Abstract
The zinc-protease a disintegrin-like and metalloprotease with thrombospondin type I repeats (ADAMTS13) cleaves the Tyr1605-Met1606 peptide bond of von Willebrand factor (VWF), avoiding the accumulation of ultra large VWF multimers. Hydrolysis by ADAMTS13 of a VWF analog (Asp1596-Arg1668 peptide, fluorescence energy transfer substrate [FRETS]-VWF73) was investigated by a fluorescence quenching method (FRETS method) from 15°C to 45°C and pH values from 4.5 to 10.5. The catalysis was influenced by two ionizable groups, whose pKa values were equal to 6.41 ± 0.08 (ionization enthalpy = 32.6 ± 1.7 kJ/mol) and 4 ± 0.1 (ionization enthalpy = 3.8 ± 0.4 kJ/mol), whereas these values were equal to 6 ± 0.1 and 4.1 ± 0.1, respectively, in Co2+-substituted ADAMTS13. The catalytic process of FRETS-VWF73 hydrolysis showed negative activation entropy (−144 kJ/mol), suggesting that the transition state becomes more ordered than the ground state of the reactants. The kcat/Km values were not linearly correlated with temperature, as expression of change of the kinetic “stickiness” of the substrate. The Met1606-Arg1668 peptide product acted as hyperbolic mixed-type inhibitor of FRETS-VWF73 hydrolysis. Asp1653, Glu1655, Glu1660, Asp1663, together with the hydrophilic side chain of Thr1656 were shown to form a “hot spot” in the VWF A2 sequence, which drives the molecular recognition and allosteric regulation of binding to ADAMTS13. The interaction of the Met1606-Arg1668 region of VWF with ADAMTS13 involves basic residues of the protease and is thus progressively inhibited at pH values >8.50. A molecular model of the FRETS-VWF73 showed that the substrate can fit into the active site only if ADAMTS13 assumes a C-like shape and, interacting with the acidic 1653–1668 region of VWF, properly orients the Tyr1605-Met1606 peptide bond for the cleavage by the zinc-aquo complex in the active site.
INTRODUCTION
A disintegrin-like and metalloprotease with thrombospondin type I repeats (ADAMTS13) is a member of the ADAMTS family of metalloproteases (1) constituted by a multidomain enzyme that cleaves von Willebrand factor (VWF) at the peptide bond between Tyr1605 and Met1606, located in the VWF A2 domain. On cleavage, one N-terminal and one C-terminal fragment are generated from the VWF monomer (2–5). Pathological perturbations of the ADAMTS13/VWF proteolytic interaction are responsible for either thrombotic microangiopathies or hemorrhagic syndromes in type 2A von Willebrand diseases (6–11). Modulation of the ADAMTS13/VWF interaction is critical for an efficient proteolysis and involves both VWF and ADAMTS13. The latter binds to VWF under static conditions and under conditions of venous (2.5 dyn/cm2) and arterial (30 dyn/cm2) shear stress. This interaction, however, is unproductive for proteolysis unless shear stress is high enough to stretch VWF and expose the buried A2 domain for cleavage (12–15). Under static conditions, ADAMTS13 cleaves VWF only under denaturing conditions (8), whereas under conditions of high shear stress found in the microvasculature VWF proteolysis is extremely rapid and occurs without denaturing agents (3,16,17). Fluid shear stress alters the conformation of VWF so that the binding and catalysis of ADAMTS13 takes place at the VWF A2 domain (13). The Tyr1605-Met1606 peptide bond is in fact buried within the VWF A2 domain under static conditions and cannot be recognized by ADAMTS13 (12,14,15). Recent studies have also shown that the binding of the platelet receptor of VWF, i.e., the Glycoprotein Ib (GpIbα), is able to induce conformational changes in the A1–A2 domains of VWF, responsible for an enhanced rate of cleavage by the metalloprotease (18,19). On the contrary, binding of chloride ions to the A1 domain of VWF inhibits proteolysis by ADAMTS13, inducing conformational transitions in the A1–A2 VWF domains, which make the cleavable peptide bond in the A2 domain unavailable to proteolysis (8,20,21). Thus, only in the presence of specific biophysical or biochemical factors, VWF molecules assume a proper conformational state available to proteolytic attack by ADAMTS13. Knowledge of the factors that can modulate this interaction is particularly relevant, as it may help to unravel the mechanisms through which the reaction is inhibited in several clinical settings. Among biochemical factors, protons play a relevant role in modulating the activity of metalloproteases and pH may change in vivo, having different values inside and outside the cellular compartment. Previous data showed that the velocity of ADAMTS13 hydrolysis of a synthetic peptide shows a maximum under acidic conditions, although no systematic study of the pH-dependence of steady state kinetic parameters was carried out (22). This pH-dependent activity of ADAMTS13 seems rather unusual if compared to other zinc-proteases, and especially matrix metalloproteases and ADAMS (23,24), which show maximum activity at pH values >7. The scenario of the pH-dependence of the activity of ADAMTS13 is complicated further by previous findings showing that under mild denaturing conditions ADAMTS13 cleaves natural VWF multimers at a pH optimum >8 (8). Unfortunately, both the need to use denaturing agents under static conditions to investigate the ADAMTS13/VWF interaction and the multimeric nature of the substrate renders practically impossible the study of the pH- and temperature-dependence of the catalytic parameters of cleavage of native VWF multimers. The discovery of a fluorescent peptide substrate spanning from Asp1596 to Arg1668 of the A2 domain of VWF (fluorescence energy transfer substrate [FRETS]-VWF73) (22) makes feasible a systematic study of the steady-state kinetic parameters for its hydrolysis as a function of pH and temperature, that is still elusive. The use of this fluorescent substrate and the Fluorescence quenching method (FRETS method) was applied to both native Zn2+-ADAMTS13 and artificially Co2+- and Ni2+-substituted ADAMTS13 to investigate the effects of pH and temperature on the protease activity of the enzyme. Finally, the use of different peptides synthesized from the Asp1596-Arg1668 substrate sequence allowed us to investigate whether the interaction with ADAMTS13 is allosterically regulated by interactions with different regions of the substrate and whether protons play a role in such a regulation. The aspect concerning the pH-dependent modulation of ADAMTS13/VWF interaction is physiologically relevant because after extrusion from intracellular granules, VWF indeed may experience large variations in environmental conditions, as pH and ionic strength. These mechanistic studies allowed us to obtain initial informations on the recognition and cleavage mechanisms operating on the proteolytic processing of VWF by ADAMTS13.
MATERIALS AND METHODS
Purification of recombinant and plasma-derived human ADAMTS13
ADAMTS13 used in this study was either a recombinant His-tagged form, produced in HEK-293 cell line based on the reported gene sequence of the enzyme (25) and purified as previously described (20) or derived from human plasma (provided by the Transfusion Center of the Catholic University School of Medicine of Rome from outdated units of fresh frozen plasma). The latter preparation was used for experiments dealing with exchange of the catalytic zinc with cobalt ion. For this preparation the original step of the purification procedure, i.e., ammonium sulfate precipitation (20), was replaced by an affinity chromatography using an anti-ADAMTS13 polyclonal antibody. Briefly, 1 mg of rabbit anti-human ADAMTS13 polyclonal antibody, raised against amino acids 1128–1427 of the enzyme, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The purified IgGs were immobilized through the carbohydrate moiety of the Fc region to 2 mL of Affi-Gel Hz Activated Support from Bio-Rad Laboratories (Hercules, CA) according to the manufacturer's procedure. One mM (final concentration) phenyl-methyl-sulfonyl-fluoride (PMSF) was added to 1 L of outdated fresh frozen plasma, which was percolated at 4°C overnight on the Affi-Gel Hz resin coupled to anti-ADAMTS13 polyclonal antibody, at a flow rate of 1.5 mL/min. After the entire amount of plasma was percolated, the column was washed with 10 mM Hepes, 0.15 M NaCl, pH 7.40 until the absorbance at 280 nm was zeroed. The bound protein was eluted by 0.1 M glycine buffer, pH 3. A small amount (≈200 μL) of 1 M Tris-HCl buffer pH 8.5 was previously added to the collecting test tubes to neutralize the pH of the glycine buffer and obtain a final pH value of ∼7. The pooled material contained a major component of ∼180 kDa and minor additional bands, that were eliminated following the previously described chromatographic procedures (20). The final material was tested by SDS-PAGE using a 4%–12% acrylamide gradient under reducing conditions, which showed a single band with a molecular mass of ∼180 kDa. The concentration of the purified enzyme was determined using an E(280 nm) (mg/mL, 1 cm) = 1.45, calculated using the method of Pace et al. (26). The purified enzyme was aliquoted and stored at −80°C in stock solutions at ∼0.3 mg/mL containing 10 mM Hepes, 0.15 M NaCl, 5 mM CaCl2, pH 7.5.
