Abstract
Rap1b has been implicated in the transduction of the cAMP mitogenic signal. It is phosphorylated and activated by cAMP, and its expression in models where cAMP is mitogenic leads to proliferation and tumorigenesis. Akt is a likely downstream effector of cAMP-Rap1 action. cAMP elevation induced a rapid and transient Akt inhibition that required activated and phosphorylated Rap1b. However, the mechanism(s) by which cAMP-Rap regulates Akt remains unclear. Here we show that (i) upstream regulators, PIK and PDK1, are not the target(s) of the cAMP inhibitory action; (ii) constitutively active Akt and calyculin A-stimulated Akt are resistant to cAMP inhibition, suggesting the action of a phosphatase; (iii) cAMP increases the rate of Akt dephosphorylation, directly implicating an Akt-phosphatase; (iv) Epac- and protein kinase A (PKA)-specific analogs synergistically inhibit Akt, indicating the involvement of both cAMP-dependent effector pathways; (v) H89 and dominant negative Epac 279E block cAMP-inhibitory action; (vi) Epac associates in a complex with Akt and PP2A, and the associated-phosphatase activity is positively modulated by cAMP in a PKA- and Rap1-dependent manner; (vii) like its action on Akt inhibition, PKA- and Epac-specific analogs synergistically activate Epac-associated PP2A; and (viii) dominant negative PP2A blocks cAMP-inhibitory action. Thus, we uncovered a novel cAMP-Epac/PKA-Rap1b-PP2A signaling module involved in Akt regulation that may represent a physiological event in the process of cAMP stimulation of thyroid mitogenesis.
cAMP stimulates the proliferation of numerous cell types by acting synergistically with other growth factors, such as insulin/IGF-1 (1). The mitogenic effect of cAMP requires the activation of both effector pathways (2, 3) (i.e. cAMP-dependent protein kinase A (PKA)3 and Epac (exchange protein activated by cAMP)) that, in turn, modulate multiple downstream signaling processes essential for cell growth (4–6) (i.e. Erk and Akt).
Akt is activated by several agonists that stimulate phosphatidylinositol 3-kinase activity, which catalyzes the formation of the D3 phosphoinositides phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate (7). These membrane lipids bind to the Akt pleckstrin homology (PH) domain (8, 9), leading to a stable interaction of Akt with cellular membranes. This membrane recruitment primes Akt for activation by phosphorylation. Full activation of Akt requires a PDK1 (phosphoinositide-dependent kinase 1)-dependent phosphorylation of Thr308 in the activation loop of Akt (10, 11) and a second phosphorylation event at Ser473 in the hydrophobic C-terminal domain of Akt (12). Although Akt can be autophosphorylated (13), several kinases with the ability to phosphorylate Akt Ser473 have been reported (14–17). Akt phosphorylation at steady state is tightly regulated, representing a balance between kinase-activating and phosphatase-inactivating events. Although efforts were originally mainly focused on the mechanism(s) of Akt activation, a role for agonist-mediated Akt inactivation is being uncovered. Several protein phosphatases, including canonical PP1 (18, 19) and PP2A (20–31) as well as newly identified Akt phosphatases (32), were recently reported to bind and dephosphorylate Akt in an agonist-dependent manner.
cAMP can either stimulate (33–43) or inhibit (44–50) Akt activity. We have previously reported that cAMP inhibited Akt activity in PCCL3 thyroid cells via the GTP-binding protein Rap1b (47) and suggested that it played a role in TSH mitogenic action in these cells. cAMP rapidly promotes activation of Rap1b via the guanine exchange factor Epac (3) and phosphorylation of Rap1b-Ser179 through PKA (52). Active and phosphorylated Rap1b is strictly required for TSH stimulation of DNA synthesis (2, 3, 53) as well as inhibition of Akt activity (47). However, the molecular mechanisms by which cAMP inhibits Akt activity remain largely undefined. Here we report that cAMP-dependent inhibition of Akt in PCCL3 thyroid cells is mediated by PP2A, the identification of a novel and stable Epac-PP2A signaling complex, and the modulation of its phosphatase activity by Rap-GTP and PKA.
EXPERIMENTAL PROCEDURES
Materials—Forskolin, isobutylmethylxanthine, and H89 were obtained from Calbiochem. Phosphatase inhibitors were obtained from Alexis. Phosphorylation-specific (Ser473, Thr308) and phosphorylation state-independent rabbit polyclonal anti-Akt antibodies were purchased from New England Biolabs (Beverly, MA). Monoclonal antibodies against HA (HA.11) and Myc (9E10) were from Covance Research Products. [γ-32P]ATP (3000 Ci/mmol) and [32P]orthophosphoric acid (carrier-free) were from MP Biomedicals. Crosstide and anti-phosphotyrosine antibodies were from Upstate Biotechnology, Inc. (Lake Placid, NY). Protein G- and GSH-Sepharose were from GE Healthcare. Phosphatidylinositol, ATP, cAMP, and digitonin were from Sigma. DiFMUP was obtained from Invitrogen.
Cell Culture—PCCL3 cells were cultured as previously described (3) in Coon's modified Ham's F-12 medium supplemented with 5% fetal bovine serum and four hormones: TSH (1 milliunits/ml), insulin (10 μg/ml), transferrin (5 μg/ml), and hydrocortisone (1 nm) at 37 °C in an atmosphere of 5% CO2 in air. HEK293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum.
Plasmids and Transfection—All tagged Rap1 plasmids have been described elsewhere (52). Akt constructs were a gift from Dr. Toker (Beth Israel Deaconess, Boston, MA). FLAG-tagged PDK1 constructs were a gift from Dr. Liu (University of Texas Health Science Center), wild type and dominant negative PP2A-L309A were provided by Dr. J. Gotz (University of Zurich, Switzerland), and HA-GRP1-PH was a gift from Dr J. Klarlund (University of Pittsburgh). PCCL3 cells, plated in 6-well plates, were transfected with the indicated plasmids (1.0–1.5 μg of DNA/well) using Fugene 6 reagent according to the supplier's protocol (Roche Molecular Biochemicals). After a 24-h recovery, cells were starved in Coon's medium containing 0.2% bovine serum albumin and transferrin (5 μg/ml) for 16 h before treatment and harvest.
Immunoprecipitation and Western Blotting—For immunoprecipitation, extracts were prepared in lysis buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 10 mm EDTA, 10 mm Na4P2O7, 2 mm sodium orthovanadate, 100 mm NaF, 0.5 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1% Triton X-100, followed by a centrifugation step for 30 min at 4 °C. For total lysates, cells were directly harvested in SDS sample buffer. Samples were resolved by SDS-PAGE followed by transfer onto polyvinylidine difluoride membranes. After blocking in 5% nonfat milk, membranes were probed with the appropriate primary antibodies. Immunoreactive proteins were detected by a chemiluminescence detection kit (Pierce), using horseradish peroxidase-conjugated secondary antibodies. Specific bands were quantified by image analysis using Bio-Rad Molecular Dynamics software.
Immunofluorescence Staining and Confocal Microscopy—Cells were cultured on coverslips in 6-well plates and transfected with the indicated constructs. For immunofluorescent staining, cells were rinsed with phosphate-buffered saline (PBS; pH 7.4), fixed in 3.7% formaldehyde in PBS for 10 min at room temperature, permeabilized in 0.5% Triton X-100 in PBS for 20 min, and washed in 0.1 m glycine in PBS for 10 min. The coverslips were washed five times for 5 min with PBS and blocked with 1% bovine serum albumin, 1% goat serum in PBS for 30 min. The cells were incubated with fluorescein isothiocyanate-coupled HA antibody or Cy3-coupled Myc antibodies for 60 min at room temperature and washed five times in 1% bovine serum albumin in TBS for 10 min. The coverslips were mounted in PermaFluor (Thermo Electron Corp.), and samples were examined under confocal microscopy.
