated times by removal of medium and cell lysis with 65% ethanol. Cell lysates were then dried and assayed using the cAMP Biotrak EIA kit (Amersham Biosciences).
Assay of Activated Rho—Cells (1 × 106) were plated on 60-mm tissue culture dishes 1 day prior to the assay. On the day of assay, cells were cultured in serum-free medium for 1 h. Ligands were then added to stimulate the cells. Reactions were stopped after 1 min of stimulation by rapid removal of medium and placement on ice; the cells were immediately washed with ice-cold 1× phosphate-buffered saline and lysed with 110 μl of lysis buffer. Cell lysates were cleared by brief centrifugation and assayed using the G-LISA RhoA activation assay kit (Cytoskeleton Inc.).
RESULTS
In the murine macrophage-like cell line, RAW 264.7, activation of β-adrenergic receptors and the Gs pathway with isoproterenol (ISO) increases concentrations of intracellular cAMP. Addition of sphingosine 1-phosphate (S1P) either simultaneously or sequentially greatly enhances this cAMP response induced by ISO. We have shown previously that this synergistic effect is mediated by the S1P2 receptor and the heterotrimeric G protein, G13 (16). Several experiments also indicate that this interaction occurs at the level of cAMP synthesis and downstream of the receptors. Therefore, a likely hypothesis is that this synergism between the Gs and G13 pathways occurs through regulation of a specific isoform of adenylyl cyclase.
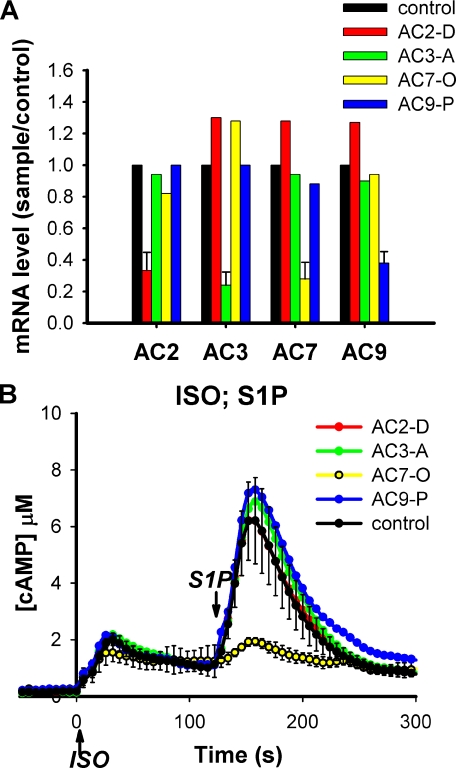
Knockdown of the AC7 Isoform of Adenylyl Cyclase Attenuates the Effect of G13 on cAMP—RAW 264.7 cells express four isoforms of transmembrane AC according to RT-PCR: AC2, AC3, AC7, and AC9. To determine whether the regulation of cAMP response by the G13 pathway is mediated by a specific isoform of AC, RNAi was used to knockdown each of the four ACs individually. Based on quantitative RT-PCR, mRNA for each isoform could be specifically knocked down by at least 60% with essentially no effect on other isoforms (Fig. 1A). Knockdown of AC2, AC3, or AC9 did not produce a significant effect on cAMP responses to either ISO or S1P. However, knockdown of AC7 greatly reduced the synergistic effect of S1P on intracellular cAMP (Fig. 1B). When S1P was added following addition of ISO, the response (second peak) in cells transfected with siRNAs targeting AC7 was reduced to less than 20% of control cells. The specificity of the RNAi effect was verified by using several siRNAs and an shRNA targeting different regions of the AC7 gene (supplemental Fig. S1) and was supported by the fact that AC7-specific RNAi oligos did not affect the expression of other ACs in RAW cells.
FIGURE 1.
Specific knockdown of AC isoforms in RAW 264.7 cells identifies AC7 as a key regulator for integration of the S1P/G13 effect on cAMP. Each AC isoform was knocked down individually in RAW cells that stably express the CAMYEL sensor either using retrovirus carrying shRNA (for AC2 and AC3) or by transient transfection of siRNA (for AC7 and AC9). Control cells carrying stable expression of nonspecific shRNA or transient expression of nonspecific siRNA were generated in parallel. Matching control cells were used for qRT-PCR analysis. cAMP responses in both control cells were similar and the former is shown. A, the effectiveness and isoform specificity of knockdowns was determined by qRT-PCR. B, cells treated for knockdowns as indicated were stimulated with 16 nm ISO at time 0, followed by addition of 10 nm S1P at 120 s. Intracellular cAMP was measured by BRET assay using CAMYEL. Error bars for the control response represent the standard deviation of results from three independent experiments. Errors were similar for the other conditions but left out for clarity.
The rise in cAMP caused by ISO alone was only slightly reduced in cells with reduced AC7 and was not affected by the knockdown of other AC isoforms. This is likely due to the redundancy of the several AC isoforms, which suggests that stimulated Gs in these cells activates multiple AC isoforms to raise intracellular cAMP, whereas the regulation from the G13 pathway is largely dependent on a single isoform, AC7.
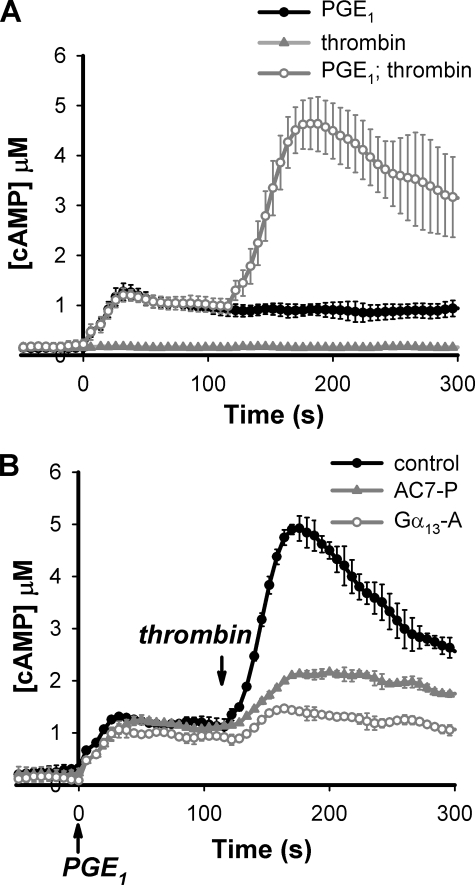
The Effect of Thrombin on Gs-stimulated cAMP Response in HEL Cells Is Mediated by G13 and AC7—Is the regulation of Gs-stimulated cAMP responses by the G13 pathway via AC7 a conserved signaling motif utilized by other cell lines? We tested this possibility by examining the cAMP responses in cultured HEL. It has been reported that thrombin greatly enhances the Gs-stimulated cAMP response in HEL cells (21, 22). Although it was not clear how thrombin effects this regulation it did not seem to be mediated by the Gi or the Gq/Ca2+ pathways. A second observation of interest was the original cloning of AC7 cDNA from HEL cells (23), an indication that this isoform of AC was highly expressed in these cells.
