Abstract
PtK1 cells containing two independent mitotic spindles can cleave between neighboring centrosomes, in the absence of an intervening spindle, as well as at the spindle equators. We used same-cell video, immunofluorescence, and electron microscopy to compare the structure and composition of normal equatorial furrows with that of ectopic furrows formed between spindles. As in controls, ectopic furrows contained midbodies composed of microtubule bundles and an electron-opaque matrix. Despite the absence of an intervening spindle and chromosomes, the midbodies associated with ectopic furrows also contained the microtubule-bundling protein CHO1 and the chromosomal passenger protein INCENP. However, CENP-E, another passenger protein, was not found in ectopic furrows but was always present in controls. We also examined cells in which the ectopic furrow initiated but relaxed. Although relaxing furrows contained overlapping microtubules from opposing centrosomes, they lacked microtubule bundles as well as INCENP and CHO1. Together these data suggest that the mechanism defining the site of furrow formation during mitosis in vertebrates does not depend on the presence of underlying microtubule bundles and chromosomes or on the stable association of INCENP or CHO1. The data also suggest that the completion of cytokinesis requires the presence of microtubule bundles and specific proteins (e.g., INCENP, CHO1, etc.) that do not include CENP-E.
INTRODUCTION
Accurate cell division requires the coordinated segregation of the chromosomes (karyokinesis) as well as division of the cytoplasm (cytokinesis). The latter is achieved in animal cells by the formation of an actin and/or myosin contractile ring (reviewed in Satterwhite and Pollard, 1992; Fishkind and Wang, 1995). Despite much study, the signals that determine the position of the contractile machinery and the underlying molecular mechanism responsible for completing cytokinesis remain ambiguous.
Normally cytokinesis is coordinated with the position of the mitotic spindle so that it occurs along the spindle equator, between the groups of separated chromosomes. Currently there are two hypotheses for how this coordination is achieved. In a now classic experiment, Rappaport (1961) found that sea urchin and starfish zygotes cleave between two independent spindles when they are manipulated so that the asters of opposing spindles can interact. The formation of a furrow between two radial or “astral” arrays of microtubules (Mts), in the absence of an intervening spindle and chromosomes, led to the idea that the asters release a factor responsible for determining the site of cleavage (reviewed in Oegema and Mitchison, 1997), either by causing the cortex to relax at the polar regions (White and Borisy, 1983) or by inducing contraction at the spindle equator (Devore et al., 1989). Alternatively, observations on cytokinesis in tissue culture cells have led to the hypothesis that the signal(s) determining furrow position come from the chromosomes and/or the spindle midzone (reviewed in Oegema and Mitchison, 1997; Glotzer, 1997). For example, tripolar spindles with a V-shaped metaphase plate undergo cytokinesis along each arm of the “V” but rarely between the arms (Wheatley and Wang, 1996; Eckley et al., 1997). This suggests that the chromosomes and/or something associated with the spindle midzone dictate the position of the cleavage furrow. In this regard it has been proposed that “chromosomal passenger proteins”, including INCENP (Cooke et al., 1987), TD-60 (Andreassen et al., 1991), CENP-E (Yen et al., 1991), and others (reviewed in Earnshaw and Bernat, 1991; Rattner, 1992), are involved in defining the site of cytokinesis. These proteins are associated with the centromeric region of the chromosome during spindle formation and redistribute to the forming cleavage furrow during anaphase. Other spindle proteins that end up in the midbody during cytokinesis, including several kinesin-like proteins (CHO1/MKLP1/ZEN-4, KLP3A), have also been proposed to be involved in furrow formation and function (Sellitto and Kuriyama, 1988; Nislow et al., 1992; Williams et al., 1995; Raich et al., 1998).
Despite the correlation between chromosome position and cytokinesis, the role of chromatin and its passenger proteins in this process remains unclear. The recent observations that cytokinesis fails in cells overexpressing an INCENP:CENP-B chimera that is tethered at centromeres (Eckley et al., 1997), or a truncated nonmicrotubule binding form of INCENP, provides experimental support for the idea that at least one passenger protein is involved in this process (Mackay et al., 1998). However, Zhang and Nicklas (1996) observed normal cytokinesis in grasshopper spermatocytes after all chromosomes were removed by micromanipulation. This implies that the signal(s) controlling furrow positioning is intrinsic to the spindle but not the chromosomes. That even the spindle itself is not needed for furrow positioning is evident from the report of Rieder et al. (1997). These workers found, as noted previously by Rappaport (1961) for echinoderm zygotes, that vertebrate (PtK1) cells containing two independent spindles can also form functional furrows between two centrosomes that lack an intervening spindle and chromosomes. This observation raises the question of whether chromosome and spindle-associated proteins are required for cytokinesis in vertebrates.
The observation that functional cleavage furrows can form in vertebrate cells between two centrosomes, which are not connected by an intervening spindle or chromosomes, supports the hypothesis (discussed above) that the site of cytokinesis is dictated by where the radial “astral” arrays of Mts growing from each opposing centrosome overlap. However the centrosomes contribute not only the Mts that form the aster and spindle but also those interpolar Mts that ultimately form the interzone and midbody between the separating chromosomes during late anaphase and telophase (see Mastronarde et al., 1993). In this respect there is a growing body of evidence that bundles of interpolar Mts play an important role not only in establishing where cytokinesis will occur (e.g., Williams et al., 1995; Cao and Wang, 1996; Giansanti et al., 1998) but also in stabilizing the furrow after it forms (Wheatley and Wang, 1996; Wheatley et al., 1998; Raich et al., 1998). Although the mechanism by which these microtubule bundles form remains to be resolved, it clearly involves one or more kinesin-like proteins (e.g., Nislow et al., 1992; Williams et al., 1995; Raich et al., 1998) and perhaps INCENP (Earnshaw and Cooke, 1991).
To evaluate the hypothesis that the positioning of a cytokinetic furrow involves Mt bundles, CHO1, CENP-E, and/or INCENP, we have thoroughly analyzed the structure and composition of ectopic furrows that form during mitosis between two independent spindles in PtK1 cells. Because these furrows can produce two daughter cells (Rieder et al., 1997), they contain all of the machinery required for initiating and completing cytokinesis. The fact that they form between centrosomes that lack an intervening spindle and chromosomes provides a unique opportunity to identify spindle and chromosomal proteins that are normally found in the cleavage furrow but that are not required for cytokinesis in vertebrates.
MATERIALS AND METHODS
Cell Culture and Electrofusion
PtK1 cells were grown on coverslips and electrofused with a ProGenetor II electroporator (Hoeffer, San Francisco, CA) as described previously (Rieder et al., 1997). Briefly, coverslip cultures that were 60–80% confluent were placed in fusion buffer (280 mM sucrose, 2 mM HEPES, 1 mM MgCl2, pH 6.9) between two platinum electrodes separated by ∼10 mm. They were then exposed to a 350 V pulse for 2 ms. Exposed cultures were then returned to conditioned medium for 1 h at 37°C. Coverslip cultures containing fused cells were then mounted in Rose chambers in fresh complete L-15 media and scanned 2 or more hours later for cells containing two independent mitotic spindles. For cytochalasin D experiments, coverslip cultures were incubated overnight in conditioned media containing 1 μg/ml cytochalasin D. On the following day they were mounted in Rose chambers containing the same medium and followed by video phase-contrast microscopy.