Phosphatidylinositol Kinase Activity—After appropriate stimulation, cell extracts were immunoprecipitated with anti-phosphotyrosine or anti-cPI3K antibodies; after several washes, beads were incubated with sonicated phosphatidylinositol and [γ-32P]ATP, and the reaction products were analyzed by TLC as previously described (54).
In Vitro Akt Kinase Activity Assay—After treatment with the indicated agents, PCCL3 cells extracts were prepared in lysis buffer (see above). The lysate was cleared by centrifugation at 15,000 × g for 10 min at 4 °C, and HA-Akt was immunoprecipitated with an anti-HA antibody, followed by protein G-Sepharose purification. After washing the immunocomplexes, samples were separated into two equal parts, used for Western blot analysis and kinase activity assay, respectively. Kinase activity was determined as previously described (47) using crosstide as substrate.
Digitonin Permeabilization and Rate of Phospho-Akt Dephosphorylation—PCCL3 cells transfected with HA-Akt WT were starved for 16 h, followed by a 2-h incubation with [32P]orthophosphoric acid in phosphate-free Coon's modified Ham's F-12 medium. Cells were washed twice in PBS and then stimulated for 10 min at 37 °C with insulin (1 μg/ml). After two washes in ICB buffer (20 mm Hepes, pH 7, 10 mm NaCl, 140 mm KCl, 2.4 mm MgCl2), cells were permeabilized with digitonin (40 μg/ml) in ICB buffer containing 5 mm ATP and 20 μm cAMP. At the indicated time points, cells were lysed and immunoprecipitated with HA antibody. HA-[32P]Akt was visualized by SDS-PAGE and autoradiography.
Microcystin-Sepharose Pull-down Assay—Upon appropriate stimulation, cells were harvested in lysis buffer (500 μl/well of a 6-well plate), and debris was eliminated by centrifugation. Microcystin-Sepharose beads (Upstate Biotechnology) were then added to the supernatant (∼5 nm bound microcystin final) and incubated for 1 h at room temperature. Upon extensive washes, associated proteins were eluted in SDS buffer and analyzed by Western blot. Control experiments were performed by the addition of soluble microcystin-LR (∼50 nm) to lysates 30 min before incubation with the microcystin-Sepharose beads.
Fluorescence-based in Vitro Phosphatase Assay—For the in vitro phosphatase assay, glutathione S-transferase (GST)-Epac complexes were prepared by GST pull-down from GST-Epac transfected cells. Cells were starved for 16 h and stimulated with DMSO control or 10 μm forskolin. Cells were harvested in lysis buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5 mm MgCl2, protease and phosphatase inhibitors). After a 1-h incubation with GSH-Sepharose, beads were extensively washed with lysis buffer (without phosphatase inhibitors) and twice with phosphatase assay buffer (50 mm Tris-HCl, pH 7.0, 0.1 mm MnCl2). Phosphatase assay was initiated by adding a phosphatase assay mixture containing 10 mm DiFMUP as substrate, and activity was monitored by fluorescence in a 96-well plate reader (Wallac Victor; PerkinElmer Life Sciences).
RESULTS
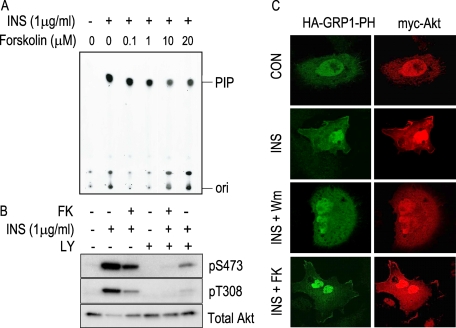
Upstream Akt Regulatory Elements Are Not the Target(s) for cAMP-mediated Inhibition—In PCCL3 thyroid cells, elevation of cAMP transiently inhibits basal and insulin-mediated Akt phosphorylation and activation (47). To start dissecting the inhibitory target site, the effect of forskolin stimulation on the upstream elements in the pathway were investigated. PIK activation represents a receptor-proximal event required for Akt activation (55). An immune complex PIK activity assay was performed using anti-phosphotyrosine antibodies, and the reaction products were analyzed on TLC plates (Fig. 1A). Although insulin stimulated PIK activity, no effect of forskolin was observed even at concentrations that maximally inhibited Akt activity (IC50 ∼ 0.1 μm) (49). Moreover, forskolin had an effect on a sample treated with saturating amounts of LY294002, a PIK inhibitor (Fig. 1B). Similar effects were observed on both critical phosphorylation sites, Ser473 and Thr308. To further confirm that cAMP levels did not have an effect on PIK activity, GRP1-PH was used as a highly specific phosphatidylinositol 1,4,5-trisphosphate probe (56) under different conditions of stimulation. As shown in Fig. 1C, translocation of both Myc-Akt and HA-GRP1-PH to a membrane compartment was observed by immunofluorescence upon insulin stimulation. However, although PIK inhibitor wortmannin blocked this translocation event, forskolin stimulation did not have any effect. Thus, the combined results of Fig. 1 clearly establish that cAMP inhibitory action on Akt lies downstream of the receptor-PIK signaling module.
FIGURE 1.
cAMP does not inhibit phosphatidylinositol 3-kinase activity. Upon a 16-h starvation period, PCCL3 cells were stimulated 5 min with forskolin at the indicated concentrations followed by a 5-min incubation in the presence of insulin. Phosphatidylinositol 3-kinase was immunoprecipitated using anti-phosphotyrosine antibody, and after extensive washes, it was subjected to a lipid kinase assay. Sonicated phosphoinositol was used as substrate in the presence of [γ-32P]ATP, and reaction products were assessed by TLC, as described (A). Phosphorylation of endogenous Akt was analyzed by Western blots with the indicated antibodies (B). Localization of HA-GRP1-PH and Myc-Akt was analyzed by immunofluorescence with fluorescein isothiocyanate-anti-HA and Cy3-anti-Myc antibodies, respectively (C). When indicated, samples were pretreated with PIK inhibitors LY294002 (LY;10 μm) and wortmannin (Wm; 50 nm) for 1 h. Con, control. FK, forskolin; INS, insulin.
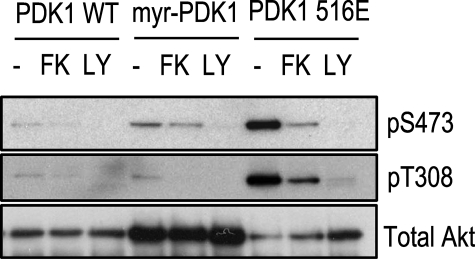
PDK1, a kinase activated by products of PIK, phosphorylates Akt at Thr308, an event required for its maximal activity (10, 11). PDK1 translocates upon PIK stimulation from a cytosolic to a membrane-bound compartment, and it has recently been suggested that cAMP is able to inhibit this translocation event in COS cells (45). To address if this represents the inhibitory target of cAMP in PCCL3 cells, a myristoylated PDK1 construct that is constitutively targeted to the plasma membrane was co-transfected with Akt. Similarly, co-transfection with the constitutively active PDK1 516E, was performed to address whether cAMP could inhibit Akt under those conditions. If translocation represents the inhibitory cAMP target or there was a cAMP inhibitory action on PDK1 activity, it is expected that the myristoylated construct will bypass this step, rendering Akt resistant to cAMP. As can be seen in Fig. 2, forskolin effectively inhibited insulin-stimulated Akt under these conditions. Consistent with these results, forskolin stimulation did not affect PDK1 activity, as assessed by an anti-phospho-PDK1 (Ser241) antibody (not shown). Thus, neither PIK nor PDK1 represents the target for the inhibitory action of cAMP on Akt.
FIGURE 2.
