Abstract
We investigated the effects of aging on Drosophila melanogaster indirect flight muscle from the whole organism to the actomyosin cross-bridge. Median-aged (49-day-old) flies were flight impaired, had normal myofilament number and packing, barely longer sarcomeres, and slight mitochondrial deterioration compared with young (3-day-old) flies. Old (56-day-old) flies were unable to beat their wings, had deteriorated ultrastructure with severe mitochondrial damage, and their skinned fibers failed to activate with calcium. Small-amplitude sinusoidal length perturbation analysis showed median-aged indirect flight muscle fibers developed greater than twice the isometric force and power output of young fibers, yet cross-bridge kinetics were similar. Large increases in elastic and viscous moduli amplitude under active, passive, and rigor conditions suggest that median-aged fibers become stiffer longitudinally. Small-angle x-ray diffraction indicates that myosin heads move increasingly toward the thin filament with age, accounting for the increased transverse stiffness via cross-bridge formation. We propose that the observed protein composition changes in the connecting filaments, which anchor the thick filaments to the Z-disk, produce compensatory increases in longitudinal stiffness, isometric tension, power and actomyosin interaction in aging indirect flight muscle. We also speculate that a lack of MgATP due to damaged mitochondria accounts for the decreased flight performance.
INTRODUCTION
Aging is a natural biological process that results in a progressive loss of cellular, tissue, and organismal function with the passage of time. One of the common effects of aging is loss of mobility and locomotive activity. Age-related reductions in human muscle mass and performance (sarcopenia) severely reduce the ability of the elderly to perform day-to-day tasks (1) and has been shown to contribute to disability (1,2), greater risk for falls and fractures (2), increases in mortality (3), and, in general, a poorer quality of life. Human sarcopenia may be caused by a variety of factors, including neural degeneration, mitochondrial dysfunction, alterations to the molecular components of muscle (such as myosin or actin), decreased protein synthesis, and an age-related decrease in activity (reviewed by Degens (4)).
Characterization and mechanistic determination of age-related changes in muscle function and performance in model organisms such as Caenorhabditis elegans or Drosophila melanogaster advance our basic understanding of muscle aging. Comparative studies between humans, other animals, worms, and insects have been particularly adept at uncovering fundamental mechanisms of aging. For instance, expression of the electron transport chain gene pathway has been found to decrease in humans, mice, and flies, suggesting that there are evolutionarily conserved pathways between species (5). Although human skeletal and Drosophila indirect flight muscle (IFM) show major differences in muscle fiber type, regenerative ability, and innervation, both sets of muscles are postmitotic and rely upon similar contraction and energy supply mechanisms. Drosophila IFM, like human muscle, have age-related decrements in functional performance (reviewed by Grotewiel et al. (6)), protein expression (7), and mitochondrial function (8,9), as well as age-related changes in gene expression (10). Notably, flies provide an excellent model system for aging research due to their short life span, ease of maintenance and powerful genetics; in addition, in-depth knowledge of numerous aging processes is available due to the multitude of studies performed in this species.
Although aging has been widely studied in Drosophila, relatively few studies have examined age-related changes in their IFM structure and function. Structural studies have produced conflicting results, finding either no major degeneration (11) or severe degeneration (12) in muscle fibers with age. However, multiple studies found age-related damage to mitochondrial structure (11–13). IFM function of mature flies decreased with age in terms of wingbeat frequency (14), flight duration (15), flight activity (16), and the percentage of flies flying (17). An eventual loss of flight ability has been noted in Drosophila near the end of their lifespan (12,16). Although these studies indicate that Drosophila IFM function, and possibly structure, decline with age, the question of how aging affects these muscles remains unresolved, especially at the myofilament level. This study was designed to examine the effects of aging on Drosophila IFM structure and function from the whole organism to the actomyosin cross-bridge.
