Abstract
The Kv7.2 subunits are the main molecular determinants of the M-current, a widespread K+ current regulating neuronal excitability. Mutations in the Kv7.2 gene cause benign familial neonatal seizures, an autosomally inherited human epilepsy. The benign familial neonatal seizure-causing mutations include those at arginine residues at positions 207 and 214 in the S4 segment of Kv7.2. In this study, each of the six S4 arginines was individually replaced with neutral glutamines, and the functional properties of mutant channels were studied by whole-cell and single-channel voltage-clamp measurements. The results obtained suggest that each S4 arginine residue plays a relevant role in the voltage-dependent gating of Kv7.2 channels. In particular, a decreased positive charge at the N-terminal end of S4 stabilized the activated state of the voltage-sensor, whereas positive-charge neutralization at the C-terminal end of S4 favored the resting conformation. Strikingly, neutralization of a single arginine at position 201 was sufficient to cause a significant loss of voltage dependence in channel activation. Moreover, by comparing the functional properties of glutamine versus tryptophan substitution, we found steric bulk to play a relevant role at position 207, but not at position 214, in which the main functional effect of this disease-causing mutation seems to be a consequence of the loss of the positive charge.
INTRODUCTION
Potassium (K+) currents play critical roles in a wide range of physiological processes such as the propagation of electrical signals by nerve cells, muscle contraction, cell volume regulation, and secretion of hormones and neurotransmitters (1). A wide variety of K+ currents has been described, each showing distinct tissue distribution and subcellular localization, often with peculiar biophysical, pharmacological, and modulatory properties. Several factors are involved in generating such extraordinary functional heterogeneity; the primary factor involves the large diversity in genes encoding for K+ channel subunits.
In voltage-gated K+ channels (Kv channels), which represent the largest family of K+ channels, specific conformational transitions triggered by membrane potential changes regulate the probability of channel opening. The Kv channels assemble as tetramers of identical or compatible subunits, each containing six transmembrane segments (S1–S6). Within each subunit, the S5–S6 domain contributes to the formation of the ion-selective pore and the inner pore gate, whereas the S1–S4 region forms the voltage sensor domain (VSD).
The recently solved structure of three bacterial nonvoltage-gated K+ channels, KcsA (2), MthK (3,4), and KirBac1.1 (5), whose membrane core of each subunit only contains the regions corresponding to the S5–S6 domain and the intervening linker, has provided a valuable structural model to explain the molecular mechanisms of ion permeation, selectivity, and pore opening/closing behavior. In Kv channel subunits, pore opening is controlled by the VSD domain. Within this region, a critical gating role has traditionally been assigned to the S4 segment that contains several positively charged residues spaced by mostly hydrophobic residues, and whose movement through the membrane electric field appears to represent the first gating transition in response to changes in membrane voltage (6,7). The crystal structure of the first voltage-gated K+ channel subunits containing six transmembrane segments including a VSD, i.e., the bacterial KvAP (8) and the mammalian Kv1.2 (9,10), seems to support such a view, although the intimate details of such movement, including the position of the VSD in the closed-channel configuration, the extent of VSD dislocation during activation (ranging from 2 Å to 15–20 Å), the relative role of the hydrophobic membrane interface, and the coupling of such movement to the inner pore gate, remain highly controversial (11).
Because of their fundamental role in regulating cellular excitability and ion distribution across the plasma membrane, Kv channels are implicated in several human disease conditions, including epilepsy, pain, migraine, arrhythmias, sensory dysfunction, and metabolic illnesses. In particular, mutations in four of the five members of the Kv7 gene family (Kv7.1–Kv7.5) were associated with human channelopathies. Thus, gene defects affecting Kv7.1, which is mainly expressed in the heart, gastrointestinal epithelia, and inner ear, but not in the brain, are responsible for the chromosome 11-linked form of long QT syndrome, whereas those targeting Kv7.4 were found in families affected by a rare form of nonsyndromic autosomal-dominant hearing loss (DFNA2). Mutations in Kv7.2 and more rarely Kv7.3 genes were identified in families affected by an autosomally dominantly inherited epilepsy of the newborn defined as benign familial neonatal seizures (BFNS). Neuron-specific Kv7.2 and Kv7.3 subunits can form either homomeric or heteromeric K+ channels underlying the so-called M-current (IKM) (12), a K+ current which regulates neuronal excitability, functioning as a brake for repetitive action potential firing and as a major determinant of spike frequency adaptation (13). It is widely thought that mutation-induced reduction in IKM function can increase neuronal excitability, leading to epileptic phenotypes. Consequently, IKM is regarded as a primary target for pharmacological intervention against hyperexcitability diseases (14,15).
Disease-causing mutations often indicate functionally relevant domains in the proteins affected. In Kv7.2, most BFNS-causing mutations are localized either in the large C-terminal domain, a critical region for subunit assembly and channel regulation by intracellular molecules, and in the VSD. In the VSD, mutations causing the substitution of two arginine (R) residues at positions 207 and 214 with tryptophan (W) were described in BFNS patients, highlighting their key role in Kv7.2 subunit function (16,17).
In this study, mutagenesis, macroscopic and single-channel electrophysiology, and molecular modeling experiments were performed to evaluate the role of each of the six R residues present in the S4 segment in the gating of Kv7.2 channels, by replacing them individually with neutral glutamines (Q). Moreover, to clarify the possible role of steric bulk of the residues introduced at positions 207 and 214 in BFNS pathogenesis, the properties of the channels carrying smaller Q residues at positions 207 and 214 were compared with those of channels in which the same positions were occupied by bulkier W residues.
MATERIALS AND METHODS
Mutagenesis and heterologous expression of Kv7.2 cDNAs
Mutations were engineered in human Kv7.2 cDNA (cloned into pcDNA3.1) by sequence overlap extension polymerase chain reaction (PCR), using Pfu DNA polymerase, as previously described (17). After PCR, mutation-containing fragments were cloned into Kv7.2, using NotI and PmlI restriction enzymes. All sequences were verified with the Big Dye Terminator Cycle Sequencing Kit in an ABI Prism 310 automated sequencer (Applied Biosystems, Foster City, CA). Wild-type (wt) and mutant cDNAs were expressed in Chinese hamster ovary (CHO) cells by transient transfection. The CHO cells were grown in 100-mm plastic petri dishes in DMEM containing 10% fetal bovine serum, nonessential amino acids (0.1 mM), penicillin (50 U/mL), and streptomycin (50 μg/mL) in a humidified atmosphere at 37°C with 5% CO2. For electrophysiological experiments, cells were seeded on glass coverslips (Carolina Biological Supply Company, Burlington, NC) and transfected the next day, using Lipofectamine 2000 (whole-cell recordings; Invitrogen, Milan, Italy) or Polyfect (single-channel recordings; Qiagen, Valencia, CA), according to the manufacturer's protocols. A plasmid encoding for enhanced green fluorescent protein (Clontech, Palo Alto, CA) was used as a transfection marker, with total cDNA in the transfection mixture kept constant at 4 μg.