Translocation of PDK-1 is not the cAMP-sensitive step. WT HA-Akt was co-expressed with WT, myristoylated PDK1 (myr-PDK1), or PDK1 516E in PCCL3 cells. Cells were starved, and stimulation was as described in the legend to Fig. 1. Upon lysis, HA-Akt was immunoprecipitated, and its phosphorylation level was assessed by Western blot using the indicated antibodies. FK, forskolin; LY, LY294002.
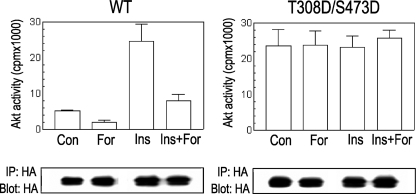
Constitutively Active Akt Is Resistant to cAMP—Steady-state phospho-Akt represents a balance between its rate of phosphorylation mediated by upstream kinases and its rate of dephosphorylation by phosphatases. Our results (Figs. 1 and 2) indicate that upstream kinase events do not represent the target for cAMP inhibition. Accordingly, a set of experiments was designed to assess the possibility that cAMP impacts the rate of Akt dephosphorylation. Conversion of Ser473 and Thr308 (the activating phosphorylation sites) to the phosphomimetic residue aspartic acid renders a constitutively active Akt protein (57). If cAMP increases the rate of Akt dephosphorylation, this constitutively active S473D/T308D protein (DD-Akt) should become resistant to the inhibitory effects of forskolin. As shown in Fig. 3, although forskolin inhibited both basal and insulin-stimulated WT Akt activity, no effect was observed on the maximally stimulated constitutively active DD-Akt protein. These results are consistent with a role of the phosphorylation sites as targets for cAMP action and suggest an Akt phosphatase as a molecular player responsible for cAMP-dependent inhibition.
FIGURE 3.
Constitutively active Akt (T308D/S473D) is resistant to cAMP inhibition. HA-Akt (WT and T308D/S473D) was expressed in PCCL3 cells. Cells were starved and treated as described in previous figure legends. Upon HA immunoprecipitation, an immune complex kinase assay was performed as described under “Experimental Procedures,” utilizing crosstide as substrate. IP, immunoprecipitation; IB, immunoblot.
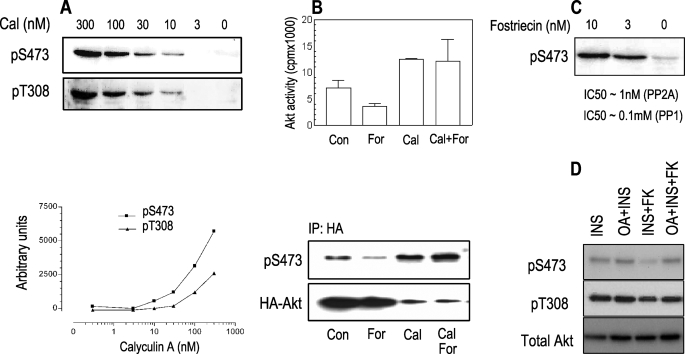
Calyculin A- and Okadaic Acid-activated Akt Is Resistant to cAMP—Phosphatase inhibitors (i.e. okadaic acid and calyculin A) increase the steady-state levels of phospho-Akt (58). If an Akt phosphatase is the target for cAMP, inhibition of its activity should render Akt resistant to cAMP inhibition, as observed above for the DD-Akt protein. Treatment of PCCL3 cells with calyculin A for 20 min increased the phosphorylation of both Ser473 and Thr308 (Fig. 4A) in a dose-dependent manner, with a concomitant increase in its kinase activity (Fig. 4B). However, although basal Akt activity was inhibited by forskolin, calyculin A-stimulated Akt was completely resistant to the inhibitory effects of forskolin (Fig. 4B). Similar results were observed with fostriecin (not shown) and okadaic acid treatment, at concentrations that specifically inhibited PP2A without affecting PP1 activity (Fig. 4, C and D). Therefore, by abolishing phosphatase activity, the ability of cAMP to inhibit Akt is lost, indicating an Akt phosphatase might represent the cAMP-inhibitory target.
FIGURE 4.
Calyculin A- and okadaic acid-mediated activation of Akt is resistant to cAMP inhibition. PCCL3 cells were starved for 16 h, followed by a 20-min incubation with calyculin A (cal) at the indicated concentration. Cell lysates were assayed for phosphorylated Akt by specific antibodies and quantified by densitometry (A). Cells expressing WT-HA-Akt were preincubated for 20 min with 300 nm calyculin A, followed by a 5-min incubation with forskolin (10 μm). Upon HA immunoprecipitation (IP), aliquots were analyzed by Western blot (anti-phospho-Ser473) and kinase activity, as described under “Experimental Procedures” (B). A decrease in total Akt upon calyculin A treatment was consistently observed but not pursued in this study. A fostriecin dose response was also assessed by Western blot with anti-phospho-Ser473 serum (C). The effect of okadaic acid (OA; 15 nm) on the inhibitory action of forskolin (FK; 10 μm) on insulin (INS; 1 μg/ml)-stimulated Akt was assessed by Western blot with the indicated antibodies (D). Con, control; For, forskolin; Cal, calyculin A.
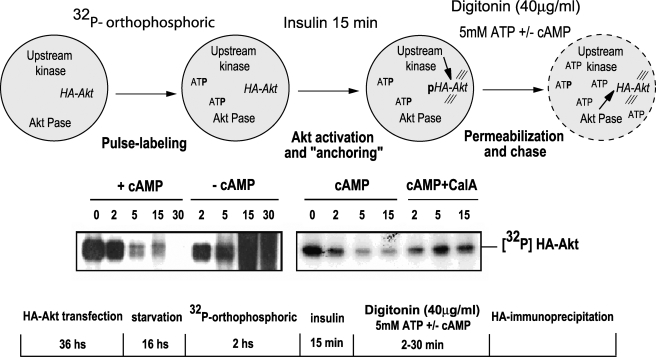
cAMP Stimulates the Rate of Akt Dephosphorylation—To assess whether phospho-Akt is a direct substrate of the putative cAMP-stimulated phosphatase activity, a pulse-chase experiment was designed to directly measure the rate of Akt dephosphorylation upon cAMP stimulation. The rationale of the experiment was to radiolabel the pool of Akt upon insulin treatment, followed by a chase phase with 5 mm cold ATP to decrease specific activity and therefore avoid any interference from de novo upstream phosphorylation events. Since ATP is nonpermeable, a digitonin permeabilization protocol was devised, and the effect of 20 μm cAMP on Akt phosphorylation was assessed by measuring 32P-labeled phospho-Akt upon immunoprecipitation. Under basal nonstimulated conditions, digitonin led to a leak out of Akt from the cells (not shown). Therefore, stimulation with insulin before permeabilization was strictly required and served a dual role: phosphorylation of the Akt pool and, most importantly, “trapping” phospho-Akt intracellularly during permeabilization. Under these experimental conditions (Fig. 5, top), the addition of cAMP during the chasing phase induced Akt dephosphorylation with a t½ of ∼5 min, consistent with the time frame of physiological inhibition by TSH or forskolin (47). No Akt dephosphorylation was observed in the absence of cAMP, and the addition of calyculin A completely abrogated the cAMP-inhibitory effect (Fig. 5). These results demonstrate that cAMP stimulation directly affects the rate of Akt dephosphorylation in a phosphatase-dependent manner.
FIGURE 5.
cAMP increases the rate of Akt dephosphorylation. Top, a scheme of the protocol used (see “Results” for details). Briefly, starved WT HA-Akt cells were labeled with [32P]orthophosphoric acid, followed by a 15-min insulin (1 μg/ml) stimulation. After a washing step, cells were permeabilized with digitonin (40 μg/ml) in the presence of 5 mm ATP, 20 μm cAMP, and calyculin A (calA; 300 nm), as indicated. Upon lysis and HA-immunoprecipitation, HA-AKT phosphorylation level was assessed by SDS-PAGE/autoradiography (bottom). IP, immunoprecipitation.