MATERIALS AND METHODS
Lifespan
A total of 200 newly eclosed female Drosophila melanogaster (Oregon-R strain) were placed in groups (25 flies per group) in 35-ml plastic vials containing standard cornmeal fly food and maintained at 25°C, 70% humidity, and a 12/12 h light/dark cycle. Flies were moved to new food vials every other day, and the number living was recorded. Flies that died from unnatural causes were subtracted from the overall total.
Flight performance
Flight measurements were performed at room temperature (22°C). Individual flies were released from the center of a Plexiglas flight chamber with a light source at the top (18), and their flight path scored as up (U), horizontal (H), down (D), or not at all (N). Flight index was determined using the formula: 6 × U/T + 4 × H/T + 2 × D/T + 0 × N/T, where U, H, D, and N are the number of flights in each category and T is the total number of flies tested (19). Wingbeat frequency was measured on tethered flies using an optical tachometer as described previously (20).
Electron microscopy
Fly thoraces were fixed, treated, and imaged as described previously (21). Percentage of total fiber area occupied by myofibrils was determined by coloring each myofibril area in a cross-sectional image black and using ImageJ software (v.1.36b; National Institutes of Health, Bethesda, MD) to threshold the scanned image and measure the percentage of black area in 18 × 18 μm sections.
Single muscle fiber solutions
Solution composition was as previously described (22), except 300 U/ml of creatine phosphokinase (CPK) was used.
X-ray diffraction
Live flies were prepared for x-ray diffraction as described previously (23). Single dorsolongitudinal muscle fibers were demembranated in skinning solution for 1 h at 4°C, clipped with aluminum T-clips ∼300 μm apart, and transferred to storage solution at −20°C. Fibers were secured between adjustable hooks in a perfusion chamber filled with relaxing solution. Resting (wings folded) live fly muscle and single fiber x-ray diffraction patterns were obtained on the Biophysics Collaborative Access Team (BioCAT) beam-line at the Advanced Photon Source (Argonne, IL) and analyzed as previously described (23). The peak intensity, widths, and peak separations for the 1,0 and 2,0 equatorial reflections were estimated using a nonlinear least squares fitting procedure. The separation of the 1,0 equatorial reflections was transformed into the distance between the lattice planes of the thick filaments (d1,0) and then converted to interfilament spacing 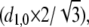 providing the center-to-center distance between thick filaments. Interfilament spacing in single fibers was measured in the presence of 0–10% (w/v) dextran T500 to determine the effect of osmotic compression on the myofilament lattice.
providing the center-to-center distance between thick filaments. Interfilament spacing in single fibers was measured in the presence of 0–10% (w/v) dextran T500 to determine the effect of osmotic compression on the myofilament lattice.
Single muscle fiber mechanics
Single muscle fibers were prepared and stored in the same manner as described for x-ray diffraction, except fibers were split in half lengthwise (to ∼100 μm diameter) to facilitate quicker diffusion of solutions. Fibers were mounted between a piezoelectric motor (Physik Instrumente, Auburn, MA) and a strain gauge (SensorNor, Horten, Norway) (24), and lowered into 30 μL of relaxing solution maintained at 15°C. Fibers were stretched until maximal oscillatory work was produced (pCa 5) and then progressively calcium-activated (from pCa 8.0 to pCa 4.5) as described previously (22). To perform sinusoidal analysis, small amplitude sinusoidal length changes (0.125% muscle length) were applied to the fiber at 47 frequencies (0.5–1000 Hz) while measuring the force response (24). Myosin attachment time was calculated from sinusoidal analysis curve-fitting techniques (25).
Fiber cross-linking experiments were performed by adding 10 mM 1-ethyl-3-[3-(dimethyl-amino)proyl]-carbodimide (EDC) to the activating solution (26). Sinusoidal analysis was performed at various time points, from 5 min up to 2 h, while the fiber remained in the activating solution plus EDC to assess the effects of differing amounts of cross-linking on single fiber mechanical properties.