Video Microscopy
Cells containing two spatially separated mitotic spindles were followed by time-lapse video microscopy on an inverted Nikon Diaphot microscope, equipped with a Dage MTI VE-1000 video camera, using either a 40× (numerical aperture [N.A.] of 0.70) or a 20× (N.A. of 0.75) phase-contrast objective. The cells were maintained on the microscope stage at 37°C using a custom-built Rose chamber heater (Rieder and Cole, 1998) and were illuminated with heat-filtered 546-nm (green) light that was shuttered between exposures. Recordings were made at 4–15 s intervals onto either super VHS tape or optical memory disks, and background subtraction and averaging were conducted using either an ARGUS-10 or a Hamamatsu C2400 image processor. Selected frames were digitized using Scion Image (Scion, Frederick, MD) frame-grabbing software and were processed with Adobe Photoshop (Adobe Systems, Mountain View, CA).
Immunofluorescence Microscopy
PtK1 cells followed in vivo were fixed at the desired time in −20°C methanol for 10 min. They were then rinsed three times in phosphate-buffered saline containing 0.05% Tween 20 (PBST). The coverslips were then incubated for 1 h at 37°C in primary antibodies against either INCENP (pab1186 [see Eckley et al., 1997]), CHO1 (Kuriyama et al., 1994) (courtesy of Dr. R. Kuriyama, University of Minnesota, Minneapolis, MN), or CENP-E (HX-1 [Schaar et al., 1997]) (courtesy of Dr. T. Yen, Fox Chase Cancer Center, Philadelphia, PA) diluted in PBST to 1:500, 1:500, and 1:250, respectively. After primary antibody staining, coverslips were rinsed three times at 5 min each in PBST. They were then incubated at 37°C for 1 h in TRITC-labeled goat anti-rabbit secondary antibodies diluted 1:50 in PBST.
Mts were labeled with a monoclonal α-tubulin antibody (T-5168, Sigma, St. Louis, MO) at 1:300 dilution in PBST and were treated as described above with the exception that a FITC-labeled goat anti-mouse secondary antibody was used. Cells treated with cytochalasin D were preextracted in 1% Triton X-100 in PHEM (60 mM piperazine-N,N′-bis[2-ethanesulfonic acid], 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 6.9) for 1 min. They were then fixed for 10 min in 1% glutaraldehyde in PHEM, reduced in NaBH4 (0.1 mg/ml in H2O), and labeled as described above.
After DNA labeling with Hoechst 33342, coverslips were mounted in glycerol/PBS (1:1) supplemented with 1 mg/ml p-phenylenediamine to prevent photobleaching. Selected cells were viewed by epifluorescence using a Nikon Optiphot microscope equipped with 60× (N.A. of 1.4) and 100× (N.A. of 1.4) objectives. Images were captured with a cooled charge-coupled device camera (KAF-1400, Photometrics, Tucson, AZ) and processed using Adobe Photoshop.
Electron Microscopy
Cells followed in vivo were fixed for electron microscopy by perfusion with 2.5% glutaraldehyde in 0.1 M phosphate buffer (see Khodjakov et al., 1997). They were then post-fixed in 2% OsO4 for 60 min at 4°C, washed in buffer, and treated with tannic acid (0.15% in phosphate buffer) for 1 min. Cultures were stained en bloc for 60 min at 4°C in 1% uranyl acetate, dehydrated in ethanol, and flat embedded in Epon (see Rieder and Cassels, 1999). Serial thin (90-nm) sections were photographed on a Zeiss 910 TEM, and the negatives were subsequently digitized and processed in Adobe Photoshop.
RESULTS
Midbodies Are Associated with Ectopic Furrows
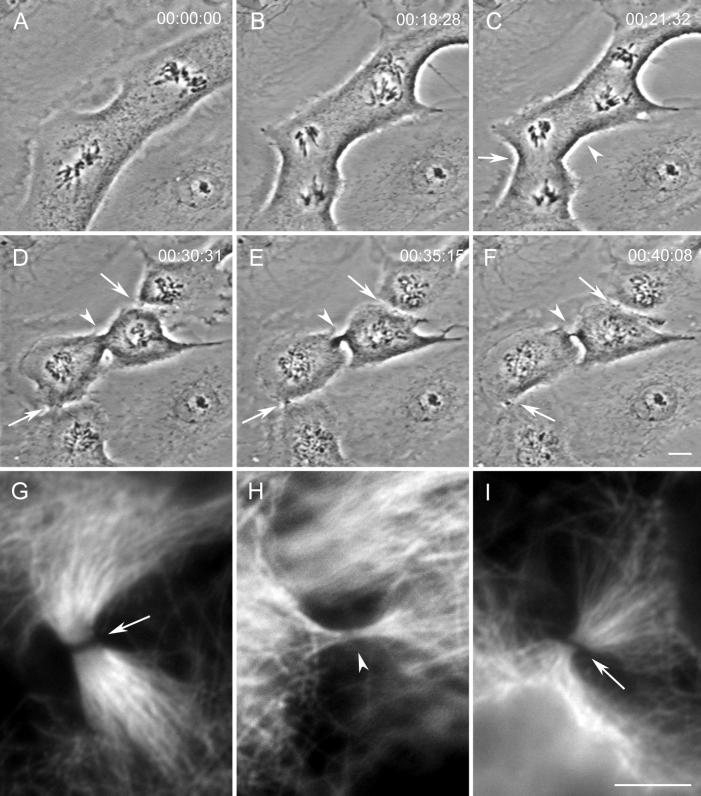
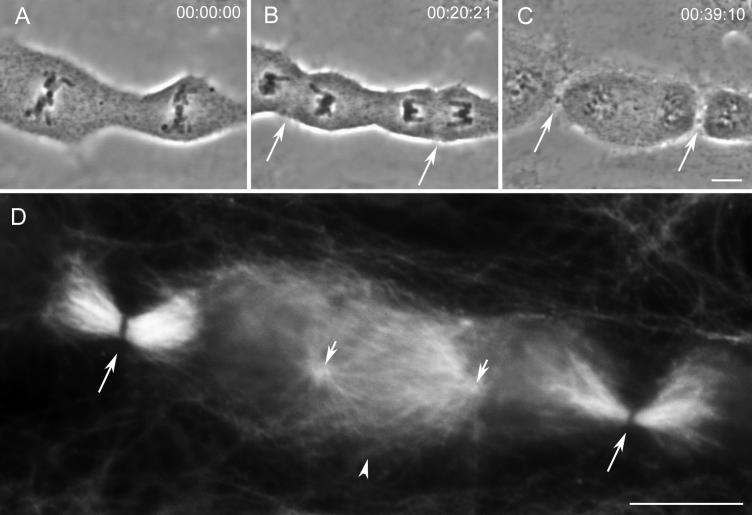
We followed 432 cells containing two independent mitotic spindles, separated on average by 35 μm (range of 18–75 μm), throughout mitosis by time-lapse light microscopy (LM). Ectopic furrows were initiated in 102 cells (24%). Of these 102 ectopic furrows, 30 (29%) relaxed before reaching the midbody stage (see below). We found no correlation between the distances that separated the closest poles of neighboring spindles and the success of either ectopic furrow initiation and/or completion. We also found no correlation between the asynchrony between the two spindles in anaphase onset (which could be >30 min) and the probability of forming an ectopic furrow. Ectopic furrows appear to form in a manner similar to that of control furrows (Figure 1).