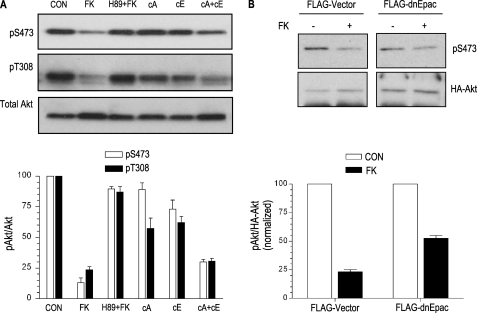
Synergistic Action of PKA and Epac on Akt Dephosphorylation—We have previously implicated both cAMP effector branches, PKA and Epac, in the action of forskolin on phospho-Akt (47). Newly identified specific Epac and PKA-activating cAMP analogs (59, 60) were used to directly assess their involvement on cAMP inhibition of Akt. The analog specific for Epac activated Rap, and that for PKA phosphorylated CREB (2). Although forskolin, in a PKA-dependent manner, inhibited Akt phosphorylation and its kinase activity, stimulation of either Epac or PKA alone was not sufficient to mimic cAMP action on Akt; however, co-stimulation with both analogs recapitulated the response of cAMP (Fig. 6A). To confirm the role of Epac, dominant negative EpacR279E was co-transfected with Akt (Fig. 6B). Similar to the effects of PKA inhibition by H89, co-transfection of dominant negative Epac R279E also blocked the ability of forskolin to inhibit Akt.
FIGURE 6.
PKA and Epac pathways are required for cAMP-mediated Akt inhibition. Specific PKA (300 μm N6-benzoyladenosine-3′,5′-cyclic monophosphate; cA) and Epac (300 μm 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate; cE) analogs were used alone or in combination to assess their relative contribution to cAMP-mediated Akt inhibition. Bottom, quantitation (phospho-Akt/total Akt) from three independent experiments (A). EpacR279E was co-transfected with HA-Akt. Upon stimulation, HA-Akt was immunoprecipitated, and its phosphorylation state was analyzed with the indicated antibody (B). Quantitation (phospho-Ser473-Akt/HA-Akt) from two independent experiments is shown. FK, forskolin.
Taken together, these results confirm the involvement of both effectors, Epac and PKA, on cAMP-dependent Akt dephosphorylation.
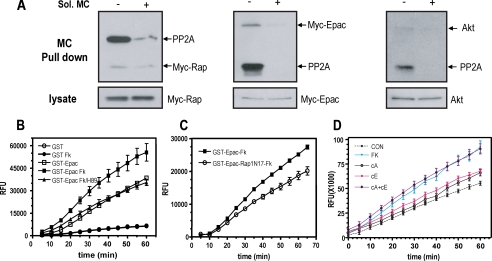
Association of Epac1 and Rap1b with a cAMP-stimulated Akt Phosphatase Activity—Genetic (N17-Rap and RapGAP) (47) and pharmacological (cAMP analogs specific for Epac or PKA) (Fig. 6) intervention clearly demonstrated a role for Rap-GTP in the phosphatase-mediated cAMP inhibition of Akt. As a first attempt at understanding some mechanistic aspects of the cAMP-Rap-phosphatase pathway, we asked whether a pool of Epac-Rap1 is present in the cell in association with a phosphatase. For this, we used microcystin-Sepharose, an affinity reagent used to enrich PP1- and PP2A-containing complexes from cell extracts. Epac, Rap, and Akt (Fig. 7A) were observed upon a single microcystin affinity step, and this steady-state association was not affected by forskolin stimulation (not shown). Binding was specific as assessed by co-incubation in the presence of soluble microcystin (Fig. 7A), and no association was observed with the okadaic-insensitive HA-PHLPP Akt phosphatase (not shown). Moreover, Epac and Akt co-localized in a cortical region of the cell when assessed by confocal microscopy, and this compartmentalization was not modified by forskolin stimulation (not shown). Next, we addressed whether a phosphatase activity could be found in association with Epac/Rap1. GST control and GST-Epac were pulled down from transfected cell extracts, and the washed beads were assessed for phosphatase activity using the fluorescent DiFMUP substrate. Results from this experiment are presented in Fig. 7B; a phosphatase activity was detected specifically in GST-Epac-containing beads, and its activity was positively modulated in a forskolin-dependent manner. The effect of forskolin on the phosphatase activity could be blocked by H89, indicating the involvement of PKA. Interestingly, a similar behavior was observed when N17-Rap was co-transfected along with GST-Epac; dominant negative N17-Rap did not significantly affect basal Epac-associated phosphatase activity but blocked its forskolin-mediated stimulation (Fig. 7C). In order to assess the role of the different cAMP effectors on Epac-bound phosphatase activity, specific Epac/PKA analogs were utilized (Fig. 7D). Consistent with the effects on Akt inhibition (Fig. 6A), stimulation of each effector pathway independently showed only a small effect; however, co-stimulation rendered a synergistic activation comparable with forkolin action. These results indicate the presence of a cAMP-stimulated Epac-associated phosphatase, whose activity could be modulated synergistically by PKA, Epac, and Rap1 action.
FIGURE 7.
Epac associates with a phosphatase activity: synergistic regulation by Epac, Rap1, and PKA. A, cells were transfected with either Myc-Rap1b or Myc-Epac1 expression plasmids. Lysates from exponentially growing cells were prepared and incubated with microcystin-Sepharose, and after extensive washes, they were analyzed by Western blot for endogenous PP2A and Akt or with 9E10 (anti-Myc) for Rap1b and Epac. Soluble microcystin (MC; 50 nm) was added during the affinity step, as indicated. Aliquots from the lysates before the affinity step are also shown. B, cells were transfected with GST or GST-Epac expression plasmids as indicated. Upon a 16-h starvation, cells were stimulated with forskolin (10 μm) for 10 min (Fk) with or without a 20-min preincubation with 20 μm PKA inhibitor (H89), followed by an affinity step with glutathione-Sepharose. Upon extensive washes, associated phosphatase activity was assayed with DiFMUP as substrate and monitored on a fluorescent reader. Results are expressed as average ± S.E. from three independent experiments. C, cells were transfected with GST-Epac and N17-Rap, stimulated with forskolin, and processed as in B for associated phosphatase activity. Results are expressed as average ± S.E. from three independent experiments. D, GST-Epac-associated phosphatase activity was measured as in B upon stimulation with the indicated drugs (FK, forskolin; cA, PKA-specific analog; cE, Epac-specific analog). Results are expressed as average ± S.E. from seven independent experiments. CON, control.
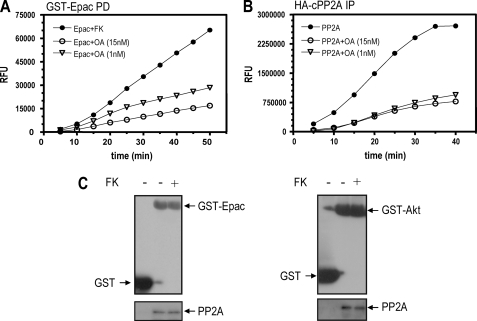
PP2A Is the Epac-associated Phosphatase Activity—GST-Epac-associated phosphatase activity could be blocked by fostriecin (not shown) and okadaic acid (Fig. 8A) at concentrations that inhibit PP2A activity (Fig. 8B), suggesting that the phosphatase activity in the Epac complex is a member of the PP2A family. To directly assess this possibility, GST-Epac pull-down material was blotted with antibodies specific for the catalytic PP2A subunit. As can be observed in Fig. 8C, catalytic PP2A is present in a complex with Epac. Consistent with the microcystin-Sepharose affinity results, forskolin treatment did not significantly alter the amount of catalytic PP2A present in the complex, indicating that forskolin action on PP2A represents an activating event rather than an association/recruitment step. Similar results were observed with Akt-associated PP2A (Fig. 8C). Thus, these results indicate that a pool of Epac in the cell is present in a complex with a phosphatase, identified pharmacologically and immunologically as PP2A, whose activity could be modulated by cAMP in a PKA- and Rap1-dependent manner.