Protein analysis
IFM fibers from 3 flies aged 3 days, 28 days, and 49 days were dissected in York modified glycerol (YMG: 20 mM KPi, 2 mM MgCl2, 1 mM EGTA, 8 mM DTT, 50% glycerol (27)). Protease inhibitors (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA) were added just before dissection. Samples were transferred to 50 μL of YMG containing 2% (v/v) Triton X-100 and protease inhibitors and demembranated overnight at −20°C. Samples were centrifuged at 14,000 rpm for 10 min at 4°C; the supernatant was removed and the fiber pellet was resuspended in 50 μL of YMG wash buffer (YMG without glycerol) containing protease inhibitors. Fibers were collected by centrifugation as before and washed two more times. The fiber pellet was homogenized in 60 μL of gel buffer (62.5 mM Tris (pH 6.8), 10% (v/v) glycerol, 50 mM DTT, 2% (v/v) SDS) containing protease inhibitors. The protein concentration of each sample was measured using the BCA protein assay kit-reducing agent compatible (Pierce, Rockford, IL). Equal amounts of each sample (∼5 μg) were loaded on 5%, 6%, or 10% SDS polyacrylamide gels, and electrophoresis performed as described previously (27), except gels were cooled to 5°C with a circulating water bath. Each sample was loaded in triplicate.
For analysis of protein expression, gels were stained overnight with Sypro Ruby (Bio-Rad, Hercules, CA) after fixation in 10% methanol and 7% acetic acid for 1 h with gentle agitation and were washed the next day using the same method. Gels were washed with distilled, deionized water before viewing in an ultraviolet light box; they were then photographed with a digital camera (Kodak DC290 Zoom; Kodak, Rochester, NY) and analyzed using the software package Phoretics 1D (v.5.10; Nonlinear Dynamics, Newcastle upon Tyne, UK). Bands were detected using the automatic detection function with minimum slope 100, noise reduction 5, and percent maximum peak 5. The background was subtracted using the method of minimum profile. The band matching function was used to align bands across lanes and was inspected visually; adjustments were made if necessary before matching bands between gels. To determine changes in protein abundance, average band intensity was calculated from the values of the three lanes. The relative protein abundance was expressed as [(x − 3d)/3d] where x is the average expression value of the band at 28 days or 49 days and 3d is 3 days.
Mass spectrometry
Mass spectrometry and data analysis were performed as previously described (28).
Statistical analysis
All data are reported as mean ± SE. Statistical analyses were performed using SPSS statistical software (v.14.0; SPSS, Chicago, IL) and were considered significant at p < 0.05. For data comparing more than two age groups, a one-way analysis of variance (ANOVA) followed by a Student-Neuman-Keuls post hoc test was used. For data comparing only two age groups, t-tests were performed to determine age differences, except for variables examined across oscillation frequencies. In this case, a repeated measures ANOVA with frequency as the repeated measure was performed first; if significant, t-tests were performed at each frequency.
RESULTS
Age-related changes were examined through the use of comparisons with fully mature 3-day-old flies (young flies). Structural and mechanical measurements were conducted on 49-day-old (median-aged) flies that could beat their wings to select for muscles that were functionally competent (Old flies, 56-day-old, could not beat their wings).
Lifespan
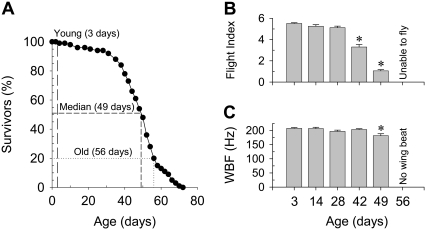
The survival curve remained relatively flat until flies reached 28 days of age, with 100% of the flies surviving at 3 days (Fig. 1 A). The most significant drop in the percentage of survivors was observed between 42 to 56 days, suggesting major age-related changes occurred within this timeframe. Approximately 50% of the flies remained alive at 49 days (median-aged), and 20% remained alive at 56 days (old age); the maximum lifespan was 70 days.
FIGURE 1.
Age-related changes in lifespan (A), flight index (B), and wingbeat frequency (WBF) (C) of female fruit flies. Dashed lines in the lifespan curve (A) indicate the three ages (3-, 49-, and 56-day-old) studied in this article. Asterisks indicate a significant difference (p < 0.05) from 3-day-old. Temperature is 25°C for lifespan curves. Temperature is 22°C (room temperature) for flight index and WBF.