For real time measurement of cAMP responses in HEL cells, we generated an HEL cell line that stably expressed the cAMP BRET sensor, CAMYEL (16). Addition of prostaglandin E1 (PGE1) elevates intracellular cAMP via Gs activation in these cells, whereas thrombin alone produces no cAMP response (Fig. 2A). However, when thrombin was added to the cells following addition of PGE1, it elicited a large second peak of cAMP. This response is transient, similar to the effect of S1P in RAW cells, and diminishes within 10 min. We verified that this effect of thrombin was not mediated by the Gi or the Gq/Ca2+ pathways (data not shown) as reported previously (21, 22). When the α subunit of G13 in the HEL cells was knocked down by transfection with siRNA, the second peak response of cAMP triggered by thrombin was greatly reduced (Fig. 2B). Specific knockdown of AC7 in the HEL cells produced a similar result. Therefore, the synergistic regulation of cAMP by thrombin in this system also utilizes the G13 pathway and regulation of AC7 activity.
FIGURE 2.
The effect of thrombin on cAMP responses in HEL cells is also mediated by Gα13 and AC7. A, an HEL cell line that stably expresses the CAMYEL sensor was established through retroviral infection and selection. The cells were treated with 100 nm PGE1 or 1 unit/ml of thrombin at time 0, or with 100 nm PGE1 at time 0 followed by 1 unit/ml of thrombin at 120 s. Intracellular cAMP was measured using the BRET assay. B, HEL cells carrying the CAMYEL sensor were transiently transfected with control oligos or siRNA oligos targeting Gα13 (a single oligo designated as -A) or AC7 (a pool of 4 oligos designated as -P). Cells were assayed for cAMP responses to sequential addition of 100 nm PGE1 at time 0 followed by 1 unit/ml of thrombin at 120 s as indicated. Knockdown of Gα13 was 64% as assessed by Western blot and knockdown of AC7 was 53% as assessed by qRT-PCR. Error bars represent the standard deviation of results from three independent experiments.
The G-protein-coupled receptors that activate the Gs and G13 pathways in HEL cells, EP receptor for PGE1 and PAR for thrombin, are both different from the β-adrenergic and S1P2 receptors used in RAW cells. This is consistent with our previous assertion that the interactions between the Gs and the G13 pathways occur downstream of receptor activation. In fact, when the intracellular cAMP concentration was elevated by treatment with cholera toxin to directly activate the Gs protein and bypass activation of receptors, stimulation of the G13 pathway was still able to induce a large increase in cAMP (supplementary Fig. S2).
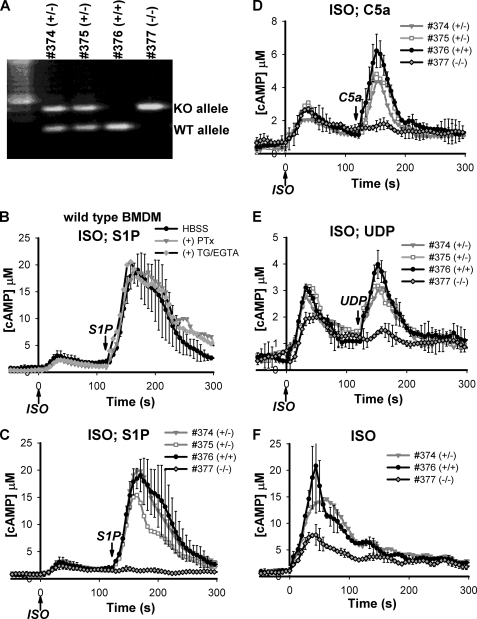
BMDMs from AC7-/- Mice Are Devoid of an S1P Effect on cAMP Stimulated by Gs-dependent Pathways—BMDMs offer an opportunity to examine regulation of AC7 by the G13 pathway in primary cells. We were able to deliver the CAMYEL sensor into freshly isolated BMDM cells using retroviral infection for subsequent examination of cAMP responses in the cultured cells (see “Experimental Procedures”). Like RAW cells, BMDMs elevate intracellular cAMP in response to stimulation with ISO. Addition of S1P alone elicited a significant change in the concentration of intracellular cAMP (supplementary Fig. S3), but induced a much larger second peak of cAMP when added after addition of ISO (Fig. 3B). The synergy seen in BMDMs appears to be greater than that observed in RAW cells. All of these response profiles are consistent with previous results when total cAMP was measured using a traditional EIA (16).
FIGURE 3.
Absence of an S1P effect on cAMP responses in AC7-/- BMDMs. A, BMDM cells were isolated from 6-week-old female mice of the same litter. The genotype of the BMDMs was determined by PCR. B-F, freshly isolated BMDMs were infected with retrovirus carrying the CAMYEL sensor. cAMP responses were measured using the BRET assay. B, wild type BMDMs were treated with buffer alone, 100 ng/ml pertussis toxin (PTx) for 20 h, or 10 μm thapsigargin and 2 mm EGTA (TG/EGTA) for 2 min as indicated and stimulated with sequential addition of 4 nm ISO at time 0 followed by 4 nm S1P at 120 s. C-E, three genotypes of BMDM cells were treated with sequential addition of 4 nm ISO at time 0 followed by 4 nm S1P (C), 50 nm C5a (D), or 500 nm UDP (E) at 120 s as indicated by arrows. F, three genotypes of BMDM cells were stimulated with 160 nm ISO at time 0. Representative error bars for selected traces are the standard deviation of results from three experiments.