Figure 1.
During mitosis ectopic cleavage furrows and midbodies can form between spindles in a PtK1 cell containing two independent spindles. (A–F) Selected images from a time-lapse recording of a cell containing two independent mitotic spindles. During telophase (C and D), cytokinesis occurs as expected between the two groups of separating chromosomes (arrows in D) but also between the two spindles (arrowhead in D). This cell was fixed soon after the image in F when all three furrows contained phase-dense intercellular bridges. (G–I) Immunofluorescence images of tubulin distribution in the three intercellular bridges pictured in F. The control midbodies, formed between groups of separating chromosomes, are pictured in G (lower left-hand bridge in F) and I (upper right-hand bridge in F). The ectopic bridge (H and arrowhead in F) also contains microtubules. Bars: A–F, 10 μm; G–I, 5 μm.
An analysis of our video records revealed that ectopic furrows that did not relax always formed, within a 10–15 min period, a thin phase-dense intercellular bridge similar to those formed by control furrows in the same cell (compare Figure 1F, arrows and arrowhead). Anti-tubulin indirect immunofluoresence (IMF) analysis revealed that, as in controls, these intercellular bridges always contained an Mt bundle that varied widely in its Mt content (n = 33; compare Figure 1, G–I). The Mt bundles found in the intercellular bridge at the completion of furrowing in controls displayed a prominent nonstaining central gap (e.g., Figure 1, G and I) that has been shown by others to correspond at the electron microscopy (EM) level to the presence of an electron-opaque matrix material (Sellitto and Kuriyama, 1988). The Mt bundle within the intercellular bridge formed by an ectopic furrow also contained a similar staining gap, but in some cases it was barely discernable (e.g., Figure 1H; see also Figures 7E and 9E).
Figure 7.
Midbodies formed in association with ectopic furrows contain the CHO1 protein. (A–D) Images from a time-lapse recording of a cell that formed an ectopic furrow between a tripolar spindle and a normal, bipolar spindle. Arrows in C and D indicate control furrows. The arrowhead in C indicates the forming ectopic furrow, and that in D indicates the ectopic midbody. (E–G) Immunofluorescence images of the same cell shown in A–D, fixed soon after the image in D. (E) Comparison of tubulin distribution between the control midbodies (arrows) and the ectopic midbody (arrowhead). This ectopic midbody exhibits the nonstaining central region found at the controls. (F) Distribution of the CHO1 protein. Arrowhead indicates the ectopic furrow. Although weaker in intensity, the location and localization patterns of the signal are identical to those of the controls. Note that the tripolar spindle region in the upper portion of the panel only shows a signal at one site, suggesting that two and not three daughter cells would have resulted upon completion of cleavage. (G) DNA distribution. Bars: A–D and E–G, 10 μm.
Figure 9.
CENP-E is not found in midbodies formed from ectopic furrows. (A–D) Images from a time-lapse sequence from a cell that formed an ectopic furrow (arrowhead in C and D). (E and F) Immunofluorescence localization of microtubules (E) and CENP-E (F) in the same cell fixed soon after the image in D. At this time the ectopic furrow (arrowhead) had progressed to a point between the two control furrows (arrows), one of which (upper) had not yet formed a midbody (see text for details). All three furrow sites contain microtubule bundles and a nonstaining central band (E). Although the two control furrows vary in their state of progression, both contain CENP-E (arrows in F), but this protein is not found in the ectopic furrow (arrowhead in F). Bars: A–D and E and F, 10 μm.
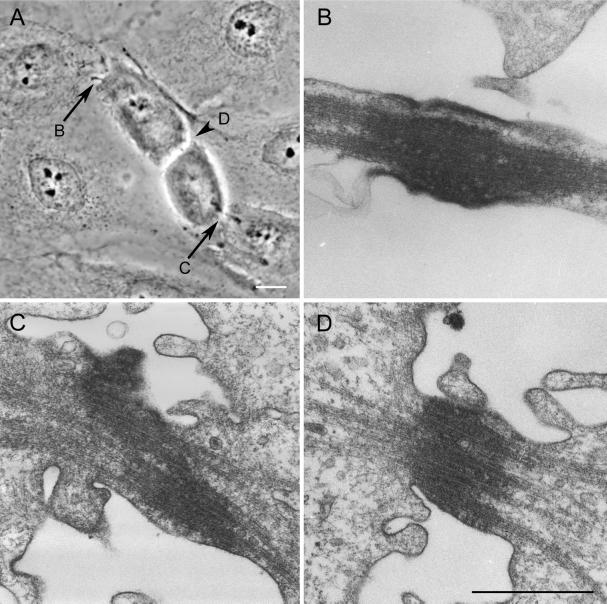
We used serial-section EM to examine the ultrastructure of the intercellular bridge produced by ectopic furrows (Figure 2A–D). In all cells (n = 9) this bridge contained a variable number of tightly packed Mts as well as an electron-opaque matrix material (Figure 2D). Because the structure of this bridge was indistinguishable from the bundle of “midbody” Mts (e.g., see Buck and Tisdale, 1962) associated with the control intercellular bridges in the same cell (Figure 2, B and C), we conclude that midbodies are always present in the thin intercellular bridges formed from the activity of an ectopic furrow. Our EM analysis also revealed that the midbodies associated with ectopic furrows sometimes contained a large number of 10-nm filaments (our unpublished observations) that are likely keratin filaments that are known to surround the spindle during mitosis in epithelia (e.g., Mandeville and Rieder, 1990).
Figure 2.
Midbodies formed in association with ectopic cleavage furrows are structurally similar to control midbodies. (A) Phase-contrast image of a cell containing two control midbodies (arrows) and one ectopic midbody (arrowhead) just before fixation for serial-section EM. (B–D) Selected electron micrographs through the midbodies pictured in A. The letter by the arrow or arrowhead in A indicates the respective micrograph depicting its ultrastructure. The midbody formed in association with the ectopic furrow (D, also arrowhead in A) is structurally similar to those of the controls (B and C). All contain bundles of microtubules that are embedded in an electron-opaque matrix. Bars: A, 10 μm; B–D, 0.75 μm.
Of the 432 cells followed for this study, 330 (76%) failed to initiate furrows between the independent spindles. We fixed and stained 15 of these cells, after the control furrows had formed midbodies, for an IMF analysis of Mt distribution (e.g., Figure 3). In all cases the region between the two spindles where an ectopic furrow would be predicted lacked Mt bundles. However, it always contained large numbers of Mts derived from the two opposing late telophase centrosomes (Figure 3D, short arrows).
Figure 3.
Cells that fail to initiate an ectopic furrow between the spindles contain numerous microtubules, but not microtubule bundles, between the opposing spindles. (A–C) Selected images from a time-lapse recording of a cell that did not initiate cleavage between separate mitotic spindles. Arrows in B indicate furrow initiation at the location of the metaphase plates, whereas those in C indicate the resultant midbodies. (D) Immunofluorescence image of microtubule distribution in this same cell fixed soon after the image in C. The control midbodies (long arrows) contain characteristic microtubule bundles bisected by a nonstaining region. By contrast, the region between the two spindles (arrowhead) contains numerous microtubules derived from the opposing centrosomes (short arrows) but no microtubule bundles. Bars: A–C and D, 10 μm.