Whole-cell electrophysiology
Currents from CHO cells were recorded at room temperature (20–22°C) 1 day after transfection, with an Axopatch 200A (Molecular Devices, Union City, CA), using the whole-cell configuration of the patch-clamp technique, with glass micropipettes of 3–5 MΩ resistance. The extracellular solution contained (in mM): 138 NaCl, 2 CaCl2, 5.4 KCl, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, with NaOH. The pipette (intracellular) solution contained (in mM): 140 KCl, 2 MgCl2, 10 EGTA, 10 HEPES, 5 Mg-ATP, 0.25 cAMP, pH 7.3–7.4, with KOH. We used pCLAMP software (version 6.0.4, Molecular Devices) for data acquisition and analysis. To generate conductance/voltage (G/V) curves, cells were held at −80 mV and then depolarized for 3 s from −80 to +20/+70 mV in 10-mV increments, followed by an isopotential pulse at 0 mV of 350-ms duration. Current values recorded at the beginning of the 0-mV pulse were normalized and expressed as a function of the preceding voltages. The data were fit to a Boltzmann equation of the following form: y = max/[1 + exp(V1/2 − V)/k], where V is the test potential, V1/2 is the half-activation potential, and k is the slope factor. To analyze current-activation kinetics, the current traces recorded in response to incremental voltage steps were fitted to a single-exponential function of the following form: y = amp exp(−t/τ) + c, where amp indicates the amplitude of the exponential component, and τ indicates the time constant. The tetraethylammonium (TEA) blockade was quantified by measuring the percentage of current inhibition at 0 mV produced by a 2-min drug application.
Single-channel electrophysiology
For single-channel recordings, channel activity in cell-attached patches was measured 48–96 h after transfection. Pipettes had resistances of 7–15 MΩ when filled with a solution that contained (in mM): 105 NaCl, 50 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4, with NaOH. Cells were bath-perfused with a solution containing (in mM): 175 KCl, 4 MgCl2, and 10 HEPES, pH 7.4, with KOH. This high (K+) solution served to clamp the resting membrane potential near 0 mV. Recording and analysis methods were similar to those described previously (18,19). Currents were recorded using an Axopatch 1-D amplifier (Molecular Devices). The data were acquired using Pulse software (HEKA Electronik, Lambrecht, Germany), sampled at 5 kHz, and filtered at 500 or 200 Hz. Single-channel data were analyzed using PulseFit and TAC (Bruxton, Seattle, WA). Open and closed events were analyzed using the “50% threshold criterion.” All events were carefully checked visually before being accepted. Open probability (po) histograms were generated using TACFit (Bruxton). The total number of channels in a given patch was estimated on the basis of two common assumptions: 1), that all of the channels in a patch behaved in an identical manner, i.e., they were homogeneous; and 2), that the Po of one channel did not depend on the gating state of the other(s), i.e., they were independent. Under these assumptions, only one channel in the patch was considered to be present if no superimposed openings were observed for a sufficiently long period of time that depended on the Po of any given channel. In the case of multiple channels in the patch, the number of open channels was governed by the binomial distribution (20). In our case, we evaluated the total number of channels in the patch by continuously recording for >1 min at strongly depolarized potentials, at which Po was the highest (∼0.2). Using this method, we estimated a maximal error rate of 3.8%, which is within the error of the pooled measurements. When superimposed openings were observed, the total number of channels in the patch was estimated from the maximal number of superimposed openings. At any given potential, the single-channel amplitude (i) was calculated by fitting all-point histograms with single or multi-Gaussian curves. The difference between fitted “closed” and “open” peaks was taken as i. Distributions of open and closed times were logarithmically binned and fitted with exponential densities by the method of maximum likelihood, as previously described (18). Single-channel conductance was calculated from the slope of the I/V chord fitted by linear regression, using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA).
Homology modeling
Three-dimensional models of wt and mutant Kv7.2 subunits were generated by homology modeling, using known structures of Kv channel subunits available in the Protein Data Bank (PDB), using SWISS-MODEL, a program that performs automated sequence-structure comparisons (21). The model generated was analyzed using both the DeepView module of the Swiss-PDBViewer (version 3.7, available at http://www.expasy.ch/spdbv/) and PyMOL (available at http://pymol.sourceforge.net/).
The Kv7.2 subunit sequence showed homology with a recently described chimeric channel in which the voltage-sensor paddle (corresponding to the S3b–S4 region) of Kv2.1 was transferred into the Kv1.2 subunit (22) (PDB accession number 2R9RH; 29% of sequence identity) and to Kv1.2 (10) (PDB accession number 2A79B; 26% of sequence identity). In this study, the homology model was built using the 2R9RH structure as template.
Statistics
Data are expressed as mean ± SE. Statistically significant differences between data were evaluated using Student's t test.
RESULTS
Biophysical properties of Kv7.2 channels carrying neutralizations in S4 arginines
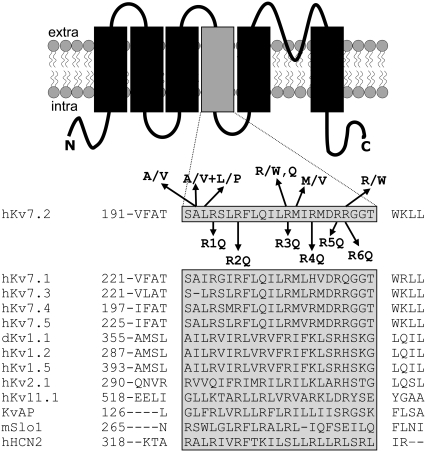
The upper panel of Fig. 1 shows a schematic representation of a single Kv7.2 subunit, indicating the six transmembrane segments, the intervening linkers, and the intracellular N and C termini. An alignment of the primary sequence of S4 and surrounding regions of all Kv7 family members and other K+ channel subunits is shown in the lower part of Fig. 1, revealing a variable number of positive charges, ranging from seven in Shaker, six in Kv7.2–7.5 channels, and only four in Kv7.1 subunits. Each of the six charged arginine residues is numbered from R1 to R6 according to their relative position in the linear sequence. For Kv7.2, the six R residues corresponded to residues R198, R201, R207, R210, R213, and R214. In all Kv7 subunits, the positively charged R corresponding to position 204 in Kv7.2, which is located in the middle of the S4 segment and is highly conserved in most ion channel subunits, is replaced by an uncharged Q residue. To investigate the relative contribution of each S4 R residue in Kv7.2 voltage-dependent gating, we engineered mutant constructs in which each of the six R residues was replaced with Q, thus generating the mutant subunits which were named R1Q, R2Q, R3Q, R4Q, R5Q, and R6Q (corresponding to the mutations R198Q, R201Q, R207Q, R210Q, R213Q, and R214Q, respectively). These constructs were expressed in CHO cells, and the functional properties of the channels generated were measured using the whole-cell configuration of the patch-clamp technique.