FIGURE 8.
Epac-associated phosphatase is PP2A. A, GST-Epac-transfected cells were incubated with forskolin (Fk) as described before in the presence of different amounts of okadaic acid (OA). Samples were processed as described before for phosphatase activity. B, HA-cPP2A-transfected cells were incubated with okadaic acid, as indicated. After immunoprecipitation with HA11 antibody, phosphatase-associated activity was assayed as described. C, GST-, GST-Epac-, and GST-Akt-transfected cells were stimulated with forskolin as indicated before. Upon a glutathione-Sepharose affinity step, associated proteins were resolved on SDS-PAGE and visualized by Western blot with anti-GST and PP2A antibodies.
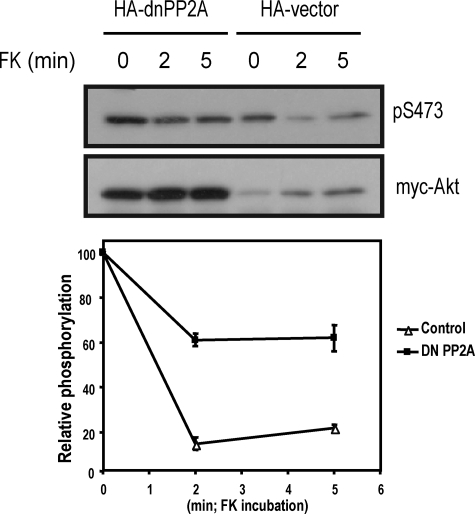
PP2A Is the cAMP-sensitive Akt Phosphatase—If PP2A is the phosphatase responsible for Akt dephosphorylation downstream of Epac-Rap1b and PKA, its specific inhibition should make the system resistant to cAMP inhibition. In order to specifically block PP2A activity, a dominant negative PP2A construct was utilized (cPP2A-L309A) (61), and its effect on forskolin-mediated Akt inhibition was determined. As can be seen in Fig. 9, forskolin treatment inhibited insulin-stimulated Akt phosphorylation as previously described (47). Co-transfection with dominant negative PP2A-L309A increased steady-state Akt phosphorylation and significantly blocked the inhibitory effect of forskolin. These results confirmed the previous observations based on pharmacological inhibitors (Fig. 4D); moreover, they demonstrate that PP2A is the Akt phosphatase responsible for the Epac-, Rap1b-, and PKA-dependent cAMP inhibition of Akt.
FIGURE 9.
PP2A is the Akt phosphatase mediating cAMP inhibition. PCCL3 cells were co-transfected with Myc-Akt and HA-cPP2A-L309A (dnPP2A) or pcDNA3.1 as control (Vector). Upon a 16-h period of serum starvation, cells were stimulated with forskolin (10 μm) for the indicated times, followed by insulin (1 μg/ml for 5 min). Upon lysis, Myc-Akt was immunoprecipitated from the extracts (9E10 antibody), and proteins were resolved on SDS-PAGE and assayed for Myc (bottom) and Akt phosphorylation (anti-phospho-Akt Ser473) (top). For unknown reasons, opposite to the observation made with calyculin A, HA-cPP2A-L309A consistently increased the steady-state levels of Myc-Akt. Quantitation (phospho-Ser473-Akt/Myc-Akt) from two independent experiments is shown.
DISCUSSION
Thyroid cell proliferation is triggered by the synergistic action of TSH and growth factors of the insulin/IGF1 family (62). We have recently demonstrated an active role for both cAMP effectors, Epac and PKA (2), in TSH-mediated mitogenesis; however, the molecular players downstream of cAMP responsible for this synergism are for the moment unknown. We have previously identified Akt activity as a downstream target of TSH-cAMP signaling in thyroid cells (47). TSH/cAMP induced a rapid and transient Akt inhibition, a process that required both PKA and active Rap1 (47). In the present study, we have demonstrated that cAMP, acting via a synergistic action of Epac, PKA, and Rap1b, activates PP2A, increasing the rate of phospho-Akt dephosphorylation, leading to inhibition of its activity.
cAMP modulates Akt in different cell types, including fibroblasts (33, 34, 45, 63), hepatocytes (42), adipocytes (41, 64), cardiomyocytes (40), skeletal muscle (43), neurons (48), Schwann (39), endothelial (46), and endocrine cells (38, 65). Specifically, in thyroid cells, the role of Akt in TSH-mediated proliferation of nontransformed cells is still controversial; either no effects (66) or both positive (67–69) and negative (47) effects of TSH on Akt phosphorylation/activity have been reported. The stimulation of Akt activity by cAMP is of small magnitude (1.5–2-fold), and it occurs with relatively slow kinetics (69). In contrast, cAMP elicits a robust (5–10-fold), rapid and transient inhibition of Akt activity with half-maximal effects developing within 2 min (47), returning to basal levels in 30–45 min. Since the transient inhibitory effect of cAMP on basal Akt activity is not always easily observed, as opposed to the effects on insulin-stimulated Akt activity, it is therefore possible that the slow positive action of cAMP on Akt reported by others represents the slow recovery phase upon inhibition.
Both cAMP effector pathways, Epac/Rap1 and PKA, seem to be actively involved in Akt regulation. A dual cAMP action on Akt was recently proposed, where a finely tuned regulation is achieved by a balance between opposite Epac/Rap1-dependent and PKA-dependent actions (63). It has recently been suggested that distinct cAMP pools, shaped by the action of phosphodiesterases, might be responsible for these specific cAMP effector responses (64). Our results in thyroid cells differ markedly with this model; effector-specific cAMP analogs do not show any effect on Akt when added independently, despite the fact that Epac- and PKA-selective analogs activated Rap1b and CREB phosphorylation, respectively (2). Importantly, when added together, Epac- and PKA-selective agents fully mimicked forskolin or TSH action. Moreover, inhibitors of either effector branch completely abrogated cAMP-dependent Akt inhibition (47). Our results clearly indicate that a synergistic action of Epac and PKA is responsible for cAMP inhibition of Akt in a manner that depends on Rap1b.
From a mechanistic point of view, the effects of cAMP on steady-state phospho-Akt could represent a regulatory action in any of the upstream kinase-activating events (i.e. PIK/PDK-1/mTORC2) or, alternatively, an effect on the regulation of its dephosphorylation. Moreover, a series of Akt binding partners were reported to modulate its phosphorylation and activity; whereas JIP-1 (70), Ft-1 (71), and TCL-1 (72) can directly modulate Akt activity, others, like Hsp90, act indirectly by protecting it from the action of inactivating phosphatases (73). Interestingly, Hsp90 was recently identified as a PKA substrate, and its phosphorylation negatively affects its ability to associate with endothelial nitric-oxide synthase (74). Whether an analogous PKA-dependent effect on Hsp90/Akt accounts for the inhibitory action of cAMP is for the moment unknown. Our findings described here are consistent with a model in which cAMP deactivates Akt by increasing an Akt phosphatase activity. Contrary to previous reports (45), cAMP did not affect in our system either PIK or PDK1 activity/translocation; constitutively active and calyculin A-stimulated Akt were both resistant to the inhibitory action of cAMP, and, most directly, cAMP affected the rate of Akt dephosphorylation (Fig. 5), thus indicating an Akt phosphatase as the cAMP target. Most importantly, we identified a new Epac-PP2A signaling module, whose phosphatase activity can be positively modulated by cAMP in a PKA- and Rap1-dependent manner. Consistent with the effects on Akt, a synergistic action of both cAMP effector branches was observed at the level of Epac-associated PP2A activity.