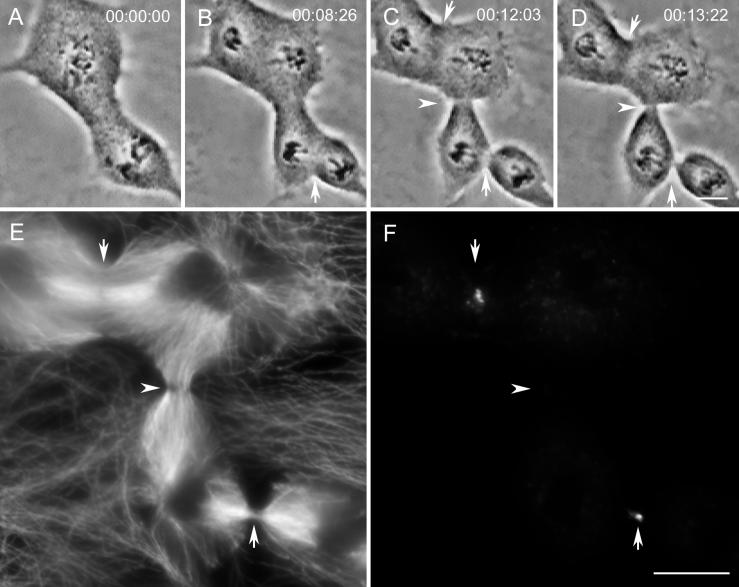
With few exceptions (<1%) after a control furrow became detectable by video LM, it proceeded without interruption to form, over a 10–15 min period, a midbody. In contrast, we found that 29% of the ectopic furrows that started between two spindles ultimately relaxed before midbody formation. As a rule in our experimental cells, the ectopic furrow began to form at approximately the same time as the control furrows. However, at variable times thereafter (and before midbody formation), ectopic furrows that were destined to relax began to increase steadily in diameter, and 5–10 min later they were no longer detectable (e.g., Figures 4, A–D, and 5, A–D). To determine how Mts are distributed in the region of relaxing ectopic furrows, we followed 10 cells containing two spindles through anaphase and fixed them for IMF 5 min after their associated ectopic furrows had begun to relax. An analysis of these cells revealed that the region of the relaxing ectopic furrow always contained numerous Mts but lacked the distinct Mt bundles characteristic of progressing control and ectopic furrows (see and compare Figures 4F and 5F with 1H and 9E; see also below).
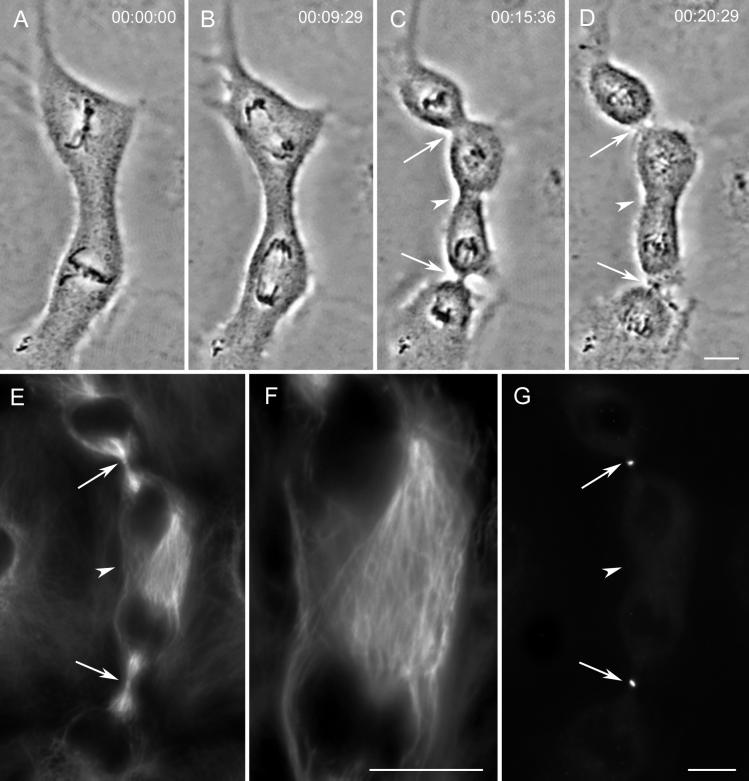
Figure 4.
Relaxing ectopic furrows lack prominent microtubule bundles and the CHO1 protein. (A–D) Images from a time-lapse recording of a cell that initiated an ectopic furrow between the independent spindles (arrowhead in C). This furrow subsequently and rapidly relaxed (arrowhead in D). The control furrows (arrows) progressed to completion without incident and formed midbodies (D). (E–G) This cell fixed soon after the image in D for an immunofluorescence analysis of microtubules (E and F) and CHO1 (G). Note that the region of the relaxing furrow contains a high density of microtubules (arrowhead in E) that at a higher magnification (F) do not appear to be organized into conspicuous bundles. Although each control furrow (arrows) also contains CHO1, this protein is not found in the region of the relaxing furrow (arrowhead in G). Bars: A–D, E and G, and F, 10 μm.
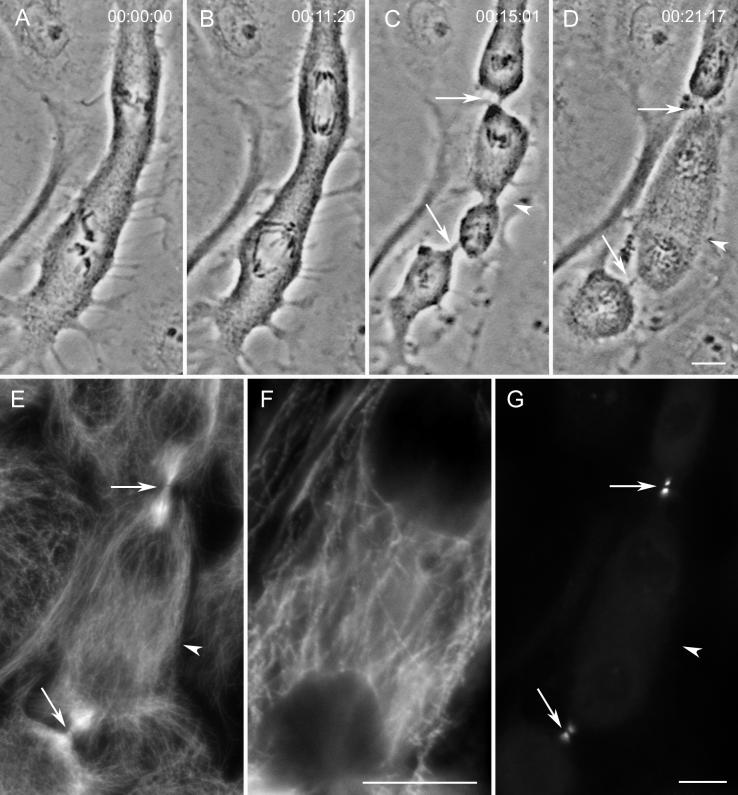
Figure 5.