FIGURE 1.
Schematic topology of a Kv7.2 subunit and sequence alignment of the S4 region among voltage-gated K+ channels. Shaded area corresponds to the S4 sequence. On top of the Kv7.2 sequence, amino-acid substitutions associated with BFNS are shown. Below the Kv7.2 sequence are the positions and nomenclatures of the mutations investigated.
Cells heterologously expressing homomeric Kv7.2 subunits (wtKv7.2) displayed voltage-dependent, K+-selective currents characterized by a rather slow time course of activation and deactivation, and a threshold for current activation around −60 mV (Fig. 2). When conditioning depolarizing pulses from −80 mV to +20 mV were followed by an isopotential test pulse to 0 mV (as described in Materials and Methods), the instantaneous current amplitude of the test pulse was saturated at 0 mV. Heterologous expression of all homomeric Kv7.2 subunits carrying the indicated mutations in S4 yielded currents comparable in size to the currents recorded from cells expressing wtKv7.2 channels. The current density, expressed in pA/pF, and measured at potentials in which the conductance was saturated (+20/+70 mV, see below), was 50 ± 6, 35 ± 12, 46 ± 12, 47 ± 8, 40 ± 10, 41 ± 13, and 33 ± 7 for wtKv7.2, R1Q, R2Q, R3Q, R4Q, R5Q, and R6Q, respectively (n = 6–18). As expected, all of the mutant channels retained their selectivity for K+ over Na+ ions. Using standard intracellular and extracellular recording solutions (see Materials and Methods), the current reversal potentials (expressed in mV) were −78 ± 1, −76 ± 1, −79 ± 1, −77 ± 1, −75 ± 1, −75 ± 1, and −79 ± 1, for wtKv7.2, R1Q, R2Q, R3Q, R4Q, R5Q, and R6Q, respectively (n = 3–8).
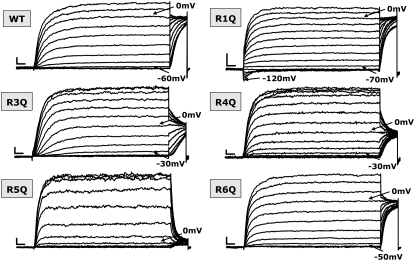
FIGURE 2.
Representative current traces of wt and R1Q, R3Q, R4Q, R5Q, and R6Q Kv7.2 mutants expressed in CHO cells. Each family of currents was recorded from a different cell held at −80 mV and then depolarized for 3 s from −120 to +20/+70 mV in 10-mV increments, followed by an isopotential pulse at 0 mV of 350-ms duration. For each set of recordings, arrows indicate current traces corresponding to the threshold potential and the 0-mV pulse. Current scale, 200 pA; timescale, 0.2 s.
The voltage dependence of activation was markedly affected in Kv7.2 channels carrying the neutralization of each of the S4 R residues (Fig. 2). In particular, when compared to wt channels, the currents carried by the R1Q mutant Kv7.2 channels activated at lower membrane potentials, and were already significantly activated at the holding potential of −80 mV. In fact, hyperpolarizing pulses from −80 mV to −120 mV caused the R1Q channels to deactivate, generating inwardly directed currents that relaxed toward zero. Moreover, during the isopotential test pulse to 0 mV that followed the conditioning pulses from −120 mV to +20 mV, the instantaneous currents saturated at conditioning pulses below −20 mV. On the other hand, homomeric R3Q, R4Q, R5Q, and R6Q channels displayed currents with more positive activation thresholds. The R5Q mutant displayed the most dramatic effect, whereas the R6Q mutant was only slightly different from wtKv7.2 channels.
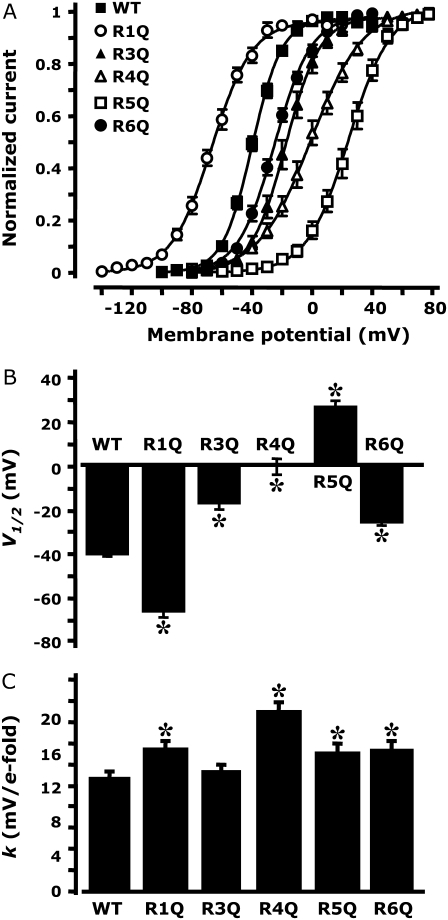
To quantify the relative changes prompted by each of the S4 mutations on the voltage dependence of the activation of Kv7.2 channels, we plotted the normalized current amplitudes at the start of the 0-mV isopotential pulse as a function of membrane potential (Fig. 3 A). These data were fit to a Boltzmann equation to obtain the half-activation potential (V1/2; Fig. 3 B) and the slope factor (k; Fig. 3 C) for each channel type. The resulting V1/2 values (expressed in mV) were −39.5 ± 1.2, −66.0 ± 2.3, −16.9 ± 2.7, 0.0 ± 3.6, 24.8 ± 3.1, and −5.5 ± 1.7, for channels composed of wt, R1Q, R3Q, R4Q, R5Q, and R6Q Kv7.2 subunits, respectively. The k values (expressed as mV/e-fold) were: 10.7 ± 0.6, 13.4 ± 0.7, 11.3 ± 0.6, 16.9 ± 1.0, 13.1 ± 0.9, and 13.3 ± 1.0, for channels composed of wt, R1Q, R3Q, R4Q, R5Q, and R6Q Kv7.2 subunits, respectively. Collectively, these results suggest that each of the R residues in the Kv7.2 S4 segment plays an important role in channel gating. In particular, neutralization of the R1 residue located at the N-terminal end of S4 caused a hyperpolarizing shift in the voltage dependence of activation, whereas neutralization of the R3, R4, R5, and R6 residues, positioned toward the C-terminal end of the S4 segment, caused gating changes in the opposite direction, i.e., a positive shift of the voltage dependence of activation.