Flight performance
Flight index began decreasing at 42 days and continued until complete loss of flight ability at 56 days (Fig. 1 B). At younger ages, 100% of the flies were flight capable; by 49 days of age, 67% were unable to fly. Wing beat frequency decreased modestly at 49 days and was nonexistent in flightless, 56-day-old flies (Fig. 1 C).
Flight muscle structure
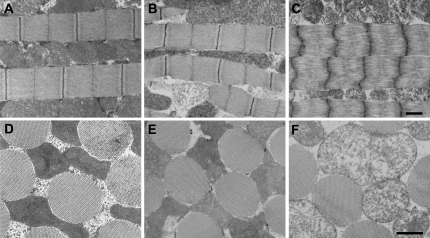
At all ages examined, thick and thin filaments formed organized hexagonal arrays with a well-defined cylindrical shape (Fig. 2, D–F), and myofibrils maintained a consistent number of thick filaments (young (Y): 36.1 ± 0.4, median-aged (M): 36.5 ± 0.5, old (O): 38.1 ± 0.5 thick filaments/myofibril diameter). Longitudinally, median-aged fibers (Fig. 2 B) had an ultrastructure similar to young (Fig. 2 A), although sarcomere length decreased slightly (Y: 3.43 ± 0.02, M: 3.33 ± 0.02 μm). Old fibers showed deteriorated longitudinal ultrastructure (Fig. 2 C) with greatly reduced sarcomere length (O: 2.41 ± 0.03 μm) compared with young and median-aged. Initial stages of mitochondrial deterioration were occasionally found in median-aged fibers (Fig. 2 B). Severe mitochondrial deterioration, including large loss of cristae and numerous enlarged mitochondria, was evident in old flies (Fig. 2, C and F). The percentage of total fiber area occupied by myofibrils, or percent myofibril area, decreased at each age level examined (Y: 55.7 ± 0.8, M: 49.7 ± 1.1, O: 40.5 ± 1.8%). As expected, chemically skinned young and median-aged fibers (Y: 36.6 ± 1.4, M: 40.1 ± 1.6%) had a decreased percent myofibril area compared to intact because the interfibrillar distance increased due to mitochondria solubilization. The percent myofibril area in intact old fibers was similar to chemically skinned young and median-aged fibers, indicating that the decrease in percent myofibril area in intact old fibers is due to mitochondrial disintegration and enlargement.
FIGURE 2.
Electron microscopy shows age-related changes in IFM structure. In longitudinal sections, young (A) and median-aged (B) flies have normal ultrastructure, although sarcomere length has decreased slightly with age, whereas old flies (C) have deteriorated sarcomeres. In cross-section, myofibrils of young (D), median-aged (E), and old (F) flies have similar cylindrical shape and diameter, with thick and thin filaments arranged in regular hexagonal arrays. The mitochondrial abnormality is apparent in the old (C and F) fibers compared to the younger ages (A, B, D, and E). Bars indicate 1 μm.
Myofilament lattice properties
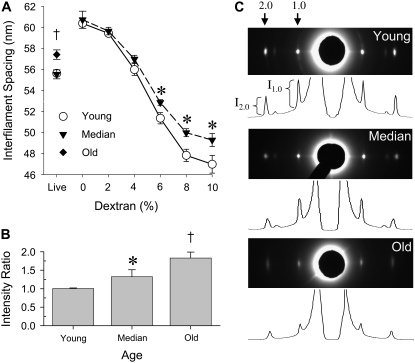
IFM interfilament spacing (thick filament center-to-center distance) in live flies was almost identical for young and median-aged flies but larger for old flies (Fig. 3 A), most likely due to the deteriorated ultrastructure found in old flies. The interfilament spacing of single skinned IFM fibers in relaxing solution (0% dextran T500) was 9% greater than in live flies due to the loss of osmotic constraints with plasma membrane removal. Adding dextran T500 osmotically compresses the filament lattice, reducing interfilament spacing. Interfilament spacing was similar in young and median-aged fibers at low osmotic pressures (up to 4% dextran T500 solution) (Fig. 3 A). At high osmotic pressures (6–10% dextran T500 solution), which compressed the lattice beyond in vivo or live spacing, interfilament distance was greater in median-aged fibers compared to young fibers at equivalent osmotic pressures (dextran concentrations). The added resistance to compression, reflected by the larger interfilament spacing, indicates that the fiber's radial stiffness increased with age.