Relaxing ectopic furrows lack bundled microtubules and INCENP. (A–D) Selected images from a time-lapse recording of an ectopic furrow (arrowhead in C) that goes almost to completion (i.e., the midbody stage) before rapidly relaxing (D). (E–G) This cell fixed soon after the image in D for an immunofluorescence analysis of microtubules (E and F) and INCENP (G). Unlike the control midbodies (arrows), each of which contains a prominent bundle of Mts (E), the region of the relaxing ectopic furrow (arrowhead in E and shown at a higher magnification in F) is rich in microtubules that are not bundled. Note also that the INCENP antigen is present in both control furrows (arrows) but absent from the relaxing ectopic furrow (arrowhead in G). Bars: A–D, E and G, and F, 10 μm.
Inhibiting Furrow Formation with Cytochalasin D Does Not Inhibit the Bundling of Interzonal Microtubules
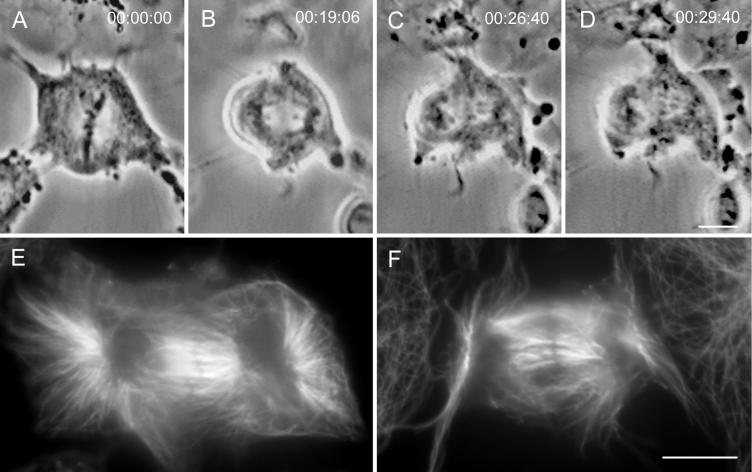
To investigate whether the absence of Mt bundles in relaxing ectopic furrows is caused by the regression of the furrow or by the absence of some internal activity required for bundling, we treated PtK1 cells with cytochalasin D. This drug specifically inhibits actin polymerization and thus cytokinesis but not progression through mitosis (e.g., Aubin et al., 1981). As expected, PtK1 cells treated with cytochalasin D at 1 μg/ml showed no evidence of furrow formation (Figure 6, A–D). An IMF analysis of the interzonal Mts in these cells (n = 7), which were fixed when untreated cells contain midbodies (30–35 min after anaphase onset), revealed that even in the absence of a constricting activity many of the interpolar Mts were bundled (Figure 6, E and F). As in the midbodies of untreated cells, these bundles also contained a central equatorial region that failed to stain with anti-tubulin antibody. Thus the formation of interzone Mt bundles during telophase does not depend on furrowing activity. Because these bundles have been shown to be extremely stable after they are formed (e.g., Salmon et al., 1976; Mullins and Biesele, 1977), the lack of Mt bundles in relaxing ectopic furrows is caused by the absence of an internal component needed for bundling and not by the relaxation of the furrow.
Figure 6.
Interpolar microtubules between the separating groups of anaphase chromosomes form bundles even when furrow formation is inhibited with cytochalasin D. (A–D) Images from a time-lapse recording of a cytochalasin-treated cell as it progressed from metaphase through telophase of mitosis. (E) This cell fixed soon after the image in D, and ∼30 min after anaphase onset, for an immunofluorescence analysis of microtubule distribution. Note that even when cytokinesis is inhibited, the interzonal microtubules positioned between the two separated nuclei form prominent bundles and contain a nonstaining equatorial band. (F) Microtubule distribution in another cytochalasin-treated cell that was followed by video LM until it was fixed during telophase. Again, even though this cell did not initiate furrowing, it contains prominent bundles of interzonal microtubules, many of which are bisected by a nonstaining region. Bars: A–D and E and F, 10 μm.
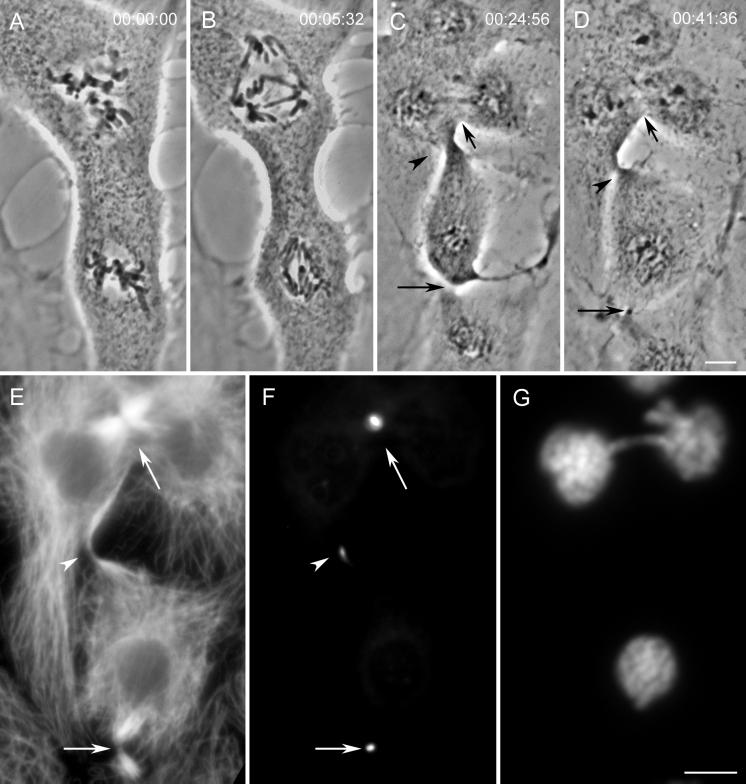
Ectopic Furrows Contain CHO1 and INCENP but Not CENP-E
The structural data presented above demonstrate that the midbodies associated with ectopic furrows are similar to those of controls. We next determined whether proteins known to be associated with control furrows and midbodies were also present in the ectopic furrows that formed in the absence of an intervening spindle and chromosomes. For these analyses we chose to assay for the presence of the kinesin-like CHO1 motor protein (e.g., Sellitto and Kuriyama, 1988; Raich et al., 1998) and two chromosomal passenger proteins, INCENP (Cooke et al., 1987) and CENP-E (e.g., Brown et al., 1996).
The distribution of CHO1 was examined in cells fixed 5 or more minutes after both the control and ectopic furrows had formed midbodies (n = 12; e.g., Figure 7). In all cases we found a variable, but clearly detectable, level of CHO1 in the ectopic midbody (Figure 7, D–F, arrowhead). As in control midbodies, CHO1 was restricted to the central region of the midbody that does not stain for tubulin by IMF (compare Figure 7, E and F, arrowheads). This is the region that corresponds to the location of the electron-dense matrix material (Sellitto and Kuriyama, 1988).
Ectopic midbodies formed in cells containing two spindles were also examined for the presence of the chromosomal passenger protein INCENP (n = 12). Because no chromosomes were present in this region, we were surprised to detect INCENP consistently in the ectopic midbodies (e.g., Figure 8). In controls INCENP was found, as reported by others (reviewed in Rattner, 1992), on opposite sides of the CHO1-containing matrix material that excludes Mt staining (Figure 8, B, B′, C, and C′). Although in most cells this was also the case for the ectopic midbodies, in some INCENP appeared to be distributed in an uninterrupted linear pattern (Figure 8, D and D′).