Unlike PP1, PP2A is not a classical target for cAMP action, although reports exist describing cAMP-dependent PP2A activation (75, 76); however, the mechanistic details for cAMP stimulation of PP2A are still unknown. PP2A is a major serine/threonine phosphatase involved in cell signaling. It forms a heterotrimeric complex composed of A, B, and C subunits (77, 78). The 37-kDa catalytic subunit (C) and 65-kDa scaffold A subunit/PR65, form the PP2A core structure. This A/C core associates with a member of a large family of nonrelated regulatory/targeting B subunits (B/PR55, B′/B56/PR61, B″/PR72, B″′/PR93/PR110), which modulate subcellular localization, substrate specificity, and catalytic activity (79). Recent studies by independent groups suggest that members of the B family (20, 31, 80, 81) and PP2A-B′/B56 heterotrimers might be specifically involved in Akt regulation (27, 29, 82). More specifically, it has been recently shown that cAMP could activate PP2A via PKA-dependent phosphorylation of B56δ (83); however, dominant negative B56δ did not block the effect of cAMP on Akt.4 The identity of the PP2A regulatory subunit involved in Epac-PP2A-Akt is under investigation.
PP2A activity is modulated by post-translational modification. cAMP-dependent regulation of any of the PP2A subunits and/or the enzyme machinery responsible for their post-translational modifications therefore represent potential targets. For instance, it has been reported that PKA phosphorylates a B regulatory subunit, altering substrate specificity (84). Catalytic C subunit possesses a unique C-terminal sequence that binds to a groove in the interface between A and B subunits (77, 78). This C-tail is subjected to tyrosine phosphorylation (85, 86) and carboxymethylation (87, 88). Tyrosine phosphorylation of Tyr307 at its C terminus inactivates PP2A (85), potentially by disrupting its interaction with B subunit (77); however, preliminary data from our laboratory suggest that cAMP does not modify the levels of Tyr307 phosphorylation.4 Carboxymethylation of Leu309 is considered necessary for the recruitment of the B regulatory subunit (89–93). However, new in vitro studies using a C subunit deficient in the last 15 residues did not interfere with the ability to form the holoenzyme (78), indicating that methylation might facilitate in vivo heterotrimer assembly, but it is certainly not required in vitro. Since cAMP increased carboxymethylation of PP2A in Xenopus eggs (94), the possibility that cAMP might affect the phosphatase methyltransferase or methylesterase activity deserved further investigation. In any case, the mechanisms involved should take into account the synergistic behavior of both cAMP effector branches, PKA and Epac.
Compartmentalization via formation of macromolecular complexes is an efficient way to attain signaling specificity (95), and particularly the association of Akt and phosphatases in such complexes was reported in several models. Integrin activation induces the formation of a complex leading to PP2A activation and Akt dephosphorylation (22, 96); dopaminergic responses in striatum are mediated by an Akt·β-arrestin 2·PP2A complex (20); p38α-dependent caveolin-1/PP2A association in cardiomyocytes leads to Akt dephosphorylation (31); TPA-mediated PKCδ and PKCε activation in keratinocytes induces PP2A association and Akt dephosphorylation (97). All of these studies suggest that local recruitment and/or activation of subpopulations of PP2A in specific complexes confers specificity to Akt regulation by upstream signals. Our studies provide a framework for cAMP regulation of Akt in the context of a new Epac-mediated complex, integrating Rap- and PKA-dependent events. Interestingly, in cardiomyocytes, an AKAP-tethered complex containing Epac, PKA, phosphodiesterases, PP2A, and members of the Erk family coordinates the integration of cAMP-dependent events (51). Whether an analogous AKAP-mediated complex is involved in the Epac-PP2A-Akt regulation is currently under investigation.
Acknowledgments
We thank Drs. J. Bos, A. Toker, J. Gotz, A. Newton, J. Klarlund, and F. Liu for vector constructs used in this study and Dr. G. Romero for help with confocal microscopy. We are grateful to C. Cirio and K. Kovacs de Ostrovich for participation at early stages of this study and other laboratory members for helpful discussions and critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Public Health Service, NCI, Grant CA071649. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PKA, protein kinase A; HA, hemagglutinin; PBS, phosphate-buffered saline; GST, glutathione S-transferase; PH, pleckstrin homology; DiFMUP, 6,8-difluoro-4-methylumbelliferyl phosphate.
K. Hong and D. L. Altschuler, unpublished data.
References
- 1.Roger, P. P., Reuse, S., Maenhaut, C., and Dumont, J. E. (1995) Vitam. Horm. 51 59-191 [DOI] [PubMed] [Google Scholar]
- 2.Hochbaum, D., Hong, K., Barila, G., Ribeiro-Neto, F., and Altschuler, D. L. (2008) J. Biol. Chem. 283 4464-4468 [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro-Neto, F., Urbani, J., Lemee, N., Lou, L., and Altschuler, D. L. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 5418-5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumaz, N., and Marais, R. (2005) FEBS J. 272 3491-3504 [DOI] [PubMed] [Google Scholar]
- 5.Houslay, M. D., and Kolch, W. (2000) Mol. Pharmacol. 58 659-668 [PubMed] [Google Scholar]
- 6.Stork, P. J., and Schmitt, J. M. (2002) Trends Cell Biol. 12 258-266 [DOI] [PubMed] [Google Scholar]
- 7.Toker, A., and Cantley, L. C. (1997) Nature 387 673-676 [DOI] [PubMed] [Google Scholar]
- 8.Franke, T. F., Kaplan, D. R., Cantley, L. C., and Toker, A. (1997) Science 275 665-668 [DOI] [PubMed] [Google Scholar]
- 9.Frech, M., Andjelkovic, M., Ingley, E., Reddy, K. K., Falck, J. R., and Hemmings, B. A. (1997) J. Biol. Chem. 272 8474-8481 [DOI] [PubMed] [Google Scholar]
- 10.Alessi, D. R., James, S. R., Downes, C. P., Holmes, A. B., Gaffney, P. R., Reese, C. B., and Cohen, P. (1997) Curr. Biol. 7 261-269 [DOI] [PubMed] [Google Scholar]
- 11.Stokoe, D., Stephens, L. R., Copeland, T., Gaffney, P. R., Reese, C. B., Painter, G. F., Holmes, A. B., McCormick, F., and Hawkins, P. T. (1997) Science 277 567-570 [DOI] [PubMed] [Google Scholar]
- 12.Alessi, D. R., Andjelkovic, M., Caudwell, B., Cron, P., Morrice, N., Cohen, P., and Hemmings, B. A. (1996) EMBO J. 15 6541-6551 [PMC free article] [PubMed] [Google Scholar]