Preparation of Co2+-substituted ADAMTS13
Apo-ADAMTS13 was prepared by treating a solution of 100 μg of ADAMTS13 in 10 mM sodium acetate, pH 5.5, with 5 mM-I,10-phenanthroline for 1 h at 4°C (final volume, 1 mL). The enzyme solution was then dialyzed against 2 L of 10 μM of 1,10-phenanthroline for 2 h, and then against 4 L of 1 mM CoCl2 (99.999% pure; Sigma-Aldrich, Milano, Italy) overnight. This CoCl2 solution and all subsequent buffers were made in glass-distilled deionized water that had been additionally treated with Chelex100 (Bio-Rad). The efficiency of incorporation of Co2+ into apo-ADAMTS13 was checked by measuring the ratio between the concentration of ADAMTS13, determined both spectrophotometrically (at 280 nm) and by a commercial ELISA assay (Imubind ADAMTS13 antigen, American Diagnostica, Instrumentation Laboratory, Milan, Italy), and its protease activity measured by FRETS-VWF73. The concentrations of Co2+- and Zn2+-ADAMTS13 measured spectrophotometrically and by ELISA were in good agreement (difference within 8% deviance). After the extraction of Zn2+ ions from ADAMTS13 and elimination of phenanthrolene by extensive dialysis, the activity of the enzyme toward FRETS-VWF73 was completely absent. The lack of any artificial inactivation of ADAMTS13 by the process of extracting zinc was checked by re-incorporating zinc ions by extensive dialysis into the catalytic site of the apo-enzyme and measuring its catalytic properties in comparison with the untreated enzyme. Co2+-ADAMTS13 preparation was kept in buffer solutions containing a final concentration of 100 μM CoCl2 and 5 mM CaCl2. Before any functional assay, Co2+-ADAMTS13 solutions were freed from Co2+ ion by gel filtration on DG-10 columns (Bio-Rad) equilibrated with appropriate buffer solution without cobalt chloride.
Determination of kinetic parameters of FRETS-VWF cleavage by FRETS method
The substrate used for the enzymatic assays was DRE-A2pr(Nma)-APNLVYMVTG-A2pr(Dnp)-PASDEIKRLPGDIQVVPIGVGPNANVQELERIGWPNAPILIQDFETLPREAPDLVLQR, corresponding to the region from D1596 to R1668 of VWF. Called FRETS-VWF73, the substrate was synthesized by Thermo Electron Corporation GmbH (Ulm, Germany). When ADAMTS13 cleaves the bond between Y10 and M11 in FRETS-VWF73, the fluorescence at λem between 420 and 460 nm and λex = 340 nm increases in proportion to the release of Nma fluorophore from the internal Dnp quencher (see Fig. S1 in Supplementary Material, Data S1). Other shorter not fluorescent peptides, that are Met1606-Phe1654, Met1606-Leu1657, Met1606-Ala1661, Met1606-Leu1664, and Met1606-Arg1668 were synthesized by PRIMM s.r.l. (Milano, Italy). The fluorescent substrate was dissolved in 50% dimethylsulfoxide at a concentration of 5 mM. In the functional assays, the final concentration was always ≤0.05% (w/v), that did not affect the protease activity. The hydrolyzed peptide was monitored by exciting the substrate at 340 nm and measuring the fluorescence at 450 nm (bandwidth of 5 nm at both excitation and emission wavelength). Assays were carried out in 5 mM Na-acetate, 5 mM Bis-Tris, 5 mM Tris, 10 mM CHES, 150 mM NaCl, 25 mM CaCl2, over the pH range spanning from 4.5 to 10.5. This four buffer system allowed to keep nearly constant the ionic strength of the solution over the entire pH range. The Michaelis constants of FRETS-VWF73 hydrolysis were calculated using 0.5–2.5 nM purified human ADAMTS13 and 1–20 μM FRETS-VWF73. The initial velocity of FRETS-VWF73 hydrolysis by ADAMTS13 was computed by a linear regression of the initial 20–40 points (r > 0.97) and always when not more than 5% (1 μM) of the substrate was hydrolyzed, according to the principles of steady-state enzyme kinetics (27). Thus, the reference curve for the conversion of the fluorescence signal into concentration of cleaved substrate was carried out constructing a reference curve (8 dilutions, dilution factor = 1.5) with a solution of FRETS-VWF73 at known concentration (1 μM) exhaustively hydrolyzed for 24 h by 0.5 nM ADAMTS13. Furthermore, the emission spectrum of the hydrolyzed FRETS-VWF73 was analyzed between 400 and 500 nm at pH values spanning from 4.5 to 10.50, and at different temperatures ranging from 15°C to 45°C, to assess any influence of these parameters on the fluorescence properties of the product. The kinetic parameters, kcat and Km, were determined as the mean of at least two independent measurements. The interassay CV was ∼8%.
Fluorescence emission spectra at 450 nm (λex = 340 nm) were recorded in a 1-cm quartz cell, using an Eclipse spectrofluorometer (Varian, Leini, Italy), equipped with a thermostated cell holder connected to a Varian Cary Peltier temperature controller.
Kinetic mechanism of ADAMTS13 activity studied as a function of temperature
The proteolytic cycle of ADAMTS13 should follow Scheme 1:
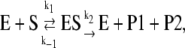 |
(Scheme 1) |
where E and ES are the free and Michaelis forms of ADAMTS13, whereas P1 and P2 are the tyrosine-containing and methionine-containing released peptide products, respectively. According to the classical Michaelis-Menten scheme we have (27):
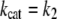 |
(1) |
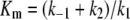 |
(2) |
hence:
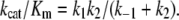 |
(3) |
The k2/k−1 ratio is the “stickiness” of the substrate, that expresses the tendency of the substrate to dissociate more slowly from its complex formed with the enzyme than it reacts to yield products, i.e., k2 > k−1. The temperature-dependence of the rate constants contained in Eqs. 1 and 3 was studied using the Arrhenius relations (28):
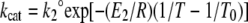 |
(4) |
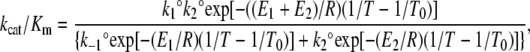 |
(5) |
where k1° and k2° are the values of k1 and k2 at the reference temperature, E1 and E2 are the activation energies associated with k1 and k2 and R is the gas constant (8.314 J/K mol). From the simultaneous analysis of the temperature dependence of kcat/Km and kcat parameters the values of k1, k−1, and k2 can be obtained together with the corresponding activation energies. Because T0 can be set to any value, the parameters can be calculated over the entire temperature range studied.
Further analysis according to the Eyring's transition state theory led to calculation of the values of the activation enthalpy and entropy pertaining to the formation of the transition state leading to cleavage of the FRETS-VWF73 substrate. Accordingly:
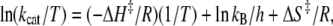 |
(6) |
where kB = Boltzmann's constant = 1.381 × 10−23 J K−1, h is the Planck's constant (6.626 × 10−34 J sec), ΔH‡ = activation enthalpy (kJ × mol−1) and ΔS‡ = activation entropy (J × mol−1 × K−1). Hence the free energy was calculated using the relation (29):
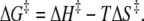 |
(7) |
In our experiments only steady-state initial velocities of FRETS-VWF73 hydrolysis (hydrolyzed peptide <10% total substrate) were analyzed to avoid the problem of inhibition of the ADAMTS13 activity by high concentrations of released P2 (see below). The FRETS-VWF73 concentrations used in the temperature experiments were between 0.3 and 20 μM, that allowed in all cases to compute the Michaelis parameters, avoiding the phenomenon of the inner filter effect (30), which was evident at concentrations >20 μM. Control experiments were carried out to measure the temperature-dependence of the emission spectrum of the cleaved FRETS-VWF73. The temperature-dependence of the Michaelis parameters was studied from 15°C to 45°C in the four-buffer system described above at pH 6.00, where the maximum activity was observed.
Effect of pH on FRETS-VWF73/ADAMTS13 interaction
The effect of pH on FRETS-VWF73 hydrolysis by ADAMTS13 was investigated using substrate concentration <Km of the reaction. Under this condition, the kinetics of substrate hydrolysis was followed at selected time intervals to compute the pseudo-first order rate constant of the reaction, kobs, that was equal to the relation kobs = (kcat/Km)e° (27), where e° is the total concentration of ADAMTS13. The fluorescence signals were thus transformed in product concentration observed at each time point, Pt, as indicated above. These values were fitted to the equation Pt = Pmax (1 − exp[−kobs × t]), where Pmax is the maximum concentration of released product at time = ∞.