To examine if the effect of S1P was mediated by Gi or the Gq/Ca2+ pathways, cells were treated, respectively, with either pertussis toxin to disable Gi or thapsigargin and EGTA to deplete intracellular and extracellular Ca2+. Neither of these perturbations affected enhancement by S1P of the Gs-stimulated cAMP response in wild type BMDMs, as measured by the CAMYEL sensor (Fig. 3B). The effectiveness of the chemical perturbations was confirmed by loss of modulation of Gs-induced cAMP by complement factor C5a or uridine 5′-diphosphate (UDP) (data not shown), which have been previously shown to couple through the Gi or Gq pathways, respectively (16). This result indicates that the effect of S1P in BMDMs is mediated by a G protein other than Gi or Gq.
Mice with knock-out of either Gna13 (Gα13) or Adcy7 (AC7) have been generated (24, 25). To determine the involvement of G13 and AC7 in the regulation of cAMP response by the S1P pathway in BMDMs, it would be ideal to obtain BMDMs from the knock-out animals and examine their cAMP responses to stimulations with ISO and S1P. Unfortunately Gα13 knock-out animals die during early embryogenesis (25). Although the majority of AC7-deficient animals are also embryonic lethal, ∼6% of AC7-/- mice do survive to adulthood (24)4 and this leaky phenotype provided an opportunity to examine the role of AC7 in BMDM cells. As shown in Fig. 3C, AC7-deficient BMDMs failed to generate any second cAMP peak in response to S1P following stimulation with ISO. BMDMs isolated from wild type or AC7+/- heterozygous litter mates displayed normal second peak responses to S1P. Identical results were obtained with a different cohort of mice and when the cAMP responses were also measured with an EIA (supplementary Fig. S4).
Regulation of the Gs-stimulated cAMP responses by the Gi and Gq/Ca2+ pathways were also examined in the AC7-deficient BMDMs. Addition of C5a or UDP following that of ISO induced 2nd cAMP peak responses in wild type and AC7+/- heterozygous BMDMs (Fig. 3, D and E). However, these 2nd peak responses were all abolished in AC7-deficient BMDMs. This result suggests that AC7 is the key isozyme for integration of multiple signals that regulate cAMP in BMDMs.
Despite the complete lack of second peak responses induced by the non-Gs pathways, AC7-/- BMDMs displayed only slightly reduced cAMP responses to stimulation with low doses of ISO alone (Fig. 3, C-E). When a high dose of ISO (160 nm) was used to stimulate the cells, the cAMP response in AC7-/- BMDMs was reduced to about 50% of that observed in wild type cells (Fig. 3F). Again, residual activity is presumably due to the presence of other AC isoforms in the BMDMs. However, integration of inputs from the non-Gs pathways appears to depend totally on AC7.
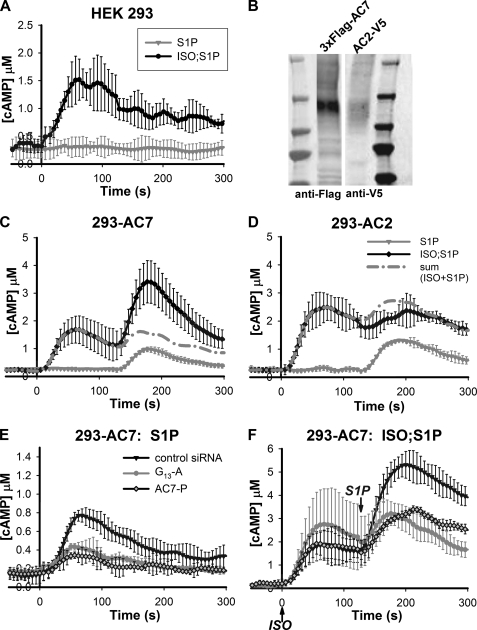
Exogenous Expression of AC7 Is Sufficient to Recapitulate the S1P/G13 Effect on Intracellular cAMP Responses—Reduction of AC7 expression in the hematopoietic cell lines described above largely attenuated the effect of the G13 pathway on cAMP responses stimulated by Gs. One possible explanation is that AC7 is the major isoform of adenylyl cyclase in those cells and therefore the fortuitous recipient for the signal integration measured. To further address this issue, we attempted to recapitulate this regulation in HEK 293 cells by exogenous expression of specific AC isoforms.
Wild type HEK 293 cells increase cAMP in response to stimulation with ISO but not with S1P. In addition, application of S1P simultaneously with or sequentially after addition of ISO did not produce any enhancement of the ISO response alone (Fig. 4A). Expression of AC7 in these cells caused a small rise of intracellular cAMP concentration to stimulation with S1P alone (Fig. 4C). This response to S1P alone is Gs-dependent because knockdown of Gs abolished this response (data not shown). When these cells with exogenous expression of AC7 were challenged with S1P following ISO addition, a second peak of response was observed (Fig. 4C). The response to S1P in the presence of ISO was much larger than that observed with S1P alone, thus emulating the synergism seen in the hematopoietic cells. HEK 293 cells express several isoforms of ACs endogenously that contribute to the response to ISO alone. Exogenous expression of AC7 in these cells contributed little to no increase in the response of cAMP to ISO alone; this suggests that the synergistic action of S1P on the actual activity of AC7 could be much larger than observed in context of the total activity measured in the cells.
FIGURE 4.
Overexpression of AC7 but not AC2 in HEK 293 cells recapitulates the S1P/G13 effect on cAMP response. HEK 293 cells that stably express the CAMYEL sensor were established through retroviral infection and selection. A, intracellular cAMP was measured in these cells using the BRET assay. The cells were stimulated with 50 nm S1P at time 0 (gray trace) or with 4 nm ISO at time 0 followed by 50 nm S1P at 120 s (black trace). B-D, HEK 293 cells carrying CAMYEL sensor were transiently transfected with cDNA encoding epitope-tagged AC2 or AC7. B, expression of the proteins was verified by Western blot using antibodies against the specific epitopes. C and D, cAMP responses were measured when the cells were stimulated with 50 nm S1P at 120 s (gray trace) or with 4 nm ISO at time 0 followed by 50 nm S1P at 120 s (black trace). The dashed trace represents the predicted sum of responses to ISO alone (not shown) and S1P alone. E and F, HEK 293 cells that stably express both the CAMYEL sensor and AC7 were established through retroviral infection and selection. The cells were then transiently transfected with control siRNA oligos or siRNA oligos targeting Gα13 (a single oligo designated as -A) or AC7 (a pool of 4 oligos designated as -P). Knockdown of either protein resulted in reduced cAMP responses to stimulation with 50 nm S1P alone at time 0 (E) or at 120 s following addition of 10 nm ISO at time 0 (F) as indicated. Error bars represent the standard deviation of results from three experiments.