FIGURE 3.
Analysis of gating properties of wt and R1Q, R3Q, R4Q, R5Q and R6Q Kv7.2 channels. (A) Steady-state activation curves, obtained by plotting normalized currents at the beginning of the 0-mV pulse as a function of the preceding conditioning potential. Continuous lines represent Boltzmann fits of the experimental data. V1/2 (expressed in mV, B) and k (expressed in mV/e-fold, C) values obtained for each indicated channel from the analysis of the data in A. *Values significantly different (p < 0.05) from the corresponding value of wtKv7.2 channels.
Removal of the charge at the R2 position in S4 causes a marked loss of voltage-dependent gating in Kv7.2 channels
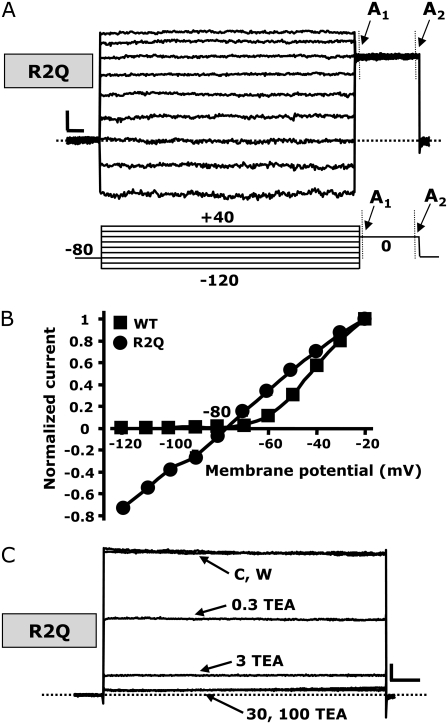
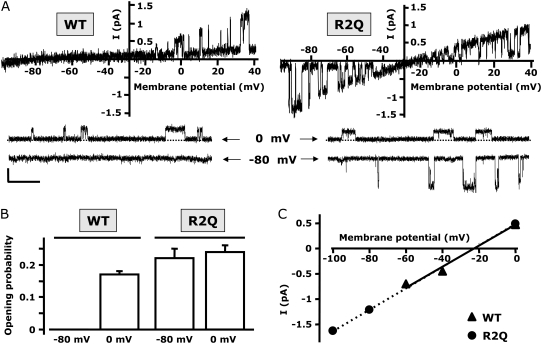
The S4 mutants described so far displayed varying shifts in the voltage dependence of activation, but all still behaved qualitatively similar to wtKv7.2 channels. However, the replacement of the R2 residue with a glutamine residue (R2Q) unexpectedly yielded channels that displayed a significant loss of time-dependent kinetics (Fig. 4 A). The R2Q channels behaved as K+-selective leak channels that were largely open at all test potentials. Indeed, the recorded R2Q currents had a reversal potential of a K+-selective pore (−79 ± 1 mV). When the normalized current amplitudes, measured at the end of test pulses from −120 mV to +40 mV, were plotted against the membrane potential for R2Q Kv7.2 channels, the I/V was completely linear within the potential range from −120 mV to −20 mV, suggesting that the channels carrying the R2Q mutation largely lost their voltage-dependent gating (Fig. 4 B). Using the same protocol, the normalized I/V of wtKv7.2 channels failed to show an inward current component at potentials more negative than −60 mV, because of the strong tendency of channels to close at such potentials (Fig. 4 B). A slight inward rectification of the I/V relationship, suggestive of a decrease in conductance at depolarizing pulses more positive than −20 mV, was observed for R2Q channels (Fig. 4 A), and was similarly observed in wtKv7.2 channels studied at the macroscopic or single-channel level (18,19).
FIGURE 4.
Biophysical and pharmacological properties of mutant R2Q Kv7.2 channels. (A) Representative current traces recorded using a voltage protocol in which the cell was held at −80 mV and then depolarized for 1.5 s from −120 to +40 mV in 20-mV increments, followed by an isopotential pulse at 0 mV of 350-ms duration. Below the traces is a schematic drawing of the voltage pulse protocol applied. A1 and A2 values refer to the beginning and end of 0-mV pulses, where current amplitudes were measured to calculate the time-dependent component (A2–A1/A2). (B) Plot of instantaneous currents normalized at the value obtained at −20 mV for both wt (squares, n = 20) and R2Q (circles, n = 10) Kv7.2 channels. (C) Currents from CHO cells expressing R2Q Kv7.2 channels were recorded using 2-s voltage steps to 0 mV, delivered at a frequency of 0.1 Hz from a holding voltage of −80mV. As indicated, the cell was sequentially exposed to control solution (C) and to 0.3, 3, 30, and 100 mM TEAe (each for ∼2 min), followed by washout (W). In both A and C, the current scale is 100 pA, and the timescale is 0.1 s.
To estimate the fraction of the current displaying slow time-dependent activation at depolarizing voltages in R2Q channels, we measured the current amplitudes at the beginning (A1, Fig. 4 A) and end (A2, Fig. 4 A) of the 0-mV pulse (350-ms duration), delivered after prepulses to −120 or −100 mV. When averaging these values from several cells, we found the A2–A1/A2 ratio to be 9.1% ± 1.0% (n = 4) and 6.9% ± 1.1% (n = 18) of the total current, respectively. This result suggested that a rather small fraction of the R2Q macroscopic current retained time-dependent kinetics; more than 90% of the macroscopic current between −120 mV and 0 mV appeared to be completely time-independent. We were unable to verify whether hyperpolarizing pulses more negative than −120 mV caused further R2Q channel deactivation because of the poor patch stability after long pulses at these negative voltages.
To confirm that these time-independent currents were carried by mutant R2Q Kv7.2 channels, we tested their sensitivity to the pore-blocker tetraethylammonium, applied extracellularly (TEAe). Homomeric wtKv7.2 channels are highly sensitive to TEAe (12). Perfusion with increasing concentrations of TEAe (0.3–100 mM) caused a similar dose-dependent and reversible blockade of currents in cells expressing wt or R2Q Kv7.2 channels (Fig. 4 C). The percentage of current blocked at 0 mV in homomeric wt and R2Q Kv7.2 channels was 63% ± 9% and 57% ± 3%, respectively, using 0.3 mM TEAe, and 89% ± 2% and 88% ± 2%, respectively, using 3 mM TEAe (n = 5). The similar TEAe sensitivity of wt and R2Q mutant Kv7.2 channels strongly suggested that the observed time- and voltage-independent currents are effectively carried by mutant R2Q Kv7.2 channels, and that the mutation did not affect the pore properties of these channels.