The effect of pH on the observed kcat/Km values of FRETS-VWF73 hydrolysis was analyzed, assuming the existence of two ionizable groups involved in catalysis. Accordingly (27):
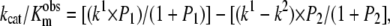 |
(8) |
where k1 is the maximum value of kcat/Km corresponding to the middle pH form, k2 is the value of kcat/Km corresponding to the high pH form, P1 = alog(pH − pKa1) and P2 = alog(pH − pKa2) and pKa1 and pKa2 are the acidic and basic pKa values controlling the catalytic activity of ADAMTS13. In this analysis, it was assumed that low pH species has no activity. This assumption was based on the known function of His residues that chelate the catalytic Zn2+. In fact, at pH <5 the protonation of the His imidazole side chain affects the interaction with Zn2+, blocking the catalytic cycle
The values of kcat/Km were then analyzed as a function of pH at six different temperatures ranging from 15°C to 37°C by a nonlinear least-square fitting procedure. The pKa values of the ionizable groups derived from the fitting procedure were analyzed by the Van t' Hoff relation (31) to calculate the values of the ionization enthalpy of the groups involved in the catalytic process. All analyses were carried out using the software Grafit (Erithacus, Horley Surrey, UK).
Experimentally, the pH-dependent changes of kcat/Km values were studied using a micromethod using 200 μl of total solution containing 0.5 nM ADAMTS13 and 1–2 μM of the FRETS-VWF73 substrate in the four-buffer system described above, analyzing 25 different pH values, ranging from 4.50 to 10.50. The stability of the purified ADAMTS13 over the pH range studied was confirmed previously through incubation of the enzyme for 180 min in buffer at a given pH. The initial velocity of hydrolysis of 1 μM FRETS-VWF73 by ADAMTS13 without previous incubation and after 180 min incubation in the four buffer system at pH spanning from 4.50 to 10.5 showed that only at extreme pH values (4.50 and 10.5).the activity of the incubated enzyme was ≈7% lower than that observed in not incubated ADAMTS13. Based on the limited loss of activity, no systematic correction was applied to the kinetic data. All the enzymatic assays were followed in a Varian Eclipse spectrofluorometer, equipped with a microplate reader, using 96-well polystyrene microplates (Corning nonbinding surface). This experimental strategy allowed us to follow simultaneously the course of the substrate's hydrolysis under different pH values, thus minimizing the error arising from interassay variability.
Effect of the Met1606-Arg1668 peptide on the hydrolysis of the FRETS-VWF73 by ADAMTS13
Recent findings showed that the C-terminal region of the VWF-73 peptide spanning from Glu1660 to Arg1668 is engaged in interaction with the spacer domain of ADAMTS13 and inhibits the hydrolysis of the VWF-73 peptide (32,33). This effect was investigated in this study as a function of pH, using purified different synthetic peptides contained in the VWF-73 substrate, such as Met1606-Phe1654, Met1606-Leu1657, Met1606-Ala1661, Met1606-Leu1664, and Met1606-Arg1668. Based on the experimental findings (see Results), the effect of the Met1606-Arg1668 peptide on FRETS-VWF73 was best analyzed using an expanded form of Scheme 1, as follows:
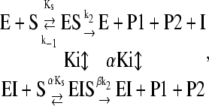 |
(Scheme 2) |
where α and β are the factors by which Ki and k2 change, respectively, when the ternary complex E·I·S is formed, and Ks is the dissociation binding constant of the substrate. Notably, for the principle of thermodynamic balance we obtain that Ks × αKi = Ki × αKs. Thus, when Km of FRETS-VWF73 hydrolysis approaches Ks (for instance at T ≥ 25°C), the former parameter changes as a function of I concentration by the same factor α, as Ki does as a function of FRETS-VWF73 concentration. This effect was verified in our experimental set up at pH 7.50 and 25°C. Thus, the initial velocities of VWF-73 hydrolysis by ADAMTS13 were fitted to the following equation, according to a classical scheme of hyperbolic mixed-type inhibition (34):
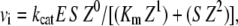 |
(9) |
where
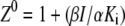 |
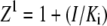 |
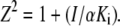 |
Hydrolysis of FRETS-VWF73 was analyzed by RP-HPLC. Briefly, at the end of incubation, stopped by 100 mM EDTA, the solutions was chromatographed on a C18 RP-HPLC column (RP-318 column, 5-μm pore size; Bio-Rad, Milano, Italy), using the following conditions: 10% CHCN3 in 0.1% TFA for 5 min, 10%–50% CHCN3 in 0.1% TFA for 45 min. The eluted peptides were detected simultaneously both by a UV/VIS spectrophotometric device at 210 nm (model 2075, Jasco, Easton, MD) and spectrofluorometric detector (FP-2020, Jasco) using a λexc = 280 nm and a λem = 340 nm. The initial velocities were taken by a linear regression of the five concentrations of FRETS-VWF73 measured as a function of time (with r > 0.92), when <5% substrate was hydrolyzed by ADAMTS13. This experimental procedure was adopted to avoid product inhibition phenomena. The reference curve for the conversion of the spectrofluorometric signals into concentration of cleaved substrate was carried out as described previously for the FRETS method. The effect of Met1606-Arg1668, over a concentration range from 0.5 to 16 μM, was studied at pH 6.0, 7.50, and 9.50 at 25°C, whereas the other peptides were studied at only pH 7.50 using a maximal concentration of 20 μM.
Modeling of FRETS-VWF73 and spacer domain of ADAMTS13
The FRETS-VWF73 peptide was modeled using its primary sequence homology to the VWF A3 domain and the coordinates for the crystal structure of this domain (Protein Data Bank, code 1atz), according to the method detailed previously (14). The model of the final three-dimensional structure was built using the modeling package MODELLER, which satisfied the spatial restraints (36). The prediction of the secondary structure of the spacer domain of ADAMTS13 was produced using its primary sequence from Ser556 to Ala685 and the PsiPred and DisoPred programs (36–38).
RESULTS
Temperature studies
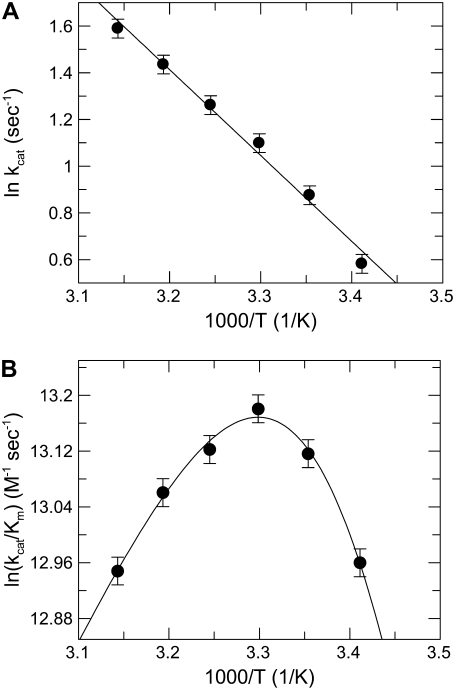
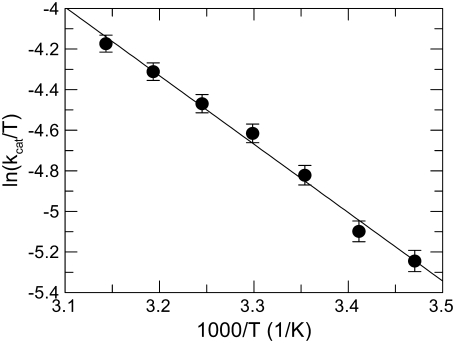
The fluorescence signal was inversely correlated to temperature, decreasing by ∼0.6 fluorescence units/1°C under the experimental conditions of the study (see Figs. S1 and S2 in Data S1). Based on this finding, the reference curve to calculate the concentration of the product from the raw fluorescence data was constructed at each temperature. In our system, at 25°C and pH 6.0 the values of kcat and Km of FRETS-VWF73 hydrolysis by ADAMTS13 were 2.54 ± 0.03 sec−1, and 4.6 ± 0.2 μM, respectively, in good agreement with previous findings (32,33,39). The kcat values were linearly correlated to temperature, as expected for a simple A → B reaction following the Arrhenius law. Thus, the kcat value reflects the k2 rate constant of Scheme 1. By contrast, this was not the case for the Km values, and consequently for kcat/Km as well, as shown in Fig. 1. Simultaneous fitting to Arrhenius equations (Eqs. 4 and 5) of experimental values of both kcat and kcat/Km of FRETS-VWF73 hydrolysis allowed the computation of the kinetic rate constants and the relative activation energies, listed in Table 1. It is conspicuous that E−1 > E2, indicating that the activation energy of FRETS-VWF73 dissociation from ADAMTS13 is higher than that needed for its hydrolysis. The “stickiness” of FRETS-VWF73 (k2/k−1) changed as a function of temperature, showing values listed in Table 1. At temperatures <25°C FRETS-VWF73 acts a sticky substrate, being the rate constant for its breakdown comparable or even higher than that for its dissociation from the active site of the enzyme. This is not the case at 37°C, as can be inferred from results reported in Table 1. At this temperature, in fact, the k2/k−1 ratio was ≈0.1 and, in virtue of Eq. 3, the value of kcat/Km is about 10-fold lower than that of k1. Moreover, being k−1 > k2 at T ≥ 25°C, in virtue of Eq. 2, Km approaches progressively the value of Kd, that is the real equilibrium dissociation constant of FRETS-VWF73 binding to ADAMTS13. Over the temperature range from 15°C to 45°C, the Eyring's plot for the reactions of FRETS-VWF73 with ADAMTS13 shown in Fig. 2, allowed us to calculate the relevant activation thermodynamic parameters pertaining to the kinetic scheme of FRETS-VWF73 cleavage. The values of these parameters were: ΔH‡ = 28 ± 1.1 kJ/mol, ΔS‡ = −144 ± 3.1 kJ/mol K. At 25°C, ΔG‡ = 70.9 ± 4 kJ/mol.