We subsequently knocked down Gα13 and AC7 in HEK 293 cells carrying stable expression of AC7 using an RNAi approach. As shown in Fig. 4F, knockdown of either Gα13 or AC7 reduced the enhanced cAMP response caused by S1P following the addition of ISO. The cAMP response to S1P alone in those cells was also decreased (Fig. 4E), suggesting that the S1P response alone is due to synergy with some tonic stimulation of AC7 by a Gs-dependent mechanism. Taken together, these data suggest that exogenous expression of AC7 in HEK 293 cells is sufficient to recapitulate the S1P/G13 effect on Gs-stimulated cAMP responses.
Type VII adenylyl cyclase belongs to the same subclass of ACs as AC2 (7, 11). These two isoforms show over 70% sequence identity in the catalytic domains and are similarly regulated by Gβγ based on in vitro assays (26, 27). As a control we chose to express AC2 in HEK 293 cells for comparison of responses with those seen by expression of AC7. Cells with exogenous expression of AC2 also gave a small rise of intracellular cAMP when stimulated with S1P alone (Fig. 4D). This response is Gs-dependent and partially sensitive to pertussis toxin treatment (data not shown). When S1P was applied following addition of ISO, a 2nd peak response was observed (Fig. 4D). However, in contrast to responses seen with AC7, this second response in the AC2 overexpressing cells was similar to stimulation seen with S1P alone, an additive response.
The fact that only overexpression of AC7, but not AC2, recapitulated regulation on cAMP by the S1P/G13 pathway indicates that these two ACs can provide highly differential responses in cells. This contrasts with past characterization that showed only similar regulatory properties for members of this AC subclass.
G13 Regulation of AC7 Activity Is Mediated by Its α Subunit—The type II subclass of ACs, which includes AC2, AC4, and AC7, can be regulated by Gβγ subunits. Once activated by Gs, their activities can be further enhanced by direct binding of βγ subunits from the Gi pathway. Although βγ subunits derived from activation of G12/13 have not been shown to have any downstream function, they could affect AC activities as do the βγ subunits from Gi. However, the magnitude of the synergy and its specificity for AC7 argue against this possibility.
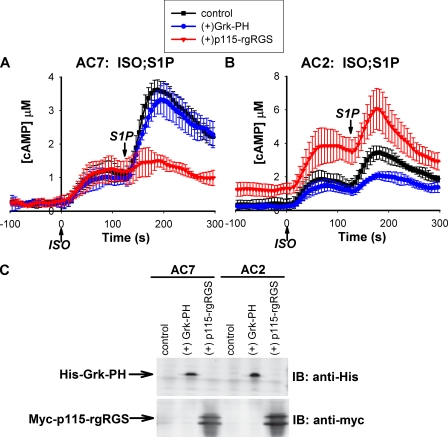
To test this directly, we overexpressed functional domains that can specifically block the actions of Gβγ or the α subunit of G13. The PH domain of Grk2 (G protein receptor kinase) has been shown to bind Gβγ and block its function (28). When this Grk-PH domain was expressed in 293T cells that overexpressed exogenous AC2, the cAMP response to S1P was reduced (Fig. 5B). However, the synergistic response of S1P in cells overexpressing AC7 was not affected by this PH domain (Fig. 5A).
FIGURE 5.
Regulation of AC7 is mediated by the α subunit of G13. 293T cells expressing the CAMYEL sensor and either AC7 (A) or AC2 (B) were co-transfected with control DNA, or DNA encoding epitope-tagged Grk-PH or p115-rgRGS domains. Expression of the proteins was verified by Western blot using antibodies against the specific epitopes (C). 48 h post-transfection, cells were stimulated with 4 nm ISO at time 0 followed by 5 nm S1P at 120 s; cAMP responses were measured using the BRET assay. Error bars represent the standard deviation of results from three experiments. IB, immunoblot.
In contrast, the response to S1P in cells expressing AC7 was largely attenuated by overexpression of the rgRGS domain of p115RhoGEF (Fig. 5A). This domain can block the effects of the G12/13 family by binding specifically to α subunits of G12 and G13 (29). Expression of the rgRGS domain in cells expressing AC2 appeared to cause elevation of basal cAMP levels, but did not affect the stimulation of cAMP by S1P in the presence of ISO. These data are consistent with the notion that in cells expressing AC2, stimulation by S1P is mediated by the Gi pathway via direct interaction of its βγ subunits with AC2. In the case of AC7, modulation by S1P is mediated by the α subunit of G13 rather than its βγ subunits. This is similar to other downstream effects of this G protein that have been sufficiently characterized (30). Similar experiments have been attempted in RAW 264.7 cells, but apparent lower expression of the domains curtailed any significant effects, including attenuation of known responses such as the Gi-mediated stimulation of Ca2+ by C5a through its βγ subunits.
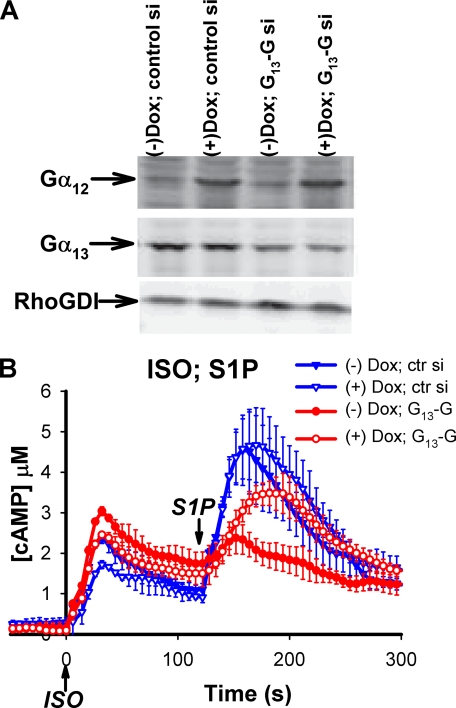
Overexpression of Wild Type Gα12 Rescues Knockdown of Gα13—The Gα12 and Gα13 proteins belong to the same subclass of heterotrimeric G proteins and share some common downstream effectors (30). Is the effect of the S1P pathway on cAMP synthesis specifically mediated by G13 or also downstream of G12? Attempts to knockdown Gα12 by RNAi in RAW cells produced only partial reduction of the protein and did not show any effect on the S1P regulation of cAMP stimulated by Gs (data not shown). It is possible that the knockdown was not adequate, that the S1P2 receptor preferentially couples to Gα13 in RAW cells, that Gα12 does not couple to AC7, or that the endogenous expression of Gα12 is insufficient to provide effective coupling in this system. We resorted to an alternative approach by testing whether overexpression of wild type Gα12 was able to rescue the phenotypes caused by knockdown of Gα13.