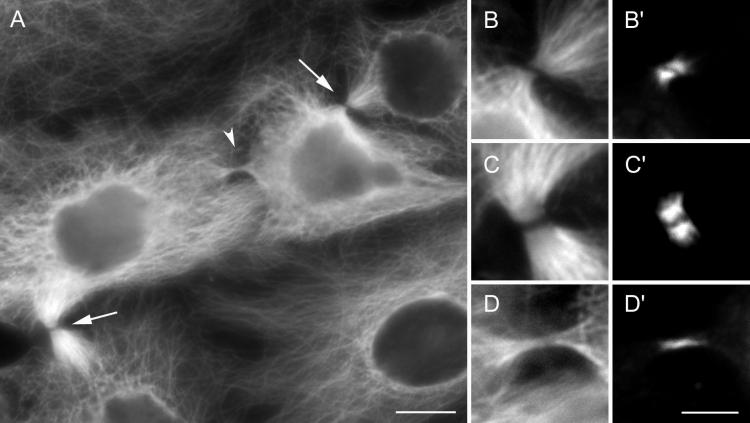
Figure 8.
Midbodies formed between spindles contain INCENP. (A) The distribution of microtubules in the cell shown in Figure 1. The arrows indicate control midbodies, whereas the arrowhead indicates the midbody formed in association with the ectopic furrow. (B–D) The microtubule patterns in these midbodies at a higher magnification. B is from the upper control midbody shown in A, C is from the lower control, and D is from the ectopic midbody. (B′–D′) The corresponding images depicting the distribution of INCENP. Note that all of the midbodies, including the ectopic one, contain this protein. Bars: A, 10 μm; B–D and B′–D′, 5 μm.
We also attempted to look at TD-60 (reviewed in Margolis and Andreassen, 1993) but were thwarted because this antibody does not recognize TD-60 in PtK1 cells, and our attempts to create cells containing two independent spindles that stain with TD-60 (e.g., CV-1) were not successful. We discovered that the independent spindles in cell types that normally round during mitosis, which include all fibroblasts and most epithelial cells, rapidly associated and fused to form a large common multipolar spindle. However, with regards to INCENP, a recent study found that the distribution of this protein and TD-60 was indistinguishable under a wide variety of conditions (Martineau-Thuillier et al., 1999).
Finally, we examined ectopic furrows for the presence of CENP-E. CENP-E is another chromosomal passenger protein that relocates during anaphase to the interzone Mts, and its location ultimately becomes restricted to the midbody. However, because it is degraded sometime after midbody formation but before the final separation of daughter cells (Brown et al., 1994), it is present in all young but not older midbodies. We therefore fixed our experimental cells (n = 9) after furrowing at the ectopic site was well underway but before midbody formation (e.g., Figure 9). Although this protein was present in all forming control furrows and midbodies (e.g., Figure 9, E and F, arrows), we never found it in ectopic furrows or forming ectopic midbodies (Figure 9, E and F, arrowhead).
Relaxing Ectopic Furrows Lack INCENP and CHO1
As noted previously, 76% of the cells followed by video LM failed to initiate cytokinesis between the two spindles after anaphase. When we examined those regions where an ectopic furrow should have formed, we always found numerous Mts that were derived from opposing centrosomes (see Figure 3D). However, in these cells we never saw INCENP or CHO1 protein at locations other than the control midbodies (our unpublished observations).
We speculate that ectopic furrows that relax are likely to be deficient in some component(s) required for the final stages of cytokinesis. One prediction is that the CHO1 protein is not present in relaxing ectopic furrows because these furrows lack Mt bundles (our unpublished observation) that require CHO1 (e.g., Kuriyama et al., 1994; Raich et al., 1998). Another prediction is that they also lack INCENP that has recently been implicated in the latter but not in early stages of cytokinesis (Mackay et al., 1998). To evaluate these predictions we examined relaxing furrows for the presence of these proteins. In every instance we found that, as predicted, these regions lacked detectable CHO1 (n = 5; Figure 4G, arrowhead) and INCENP (n = 5; Figure 5G, arrowhead).
DISCUSSION
PtK1 cells that contain two independent mitotic spindles can be produced by electrofusion. As described by Rieder et al. (1997), these spindles remain independent throughout mitosis unless they wander close enough (within ∼20 μm) for their Mt arrays to overlap, at which time they then rapidly fuse to form a single large multipolar spindle. In their original report, Rieder et al. (1997) noted that cells containing two spindles sometimes undergo cytokinesis between the spindles in a region that lacked an intervening spindle and chromosomes. Although it was noted that many of these “ectopic” furrows were fully functional in that they led to the production of two independent daughter cells, no attempt was made at the time to study their structure or composition.
The demonstration that cytokinesis can occur in vertebrate somatic cells between two centrosomes, in the absence of an intervening spindle or chromosomes, is similar to that reported earlier for echinoderm zygotes (reviewed in Rappaport, 1991). The fact that both vertebrate somatic cells and large invertebrate zygotes play by the same rules implies that cytokinesis occurs by similar mechanisms in diverse systems. Because cytokinesis can occur in the absence of a spindle and chromosomes, the role these structures play in this process remains unclear.
As emphasized by Oegema and Mitchison (1997), data concerning the structure and composition of ectopic furrows are potentially useful for evaluating models of cytokinesis. We therefore used same-cell correlative LM, IMF, and EM to characterize the structure of ectopic furrows and to determine whether they contain some of the chromosomal passenger (INCENP and CENP-E) and spindle (CHO1) proteins thought to be involved in cytokinesis.
Ectopic Furrows Lead to the Formation of Midbodies
During anaphase the interzonal region between the groups of separating chromosomes contains two overlapping interpolar Mt arrays, of opposite polarity, that are derived from the opposing centrosomes (e.g., Mastronarde et al., 1993). During late anaphase and near the initiation of cytokinesis, adjacent Mts within this interpolar array begin to coalesce into multiple bundles, each of which contains a discrete patch of an electron-opaque matrix material (Buck and Tisdale, 1962; reviewed in Rattner, 1992; Kuriyama et al., 1994). As furrowing is initiated, these multiple Mt bundles and their associated matrix coalesce into a single Mt bundle referred to as a midbody (Buck and Tisdale, 1962; reviewed in Rattner, 1992) (Figure 2, B–D). The midbody then bridges the two daughter cells until it is broken and discarded (see Mullins and Biesele, 1977).
The preliminary results of Rieder et al. (1997) suggested that ectopic furrows in PtK1 cells form in a region between the spindles devoid of Mts and lack a midbody. However, our extensive analyses of cells containing two independent spindles reveal that by telophase the region between the two spindles always contains numerous Mts (Figures 3D, 4E, and 5F). Because these Mts are not present between the spindles at the time of anaphase onset (Rieder et al., 1997), they likely arise during telophase as each of the centrosomes nucleate a new array of cytoplasmic Mts (e.g., see Vandre et al., 1984).