FIGURE 1.
Arrhenius plot of kcat (A) and kcat/Km (B) values of FRETS-VWF73 hydrolysis by ADAMTS13. The continuous lines were drawn according to Eqs. 4 and 5, with the best-fit parameter values listed in Table 1. In all the data sets the vertical bars represent the SD from two different determinations.
TABLE 1.
Kinetic and thermodynamic parameters for the reaction of ADAMTS13 with FRETS-WF73
| T (°C) | k1 (M−1 sec−1) | k−1 (sec−1) | k2 (sec−1) | k2/k−1 |
|---|---|---|---|---|
| 15 | 4.40 ± 0.2 × 105 | 0.61 ± 0.2 | 1.50 ± 0.06 | 2.45 |
| 20 | 7.90 ± 1.3 × 105 | 1.64 ± 0.6 | 1.89 ± 0.06 | 1.15 |
| 25 | 1.39 ± 0.4 × 106 | 4.10 ± 1.9 | 2.30 ± 0.05 | 0.56 |
| 30 | 2.39 ± 0.6 × 106 | 10.00 ± 5.7 | 2.86 ± 0.05 | 0.29 |
| 35 | 3.96 ± 29 × 106 | 23.70 ± 13 | 3.48 ± 0.06 | 0.15 |
| 40 | 6.70 ± 4 × 106 | 56.20 ± 38 | 4.21 ± 0.1 | 0.074 |
| 45 | 7.31 ± 5 × 106 | 70.50 ± 42 | 5.10 ± 0.16 | 0.073 |
The activation free energy of k+1 (E1), k−1 (E−1), and k2 (E2) was equal to 81.5 ± 20 kJ/mol, 135 ± 16 kJ/mol, and 30.5 ± 1.6 kJ/mol, respectively.
FIGURE 2.
Eyring plot of kcat values of FRETS-VWF73 hydrolysis by ADAMTS13. The slope (r = −0.996) and the intercept allowed to calculate the relevant activation thermodynamic parameters: ΔH‡ = 28 ± 1.1 kJ/mol, ΔS‡ = −144 ± 3.1 J/mol K. At the standard temperature of 25°C, ΔG‡ = 70.9 ± 4 kJ/mol.
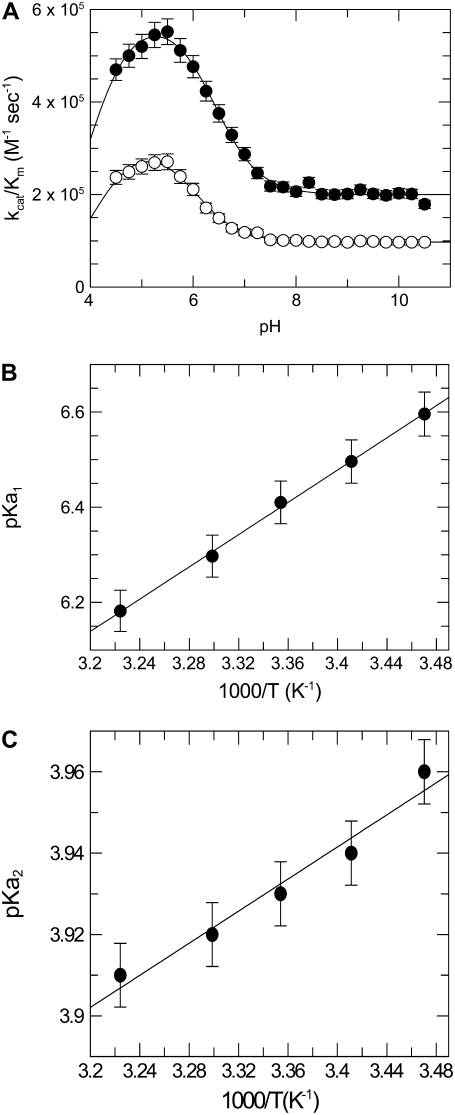
Effect of pH on kcat/Km of FRETS-VWF73 hydrolysis by ADAMTS13
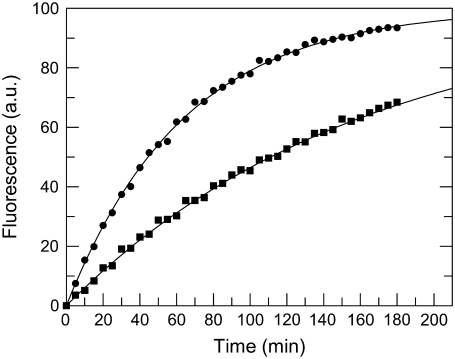
Control experiments showed that the fluorescence signals were not appreciably affected by pH ranging from 4.5 to 10.5. Under the pseudo-first conditions of this study, the time course of FRETS-VWF73 cleavage allowed to reliably calculate the kcat/Km value of the reaction, as shown in Fig. 3. Analysis of the pH-dependence of kcat/Km values at each temperature (Eq. 8) over the pH range from 4.5 to 10.5 showed that two ionizable groups in the free form of the enzyme are involved in the catalytic cleavage of the FRETS-VWF73 peptide, as shown in Fig. 4 a. For Zn2+-ADAMTS13, the calculated pKa1 value of 6.41 ± 0.08 at 25°C is compatible with a histidine group. The pKa value of this group could be theoretically assigned even to a group of unliganded FRETS-VWF73. However, the absence of a histidine residue in the sequence of the substrate allowed to rule out such an involvement. We know that three His residues are present in the catalytic site of ADAMTS13 in the sequence His224Glu-Ile-Gly-His228-Ser-Phe-Gly-Leu-His234, where His224, His228, and His234 should be engaged in the coordination of the zinc ion. The latter should be coordinated by a fourth ligand, which is hypothesized to be a water molecule bound to a zinc ion. The Van t' Hoff analysis of the pKa1 values showed that the standard enthalpy of its ionization was equal to 32.5 ± 1.8 kJ/mol, as shown in Fig. 4 b. The results of these temperature studies are consistent with the assignment of the general acid either to a H-N2(His) atom or a metal-bound water molecule (40). To overcome this uncertainty, we measured the pKa of the ionizable group involved in the catalytic mechanisms of the Co2+-substituted ADAMTS13. The process of Zn2+ extraction from the native enzyme did not alter its functional properties, as the reconstitution of the Zn2+-ADAMTS13 from apo-ADAMTS13 was reversible and generated again an enzyme with values of kcat and Km of FRETS-VWF73 hydrolysis very close to those of the native form (kcat of 2.1 ± 0.3 vs. 2.4 ± 0.2 sec−1, and Km of 4.5 ± 0.5 μM vs. 4.8 ± 0.4 μM, respectively). Co2+-ADAMTS13 showed at pH 6.00 a reduced kcat/Km compared to that of the Zn2+-ADAMTS13. This effect was caused by a minimal change of Km (5.1 vs. 4.8 μM) and a significant decrease of kcat value (1 vs. 2.4 sec−1). It is noteworthy that substitution of zinc with nickel ion caused a much more marked decrease of the peptidase activity of ADAMTS13, which was almost inactive in the FRET assay. The replacement of metal ions in the active site of ADAMTS13 in studies concerning the pH-dependent amidase activity provided useful elements to try unraveling of the assignment of this ionizable group involved in the catalytic machinery. Theoretical and experimental studies showed that a metal ion-dependent shift in pKa for metalloproteases reflects the ionization of the metal-coordinated water molecule bound to enzyme (41). On the contrary, the same pKa value for different metal-substituted metalloproteases was shown to be due to a critical amino acid at the active site of the protease involved and not to an enzyme-metal-water complex (42). As shown in Fig. 4 a, the analysis of the kcat/Km values of FRETS-VWF73 hydrolysis by Co2+-ADAMTS13 as a function of pH showed a significant change of the pKa value of the Co2+-substituted enzyme from 6.41 ± 0.08 in the Zn2+-ADAMTS13 to 6 ± 0.09 in the Co2+-substituted enzyme. Thus, both the value of the standard ionization enthalpy equal to 28 kJ/mol, which is typical of water molecules (43), and the intrinsic pKa value provided functional evidence that the pKa value of 6.41 of Zn2+-ADAMTS13 derives from the metal-bound water molecule at the active site of the enzyme.
FIGURE 3.