A RAW cell line that stably carries inducible expression of Gα12 was established using infection with lentivirus (19). Overexpression of Gα12 was induced in this cell line by addition of doxycyclin in the culture medium. Knockdown of Gα13 was achieved by transient transfection of siRNA. As shown in Fig. 6A, knockdown of Gα13 reduced the amount of Gα13 protein to ∼30% of the control, whereas overexpression of Gα12 (+Dox) increased the α subunit about 5-fold over the endogenous level. Overexpression of wild type Gα12 appeared to slightly reduce the cAMP response to ISO alone but retained the effect of S1P (2nd cAMP peak) (Fig. 6B). Knockdown of Gα13 greatly reduced the synergistic effect of S1P (2nd cAMP peak), as reported before (16). When overexpression of Gα12 was induced in the Gα13 knockdown cells, the enhancement of cAMP by S1P was largely restored. This result indicates that the G12 pathway is capable of regulating Gs-stimulated cAMP responses and shares this novel regulatory activity with G13.
FIGURE 6.
Overexpression of Gα12 rescues the cAMP phenotype caused by knockdown of Gα13 in RAW 264.7 cells. RAW cells that stably express the CAMYEL sensor were infected with lentivirus carrying Gα12 cDNA under an inducible promoter. The cells were first transiently transfected with control siRNA or siRNA oligos (-G) targeting Gα13. 24 h post-transfection the cells were split onto a 96-well plate, and cultured overnight either in the absence or presence of 0.5 μg/ml doxycyclin (Dox) as indicated. A, inducible expression of Gα12 and knockdown of Gα13 were examined by Western blot using antibodies specific to Gα12 or Gα13. B, the cells were challenged with 16 nm ISO at time 0 followed by 10 nm S1P at 120 s and cAMP was measured using the BRET assay. Error bars represent the standard deviation of results from three experiments.
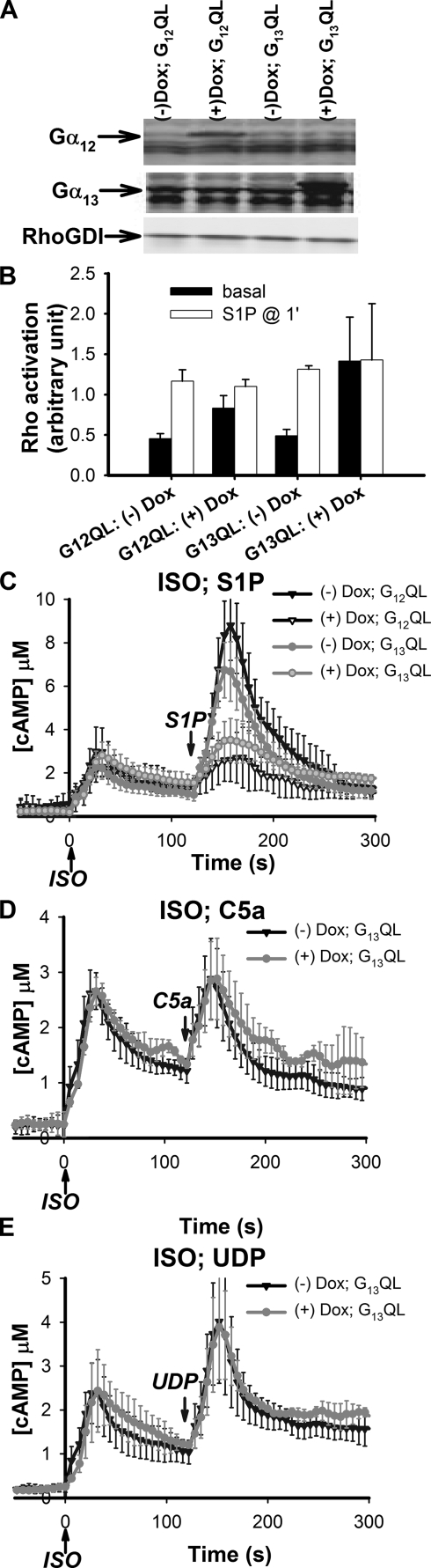
Overexpression of Constitutively Active Forms of the Gα12 and Gα13 Attenuates the Effect of S1P on cAMP Responses in RAW Cells—The effect of S1P on intracellular cAMP stimulated by Gs clearly requires activation of the G13 protein. Because this regulation is mediated by the α subunit of G13, we tested if a constitutively active mutant of Gα13, Gα13Q226L (Gα13QL), would result in constitutive sensitization of AC7 to Gs stimulation. The Gα13QL mutant lacks GTPase activity and has been shown to cause constitutive activation of RhoA, a major downstream effector of the G13 pathway (31, 32). This effect of the activated α subunit on RhoA-GTP is also observed in RAW cells (Fig. 7B). If this mutant form of Gα13 had a similar effect on cAMP responses in RAW cells, we would expect a greatly enhanced cAMP response to ISO stimulation alone in cells overexpressing the Gα13QL mutant protein. In contrast, we found that overexpression of constitutively active Gα13QL did not affect the cAMP response to ISO (Fig. 7C) alone. Surprisingly, the synergistic 2nd peak induced by S1P in the presence of ISO was diminished by overexpression of Gα13QL. Thus, this constitutively active protein gives a dominant negative phenotype with respect to cAMP regulation. Clearly, regulation of RhoA and cAMP responses by the G12/13 pathway have distinct mechanisms. Overexpression of constitutively active Gα12QL produced identical results (Fig. 7C), consistent with the notion that Gα12 and Gα13 proteins are interchangeable. Overexpression of Gα12QL or Gα13QL did not affect Ca2+ responses to various ligands that use Gi and Gq pathways (data not shown). More importantly, modulation of cAMP responses by ligands that stimulate the Gi or Gq pathways, C5a and UDP, respectively, remained unchanged (Fig. 7, D and E). This result argues that overexpression of Gα12/13QL is unlikely to regulate the activity of Gs; rather it acts to attenuate the G13 pathway that converges on AC7.