We found that the intercellular bridge formed by an ectopic furrow contained a midbody that was indistinguishable from those formed by control furrows in the same cell (compare Figure 2, B–D). In some cases the ectopic midbody contained only a few bundled Mts (e.g., Figures 1H and 7E) and little matrix material, but in others it was as robust as those of control midbodies formed between separating groups of chromosomes (e.g., Figure 2D). By contrast, although overlapping arrays of interpolar Mts were always found in the region between two independent telophase spindles, we never saw bundles of Mts or midbodies in this region in cells that failed to initiate an ectopic furrow (Figure 3D) or in those cells in which the ectopic furrow relaxed (Figures 4F and 5F). Considering the fact that after being formed, interzonal Mt bundles are extremely stable (Salmon et al., 1976; Mullins and Biesele, 1977; Mullins and McIntosh, 1982), we conclude that ectopic furrows can be initiated in the absence of Mt bundling. However, Mt bundling seems to be required for the furrow to propagate to the midbody stage because we always found bundles in the midbodies of ectopic furrows and, as evident from our cytochalasin D-treated cells, bundling is not a product of furrow constriction but an intrinsic property of the spindle interzone (e.g., Figure 6). In this context it is noteworthy that we always found the Mt-bundling protein CHO1 in both control and ectopic midbodies but never in relaxing ectopic furrows. Our conclusion that Mt bundling is required for furrow propagation but not for initiation is consistent with the recent report that furrows are initiated, but then ultimately relax, in ZEN-4 null Caenorhabditis elegans embryonic cells that also lack the capacity to bundle interzone Mts (Raich et al., 1998).
CHO1 and INCENP Are Present in Ectopic Midbodies and Are Therefore Likely Required for Cytokinesis, whereas CENP-E Is Not
The formation of midbodies between adjacent spindles occurred < 20% of the time (i.e., in 72 of 432 or 17.6% of the cells), but when these midbodies did form, they typically appeared to be both functionally and structurally normal. Their formation therefore provides a model system for analyzing the function of specific proteins during cytokinesis. In particular, proteins that are required for midbody formation must be present in both normal (control) and ectopic furrows.
Our study reveals that the kinesin-like Mt motor protein CENP-E, which becomes concentrated in control cleavage furrows and midbodies, is not essential for cytokinesis. Its location within the furrow and its association with midbody Mts have led to the suggestion that this chromosomal passenger protein is involved in this process (e.g., see Yen et al., 1991). However, this hypothesis has not been directly tested because the inhibition of CENP-E function, by antibody injection (Yen et al., 1991) or overexpressing a mutant (Schaar et al., 1997), inhibits the anaphase onset that is a prerequisite for cytokinesis. Our data reveal that although CENP-E is always present in control furrows and midbodies, it is never seen in ectopic furrows or ectopic midbodies (Figure 6). Thus we conclude that CENP-E is not required for cytokinesis.
The converse of this logic is that proteins that are always associated with both control and ectopic furrows and midbodies are excellent candidates for components that have an obligatory role in cytokinesis. Although localization data by itself cannot demonstrate a functional involvement, it is striking that two of the proteins we found to be always associated with normal and ectopic furrows have been independently suggested to be involved in cytokinesis.
The first of these is the CHO1 protein that bundles Mts in vitro (Nislow et al., 1992) and in vivo (Kuriyama et al., 1994) and is known to have human (MKLP1 [Nislow et al., 1992]) and C. elegans (ZEN-4 [Raich et al., 1998]) homologues. During spindle formation, this protein is distributed diffusely along the spindle fibers, but during anaphase, it redistributes into prominent streaks within the interzone that correspond to MT bundles. Finally during telophase, it becomes restricted to the very center of the midbody where the electron-opaque matrix associated with overlapping Mt ends is located (Sellitto and Kuriyama, 1988). Like CENP-E, CHO1 is a member of the kinesin superfamily, but unlike CENP-E, our data reveal that CHO1 is present in every ectopic midbody, suggesting that it is required for cytokinesis in vertebrates as its homologue (ZEN-4) is required in C. elegans (Raich et al., 1998). How CHO1 becomes associated with ectopic furrows, which can form many micrometers from a spindle and chromosomes, remains to be determined. Because it is a plus-end Mt motor, present in the centrosome and nucleus before spindle assembly, it is possible that it moves into the region where the ectopic furrow will form during telophase as the centrosomes begin to renucleate extensive arrays of cytoplasmic Mts.
The chromosomal passenger protein INCENP was originally suggested to play a role in cytokinesis (Cooke et al., 1987) and becomes concentrated at the site of furrow formation before myosin (Eckley et al., 1997). Recent studies reveal that cytokinesis ultimately fails in cells overexpressing different dominant-negative mutant forms of INCENP (Eckley et al., 1997; Mackay et al., 1998). However, a role for this protein in cytokinesis has been questioned because it is difficult to envision how a chromosomal protein can become positioned in ectopic furrows that form many micrometers from the closest chromosome (Oegema and Mitchison, 1997). Here we show that INCENP is present in all ectopic furrows. This result reveals that this protein does not have to be delivered by a chromosome but can move freely within the cell. Because we found INCENP in all ectopic furrows that formed midbodies, the data also strongly support the hypothesis that it plays an obligatory role in cytokinesis.
One advantage of our system is that it allows one to compare the structure and composition of completed furrows with those that began to form but then ultimately relaxed. As in echinoderm zygotes (Rappaport, 1961), some (∼30%) of the ectopic furrows formed in PtK1 cells relaxed before reaching the midbody stage (Rieder et al., 1997) (our unpublished observations). Because cytokinesis has long been known to be a multistep process (reviewed in Rappaport, 1991; Adachi et al., 1997), this result is not unexpected. It means that the conditions for forming and initiating the activity of a furrow are not the same as those for maintaining and propagating the furrow. Therefore, an analysis of regressing ectopic furrows may be useful for defining which components are needed for maintaining and stabilizing a furrow after it is formed. Our findings that CHO1 and INCENP are always present in ectopic midbodies, but never in regressing ectopic furrows, suggest that they are not involved in furrow initiation but instead stabilize the activity of the furrow until the midbody is formed. This hypothesis is consistent with the recent observation that furrowing is initiated in C. elegans zygotes lacking the CHO1 homologue (ZEN-4) but that these furrows ultimately regress (Raich et al., 1998). It is also consistent with the demonstration of Eckley et al. (1997) (see also Mackay et al., 1998) that overexpressing a mutant form of INCENP disrupts cytokinesis only after the furrow has already formed.
Although it is not known which molecules signal the location of either normal or ectopic furrows, our data permit us to conclude that the initiation of furrow formation requires more than just overlapping antiparallel arrays of Mts in a telophase cytoplasm; 70% of our cells met this condition without exhibiting any detectable signs of furrow formation. The bundles of interpolar Mts that form within the interzone during anaphase and telophase are extremely stable (see above), and their formation requires CHO1 but not the presence of a contractile furrow (e.g., see Figure 6). Recent observations of mutant Drosophila spermatocytes that fail to undergo cytokinesis suggest that the bundles of interpolar Mts formed in the interzone during anaphase are involved in positioning and initiating furrow formation (e.g., Williams et al., 1995; Giansanti et al., 1998). However, we do not find bundles of interzonal Mts in regressing ectopic furrows where, because of their extreme stability, they would be expected if they were required for furrow formation. Indeed, when the formation of interzonal Mt bundles is inhibited in C. elegans by knocking out the CHO1 homologue ZEN-4, cytokinesis is still initiated but ultimately fails (Raich et al., 1998).