Given the dramatic gating changes observed in macroscopic current recordings from R2Q Kv7.2 channels, we performed single-channel measurements to evaluate the effects of the mutation on channel po and opening/closing kinetics as a function of voltage, as well as on single-channel conductance. To this end, we used the cell-attached configuration to avoid possible perturbations of the intracellular milieu, and to retain intact the biochemical machinery required for channel modulation (23). We used a pipette (extracellular) solution containing 50 mM K+ to shift the equilibrium potential for K+ ions to about −30 mV, allowing us to measure both inward and outward currents, using pulse or ramp protocols from hyperpolarized (−100/−80 mV) to depolarized (0/+40 mV) potentials.
Figure 5 A shows representative recordings obtained from wt and R2Q Kv7.2 channels, both using ramp (Fig. 5, top) and pulse (Fig. 5, bottom) voltage protocols. When the membrane voltage was ramped from −100 mV to +40 mV, openings of wtKv7.2 channels were only detected in the outward direction (above −30 mV). In contrast, openings of R2Q channels were recorded throughout the entire voltage range, in both the inward and outward directions, according to changes in the driving force predicted for membrane potential. Similar results were also obtained using pulses to −80 mV or 0 mV. The po of wtKv7.2 channels was unmeasurable at −80 mV (because no openings were detected), and reached a maximal value of 0.17 ± 0.01 (n = 14) at a depolarized potential of 0 mV. Consistent with previous work (18,19), such po values did not increase with further membrane depolarization (data not shown). Interestingly, the po recorded at 0 mV from the R2Q mutant Kv7.2 channel was similar to that obtained from wt channels at the same potential. Moreover, we were unable to detect statistically significant differences in po values measured between −80 mV or 0 mV in R2Q mutant Kv7.2 channels (Fig. 5 B), a result consistent with the marked loss of voltage dependence observed in macroscopic current recordings within the same voltage range. Kinetic analysis of homomeric Kv7.2 channels, similar to heteromeric Kv7.2/Kv7.3 channels and native IKM, suggest the presence of at least three shut and two open states (18). The distributions of shut and open times obtained from single R2Q mutant Kv7.2 channels was also adequately fitted by three and two exponentials, respectively, at both −80 mV and 0 mV of membrane potentials. As shown in Table 1, the time constants for each of the three closed states (τc1, τc2, and τc3) and the two open states (τo1 and τo2), as well as their relative contributions, were unaltered between −80 and 0 mV, again supporting the dramatic loss of channel voltage dependence observed for Kv7.2 R2Q channels within this voltage range.
FIGURE 5.
Single-channel properties of wt and R2Q Kv7.2 channels. (A, top) Representative single-channel traces recorded using a ramp voltage protocol (from −100 mV to + 40 mV; 3-s duration). (Bottom) representative single-channel sweeps obtained using pulses to the indicated potentials (−80 mV and 0 mV). (B) Plot of open probability obtained at −80 mV and 0 mV, as indicated (n = 8–14 patches for each data set). (C) Plot of unitary current-voltage relationships of single wt and R2Q Kv7.2 channels. Straight lines represent linear fits of experimental data. Each data point derives from the analysis of 5–7 patches. Current scale, 0.5 pA; timescale, 0.5 s.
TABLE 1.
Kv7.2 R201Q single-channel kinetics
| Parameters | 0 mV | −80 mV |
|---|---|---|
| τc1 (ms) | 4.9 ± 0.6 | 4.2 ± 0.4 |
| Fraction (%) | 30.5 ± 3.5 | 32.3 ± 2.3 |
| τc2 (ms) | 30.6 ± 4.1 | 32.8 ± 4.5 |
| Fraction (%) | 37.6 ± 8.7 | 35.4 ± 5.4 |
| τc3 (ms) | 250.6 ± 11.7 | 306.1 ± 37.4 |
| Fraction (%) | 31.9 ± 3.6 | 32.3 ± 5.4 |
| τo1 (ms) | 7.9 ± 1.1 | 5.3 ± 0.6 |
| Fraction (%) | 40.9 ± 2.4 | 45.9 ± 5.0 |
| τo2 (ms) | 59.1 ± 8.4 | 55.9 ± 11.1 |
| Fraction (%) | 59.1 ± 2.4 | 54.1 ± 5.0 |
| i (pA) | 0.54 ± 0.07 | 1.17 ± 0.08 |
| n | 7 | 9 |
Data are mean ± SE.
Finally, despite these marked changes in voltage dependence of po, single-channel K+ conductance was not affected by the mutation, at 20.8 ± 1.2 pS and 21.2 ± 0.4 pS for wt and R2Q Kv7.2 channels, respectively (n = 6), further arguing against a significant effect of the mutation on the conduction pathway of the Kv7.2 channel (Fig. 5 C).
Comparison between functional properties of Kv7.2 channels carrying glutamine-substituted R3 or R6 residues and those of tryptophan substitutions at the same positions
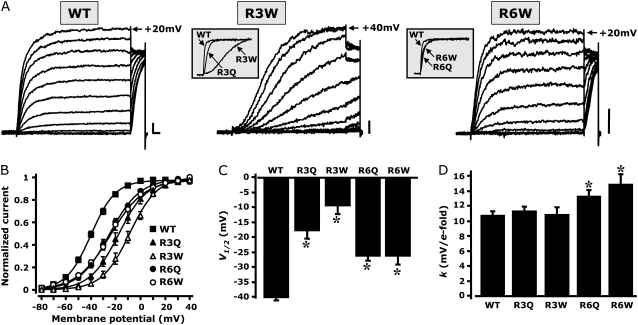
The S4 region in Kv7.2 is a “hot spot” for mutations responsible for BFNS. In fact, both uncharged and charged substitutions were identified in several families affected by the disease; these are indicated above the sequence alignment shown in Fig.1 A. In particular, Dedek et al. (16) described the substitution of the R3 residue with a tryptophan (W) in a family affected by an unusual association of BFNS and neuromuscular abnormalities (myokymia). In another BFNS family, Castaldo et al. (17) found a mutation involving the R to W replacement at position R6. In addition, while this work was in preparation, a family affected by peripheral-nerve excitability (but not BFNS) that carried the R3Q mutation in Kv7.2 was described (24). To ascertain whether the previously described functional consequences introduced in Kv7.2 channels by the R3Q and R6Q mutations are only related to the loss of the positive charge of the R residue or to the specific properties of the side chains of the R-replacing amino acid, we compared the properties of the macroscopic currents of Q-substituted (R3Q and R6Q) and W-substituted (R3W and R6W) R3 or R6 residues in Kv7.2 channels, using the whole-cell configuration of the patch-clamp technique.