FIGURE 3.
Age-related changes in interfilament spacing (A), live fly intensity ratio (I2,0/I1,0) (B), and live fly equatorial x-ray diffraction patterns and intensity profiles (C). Note that the interfilament spacing for the live median-aged flies (triangles) and live young flies (circles) are virtually identical such that the two symbols overlap. Single fiber interfilament spacing data were not collected for old fibers due to fiber degradation. Asterisks indicate a significant difference (p < 0.05) between median-aged and young. The dagger indicates a significant difference between old and young.
The 1,0 and 2,0 equatorial reflections in the x-ray diffraction patterns of live flies decreased in peak intensity and increased in peak widths with age (Fig. 3 C). The decreased intensities indicate a reduction in coherently diffracting mass. The peak width parameter Δd1,0/d1,0 (Y: 3.06 ± 0.29, M: 5.45 ± 0.01, O: 3.97 ± 0.01%), a measure of the width of distribution of lattice spacings among myofibrils, and ΔX/d1,0 (Y: 1.01 ± 0.17, M: 1.17 ± 0.27, O: 1.87 ± 0.18%), a measure of the “liquid-like” disorder within the lattice of individual myofibrils, both increase with age, indicating that the myofilament lattice becomes increasingly disordered (29). The equatorial intensity ratios (I2,0/I1,0) were increased in median-aged and old flies compared to young flies (Fig. 3 B). Because this ratio increases when myosin heads attach during contraction (30), increased intensity ratios indicate movement of the myosin heads from the thick filament backbone toward the thin filament and, consequently, increased actomyosin interactions that could explain the increased radial stiffness of median-aged flies.
Skinned muscle fiber mechanics
Old IFM fibers could not be activated by increasing the calcium concentration, which is most likely due to the extensive myofibrillar damage evident in electron micrographs and, therefore, could not be studied mechanically.
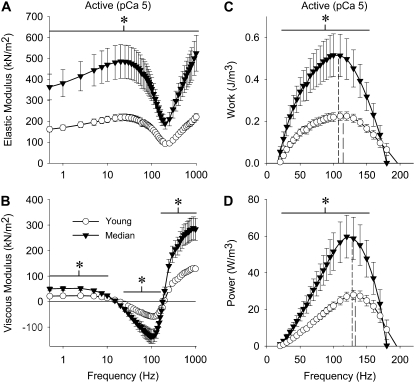
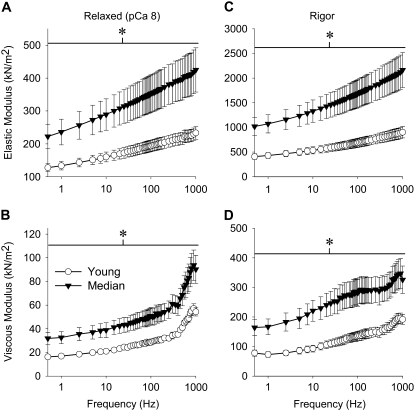
Remarkably, passive, active, and cross-bridge-dependent isometric tension was increased in median-aged fibers, typically ∼100% or double the young values (Table 1). The relationship between isometric tension and calcium concentration showed no differences in pCa50 values and Hill coefficients (Table 1), suggesting that thin filament regulation does not change with age. At peak calcium activation (pCa 5.0), median-aged fibers were markedly increased in their magnitudes of elastic and viscous moduli (ranging from 65 to 160% more, except where the viscous modulus neared and crossed zero) as well as in work and power output (125% and 110% more at maximum work and power, respectively) compared to young fibers (Fig. 4). Under relaxed conditions (pCa 8, where myosin heads are weakly attached to actin), median-aged fibers showed an increase over the entire frequency range for elastic and viscous moduli (ranging from 65 to 95% more) (Fig. 5, A and B). Under rigor conditions (no ATP, where myosin cross-bridges are strongly attached), aged fibers also showed a dramatic increase across all frequencies in elastic and viscous moduli (ranging from 70% to 150% more) (Fig. 5, C and D).