FIGURE 7.
Expression of the constitutively active mutants of Gα12 or Gα13 diminishes the S1P effect on Gs-stimulated cAMP in RAW 264.7 cells. RAW cells that stably express the CAMYEL sensor were infected with lentivirus carrying Gα12QL or Gα13QL cDNA under an inducible promoter. A, inducible expression of Gα12QL and Gα13QL proteins was achieved by addition of 0.5 μg/ml doxycyclin (Dox) in cell culture media for 1 day. Protein expression was determined by Western blot using specific antibodies. B, the effectiveness of G12QL or G13QL proteins was verified by assessment of RhoA activation. Cells were stimulated with 1 μm S1P. Reactions were stopped at 1 min and cells were lysed and assayed for activated RhoA using the G-LISA kit (see “Experimental Procedures”). C, treated cells were stimulated with 16 nm ISO at time 0 followed by 10 nm S1P at 120 s. D and E, overexpression of G13QL did not affect regulation of cAMP responses mediated through the Gi or Gq/Ca2+ pathways. Treated cells as indicated were stimulated with 16 nm ISO at time 0 and either 100 nm C5a (D) or 500 nm UDP (E) at 120 s, the respective ligands for activation of Gi or Gq/Ca2+ pathways. Intracellular cAMP was measured using the BRET assay. Error bars represent the standard deviation of results from three experiments.
DISCUSSION
Precise regulation of intracellular cAMP is crucial for this ubiquitous second messenger to control a wide variety of cellular functions, including cell proliferation and differentiation (2), cell metabolism (2), memory formation (5), cardiac contractility (4), and immune responses (1, 3). It has been shown that expression of specific isoforms, subcellular localization, and differential regulation of ACs greatly contribute to the diverse and tissue-specific regulation of cAMP (33-37). Here we report a novel regulatory pathway for control of cAMP synthesis in three hematopoietic cell lines. A G12/13 pathway can enhance Gs-dependent increases in cAMP in these cells and this synergy is mediated by a specific AC isoform, AC7.
AC7 has been grouped together with AC2 and AC4 as a subfamily of AC isozymes based on sequence similarities and their regulation by G protein βγ subunits (11, 26, 27, 38). However, our results suggest that AC7, but not AC2, is a specific AC isoform required for regulation of cAMP by the G12/13 pathway. Although HEL cells do not express AC2, RAW cells and BMDMs do express the isozyme. In the case of RAW cells, knockdown of AC2 mRNA to a similar extent as that of AC7 did not affect regulation of cAMP by the S1P/G13 pathway. In contrast, BMDMs deficient in AC7 were unable to respond to the S1P/G13 pathway in their cAMP response. More importantly, exogenous expression of AC7, but not AC2, in HEK 293 cells recapitulated regulation of cAMP responses by the G12/13 pathway. These data clearly distinguish selective actions of AC7 versus AC2. None of the cell lines examined in this study expresses AC4 and its sensitivity to the S1P/G13 pathway remains to be determined.
How does the G12/13 pathway increase intracellular cAMP? It has been shown previously that this regulation occurs via increased rates of cAMP synthesis rather than decreased rates of degradation (16). Several pieces of evidence indicate that the most likely mechanism involves direct action of the G12/13 pathway on the AC itself. First, it is clear that this regulation occurs downstream of the receptors, because this interaction exists between a variety of ligands that stimulate unique receptors coupled to the G12/13 or Gs pathways in several cell lines. Second, direct activation of Gs via modification with cholera toxin is a sufficient basis for enhancement of cAMP responses by the G12/13 pathway (supplementary Fig. S2). Third, this regulation relies on a specific isoform of AC, AC7. Fourth, overexpression of the constitutively active Gα12/13QL mutants specifically disrupts modulation of cAMP responses from the G12/13 pathway but not that from the Gi or Gq/Ca2+pathways. If indeed the G12/13 pathway directly regulates AC7 activity, a prediction is that direct activation of the AC enzyme might allow synergistic activation with the G12/13 pathway. We attempted to test this hypothesis by directly activating ACs with forskolin. Addition of forskolin to HEL cells or to 293 cells expressing AC7 elevated their intracellular cAMP, but activation of the G12/13 pathway with thrombin or S1P failed to induce a further enhanced cAMP response (data not shown). There are minimally two reasons that such an experiment may not work. First, several ACs are expressed in the cells assayed here and they have different sensitivities to forskolin. If AC7 is less sensitive to forskolin than other ACs as has been reported for AC2 (39), any synergism may get lost in the large signal from other ACs in the cells. Second, even though an AC can be activated by forskolin, the activation state of the protein appears to have a different conformation than when it is activated by Gs (40-42). Therefore, coupled regulation of the ACs may be different with the two forms of activation. For example, the activity of AC2 in vitro can be enhanced by Gβγ when AC2 is activated by Gs, but not if it is activated by forskolin.5
We have previously argued that the effect of G13 on cAMP responses is unlikely to be mediated by its βγ subunits even though Gs stimulation of selected AC isoforms can be enhanced by Gβγ (16). In this study, we provide convincing evidence that regulation of AC7 activity by the G12/13 pathway is indeed mediated by the α subunits of this class of G proteins. The direct experiment was to specifically block the function of the α or βγ subunits of G13 by overexpression of the p115-rgRGS or Grk-PH domains, respectively. Differential results were obtained in cells expressing AC2 or AC7. Blocking the function of the Gα13 subunit with overexpression of the p115-rgRGS domain specifically blunted the effect of S1P on the Gs-stimulated cAMP response in cells expressing AC7. In contrast, sequestration of Gβγ with the Grk-PH domain reduced the effect of S1P only in cells expressing AC2.
Additional results are consistent with this conclusion. First, the activities of both AC2 and AC7 can be modulated by Gβγ but the regulation by G13 is specifically mediated by AC7, not AC2. Second, overexpression of constitutively active Gα12QL or Gα13QL negated regulation of cAMP by the G12/13 pathway but did not affect other G protein pathways. Taken together, the heterotrimeric G12/13 proteins must exert this regulation of cAMP via their α subunits.