The results suggest that significant differences exist when Kv7.2 channels carrying the R3Q and the R3W substitutions are compared, with respect to both activation kinetics and steady-state properties. Although both substitutions caused a depolarizing shift in the midpoint potentials (V1/2) of steady-state activation, the effect was more dramatic when the R3 position was occupied by a W compared with the R3Q substitution (Fig. 6). Moreover, the activation kinetics were very different. The fitting of current traces recorded at +20 mV to a single exponential function yielded activation time constants of 100 ± 4 ms for wt, 255 ± 42 ms for R3Q, and 1166 ± 260 for Q3W Kv7.2 channels (n = 5). These results clearly suggest that, at position R3, both charge and side-chain size influence the gating properties of Kv7.2.
FIGURE 6.
Comparison between gating properties of Kv7.2 channels carrying Q- or W-substituted R3 or R6 residues. (A) Representative current traces recorded using same voltage protocol described in Fig. 2. Insets, Comparisons of initial parts of normalized current traces at +20 mV for the indicated channels. (B) Steady-state activation curves, obtained by plotting normalized currents at the beginning of the 0-mV pulse as a function of the preceding conditioning potential. Continuous lines represent Boltzmann fits of the experimental data. V1/2 (expressed in mV, C) and k (expressed in mV/e-fold, D) values were obtained for each indicated channel. *Values significantly different (p < 0.05) from corresponding value for wtKv7.2 channels. Current scale, 200 pA; timescale, 0.2 s.
In contrast, substitution at position R6 with either Q or W residues resulted in similar effects on Kv7.2 channel gating. In fact, both steady-state and kinetic properties of Kv7.2 channels carrying the R6Q or R6W substitution were indistinguishable, because the activation V1/2, the slope factors k, and the activation time constants (125 ± 15 ms and 199 ± 42 ms for R6Q and R6W channels, respectively; n = 4–6) were identical in homomeric channels formed by R6Q or R6W subunits (Fig. 6).
DISCUSSION
Kv7.2 subunits participate to the formation of IKM, a widespread regulator of neuronal excitability. Mutations in the Kv7.2 gene cause BFNS, a rare form of human epilepsy. Two BFNS-causing mutations in Kv7.2 affect positively charged residues (R207 and R214) in the S4 segment, whose role in the voltage-sensing of Kv channels is firmly established. In agreement with this hypothesis, gating changes were described in Kv7.2 channels carrying such mutations (16,17). This study was undertaken to dissect the contribution of each of the six arginine residues present in the S4 segment on the voltage-sensing of Kv7.2 channels. To this aim, each of these residues was substituted by glutamine residues, thus neutralizing the positive charge, and the functional properties of the mutant Kv7.2 channels were studied using whole-cell and single-channel patch-clamps upon their heterologous expression in CHO cells. The results suggest that each of the charged arginines in the S4 segment of Kv7.2 subunits plays a significant role in voltage-dependent channel gating. Strikingly, neutralization of a single R at position 201 was sufficient to cause a significant loss of voltage dependence in channel activation.
None of the mutations investigated here impeded channel function. In contrast, neutralization of the residues corresponding to Kv7.2 positions R4 or R5 in Shaker (ShB) channels (25), and R4 in hyperpolarization-activated cyclic-nucleotide gated channels (HCN2) (26), did not produce functional homomeric channels, possibly because of altered subunit trafficking and folding, leading to greatly reduced surface expression (27). Interestingly, incorporation into a tetrameric concatenamer of the rat homologue of Shaker (rRCK1) of a single subunit carrying the mutation corresponding to Kv7.2 R4Q or R5Q recovered functional channels displaying a negative and positive shift in current activation properties, respectively (28).
Neutralization of each of the R residues caused significant effects on Kv7.2 channel gating. The most striking effect on voltage dependence was seen when the R2 residue was replaced by a glutamine. The currents carried by mutant R2Q Kv7.2 channels displayed largely time- and voltage-independent activation, and behaved like those carried by K+-selective leak pores. Similar functional consequences on K+ channel gating were previously obtained in other voltage-dependent channels upon substitution of positively charged R residues in S4. However, in Shaker (ShB) channels, at least three residues, corresponding to Kv7.2 R1, R2, and R3, need to be neutralized simultaneously to produce such a dramatic effect (29). In addition, residues immediately before the S4 segment were involved in the stabilization of the activated conformation of the voltage sensor. Thus, Tang and Papazian (30) found that in Kv10.1 channels, the introduction of a negatively charged amino acid two residues before the first R in the S4 segment (A345E), a substitution which reproduced the sequence of the voltage-independent olfactory cyclic nucleotide gated channels, produced a significant negative shift in the steady-state voltage dependence of activation. Mutations affecting uncharged residues at this same position in Kv7.2 were recently found in BFNS families (31). Thus, in agreement with our findings that the R1Q mutation also increased the stability of the voltage-sensor conformation in the activated position at more negative voltages, these results suggest that the net charge of the region corresponding to the end of the S3–S4 linker and the N-terminal portion of S4 is a crucial gating determinant in Kv channels. Thus, an increased positive net charge within this region is likely to stabilize the resting (closed) state of the voltage sensor, whereas the introduction of negative charges or the removal of positive charges favors the activated (open) conformation. Such conclusions also seem valid for Nav1.2 voltage-dependent Na+ channels, for which the removal of positive charges within the N-terminal half of S4 produces negative shifts in the voltage dependence of activation, whereas more subtle and opposing effects are triggered by the neutralization of more distal positive charges (32). Moreover, direct gating-current measurements of the total gating-charge translocation in Shaker K+ channels suggest that movement of the N-terminal half, but not of the C-terminal end, of the S4 segment underlies gating currents (33).
The dramatic changes in voltage-dependent gating observed in macroscopic current recordings from R2Q mutant Kv7.2 channels were confirmed by our single-channel measurements, because mutant channels showed no significant change in po or shut and open time distributions between −80 and 0 mV. Moreover, our single-channel analysis also showed that the maximal po, which is primarily determined by voltage-independent transitions between closed and open states, did not show statistically significant differences between wt and R2Q mutant Kv7.2 channels, again suggesting that the mutation dramatically affected the conformational changes occurring between the nonconducting closed states of the channels where most of the voltage-dependent transitions occur (34). Moreover, the similar maximal po of wt and R2Q Kv7.2 channels is consistent with the hypothesis that the maximal po of Kv7 channels is not determined by voltage, but by phosphatidylinositol 4,5-bisphosphate affinity, which is apparently not affected by the S4 mutants described here (35). In addition to single-channel measurements, further support for the hypothesis that the R2Q mutation does not significantly change the open pore structure also derives from the identical TEAe sensitivity and K+ selectivity of wt and R2Q Kv7.2 channels.