In conclusion, the ability to examine the structure and composition of ectopic furrows that complete furrowing to the midbody stage and of regressing ectopic furrows provides a novel and powerful system for exploring the mechanism of cytokinesis. We have used this system to evaluate the role of several proteins thought to be involved in this process and have identified one (CENP-E) that is not. Furthermore, our data implicate the Mt-bundling CHO1 protein and INCENP in the propagation and/or stabilization of furrows but not in their formation. The assay described here should be useful in the future for determining whether proteins suspected of being involved in cytokinesis are and if so whether they are involved in the initial or latter stages of this process.
ACKNOWLEDGMENTS
We gratefully acknowledge Ms. Cindy Hughes for her tireless assistance with cell culture, Ms. Grisel Cassels for help with the EM aspects of this study, and Mr. Richard Cole for thoughtful discussions and technical input. We also thank Drs. T. Yen (Fox Chase Cancer Center, Philadelphia, PA) and R. Kuriyama (Department of Cell Biology and Neuroanatomy, University of Minnesota, Minneapolis, MN) for their generous gifts of the CENP-E and CHO1 antibodies, respectively. This work was supported by National Institutes of Health/GMS grant 40198 (to C.L.R.) and a Principal Research Fellowship from the Welcome Trust (to W.C.E.) and made use of the Wadsworth Center’s video LM core facility.
REFERENCES
- Adachi H, Takahashi Y, Hasebe T, Shirouzu M, Yokoyama S, Sutoh K. Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J Cell Biol. 1997;137:891–898. doi: 10.1083/jcb.137.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen PR, Palmer DK, Wener MH, Margolis R. Telophase disk: a new mammalian mitotic organelle bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- Aubin JE, Osborn M, Weber K. Inhibition of cytokinesis and altered contractile ring morphology induced by cytochalasins in synchronized PtK2 cells. Exp Cell Res. 1981;136:63–79. doi: 10.1016/0014-4827(81)90038-0. [DOI] [PubMed] [Google Scholar]
- Brown KD, Coulson RMR, Yen TJ, Cleveland DW. Cyclin-like accumulation and loss of the putative kinetochore motor CENP-E results from coupling continuous synthesis with specific degradation at the end of mitosis. J Cell Biol. 1994;125:1303–1312. doi: 10.1083/jcb.125.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Wood KW, Cleveland DW. The kinesin-like protein CENP-E is a kinetochore-associated throughout poleward chromosome segregation during anaphase-A. J Cell Sci. 1996;109:961–969. doi: 10.1242/jcs.109.5.961. [DOI] [PubMed] [Google Scholar]
- Buck RC, Tisdale JM. The fine structure of the midbody of the rat erythroblast. J Cell Biol. 1962;13:109–115. doi: 10.1083/jcb.13.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L-g, Wang Y-l. Signals from the spindle midzone are required for stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Heck MMS, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to the midbody during mitosis. J Cell Biol. 1987;105:2053–2064. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore JJ, Conrad GW, Rappaport R. A model for astral stimulation of cytokinesis in animal cells. J Cell Biol. 1989;109:2225–2232. doi: 10.1083/jcb.109.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Bernat RL. Chromosomal passengers: toward an integrated view of mitosis. Chromosoma. 1991;100:139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Cooke CA. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of a pathway of structural changes in the chromosomes during metaphase and early events in cleavage furrow formation. J Cell Sci. 1991;98:443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- Eckley DM, Ainsztein AM, Mackay A, Goldberg IG, Earnshaw WC. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and the inner-centromere protein position in mitotic heterokaryons and midanaphase cells. J Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkind DJ, Wang Y-l. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Giansanti MG, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, Gatti M. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, McEwen BF, Buttle K, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Dragas-Granoic S, Maekawa T, Vassilev A, Khodjakov A, Kobayashi H. Heterogeneity and microtubule interaction of the CHO1 antigen, a mitosis-specific kinesin-like protein. J Cell Sci. 1994;107:3485–3499. doi: 10.1242/jcs.107.12.3485. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Ainsztein AM, Eckley DM, Earnshaw WC. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville EC, Rieder CL. Keratin filaments restrict organelle migration into the forming spindle of newt pneumocytes. Cell Motil Cytoskeleton. 1990;15:110–120. doi: 10.1002/cm.970150207. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Andreassen PR. The telophase disk: its possible role in mammalian cell cleavage. Bioessays. 1993;15:201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- Martineau-Thuillier, S., Andreassen, P.R., and Margolis, R.L. (1999). Colocalization of TD-60 and INCENP throughout G2 and mitosis: evidence for their possible interaction in signaling cytokinesis. Chromosoma (in press). [DOI] [PubMed]
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins JM, Biesele JJ. Terminal phase of cytokinesis in D-98S cells. J Cell Biol. 1977;73:672–684. doi: 10.1083/jcb.73.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins JM, McIntosh JR. Isolation and initial characterization of the mammalian midbody. J Cell Biol. 1982;94:654–661. doi: 10.1083/jcb.94.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Oegema K, Mitchison TJ. Rappaport rules: cleavage furrow induction in animal cells. Proc Natl Acad Sci USA. 1997;94:4817–4820. doi: 10.1073/pnas.94.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Enhancement of aster-induced furrowing activity by a factor associated with the nucleus. J Exp Zool. 1991;257:87–95. [Google Scholar]
- Rattner JB. Mapping the mammalian intercellular bridge. Cell Motil Cytoskeleton. 1992;23:231–235. doi: 10.1002/cm.970230402. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cassels G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 1999;61:297–315. doi: 10.1016/s0091-679x(08)61987-1. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cole RW. Perfusion chambers for high-resolution video-light microscopic studies of vertebrate cell monolayers: some considerations and a design. Methods Cell Biol. 1998;56:253–275. doi: 10.1016/s0091-679x(08)60430-6. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Khodjakov A, Paliulis LV, Fortier TM, Cole RW, Sluder G. Mitosis in vertebrate cells with two spindles: implications for the anaphase onset checkpoint and cleavage. Proc Natl Acad Sci USA. 1997;94:5107–5112. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon ED, Goode D, Maugel TK, Bonar DB. Pressure-induced depolymerization of microtubules. II. Differential stability in HeLa cells. J Cell Biol. 1976;69:443–454. doi: 10.1083/jcb.69.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite LL, Pollard TD. Cytokinesis. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto C, Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988;106:431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandre DD, Kronebusch P, Borisy GG. The interphase-mitosis transition: microtubule rearrangements in cultured cells and sea urchin eggs. In: Borisy GG, Cleveland DW, Murphy DB, editors. Molecular Biology of the Cytoskeleton. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1984. pp. 3–16. [Google Scholar]
- Wheatley SP, O’Connell CB, Wang Y-l. Inhibition of chromosomal separation provides insights into cleavage furrow stimulation in cultured epithelial cells. Mol Biol Cell. 1998;9:2173–2184. doi: 10.1091/mbc.9.8.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y-l. Midzone microtubules are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Borisy GG. On the mechanism of cytokinesis in animal cells. J Theor Biol. 1983;101:289–313. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]
- Williams BC, Reidy MF, Williams EV, Gatti M, Goldberg ML. The Drosophila kinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Compton DA, Wise D, Zinkowski RP, Brinkley BR, Earnshaw WC, Cleveland DW. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 1991;10:1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. “Anaphase” and cytokinesis in the absence of chromosomes. Nature. 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]