TABLE 1.
Summary of isometric data from isolated IFM
| Tension (kN/m2)
|
Hill fit parameters
|
||||||
|---|---|---|---|---|---|---|---|
| Age | Active (pCa = 5.0) | Passive (pCa = 8.0) | Cross-bridge dependent (Active–Passive) | n | pCa50 | Hill coefficient | n |
| Young* | 1.2 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 11 | 6.1 ± 0.1 | 2.0 ± 0.3 | 6 |
| Median† | 2.5 ± 0.4‡ | 1.6 ± 0.3‡ | 0.9 ± 0.2‡ | 11 | 6.2 ± 0.1 | 2.6 ± 0.3 | 9 |
Values are mean ± SE. Temperature = 15°C.
Young = 3 days of age.
Median-aged = 49 days of age.
Indicates significant difference (p < 0.05) from young.
FIGURE 4.
Elastic modulus (A), viscous modulus (B), work (C), and power (D) for active IFM fibers across muscle oscillation frequencies for young and median-aged flies. Dashed lines in (C) and (D) represent frequency of maximum work and power, respectively, from the article. Asterisks indicate a significant difference (p < 0.05) between young and median-aged flies at a particular frequency range (horizontal line). Temperature = 15°C.
FIGURE 5.
Elastic and viscous modulus values for relaxed (A and B) and rigor (C and D) IFM fibers across muscle oscillation frequencies for young and median-aged flies. Asterisks indicate a significant difference (p < 0.05) between young and median-aged across the entire frequency range. Temperature = 15°C.
These results indicate that aging increases the moduli of myofibril components, regardless of whether cross-bridges are strongly attached (active and rigor conditions) or weakly attached (passive conditions), suggesting adaptations in noncross-bridge elements.
Although active stiffness and power output showed dramatic increases with age, no significant differences in active (pCa 5.0) muscle cross-bridge kinetics were observed. The oscillatory frequencies at which maximum work (Y: 115 ± 5, M: 107 ± 6 Hz) and power (Y: 133 ± 5, M: 128 ± 4 Hz) occurred, as well as myosin attachment time (Y: 289 ± 23, M: 290 ± 26 ns), were similar with age.
Cross-linking of proteins, primarily collagen, by advanced glycation end-products is a major mechanism in increased cardiovascular stiffness with age (31). We cross-linked skinned IFM fibers from young flies in situ with EDC (1-ethyl-3-[3-(dimethyl-amino)proyl]-carbodimide), which cross-links myosin to actin and the myosin rods to each other (26), in an attempt to mimic the mechanical properties of median-aged fibers. Cross-linked fibers showed an increased elastic modulus but no increase in viscous modulus (data not shown) in contrast to the observed age-related increase in elastic and viscous moduli. From these experiments, we conclude that nonspecific protein cross-linking alone cannot be responsible for the age-related changes in fiber mechanical properties.