As previously discussed, the functional consequences on voltage-dependent gating induced by the neutralization of R2 seem to be unique for Kv7.2 channels among other Kv channels. However, similar effects were also generated in the closely related cardiac Kv7.1 channels upon neutralizing the same R residue (R231A) (36). These authors interpreted such an effect as a consequence of the unique paucity of net charge present in the S4 region of this channel (+3 in Kv7.1, whereas all other Kv7 members have +5). This view is supported by the formation of voltage-independent channels when Kv7.4 subunits carry a triple mutation (R207A, H216R, and Q220R), reproducing the net charge occurring in Kv7.1 subunits. However, our data suggest that the charge at the position corresponding to R2 has a primary role in stabilizing the resting conformation of the VSD, irrespective of the sequence of the C-terminal half of the S4 segment because similar effects were produced by its neutralization in both Kv7.1 and Kv7.2 channels.
To test the role of charge removal versus steric hindrance at pathophysiologically relevant R3 and R6 sites in Kv7.2, we compared the functional properties of channels in which these positions were substituted by a Q (with a similar size to R, but missing the charge) or a W (a bulkier, uncharged amino acid). The results suggest that steric bulk plays a relevant role at position 207, but not at position 214, where the main functional effect of the mutations seems to be consequent to the loss of the charge. In fact, Kv7.2 R3W channels displayed a further positive shift in the half-activation potential when compared with R3Q channels, together with a marked slowing in current-activation kinetics. In contrast, both these parameters were identical in R6W and R6Q Kv7.2 channels. This result suggests that the skeletal muscle myokymia accompanying BFNS in patients carrying the R3W mutation may be caused by the specific introduction of the W residue at the R3 position (16). However, this view was challenged by the discovery of a novel family in which peripheral-nerve hyperexcitability with muscle myokymia was associated with an R3Q mutation in Kv7.2, in the apparent absence of other neurological symptoms, including BFNS (24). Several hypotheses can be proposed to explain the lack of BFNS in R3Q-carrying myokymic patients, including a different degree of heteromeric association with Kv7.3, a different subcellular localization of homomeric versus heteromeric channels in forebrain versus spinal motorneurons, and different degrees of dominant-negative suppression of IKM function prompted by the two mutations at the R3 site. On the other hand, despite the obvious difficulties in drawing genotype-phenotype correlations based only on these functional results (37), the fact that the R6W mutation only caused small functional changes on Kv7.2 channel-gating is compatible with the fact that patients carrying such mutation are only affected by classic BFNS, with no other peripheral signs of neuronal hyperexcitability (17).
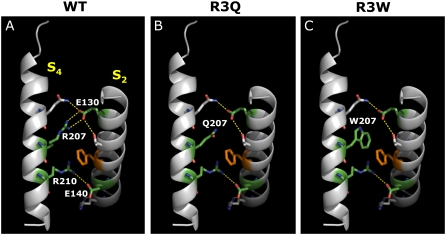
To provide further insights into the possible structural consequences of the replacement of the R3 residue with Q or W, and into the overall mechanism for the presumed effect of S4 mutations on Kv7.2 channel VSD displacement during gating, we built a homology model of the Kv7.2 VSD. As a template, we used the crystal coordinates of the activated configuration of a recently described chimeric channel in which the voltage-sensor paddle (corresponding to the S3b–S4 region) of Kv2.1 was transferred into the Kv1.2 subunit (22) (PDB accession number 2R9R). The sequence identity between Kv7.2 and the Kv1.2/2.1 chimera is 29%. In this chimeric channel, it was proposed that the resting and activated positions of the VSD are stabilized by ionized hydrogen bonds between the charged R subunits in the S4 segment and two negatively charged clusters: one facing the extracellular side of the membrane and provided by the S1 and S2 helices, and another closer to the intracellular membrane surface, involving the S2 and the S3a helices. The two clusters are separated by a highly conserved phenylalanine (F137) residue positioned in the middle of S2 (the so-called “phenylalanine gap”). As shown in Fig. 7 A, a similar spatial arrangement of charged residues within this region is evident from our Kv7.2 homology model. In particular, in the activated VSD configuration, R3 (R207) in Kv7.2 forms ionized hydrogen bonds with a negatively charged residue belonging to the external cluster (E130), whereas R4 (R210) is predicted to interact with negative charges of the inner cluster (E140 in S2; D172 in S3). Analysis of the model suggests that the replacement of R at position 207 with a neutral amino acid such as Q (Fig. 7 B) or W (Fig. 7 C) hampers this interaction. Thus, it seems plausible to hypothesize that in both R3Q and R3W channels, the activated configuration of the VSD is destabilized, explaining the positive shift in steady-state voltage dependence of activations observed in these mutants. Furthermore, as also suggested by biochemical experiments using cysteine-reacting methanethiosulfonate reagents in Shaker channels (38), the R3 residue in S4 appears to flip up around the “phenylalanine gap” on its way across the membrane during the activation process (22). Therefore, it seems possible that the presence of a bulkier W at this position delays such movement more than the smaller Q residue, leading to a substantial slowing of R3W channel opening kinetics.
FIGURE 7.
Three-dimensional homology model of Kv7.2 VSD. For clarity, only regions corresponding to the S2 and S4 segments are shown, as indicated. (A) wtKv7.2 subunit. (B) R3Q mutant Kv7.2 subunit. (C) R3W mutant Kv7.2 subunit. The peptide backbone is shown as gray ribbons. Residues at positions E130 (in S2) and R207 (in S4) are shown in green. Ionized hydrogen bonds are highlighted in yellow, and the highly conserved phenylalanine residue in S2 is shown in orange.
As previously pointed out, the R2Q substitution led to a significant destabilization of the resting VSD conformation. Although the available structures only reveal the VSD conformation in their active states, analysis of the Kv1.2/2.1 chimera suggests that in the resting VSD conformation, the R1 residue is positioned at the level of the “phenylalanine gap” (22). Therefore, one could speculate that the highly conserved R2 side chain positioned below R1 could interact with the negatively charged residues of the inner cluster, to stabilize the resting VSD conformation. Therefore, the R2Q substitution, by preventing such an interaction, would cause the VSD to be largely locked in a permanently activated position.
In conclusion, our data show that each charged residue in S4 has a different role in voltage-dependent gating of Kv7.2 channels. The N-terminal residues are more involved in stabilizing the resting conformation, and the more distal residues are more involved in controlling the activated position of the voltage-sensor. This analysis does not allow for the definition of a precise mechanism of VSD dislocation during the activation process of Kv7.2 channels. Nevertheless, by highlighting the relative functional role of each R residue in channel gating, these results provide molecular clues about the pathophysiology of BFNS and possibly other neuropsychological abnormalities associated with Kv7.2 mutations.
Acknowledgments
We are deeply indebted to Prof. Thomas J. Jentsch, Department of Physiology and Pathology of Ion Transport, Leibniz-Institut für Molekulare Pharmakologie, Berlin-Buch, Germany, for Kv7.2 cDNA, and to Dr. Paolo Ambrosino, Department of Neuroscience, University of Naples, Naples, Italy, for help with Kv7.2 homology modeling.