- 13.Toker, A., and Newton, A. C. (2000) J. Biol. Chem. 275 8271-8274 [DOI] [PubMed] [Google Scholar]
- 14.Bayascas, J. R., and Alessi, D. R. (2005) Mol. Cell 18 143-145 [DOI] [PubMed] [Google Scholar]
- 15.Dong, L. Q., and Liu, F. (2005) Am. J. Physiol. 289 E187-E196 [DOI] [PubMed] [Google Scholar]
- 16.Hresko, R. C., and Mueckler, M. (2005) J. Biol. Chem. 280 40406-40416 [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005) Science 307 1098-1101 [DOI] [PubMed] [Google Scholar]
- 18.Chen, C. S., Weng, S. C., Tseng, P. H., Lin, H. P., and Chen, C. S. (2005) J. Biol. Chem. 280 38879-38887 [DOI] [PubMed] [Google Scholar]
- 19.Xu, W., Yuan, X., Jung, Y. J., Yang, Y., Basso, A., Rosen, N., Chung, E. J., Trepel, J., and Neckers, L. (2003) Cancer Res. 63 7777-7784 [PubMed] [Google Scholar]
- 20.Beaulieu, J. M., Sotnikova, T. D., Marion, S., Lefkowitz, R. J., Gainetdinov, R. R., and Caron, M. G. (2005) Cell 122 261-273 [DOI] [PubMed] [Google Scholar]
- 21.Cazzolli, R., Carpenter, L., Biden, T. J., and Schmitz-Peiffer, C. (2001) Diabetes 50 2210-2218 [DOI] [PubMed] [Google Scholar]
- 22.Ivaska, J., Nissinen, L., Immonen, N., Eriksson, J. E., Kahari, V. M., and Heino, J. (2002) Mol. Cell Biol. 22 1352-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, W., Akhand, A. A., Takeda, K., Kawamoto, Y., Itoigawa, M., Kato, M., Suzuki, H., Ishikawa, N., and Nakashima, I. (2003) Cell Death Differ. 10 772-781 [DOI] [PubMed] [Google Scholar]
- 24.Meier, R., Thelen, M., and Hemmings, B. A. (1998) EMBO J. 17 7294-7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora, A., Sabio, G., Risco, A. M., Cuenda, A., Alonso, J. C., Soler, G., and Centeno, F. (2002) Cell. Signal. 14 557-562 [DOI] [PubMed] [Google Scholar]
- 26.Resjo, S., Goransson, O., Harndahl, L., Zolnierowicz, S., Manganiello, V., and Degerman, E. (2002) Cell. Signal. 14 231-238 [DOI] [PubMed] [Google Scholar]
- 27.Rocher, G., Letourneux, C., Lenormand, P., and Porteu, F. (2007) J. Biol. Chem. 282 5468-5477 [DOI] [PubMed] [Google Scholar]
- 28.Ugi, S., Imamura, T., Maegawa, H., Egawa, K., Yoshizaki, T., Shi, K., Obata, T., Ebina, Y., Kashiwagi, A., and Olefsky, J. M. (2004) Mol. Cell Biol. 24 8778-8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Kanegan, M. J., Adams, D. G., Wadzinski, B. E., and Strack, S. (2005) J. Biol. Chem. 280 36029-36036 [DOI] [PubMed] [Google Scholar]
- 30.Yellaturu, C. R., Bhanoori, M., Neeli, I., and Rao, G. N. (2002) J. Biol. Chem. 277 40148-40155 [DOI] [PubMed] [Google Scholar]
- 31.Zuluaga, S., Alvarez-Barrientos, A., Gutierrez-Uzquiza, A., Benito, M., Nebreda, A. R., and Porras, A. (2007) Cell. Signal. 19 62-74 [DOI] [PubMed] [Google Scholar]
- 32.Gao, T., Furnari, F., and Newton, A. C. (2005) Mol. Cell 18 13-24 [DOI] [PubMed] [Google Scholar]
- 33.Bommakanti, R. K., Vinayak, S., and Simonds, W. F. (2000) J. Biol. Chem. 275 38870-38876 [DOI] [PubMed] [Google Scholar]
- 34.Filippa, N., Sable, C. L., Filloux, C., Hemmings, B., and Van Obberghen, E. (1999) Mol. Cell Biol. 19 4989-5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita, H., Ogino, T., Kobuchi, H., Fujiwara, T., Yano, H., Akiyama, J., Utsumi, K., and Sasaki, J. (2006) Brain Res. 1113 10-23 [DOI] [PubMed] [Google Scholar]
- 36.Kagawa, T., Varticovski, L., Sai, Y., and Arias, I. M. (2002) Am. J. Physiol. 283 C1655-C1666 [DOI] [PubMed] [Google Scholar]
- 37.Machida, K., Inoue, H., Matsumoto, K., Tsuda, M., Fukuyama, S., Koto, H., Aizawa, H., Kureishi, Y., Hara, N., and Nakanishi, Y. (2005) Am. J. Physiol. 288 L860-L867 [DOI] [PubMed] [Google Scholar]
- 38.Meroni, S. B., Riera, M. F., Pellizzari, E. H., and Cigorraga, S. B. (2002) J. Endocrinol. 174 195-204 [DOI] [PubMed] [Google Scholar]
- 39.Monje, P. V., Bartlett Bunge, M., and Wood, P. M. (2006) Glia 53 649-659 [DOI] [PubMed] [Google Scholar]
- 40.Morisco, C., Condorelli, G., Trimarco, V., Bellis, A., Marrone, C., Condorelli, G., Sadoshima, J., and Trimarco, B. (2005) Circ. Res. 96 180-188 [DOI] [PubMed] [Google Scholar]
- 41.Moule, S. K., Welsh, G. I., Edgell, N. J., Foulstone, E. J., Proud, C. G., and Denton, R. M. (1997) J. Biol. Chem. 272 7713-7719 [DOI] [PubMed] [Google Scholar]
- 42.Zhao, A. Z., Shinohara, M. M., Huang, D., Shimizu, M., Eldar-Finkelman, H., Krebs, E. G., Beavo, J. A., and Bornfeldt, K. E. (2000) J. Biol. Chem. 275 11348-11354 [DOI] [PubMed] [Google Scholar]
- 43.Brennesvik, E. O., Ktori, C., Ruzzin, J., Jebens, E., Shepherd, P. R., and Jensen, J. (2005) Cell. Signal. 17 1551-1559 [DOI] [PubMed] [Google Scholar]
- 44.Grader-Beck, T., van Puijenbroek, A. A., Nadler, L. M., and Boussiotis, V. A. (2003) Blood 101 998-1006 [DOI] [PubMed] [Google Scholar]
- 45.Kim, S., Jee, K., Kim, D., Koh, H., and Chung, J. (2001) J. Biol. Chem. 276 12864-12870 [DOI] [PubMed] [Google Scholar]
- 46.Lee, H. T., and Kay, E. P. (2003) Invest. Ophthalmol. Vis. Sci. 44 3816-3825 [DOI] [PubMed] [Google Scholar]
- 47.Lou, L., Urbani, J., Ribeiro-Neto, F., and Altschuler, D. L. (2002) J. Biol. Chem. 277 32799-32806 [DOI] [PubMed] [Google Scholar]
- 48.Poser, S., Impey, S., Xia, Z., and Storm, D. R. (2003) J. Neurosci. 23 4420-4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, P. G., Wang, F., Wilkinson, K. N., Savage, K. J., Klein, U., Neuberg, D. S., Bollag, G., Shipp, M. A., and Aguiar, R. C. (2005) Blood 105 308-316 [DOI] [PubMed] [Google Scholar]
- 50.Wang, L., Liu, F., and Adamo, M. L. (2001) J. Biol. Chem. 276 37242-37249 [DOI] [PubMed] [Google Scholar]
- 51.Dodge-Kafka, K. L., Soughayer, J., Pare, G. C., Carlisle Michel, J. J., Langeberg, L. K., Kapiloff, M. S., and Scott, J. D. (2005) Nature 437 574-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altschuler, D., and Lapetina, E. G. (1993) J. Biol. Chem. 268 7527-7531 [PubMed] [Google Scholar]
- 53.Ribeiro-Neto, F., Leon, A., Urbani-Brocard, J., Lou, L., Nyska, A., and Altschuler, D. L. (2004) J. Biol. Chem. 279 46868-46875 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto, K., Altschuler, D., Wood, E., Horlick, K., Jacobs, S., and Lapetina, E. G. (1992) J. Biol. Chem. 267 11337-11343 [PubMed] [Google Scholar]
- 55.Katso, R., Okkenhaug, K., Ahmadi, K., White, S., Timms, J., and Waterfield, M. D. (2001) Annu. Rev. Cell Dev. Biol. 17 615-675 [DOI] [PubMed] [Google Scholar]
- 56.Lietzke, S. E., Bose, S., Cronin, T., Klarlund, J., Chawla, A., Czech, M. P., and Lambright, D. G. (2000) Mol. Cell 6 385-394 [DOI] [PubMed] [Google Scholar]