Examples of the kinetics of FRETS-VWF73 cleavage (1 μM) by 0.5 nM ADAMTS13 in the four-buffer system solution at pH 5.0 (▪) and 10.0 (•) and T = 25°C. For sake of clarity, the fluorescence signal at time = ∞ was set at 100% and corresponded to 1 μM cleaved FRETS-VWF73. The continuous lines were drawn according to a simple first order kinetics with the best-fit kcat/Km values equal to 5.2 ± 0.1 × 105 M−1 sec−1 and 2.03 ± 0.06 × 105 M−1 sec−1 for pH 5 and 10, respectively.
FIGURE 4.
(A) Effect of pH on kcat/Km values of FRETS-VWF73 hydrolysis by both Zn2+-ADAMTS13 (•) and Co2+-ADAMTS13 (○) at 25°C. The continuous lines were drawn according to Eq. 8, with the best-fit pKa values of 6.41 ± 0.08 and 4 ± 0.1 for Zn2+-ADAMTS13 (k1 = 5.9 ± 0.09 × 105 M−1 sec−1, k2 = 1.99 ± 0.03 × 105 M−1 sec−1) and 6.01 ± 0.09 and 4.1 ± 0.13 (k1 = 3.2 ± 0.08 × 105 M−1 sec−1 and k2 = 0.98 ± 0.02 × 105 M−1 sec−1) for Co2+-ADAMTS13. (B) Van t' Hoff plot of the pKa1 of the ionizable group involved in the catalytic cycle of ADAMTS13. The straight line was drawn with the best-fit ΔH value equal to 32.5 ± 1.8 kJ/mol. (C) Van t' Hoff plot of the pKa2 of the ionizable group involved in the catalytic cycle of ADAMTS13. The straight line was drawn with the best-fit ΔH value equal to 3.8 ± 0.4 kJ/mol.
The second ionizable group involved in ADAMTS13 catalysis exhibited a lower pKa value, equal to ∼4 for both Zn2+- and Co2+-ADAMTS13 (Fig. 4 a). The standard enthalpy of ionization was equal to 3.8 ± 0.4, as shown by a van't Hoff plot in Fig. 4 c. This value is typical for ionization of a carboxylate group (44). Due to the presence of a glutamate at position 225 of ADAMTS13, in the region engaged in the coordination of the catalytic zinc ion, we could reasonably assign to Glu225 the pKa2 value measured in these experiments.
Inhibition of ADAMTS13 by the Met1606-Arg1668 peptide
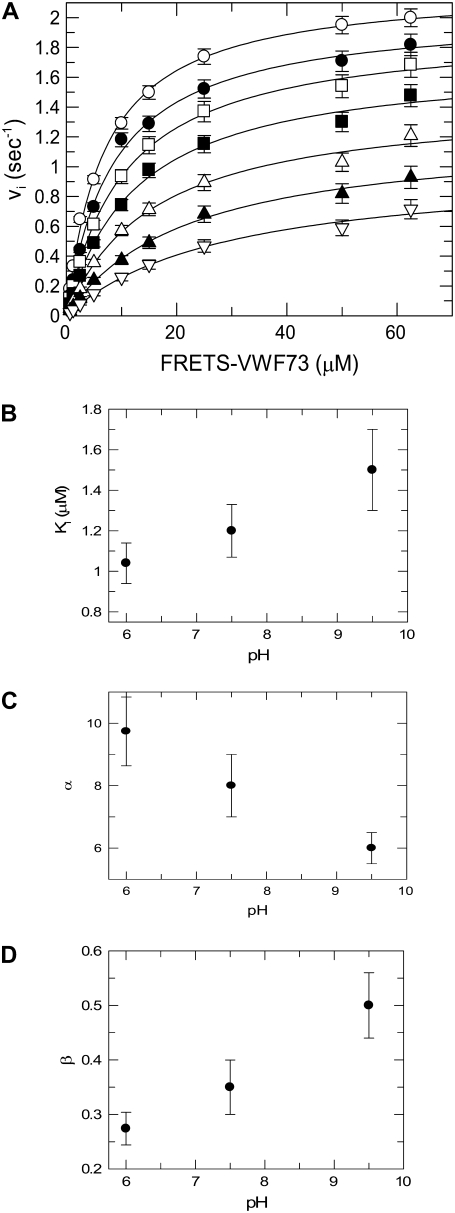
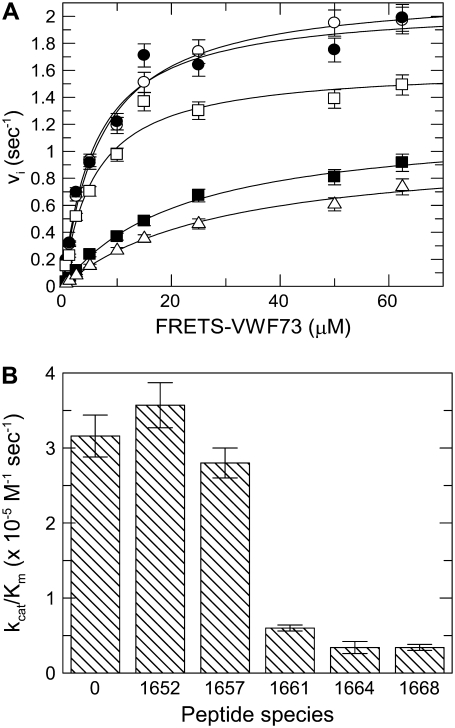
Extensive analysis of the experimental data set pertaining to the inhibitory effect of Met1606-Arg1668 peptide on FRETS-VWF73 hydrolysis by ADAMTS13 showed that this phenomenon was best described by a hyperbolic mixed-type mechanism. Two other models were analyzed: 1) a pure noncompetitive inhibition, and 2) a linear mixed-type inhibition (45). The minimization procedure provided in fact a reduced χ2 equal to 4.454 × 10−4 for the hyperbolic mixed-type, 1.6 − 10−3 for the linear mixed-type, and 4.86 × 10−3 for the noncompetitive inhibition model. F-testing of the results obtained at pH 6.0 with the hyperbolic and linear mixed-type inhibition model gave a probability value = 3 × 10−6, thus showing that Eq. 9 expresses the best model for the experimental data. The interaction of the Met1606-Arg1668 was characterized by a significant affinity, showing at pH 6.0 and 25°C a Ki value equal to ∼1 μM, whereas α and β values were equal to 10 and 0.23, respectively, as shown in Fig. 5 a. These results are in agreement with a model where binding of the Met-containing peptide allosterically decreases by a factor ≈10 the affinity of the FRETS-VW73/ADAMTS13 interaction, and reduces ≈4-fold the kcat value of the substrate cleavage. These effects are possible only if P2 in Scheme 2, binds to an exosite, distinct from the active site of the enzyme, whose ligation is able to affect the interaction with the substrate and the catalytic mechanism of the protease. The values of Ki, α, and β changed slightly but significantly as a function of pH in a range from 6.0 to 9.5 (Fig. 5, b–d). A trend to an increase of both Ki and β, and a decrease of α values was observed (Fig. 5, b–d). This result suggests that the interaction of P2 with ADAMTS13 involves the side chain of basic residues, and thus has a tendency to be weaker and to affect less efficiently the catalytic mechanisms at pH values >7.5. The use of shorter C-terminal VWF73 peptides, such as Met1606-Phe1654, Met1606-Leu1657, Met1606-Ala1661, and Met1606-Leu1664, variably inhibited the hydrolysis of FRETS-VWF73 (Fig. 6 a). In particular, a strong inhibition, approaching that one exerted by Met1606-Arg1668, was observed particularly when the peptides contained in their sequence Ala1661 and Leu1664 (Fig. 6 b).
FIGURE 5.
(A) Inhibition of ADAMTS13-catalyzed hydrolysis of FRETS-VWF73 by Met1606-Arg1668 peptide used at the following concentrations: (O) zero, (•) 0.5 μM, (□) 1 μM, (▪) 2 μM, (Δ) 4 μM, (▴) 8 μM, (∇) 16 μM. The experiment was carried out in four-buffer system (see text) at pH 7.50. Fifty-six experimental points were simultaneously fitted to a hyperbolic mixed-type inhibition equation (Eq. 9) with the best-fit parameter values kcat = 2.1 ± 0.02, Km = 6 ± 0.2 μM, α = 10 ± 1, β = 0.23 ± 0.02. The vertical bars represent the SD from two different determinations. In the other panels the effect of pH on Ki (B), α (C), and β (D) parameter values of Eq. 9. are shown. The vertical bars represent the SD from two different determinations.
FIGURE 6.