A major downstream effector of the G12/13 pathway is the RhoA GTPase (31, 43), which can be mediated by interaction of activated α subunits with a family of RGS-RhoGEFs (44). Here, we have identified AC7 as a novel downstream effector of the G12/13 pathway, either by direct interaction with the Gα12/13 proteins or via unknown intermediates. Yet, the engagement of the activated α subunits with these two effectors appears to differ. Although regulation of both effectors in vivo by extracellular stimuli apparently requires activation of the G protein and both responses are transient, long-term responses differ. In contrast to the long-term activation of RhoA by overexpression of activated Gα12QL or Gα13QL, effects on AC activity are blocked by expression of the activated subunits. This may suggest that the turn off mechanisms of these two responses are different. In the case of RhoA activation, the turn off mechanism lies upstream or at the level of G protein activation, possibly involving receptor desensitization. Due to the absence of an efficient turn off mechanism downstream, expression of a constitutively active G12/13 α subunit leads to sustained RhoA activation. However, the cAMP response was greatly attenuated by expression of the activated G protein α subunits. Thus suggests a potent turn off mechanism downstream of the receptors and G protein. This turn off mechanism is unlikely to be mediated by the primary output, cAMP, as the effect of S1P can be observed at various times after the second messenger has been elevated in cells (16). Furthermore, the transient nature of the S1P effect is also observed in the absence of cAMP; that is, the ability of the G12/13 pathway to enhance cAMP synthesis invoked by the Gs pathway is lost over 5 min if S1P is added to cells first followed by ISO at various delayed times (supplementary Fig. S5).
At this time, precise mechanisms for either stimulation of AC7 by the G12/13 pathway or desensitization of the regulation are unknown. Either may be mediated by direct interaction of the G protein α subunits with the cyclase or involve yet to be identified intermediate proteins. Attempts to examine direct interactions between Gα13 and AC7 by co-immunoprecipitation and direct regulation of AC7 by Gα13 using membrane assays have not yielded any positive result so far. Determining if an additional player is involved in this interaction and identification of such a protein may hold the key to understanding the molecular mechanism of AC7 regulation by the G12/13 pathway. Several studies have revealed various proteins that interact with the α subunit of the G12/13 proteins (45-48). Although we have shown previously that RhoA activity is not required for the effect on cAMP regulation (16), other interacting proteins could be potential candidates for mediation if required.
Supplementary Material
Acknowledgments
We thank Dr. Tamara Roach for providing the PlatE and CMG14-12 cell lines, Dr. William Singer for the G13QL construct and Gα13 antibody, and Dr. Ronald Taussig for rat AC2 and human AC7 cDNAs. We are grateful to Drs. Elliott Ross and Ronald Taussig for enlightening discussions and comments on this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants GM62114 and GM31954 (to P. C. S.). This work was also supported by grants from the Robert A. Welch Foundation (to P. C. S.) and the Alfred and Mabel Gilman Chair in Molecular Pharmacology (to P. C. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S5.
Footnotes
The abbreviations used are: AC, adenylyl cyclase; ISO, isoproterenol; PGE1, prostaglandin E1; S1P, sphingosine 1-phosphate; C5a, complement C5a; HEL, human erythroleukemia cells; BMDM, bone marrow-derived macrophage; CAMYEL, cAMP sensor using YFP-Epac-RLuc; EIA, enzyme-linked immunoassay; BRET, bioluminescence resonance energy transfer; Grk, G protein receptor kinase; RNAi, RNA interference; siRNA, small interfering RNA; HEK, human embryonic kidney; qRT, quantitative reverse transcriptase; UDP, uridine 5-diphosphate.
L. I. Jiang, unpublished data.
R. Taussig, personal communication.
References
- 1.Castro, A., Jerez, M. J., Gil, C., and Martinez, A. (2005) Med. Res. Rev. 25 229-244 [DOI] [PubMed] [Google Scholar]
- 2.Lania, A., Mantovani, G., and Spada, A. (2001) Eur. J. Endocrinol. 145 543-559 [DOI] [PubMed] [Google Scholar]
- 3.Lerner, A., and Epstein, P. (2006) Biochem. J. 393 21-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Movsesian, M. A., and Bristow, M. R. (2005) Curr. Top. Dev. Biol. 68 25-48 [DOI] [PubMed] [Google Scholar]
- 5.Wang, H., and Storm, D. R. (2003) Mol. Pharmacol. 63 463-468 [DOI] [PubMed] [Google Scholar]
- 6.Weinstein, L. S., Chen, M., Xie, T., and Liu, J. (2006) Trends Pharmacol. Sci. 27 260-266 [DOI] [PubMed] [Google Scholar]
- 7.Hanoune, J., and Defer, N. (2001) Annu. Rev. Pharmacol. Toxicol. 41 145-174 [DOI] [PubMed] [Google Scholar]
- 8.Houslay, M. D., and Milligan, G. (1997) Trends Biochem. Sci. 22 217-224 [DOI] [PubMed] [Google Scholar]
- 9.Beavo, J. A. (1995) Physiol. Rev. 75 725-748 [DOI] [PubMed] [Google Scholar]
- 10.Houslay, M. D., and Adams, D. R. (2003) Biochem. J. 370 1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunahara, R. K., and Taussig, R. (2002) Mol. Interv. 2 168-184 [DOI] [PubMed] [Google Scholar]
- 12.Beavo, J. A., and Brunton, L. L. (2002) Nat. Rev. Mol. Cell Biol. 3 710-718 [DOI] [PubMed] [Google Scholar]
- 13.Chin, K.-V., Yang, W.-L., Ravatn, R., Kita, T., Reitman, E., Vettori, D., Cvijic, M. E., Shin, M., and Iacono, L. (2002) Ann. N. Y. Acad. Sci. 968 49-64 [DOI] [PubMed] [Google Scholar]