- 57.Aoki, M., Batista, O., Bellacosa, A., Tsichlis, P., and Vogt, P. K. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 14950-14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andjelkovic, M., Jakubowicz, T., Cron, P., Ming, X. F., Han, J. W., and Hemmings, B. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5699-5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christensen, A. E., Selheim, F., de Rooij, J., Dremier, S., Schwede, F., Dao, K. K., Martinez, A., Maenhaut, C., Bos, J. L., Genieser, H. G., and Doskeland, S. O. (2003) J. Biol. Chem. 278 35394-35402 [DOI] [PubMed] [Google Scholar]
- 60.Enserink, J. M., Christensen, A. E., de Rooij, J., van Triest, M., Schwede, F., Genieser, H. G., Doskeland, S. O., Blank, J. L., and Bos, J. L. (2002) Nat. Cell Biol. 4 901-906 [DOI] [PubMed] [Google Scholar]
- 61.Schild, A., Isenmann, S., Tanimoto, N., Tonagel, F., Seeliger, M. W., Ittner, L. M., Kretz, A., Ogris, E., and Gotz, J. (2006) Mech. Dev. 123 362-371 [DOI] [PubMed] [Google Scholar]
- 62.Kimura, T., Van Keymeulen, A., Golstein, J., Fusco, A., Dumont, J. E., and Roger, P. P. (2001) Endocr. Rev. 22 631-656 [DOI] [PubMed] [Google Scholar]
- 63.Mei, F. C., Qiao, J., Tsygankova, O. M., Meinkoth, J. L., Quilliam, L. A., and Cheng, X. (2002) J. Biol. Chem. 277 11497-11504 [DOI] [PubMed] [Google Scholar]
- 64.Zmuda-Trzebiatowska, E., Manganiello, V., and Degerman, E. (2007) Cell. Signal. 19 81-86 [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Robayna, I. J., Falender, A. E., Ochsner, S., Firestone, G. L., and Richards, J. S. (2000) Mol. Endocrinol. 14 1283-1300 [DOI] [PubMed] [Google Scholar]
- 66.Coulonval, K., Vandeput, F., Stein, R. C., Kozma, S. C., Lamy, F., and Dumont, J. E. (2000) Biochem. J. 348 351-358 [PMC free article] [PubMed] [Google Scholar]
- 67.De Gregorio, G., Coppa, A., Cosentino, C., Ucci, S., Messina, S., Nicolussi, A., D'Inzeo, S., Di Pardo, A., Avvedimento, E. V., and Porcellini, A. (2007) Oncogene 26 2037-2047 [DOI] [PubMed] [Google Scholar]
- 68.Saito, J., Kohn, A. D., Roth, R. A., Noguchi, Y., Tatsumo, I., Hirai, A., Suzuki, K., Kohn, L. D., Saji, M., and Ringel, M. D. (2001) Thyroid 11 339-351 [DOI] [PubMed] [Google Scholar]
- 69.Tsygankova, O. M., Saavedra, A., Rebhun, J. F., Quilliam, L. A., and Meinkoth, J. L. (2001) Mol. Cell Biol. 21 1921-1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim, A. H., Sasaki, T., and Chao, M. V. (2003) J. Biol. Chem. 278 29830-29836 [DOI] [PubMed] [Google Scholar]
- 71.Remy, I., and Michnick, S. W. (2004) Mol. Cell Biol. 24 1493-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pekarsky, Y., Koval, A., Hallas, C., Bichi, R., Tresini, M., Malstrom, S., Russo, G., Tsichlis, P., and Croce, C. M. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 3028-3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato, S., Fujita, N., and Tsuruo, T. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10832-10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei, H., Venkatakrishnan, A., Yu, S., and Kazlauskas, A. (2007) J. Biol. Chem. 282 9364-9371 [DOI] [PubMed] [Google Scholar]
- 75.Feschenko, M. S., Stevenson, E., Nairn, A. C., and Sweadner, K. J. (2002) J. Pharmacol. Exp. Ther. 302 111-118 [DOI] [PubMed] [Google Scholar]
- 76.Moon, E. Y., and Lerner, A. (2003) Blood 101 4122-4130 [DOI] [PubMed] [Google Scholar]
- 77.Cho, U. S., and Xu, W. (2007) Nature 445 53-57 [DOI] [PubMed] [Google Scholar]
- 78.Xu, Y., Xing, Y., Chen, Y., Chao, Y., Lin, Z., Fan, E., Yu, J. W., Strack, S., Jeffrey, P. D., and Shi, Y. (2006) Cell 127 1239-1251 [DOI] [PubMed] [Google Scholar]
- 79.Janssens, V., and Goris, J. (2001) Biochem. J. 353 417-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuo, Y. C., Huang, K. Y., Yang, C. H., Yang, Y. S., Lee, W. Y., and Chiang, C. W. (2008) J. Biol. Chem. 283 1882-1892 [DOI] [PubMed] [Google Scholar]
- 81.Li, L., Ren, C. H., Tahir, S. A., Ren, C., and Thompson, T. C. (2003) Mol. Cell Biol. 23 9389-9404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen, W., Arroyo, J. D., Timmons, J. C., Possemato, R., and Hahn, W. C. (2005) Cancer Res. 65 8183-8192 [DOI] [PubMed] [Google Scholar]
- 83.Ahn, J. H., McAvoy, T., Rakhilin, S. V., Nishi, A., Greengard, P., and Nairn, A. C. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 2979-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Usui, H., Inoue, R., Tanabe, O., Nishito, Y., Shimizu, M., Hayashi, H., Kagamiyama, H., and Takeda, M. (1998) FEBS Lett. 430 312-316 [DOI] [PubMed] [Google Scholar]
- 85.Chen, J., Martin, B. L., and Brautigan, D. L. (1992) Science 257 1261-1264 [DOI] [PubMed] [Google Scholar]
- 86.Chen, J., Parsons, S., and Brautigan, D. L. (1994) J. Biol. Chem. 269 7957-7962 [PubMed] [Google Scholar]
- 87.Favre, B., Zolnierowicz, S., Turowski, P., and Hemmings, B. A. (1994) J. Biol. Chem. 269 16311-16317 [PubMed] [Google Scholar]
- 88.Kloeker, S., Bryant, J. C., Strack, S., Colbran, R. J., and Wadzinski, B. E. (1997) Biochem. J. 327 481-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bryant, J. C., Westphal, R. S., and Wadzinski, B. E. (1999) Biochem. J. 339 241-246 [PMC free article] [PubMed] [Google Scholar]
- 90.Tolstykh, T., Lee, J., Vafai, S., and Stock, J. B. (2000) EMBO J. 19 5682-5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei, H., Ashby, D. G., Moreno, C. S., Ogris, E., Yeong, F. M., Corbett, A. H., and Pallas, D. C. (2001) J. Biol. Chem. 276 1570-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu, J., Tolstykh, T., Lee, J., Boyd, K., Stock, J. B., and Broach, J. R. (2000) EMBO J. 19 5672-5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu, X. X., Du, X., Moreno, C. S., Green, R. E., Ogris, E., Feng, Q., Chou, L., McQuoid, M. J., and Pallas, D. C. (2001) Mol. Biol. Cell 12 185-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Floer, M., and Stock, J. (1994) Biochem. Biophys. Res. Commun. 198 372-379 [DOI] [PubMed] [Google Scholar]
- 95.Wong, W., and Scott, J. D. (2004) Nat. Rev. Mol. Cell Biol. 5 959-970 [DOI] [PubMed] [Google Scholar]
- 96.Pankov, R., Cukierman, E., Clark, K., Matsumoto, K., Hahn, C., Poulin, B., and Yamada, K. M. (2003) J. Biol. Chem. 278 18671-18681 [DOI] [PubMed] [Google Scholar]
- 97.Li, L., Sampat, K., Hu, N., Zakari, J., and Yuspa, S. H. (2006) J. Biol. Chem. 281 3237-3243 [DOI] [PubMed] [Google Scholar]