(A) Inhibition of ADAMTS13-catalyzed hydrolysis of FRETS-VWF73 by 20 μM of each of these VWF-derived peptides: Met1606-Phe1654 (•), Met1606-Leu1657 (□), Met1606-Ala1661 (▪), Met1606-Leu1664 (Δ), and in absence of any peptide (O). The best fit kcat and Km values were 2.09 ± 0.09 sec−1 and 5.83 ± 0.9 μM, respectively, for Met1606-Phe1654 (•), 1.63 ± 0.07 sec−1 and 5.83 ± 0.97 μM for Met1606-Leu1657 (□), 1.21 ± 0.04 sec−1 and 22 ± 1.7 μM for Met1606-Ala1661 (▪), 1.04 ± 0.06 sec−1 and 30 ± 3.7 μM for Met1606-Leu1664 (Δ). The best fit kcat and Km values were equal to 2.21 ± 0.05 sec−1 and 6.9 ± 0.06 μM in the absence of peptides (O). The vertical bars represent the SD from two different determinations. (B) Bar graph showing the measured kcat/Km values for hydrolysis of FRETS-VWF73 in the presence of 20 μM of the various C-terminal peptides of VWF73. The number reported for each peptide species refers to the number of the last amino acid in the peptide sequence, starting from Met1606. The vertical bars represent the SD from two different determinations.
DISCUSSION
Some relevant findings emerged from this study: 1), two ionizable residues control the catalytic activity of ADAMTS13 toward FRETS-VWF73 over the pH range from 4.5 to 10.5; and 2), the pKa of one group involved in the catalytic cycle can be reasonably assigned to the metal-bound water molecule, which generates the hydroxide species responsible for the cleavage of the substrate's peptide bond. Based on the findings in this study, the metal-bound water molecule is the natural candidate to act as a conduit for proton transfer (46). These results showed that the acidity of this ionizable group is dependent on the metal involved, and, from both theoretical and experimental standpoint, this behavior is typical of coordinated water molecules (41,43,47). The H2O molecule bound to the “harder” Zn2+ ion, would render the metal-aquo complex a stronger Lewis acid than coordination to the “softer” Co2+ ion. About the almost complete inhibition of ADAMTS13 caused by Ni2+ insertion into the catalytic site, we hypothesize that this effect is consistent with a distorted tetrahedral coordination geometry of the metal ion, which is incompatible with an efficient alignment with the scissile bond.
Thus, the Zn2+ ion present in the catalytic site of ADAMTS13 and coordinated by His224, His228, and His234, should be coordinated by the fourth ligand represented by a bound water molecule, which undergoes the metal-catalyzed dissociation:
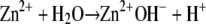 |
(10) |
The hydroxide species generated in the reaction acts as a nucleophile and attacks the peptide bond to be hydrolyzed (48). The findings that substitution of Zn2+ with Co2+ at the active site of ADAMTS13 causes a decrease of both the kcat value and the pKa value of the group involved in catalysis are in agreement with this mechanism. Ionization of His residues coordinating the zinc ion in the catalytic site could obviously contribute to the measured pKa1 value. The second ionizable group with a pKa ≅ 4 is likely represented by Glu225, contained in the active site sequence from His224 to His234 bearing the residues coordinating the zinc ion. We could predict that a favorable hydrogen bond between this glutamate and the metal-bound water molecule would make the latter more nucleophilic. Compared to cobalt and nickel, zinc was the most efficient metal to ionize the catalytic water molecule responsible for the proteolytic activity of ADAMTS13. This effect likely depends also from the coordination geometry of metal ion and the position of the metal-bound water molecule. In fact, other metalloproteases, such as carboxypeptidase A and thermolysin, showed a higher catalytic specificity in the cleavage of their substrates upon the exchange of zinc with cobalt (41,49).
Although it is very likely that the acidic pKa of the second catalytic residue could be assigned to Glu225, as indicated in the Results section, we need to carefully interpret the experimental results. In fact, at pH <5 the protonation of the His imidazole side chain could affect the interaction with the zinc ion and consequently the catalytic cycle. These studies on the effect of temperature allowed us to have an estimate of the relevant kinetic rate constants and the relative activation energies of the catalytic steps of FRETS-VWF73 hydrolysis. In particular, from data listed in Table 1, it can be deduced that FRETS-VWF73 acts as a sticky substrate at pH 6.0 under temperatures <25°C, being the rate constant for the substrate dissociation from the active site comparable or even lower than that of its cleavage. This behavior renders the value of the kcat/Km similar to that of association rate constant of the substrate binding to the active site of ADAMTS13. By contrast, at temperatures >25°C the dissociation rate is much higher than that of the cleavage rate, and the value of kcat/Km is about 10-fold lower than that of the association rate constant.
Furthermore, application of the Eyring's transition theory showed that the ΔS‡ value is strongly negative. The activation entropy is a macroscopic quantity that includes a number of microscopic contributions by both the reactants and solvating molecules. Activation entropies are in many cases dominated by solvent reorganization effects (34). Activation entropy of catalytic interaction between VWF and ADAMTS13 is strongly negative, indicating that the transition state of this bimolecular interaction becomes more ordered by reducing considerable translational and rotational degrees of freedom of motion of the reactants. This result implies that the formation of the transition state requires the enzyme and the substrate to adopt discrete and precise conformations and solvent orientation. Thus, these findings indicate that ADAMTS13 recognizes and cleaves the Tyr-Met peptide bond in the A2 domain of VWF by lowering the activation entropy of the reaction due to positioning the two reactants and ordering metal-bound water in the active site. This mechanism is in accord with the extraordinary chemical and conformational specificity, which characterizes the catalytic interaction between VWF and ADAMTS13.
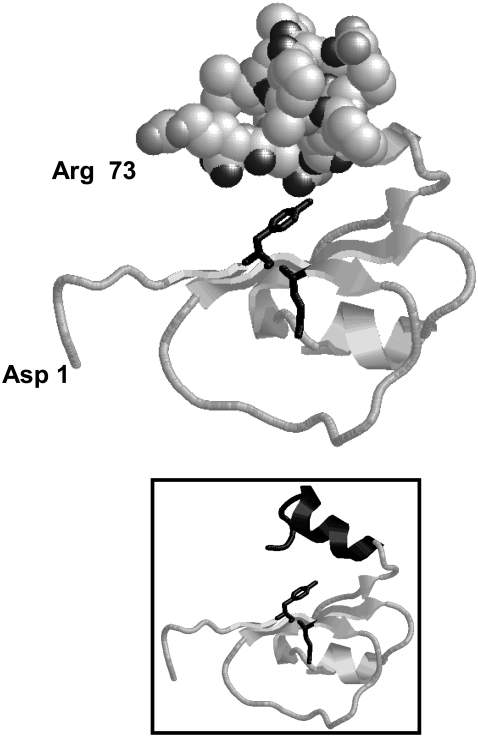
This mechanism is facilitated by the interactions of FRETS-VWF73 with exosites of the enzyme, that contribute to its correct and productive binding so that the catalytic water molecule is perfectly aligned toward the scissile bond of the substrate. Notably, the C-terminal product of ADAMTS13 hydrolysis, that is Met1606-Arg1668 peptide, is able to bind with considerable affinity to ADAMTS13 and to inhibit it with a hyperbolic mixed-type mechanism. This result is in agreement with recent findings, showing that this VWF peptide binds to the spacer domain of ADAMTS13 (32,33). The experiments reported in this study show that the binding of FRETS-VWF73 to the enzyme reduces 6- to 10-fold the affinity of the Met1606-Arg1668 peptide and vice-versa. The binding affinity of the Met1606-Arg1668 peptide tends to decrease and have lower allosteric effects on both kcat (resulting in higher β values) and Km (resulting in lower α values) at pH >7.5, as shown in Fig. 5, b–d. No histidine residues are present in the Met1606-containing peptide sequence, whereas in the spacer domain (Ser556-Ala685 sequence) two histidine residues are present at position 1588 and 1594. Although the latter groups could be involved in the pH-dependence of the Met-containing peptide binding, a significant increase of the Ki of the Met-containing peptide occurs at pH > 8.5, where such groups should be already completely not protonated. This suggests the involvement in this interaction of ionizable residues with more basic pKa values. Thus, the side chain of lysine or arginine residues may be engaged in this interaction. The molecular model of the FRETS-VWF73 and the prediction of the secondary folding of the spacer domain of ADAMTS13 provided some elements that could be useful to interpret these functional results from a structural point of view. These models showed that FRETS-VWF73 has an overall C-shaped scaffold (≈40 × ≈40 × ≈25 Å) with a helical C-terminal domain from D1653 to R1668 (Fig. 7). The FRETS-VWF73 molecule is mostly acidic (theoretical pI = 4.4), containing 6 basic and 11 acidic residues. Most of the charged residues are located after the Tyr1605-Met1606 bond, such as the cluster composed of Asp1614 (VWF numbering system; Asp19 in the sequence of the peptide), Lys1617, Arg1618, Glu1638, and Glu1640, which cover a surface area of ∼400 Å2. Likewise, another mostly acidic cluster is composed of