This study was supported by grants from Telethon GP07125, by the European Commission Strategic Research Program No. 503038 and E-Rare 2007 to M.T., and by United States National Institutes of Health grant RO1 NS043394 and American Heart Association grant-in-aid 0755071Y to M.S.S.
Editor: Toshinori Hoshi.
References
- 1.Hille, B. 2001. Ion Channels of Excitable Membranes. Sinauer Associates, Inc., Sunderland, MA.
- 2.Doyle, D. A., J. Morais Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- 3.Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- 4.Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- 5.Kuo, A., J. M. Gulbis, J. F. Antcliff, T. Rahman, E. D. Lowe, J. Zimmer, J. Cuthbertson, F. M. Ashcroft, T. Ezaki, and D. A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- 6.Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. [DOI] [PubMed] [Google Scholar]
- 7.Yellen, G. 2002. The voltage-gated potassium channels and their relatives. Nature. 419:35–42. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- 9.Long, S. B., E. B. Campbell, and R. Mackinnon. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903. [DOI] [PubMed] [Google Scholar]
- 10.Long, S. B., E. B. Campbell, and R. Mackinnon. 2005. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 309:903–908. [DOI] [PubMed] [Google Scholar]
- 11.Tombola, F., M. M. Pathak, and E. Y. Isacoff. 2005. How far will you go to sense voltage? Neuron. 48:719–725. [DOI] [PubMed] [Google Scholar]
- 12.Wang, H. S., Z. Pan, W. Shi, B. S. Brown, R. S. Wymore, I. S. Cohen, J. E. Dixon, and D. McKinnon. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. A., and P. R. Adams. 1980. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 283:673–676. [DOI] [PubMed] [Google Scholar]
- 14.McNaughton-Smith, G. A. W. A. 2006. Compounds that activate KCNQ (2–5) family of potassium ion channels. In Voltage-Gated Ion Channels as Drug Targets Series: Methods and Principles in Medicinal Chemistry. C. M. Triggle, D. J. Rampe, W. Zheng, editors. Wiley-VCH Verlag GmbH & Co., Weinheim, Germany. 355–380.
- 15.Miceli, F., M. V. Soldovieri, M. Martire, and M. Taglialatela. 2008. Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr. Opin. Pharmacol. 8:65–74. [DOI] [PubMed] [Google Scholar]
- 16.Dedek, K., B. Kunath, C. Kananura, U. Reuner, T. J. Jentsch, and O. K. Steinlein. 2001. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc. Natl. Acad. Sci. USA. 98:12272–12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaldo, P., E. Miraglia del Giudice, G. Coppola, A. Pascotto, L. Annunziato, and M. Taglialatela. 2002. Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels. J. Neurosci. 22:RC199 (1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selyanko, A. A., J. K. Hadley, and D. A. Brown. 2001. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J. Physiol. 534:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y., N. Gamper, and M. S. Shapiro. 2004. Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent. J. Neurosci. 24:5079–5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colquhoun, D., and A. G. Hawkes. 1995. The principles of the stochastic interpretation of ion-channel mechanisms. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York 589–633.
- 21.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long, S. B., X. Tao, E. B. Campbell, and R. MacKinnon. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382. [DOI] [PubMed] [Google Scholar]
- 23.Simmons, M. A., and C. R. Schneider. 1998. Regulation of M-type potassium current by intracellular nucleotide phosphates. J. Neurosci. 18:6254–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuttke, T. V., K. Jurkat-Rott, W. Paulus, M. Garncarek, F. Lehmann-Horn, and H. Lerche. 2007. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 69:2045–2053. [DOI] [PubMed] [Google Scholar]
- 25.Papazian, D. M., L. C. Timpe, Y. N. Jan, and L. Y. Jan. 1991. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 349:305–310. [DOI] [PubMed] [Google Scholar]
- 26.Chen, S., J. Wang, L. Zhou, M. S. George, and S. A. Siegelbaum. 2007. Voltage sensor movement and cAMP binding allosterically regulate an inherently voltage-independent closed-open transition in HCN channels. J. Gen. Physiol. 129:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papazian, D. M., X. M. Shao, S. A. Seoh, A. F. Mock, Y. Huang, and D. H. Wainstock. 1995. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 14:1293–1301. [DOI] [PubMed] [Google Scholar]
- 28.Tytgat, J., K. Nakazawa, A. Gross, and P. Hess. 1993. Pursuing the voltage sensor of a voltage-gated mammalian potassium channel. J. Biol. Chem. 268:23777–23779. [PubMed] [Google Scholar]
- 29.Bao, H., A. Hakeem, M. Henteleff, J. G. Starkus, and M. D. Rayner. 1999. Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shaker channels. J. Gen. Physiol. 113:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, C. Y., and D. M. Papazian. 1997. Transfer of voltage independence from a rat olfactory channel to the Drosophila ether-a-go-go K+ channel. J. Gen. Physiol. 109:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soldovieri, M. V., M. R. Cilio, F. Miceli, G. Bellini, E. Miraglia del Giudice, P. Castaldo, C. C. Hernandez, M. S. Shapiro, A. Pascotto, L. Annunziato, and M. Taglialatela. 2007. Atypical gating of M-type potassium channels conferred by mutations in uncharged residues in the S4 region of KCNQ2 causing benign familial neonatal convulsions. J. Neurosci. 27:4919–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuhmer, W., F. Conti, H. Suzuki, X. D. Wang, M. Noda, N. Yahagi, H. Kubo, and S. Numa. 1989. Structural parts involved in activation and inactivation of the sodium channel. Nature. 339:597–603. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal, S. K., and R. MacKinnon. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177. [DOI] [PubMed] [Google Scholar]
- 34.Zagotta, W. N., and R. W. Aldrich. 1990. Alterations in activation gating of single Shaker A-type potassium channels by the Sh5 mutation. J. Neurosci. 10:1799–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Y., N. Gamper, D. W. Hilgemann, and M. S. Shapiro. 2005. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25:9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panaghie, G., and G. W. Abbott. 2007. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J. Gen. Physiol. 129:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soldovieri, M. V., F. Miceli, G. Bellini, G. Coppola, A. Pascotto, and M. Taglialatela. 2007. Correlating the clinical and genetic features of benign familial neonatal seizures (BFNS) with the functional consequences of underlying mutations. Channels. 1:228–233. [DOI] [PubMed] [Google Scholar]
- 38.Larsson, H. P., O. S. Baker, D. S. Dhillon, and E. Y. Isacoff. 1996. Transmembrane movement of the shaker K+ channel S4. Neuron. 16:387–397. [DOI] [PubMed] [Google Scholar]