- 14.Cooper, D. M. (2003) Biochem. J. 375 517-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel, T. B., Du, Z., Pierre, S., Cartin, L., and Scholich, K. (2001) Gene (Amst.) 269 13-25 [DOI] [PubMed] [Google Scholar]
- 16.Jiang, L. I., Collins, J., Davis, R., Lin, K. M., DeCamp, D., Roach, T., Hsueh, R., Rebres, R. A., Ross, E. M., Taussig, R., Fraser, I., and Sternweis, P. C. (2007) J. Biol. Chem. 282 10576-10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeshita, S., Kaji, K., and Kudo, A. (2000) J. Bone Miner. Res. 15 1477-1488 [DOI] [PubMed] [Google Scholar]
- 18.Zhu, X., Santat, L. A., Chang, M. S., Liu, J., Zavzavadjian, J. R., Wall, E. A., Kivork, C., Simon, M. I., and Fraser, I. D. (2007) BMC Mol. Biol. 8 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin, K. J., Wall, E. A., Zavzavadjian, J. R., Santat, L. A., Liu, J., Hwang, J. I., Rebres, R., Roach, T., Seaman, W., Simon, M. I., and Fraser, I. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 13759-13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer, W. D., Miller, R. T., and Sternweis, P. C. (1994) J. Biol. Chem. 269 19796-19802 [PubMed] [Google Scholar]
- 21.Turner, J. T., Camden, J. M., Kansra, S., Shelton-James, D., Wu, H., and Halenda, S. P. (1992) J. Pharmacol. Exp. Ther. 263 708-716 [PubMed] [Google Scholar]
- 22.Brass, L. F., and Woolkalis, M. J. (1992) Biochem. J. 281 73-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellevuo, K., Yoshimura, M., Kao, M., Hoffman, P. L., Cooper, D. M., and Tabakoff, B. (1993) Biochem. Biophys. Res. Commun. 192 311-318 [DOI] [PubMed] [Google Scholar]
- 24.Hines, L. M., Hoffman, P. L., Bhave, S., Saba, L., Kaiser, A., Snell, L., Goncharov, I., LeGault, L., Dongier, M., Grant, B., Pronko, S., Martinez, L., Yoshimura, M., and Tabakoff, B. (2006) J. Neurosci. 26 12609-12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offermanns, S., Mancino, V., Revel, J. P., and Simon, M. I. (1997) Science 275 533-536 [DOI] [PubMed] [Google Scholar]
- 26.Diel, S., Klass, K., Wittig, B., and Kleuss, C. (2006) J. Biol. Chem. 281 288-294 [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura, M., Ikeda, H., and Tabakoff, B. (1996) Mol. Pharmacol. 50 43-51 [PubMed] [Google Scholar]
- 28.Koch, W. J., Hawes, B. E., Inglese, J., Luttrell, L. M., and Lefkowitz, R. J. (1994) J. Biol. Chem. 269 6193-6197 [PubMed] [Google Scholar]
- 29.Hart, M. J., Jiang, X., Kozasa, T., Roscoe, W., Singer, W. D., Gilman, A. G., Sternweis, P. C., and Bollag, G. (1998) Science 280 2112-2114 [DOI] [PubMed] [Google Scholar]
- 30.Kurose, H. (2003) Life Sci. 74 155-161 [DOI] [PubMed] [Google Scholar]
- 31.Buhl, A. M., Johnson, N. L., Dhanasekaran, N., and Johnson, G. L. (1995) J. Biol. Chem. 270 24631-24634 [DOI] [PubMed] [Google Scholar]
- 32.Voyno-Yasenetskaya, T., Conklin, B. R., Gilbert, R. L., Hooley, R., Bourne, H. R., and Barber, D. L. (1994) J. Biol. Chem. 269 4721-4724 [PubMed] [Google Scholar]
- 33.Ostrom, R. S., Liu, X., Head, B. P., Gregorian, C., Seasholtz, T. M., and Insel, P. A. (2002) Mol. Pharmacol. 62 983-992 [DOI] [PubMed] [Google Scholar]
- 34.Schaefer, M. L., Wong, S. T., Wozniak, D. F., Muglia, L. M., Liauw, J. A., Zhuo, M., Nardi, A., Hartman, R. E., Vogt, S. K., Luedke, C. E., Storm, D. R., and Muglia, L. J. (2000) J. Neurosci. 20 4809-4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, K. E., Gu, C., Fagan, K. A., Hu, B., and Cooper, D. M. (2002) J. Biol. Chem. 277 6025-6031 [DOI] [PubMed] [Google Scholar]
- 36.Wong, S. T., Trinh, K., Hacker, B., Chan, G. C., Lowe, G., Gaggar, A., Xia, Z., Gold, G. H., and Storm, D. R. (2000) Neuron 27 487-497 [DOI] [PubMed] [Google Scholar]
- 37.Wu, Z. L., Thomas, S. A., Villacres, E. C., Xia, Z., Simmons, M. L., Chavkin, C., Palmiter, R. D., and Storm, D. R. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 220-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang, W. J., and Gilman, A. G. (1991) Science 254 1500-1503 [DOI] [PubMed] [Google Scholar]
- 39.Sutkowski, E. M., Tang, W. J., Broome, C. W., Robbins, J. D., and Seamon, K. B. (1994) Biochemistry 33 12852-12859 [DOI] [PubMed] [Google Scholar]
- 40.Sunahara, R. K., Tesmer, J. J., Gilman, A. G., and Sprang, S. R. (1997) Science 278 1943-1947 [DOI] [PubMed] [Google Scholar]
- 41.Tang, W. J., and Gilman, A. G. (1995) Science 268 1769-1772 [DOI] [PubMed] [Google Scholar]
- 42.Zhang, G., Liu, Y., Ruoho, A. E., and Hurley, J. H. (1997) Nature 386 247-253 [DOI] [PubMed] [Google Scholar]
- 43.Sah, V. P., Seasholtz, T. M., Sagi, S. A., and Brown, J. H. (2000) Annu. Rev. Pharmacol. Toxicol. 40 459-489 [DOI] [PubMed] [Google Scholar]
- 44.Sternweis, P. C., Carter, A. M., Chen, Z., Danesh, S. M., Hsiung, Y. F., and Singer, W. D. (2007) Adv. Protein Chem. 74 189-228 [DOI] [PubMed] [Google Scholar]
- 45.Kozasa, T., Jiang, X., Hart, M. J., Sternweis, P. M., Singer, W. D., Gilman, A. G., Bollag, G., and Sternweis, P. C. (1998) Science 280 2109-2111 [DOI] [PubMed] [Google Scholar]
- 46.Meigs, T. E., Fields, T. A., McKee, D. D., and Casey, P. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 519-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu, J., Vaiskunaite, R., Suzuki, N., Kozasa, T., Carr, D. W., Dulin, N., and Voyno-Yasenetskaya, T. A. (2001) Curr. Biol. 11 1686-1690 [DOI] [PubMed] [Google Scholar]
- 48.Shi, C. S., Sinnarajah, S., Cho, H., Kozasa, T., and Kehrl, J. H. (2000) J. Biol. Chem. 275 24470-24476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.