Asp1653, Glu1655, Glu1660, Asp1663, and the polar hydroxyl residue of Thr1656. These residues, as mentioned previously, are contained in a helix located at the C terminus of the peptide and, as shown by the three-dimensional model, oppose the Tyr1605-Met1606 peptide bond cleaved by ADAMTS13 at a mean distance of ∼18/20 Å (Fig. 7, inset). The experimental findings shown in Fig. 6, a and b, showed that the product's region from Glu1660 and Arg1668 is necessary for an efficient inhibition of ADAMTS13, in agreement with recent studies (32). Thus, this helical region would bind to a part of the spacer domain using considerable ionic and polar interactions for the molecular recognition. Without known three-dimensional structures homologous to the spacer domain of ADAMTS13, just a secondary structure prediction of this region can be given in consideration of the lack of cysteine residues, and thus of constraints dictated by the disulfide-bonding connectivity. The ADAMTS13 spacer domain is positively charged (theoretical pI = 9.51), containing 15 (11.5%) Arg and three (2.3%) Lys residues. The secondary structure prediction carried out by the PsiPred program showed that this domain should be mostly composed of strands (see Fig. S3 in Data S1) with more disordered regions only at the ends of N- and C-terminal regions. As a consequence, this domain should assume mainly a stretched configuration. This finding is in agreement with the “connecting” nature of this domain, which links the metalloprotease/disintegrin/Cys-rich domain with the TSP-1 repeats of the enzyme (5). Moreover, this domain should be extensively exposed to solvent, as it represents often the main target for auto-antibodies produced in immunomediated thrombotic microangiopathies (50). At its maximal theoretical extension the spacer domain is 195 Å long, so we can predict from simple geometrical considerations that only the N-terminal region extending from Ser1556 to Ala1586 and containing three Arg and one Lys residues could interact with the acidic C-terminal region of FRETS-VWF73. Based on these biochemical and modeling results, it is likely that the enzyme has a C-shaped scaffold, so that the spacer domain should oppose the active site of the metalloprotease to accommodate the entire FRETS-VWF73 molecule that, according to the model reported in Fig. 7, has a similar and complementary C-shaped conformation. Due to dimensions of the various enzyme domains and the conformation of the VWF73 substrate, only this particular tertiary structure of ADAMTS13 could allow an efficient interaction with FRETS-VWF73. Notably, the C-shaped scaffold was also observed in the recently solved structure of ADAMTS-1, where the Cys-rich domain, flanking the spacer region of the metalloprotease in the ADAMTS family, stacks against the active site of the enzyme (51). The charged residues of both VWF and ADAMTS13 could serve to preorient correctly the enzyme and the substrate. The interaction with the spacer domain would favor the molecular recognition of VWF. This conclusion was also supported by the finding that a shorter FRETS-VWF substrate (from Asp1596 to Phe1654) was minimally recognized and hydrolyzed by ADAMTS13, at pH 6.00 and 37°C (data not shown). The involvement of this exosite favors a correct and efficient orientation of the VWF molecule toward the catalytic pocket of ADAMTS13, where the zinc-bound water molecule can properly face the scissile Tyr-Met bond, carrying out the catalytic cleavage. Moreover, once sufficient concentrations of C-terminal product is formed, binding of the C-terminal cleaved product to the spacer domain induces an inhibitory allosteric control of the ADAMTS13 activity, likely aimed at limiting an excessive loss of high molecular weight multimers of VWF. The relevance of the VWF Met1606-Arg1668 sequence for the molecular recognition by the protease is also shown by the strong inhibitory effect exerted by auto-antibodies, which interact with the spacer domain of ADAMTS13, often present in thrombotic microangiopathies (50).
FIGURE 7.
Three-dimensional molecular model of FRETS-VWF73. The residues 1 and 73 in FRETS -VWF73 refer to the primary sequence of the peptide substrate, whereas they correspond to residues Asp1596 and Arg1668 of VWF, respectively. The sequence from Thr1656 to Arg1668 in FRETS-VWF73 (VWF numbering system, Thr61 and Arg73, respectively, in the primary sequence of FRETS-VWF73) are shown as filled space. In the FRETS-VWF73 model the side chain of both Tyr1605 and Met1606 are shown as black sticks. In the inset, the model of the peptide is shown as strands in the same orientation.
Notably, a recent study with recombinant VWF constructs carrying mutations responsible for the occurrence of a type 2A Von Willebrand disease showed that these VWF molecules are characterized by an exalted hydrolysis by ADAMTS13 (7). Among these VWF molecules, the authors indicated several mutations located in the region from Met1606 to Arg1668 such as Val1607Asp, Gly1609Arg, Ile1628Thr, Gly1629Glu, Gly1631Asp, and Glu1638Lys (7). Based on the results of this study, we can hypothesize that these mutations, and especially the charge reversal at position 1638 of VWF, affect the interaction of VWF with the spacer domain of ADAMTS13, thus perturbing the negative feed back mechanism of VWF cleavage described above and favoring its degradation
In conclusion, the study of the pH- and temperature-dependence of the ADAMTS13 activity provided novel findings to address the knowledge of the intermediate steps of the catalytic cycle of the metalloprotease. Furthermore, the Met-containing peptide produced by cleavage at the Tyr1605-Met1606 peptide bond is able to allosterically inhibit the enzyme's function. In this study, we showed that pH-dependent interactions between acidic residues from Met1606 and Arg1668 of VWF A2 domain and basic residues of the spacer domain of ADAMTS13 play a central role in this allosteric mechanism. Further studies are needed to assess whether the binding of particular auto-antibodies to the spacer domain of ADAMTS13, responsible for acquired forms of thrombotic microangiopathies, could directly trigger this allosteric mechanism, inhibiting the interaction with VWF multimers.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
R.D.C. is grateful to Dr. S. Rutella (Catholic University School of Medicine, Rome) for critical reading of the manuscript and providing useful suggestions. The Protein Data Bank files of the molecular models of FRETS-VWF73 are available on request at: rdecristofaro@rm.unicatt.it.
This study was supported by the Italian Ministry of University and Research (“ex-60%”, 2006, and “PRIN 2005”).
Editor: David W. Piston.
References
- 1.Chung, D. W., and K. Fujikawa. 2002. Processing of von Willebrand factor by ADAMTS-13. Biochemistry. 41:11065–11070. [DOI] [PubMed] [Google Scholar]
- 2.Dent, J. A., S. D. Berkowitz, J. Ware, C. K. Kasper, and Z. M. Ruggeri. 1990. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc. Natl. Acad. Sci. USA. 87:6306–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai, H. M. 1996. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 87:4235–4244. [PubMed] [Google Scholar]
- 4.Fischer, B. E., K. B. Thomas, U. Schlokat, and F. Dorner. 1998. Triplet structure of human von Willebrand factor. Biochem. J. 331:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo, B. H., D. Oh, S. Y. Chung, N. K. Kim, S. Park, Y. Jang, and K. H. Chung. 2002. Deficiency of von Willebrand factor-cleaving protease activity in the plasma of malignant patients. Thromb. Res. 105:471–476. [DOI] [PubMed] [Google Scholar]
- 6.Rayes, J., A. Hommais, P. Legendre, H. Tout, A. Veyradier, B. Obert, A. S. Ribba, and J. P. Girma. 2007. Effect of von Willebrand disease type 2B and type 2M mutations on the susceptibility of von Willebrand factor to ADAMTS-13. J. Thromb. Haemost. 5:321–328. [DOI] [PubMed] [Google Scholar]
- 7.Hassenpflug, W. A., U. Budde, T. Obser, D. Angerhaus, E. Drewke, S. Schneppenheim, and R. Schneppenheim. 2006. Impact of mutations in the von Willebrand factor A2 domain on ADAMTS13-dependent proteolysis. Blood. 107:2339–2345. [DOI] [PubMed] [Google Scholar]
- 8.Furlan, M., R. Robles, and B. Lamie. 1996. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 87:4223–4234. [PubMed] [Google Scholar]
- 9.Levy, G. G., W. C. Nichols, E. C. Lian, T. Foroud, J. N. McClintick, B. M. McGee, A. Y. Yang, D. R. Siemieniak, K. R. Stark, R. Gruppo, R. Sarode, S. B. Shurin, V. Chandrasekaran, S. P. Stabler, H. Sabio, E. E. Bouhassira, J. D. Upshaw, Jr., D. Ginsburg, and H. M. Tsai. 2001. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 413:488–494. [DOI] [PubMed] [Google Scholar]
- 10.Moake, J. L. 2002. Thrombotic microangiopathies. N. Engl. J. Med. 347:589–600. [DOI] [PubMed] [Google Scholar]
- 11.Tsai, H. M., I. I. Sussman, D. Ginsburg, H. Lankhof, J. J. Sixma, and R. L. Nagel. 1997. Proteolytic cleavage of recombinant type 2A von Willebrand factor mutants R834W and R834Q: inhibition by doxycycline and by monoclonal antibody VP-1. Blood. 89:1954–1962. [PubMed] [Google Scholar]
- 12.O'Brien, L. A., J. J. Sutherland, D. F. Weaver, and D. Lillicrap. 2005. Theoretical structural explanation for Group I and Group II, type 2A von Willebrand disease mutations. J. Thromb. Haemost. 3:796–797. [DOI] [PubMed] [Google Scholar]
- 13.Dong, J. F. 2005. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. J. Thromb. Haemost. 3:1710–1716. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland, J. J., L. A. O'Brien, D. Lillicrap, and D. F. Weaver. 2004. Molecular modeling of the von Willebrand factor A2 Domain and the effects of associated type 2A von Willebrand disease mutations. J. Mol. Model. 10:259–270. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien, L. A., P. D. James, M. Othman, E. Berber, C. Cameron, C. R. Notley, C. A. Hegadorn, J. J. Sutherland, C. Hough, G. E. Rivard, D. O'Shaunessey, and D. Lillicrap. 2003. Founder von Willebrand factor haplotype associated with type 1 von Willebrand disease. Blood. 102:549–557. [DOI] [PubMed] [Google Scholar]
- 16.Dong, J. F., J. L. Moake, L. Nolasco, A. Bernardo, W. Arceneaux, C. N. Shrimpton, A. J. Schade, L. V. McIntire, K. Fujikawa, and J. A. Lopez. 2002. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 100:4033–4039. [DOI] [PubMed] [Google Scholar]
- 17.Tsai, H. M., I. I. Sussman, and R. L. Nagel. 1994. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 83:2171–2179. [PubMed] [Google Scholar]
- 18.Nishio, K., P. J. Anderson, X. L. Zheng, and J. E. Sadler. 2004. Binding of platelet glycoprotein Ibα to von Willebrand factor domain A1 stimulates the cleavage of the adjacent domain A2 by ADAMTS13. Proc. Natl. Acad. Sci. USA. 101:10578–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim, K., P. J. Anderson, E. A. Tuley, E. Wiswall, and J. E. Sadler. 2008. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 111:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Cristofaro, R., F. Peyvandi, R. Palla, S. Lavoretano, R. Lombardi, G. Merati, F. Romitelli, E. Di Stasio, and P. M. Mannucci. 2005. Role of chloride ions in modulation of the interaction between von Willebrand factor and ADAMTS-13. J. Biol. Chem. 280:23295–23302. [DOI] [PubMed] [Google Scholar]
- 21.De Cristofaro, R., F. Peyvandi, L. Baronciani, R. Palla, S. Lavoretano, R. Lombardi, E. Di Stasio, A. B. Federici, and P. M. Mannucci. 2006. Molecular mapping of the chloride-binding site in von Willebrand factor (VWF): energetics and conformational effects on the VWF/ADAMTS-13 interaction. J. Biol. Chem. 281:30400–30411. [DOI] [PubMed] [Google Scholar]
- 22.Kokame, K., Y. Nobe, Y. Kokubo, A. Okayama, and T. Miyata. 2005. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br. J. Haematol. 129:93–100. [DOI] [PubMed] [Google Scholar]
- 23.Fasciglione, G. F., S. Marini, S. D'Alessio, V. Politi, and M. Coletta. 2000. pH- and temperature-dependence of functional modulation in metalloproteinases. A comparison between neutrophil collagenase and gelatinases A and B. Biophys. J. 79:2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha, J., M. V. Pedersen, and D. S. Auld. 1996. Metal and pH dependence of heptapeptide catalysis by human matrilysin. Biochemistry. 35:15831–15838. [DOI] [PubMed] [Google Scholar]
- 25.Plaimauer, B., K. Zimmermann, D. Volkel, G. Antoine, R. Kerschbaumer, P. Jenab, M. Furlan, H. Gerritsen, B. Lammle, H. P. Schwarz, and F. Scheiflinger. 2002. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13). Blood. 100:3626–3632. [DOI] [PubMed] [Google Scholar]
- 26.Pace, C. N., F. Vajdos, L. Fee, G. Grimsley, and T. Gray. 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4:2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fersht, A. 1985. Enzyme Structure and Mechanism. Freeman, New York.
- 28.Ayala, Y. M., and E. Di Cera. 2000. A simple method for the determination of individual rate constants for substrate hydrolysis by serine proteases. Protein Sci. 9:1589–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laidler, K. 1965. Chemical Kinetics. McGraw-Hill, New York.
- 30.Di Stasio, E., P. Bizzarri, F. Misiti, E. Pavoni, and A. Brancaccio. 2004. A fast and accurate procedure to collect and analyze unfolding fluorescence signal: the case of dystroglycan domains. Biophys. Chem. 107:197–211. [DOI] [PubMed] [Google Scholar]
- 31.Di Cera, E., R. De Cristofaro, D. J. Albright, and J. W. Fenton 2nd. 1991. Linkage between proton binding and amidase activity in human alpha-thrombin: effect of ions and temperature. Biochemistry. 30:7913–7924. [DOI] [PubMed] [Google Scholar]
- 32.Gao, W., P. J. Anderson, E. M. Majerus, E. A. Tuley, and J. E. Sadler. 2006. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc. Natl. Acad. Sci. USA. 103:19099–19104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, J. J., K. Fujikawa, B. A. McMullen, and D. W. Chung. 2006. Characterization of a core binding site for ADAMTS-13 in the A2 domain of von Willebrand factor. Proc. Natl. Acad. Sci. USA. 103:18470–18474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segel, I. H. 1975. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. Wiley, New York.
- 35.Reference deleted in proof.
- 36.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195–202. [DOI] [PubMed] [Google Scholar]
- 37.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics. 16:404–405. [DOI] [PubMed] [Google Scholar]
- 38.Snider, C. E., J. C. Moore, T. E. Warkentin, C. N. Finch, C. P. Hayward, and J. G. Kelton. 2004. Dissociation between the level of von Willebrand factor-cleaving protease activity and disease in a patient with congenital thrombotic thrombocytopenic purpura. Am. J. Hematol. 77:387–390. [DOI] [PubMed] [Google Scholar]
- 39.Zanardelli, S., J. T. Crawley, C. K. Chion, J. K. Lam, R. J. Preston, and D. A. Lane. 2006. ADAMTS13 substrate recognition of von Willebrand factor A2 domain. J. Biol. Chem. 281:1555–1563. [DOI] [PubMed] [Google Scholar]
- 40.Tipton, K. F., and H. B. Dixon. 1979. Effects of pH on enzymes. Methods Enzymol. 63:183–234. [DOI] [PubMed] [Google Scholar]
- 41.Auld, D. S., and B. L. Vallee. 1970. Kinetics of carboxypeptidase A. The pH dependence of tripeptide hydrolysis catalyzed by zinc, cobalt, and manganese enzymes. Biochemistry. 9:4352–4359. [DOI] [PubMed] [Google Scholar]
- 42.Huang, D. T., M. A. Thomas, and R. I. Christopherson. 1999. Divalent metal derivatives of the hamster dihydroorotase domain. Biochemistry. 38:9964–9970. [DOI] [PubMed] [Google Scholar]
- 43.Cross, J. B., J. S. Duca, J. J. Kaminski, and V. S. Madison. 2002. The active site of a zinc-dependent metalloproteinase influences the computed pK(a) of ligands coordinated to the catalytic zinc ion. J. Am. Chem. Soc. 124:11004–11007. [DOI] [PubMed] [Google Scholar]
- 44.Kitzinger, C., and R. Hems. 1959. Enthalpies of hydrolysis of glutamine and asparagine and of ionization of glutamic and aspartic acids. Biochem. J. 71:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornish-Bowden, A. 1995. Fundamentals of enzyme kinetics. Portland Press, London.
- 46.Kluger, R., A. K. Dodds, and M. K. Wong. 1984. Variation of steric effects in metal ion catalyzed proton transfer. a probe of transition-state structure. J. Am. Chem. Soc. 106:1113–1117. [Google Scholar]
- 47.Makinen, M. W., L. C. Kuo, J. J. Dymowski, and S. Jaffer. 1979. Catalytic role of the metal ion of carboxypeptidase A in ester hydrolysis. J. Biol. Chem. 254:356–366. [PubMed] [Google Scholar]
- 48.Auld, D. S. 2004. Catalytic Mechanisms for Metallopeptidases. Elsevier Academic Press, New York.
- 49.Holmquist, B., and B. L. Vallee. 1974. Metal substitutions and inhibition of thermolysin: spectra of the cobalt enzyme. J. Biol. Chem. 249:4601–4607. [PubMed] [Google Scholar]
- 50.Klaus, C., B. Plaimauer, J. D. Studt, F. Dorner, B. Lammle, P. M. Mannucci, and F. Scheiflinger. 2004. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 103:4514–4519. [DOI] [PubMed] [Google Scholar]
- 51.Gerhardt, S., G. Hassall, P. Hawtin, E. McCall, L. Flavell, C. Minshull, D. Hargreaves, A. Ting, R. A. Pauptit, A. E. Parker, and W. M. Abbott. 2007. Crystal structures of human ADAMTS-1 show a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains. J. Mol. Biol. 373:891–902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.