Myofibril protein composition
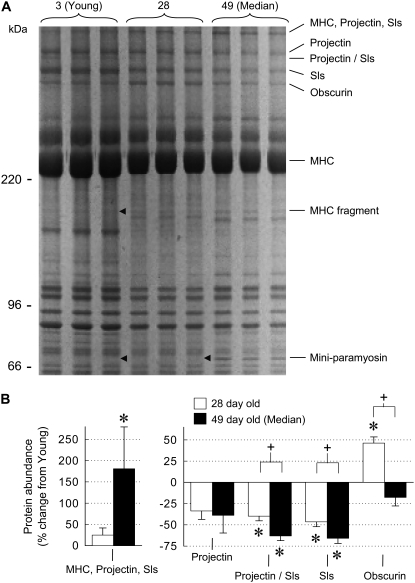
Gel electrophoresis analysis of proteins extracted from skinned IFM fibers reveal changes in the relative abundance of high molecular mass proteins (>200 kDa). Sallimus (Sls) and projectin, both of which connect the thick filament to the Z-band and are known to contribute to fiber stiffness (32), were identified by mass spectrometry in a number of protein bands. A band containing mostly myosin heavy chain (MHC), projectin, and Sls increased in median-aged fibers compared to young fibers, whereas the remaining bands containing projectin and/or Sls were downregulated in 28-day-old and median-aged fibers compared to young fibers (Fig. 6). In contrast, the expression of obscurin, an M-line-associated protein (33), increased at 28 days but remained unchanged in median-aged fibers in comparison to young fibers. The protein abundance of projectin/Sls, Sls, and obscurin were significantly different between 28-day-old and median-aged fibers, showing that protein changes occur throughout the fly's lifespan. These results indicate that aging changes the connecting filaments that anchor the thick filaments to the Z-disk and M-line-associated proteins.
FIGURE 6.
Changes in myofibrillar protein abundance with age. A representative 6% sodium dodecyl-sulfate-polyacrylamide gel stained with Sypro Ruby (A) was loaded with an equal amount of total protein (∼5 μg) in each lane. Proteins named on the right column were identified by mass spectrometry of the indicated gel bands. MHC fragment and miniparamyosin-labeled bands are found only in aged flies (arrowheads indicate ages that are missing bands). The relative quantification of high molecular weight bands (B) represents the average abundance determined from the triplicate lanes in gel in (A) and three additional gels. Although this representative gel may suggest additional protein changes besides those identified, only bands that could be matched across all 12 samples were included. Old fibers were too degraded for useful protein abundance measurements. Asterisks indicate a significant difference (p < 0.05) from young. Plus signs indicate a significant difference between 28-day-old and median-aged.
Fibers from median-aged flies showed two additional bands at ∼150 kDa (MHC fragment) and 68 kDa (miniparamyosin or paramyosin fragment) that are not present in young fibers (Fig. 6 A). MHC peptides from the amino and carboxyl terminal were identified, suggesting that the MHC fragment does not represent products from site-specific proteolysis of myosin, as documented for some mutants (34). Peptides identified by mass spectrometry for the 68 kDa band did not discriminate between paramyosin and miniparamyosin because these two proteins are encoded by the same gene (35). Based upon molecular mass, we assume the 68 kDa band is miniparamyosin, which is located at both ends of the thick filament and at the M-line (36).
DISCUSSION
We investigated the effects of aging on Drosophila melanogaster IFM, finding age-related changes from the level of the cross-bridge up to the whole organism. As discussed below, we hypothesize that changes in myofibril protein composition, primarily in the thick filaments and connecting filaments, produce an increase in their longitudinal stiffness that drives the increases in isometric tension, oscillatory power output, and actomyosin interaction in aging Drosophila IFM. We observed mitochondrial changes that may initiate or accelerate the IFM aging process, and we propose that a lack of ATP availability due to mitochondria structural damage underlies the reduced flight ability of aging flies.
Muscle structure
The myofibril ultrastructure remains relatively unchanged between young (3-day-old) and median-aged (49-day-old) flies still able to beat their wings, except for a slight age-related decrease in sarcomere length. However, the increase in skinned fiber interfilament spacing and in vivo equatorial intensity ratio at high osmotic pressure in median-aged fibers indicates that myosin heads have moved toward the thin filament (30), which is accompanied by an increase in the fiber's overall radial stiffness. Myofibrils from old flies (56-day-old), only 1 week older than median-aged flies, have greatly reduced sarcomere length, increased in vivo interfilament spacing, and increased lattice disorder, showing a loss of ultrastructural integrity and acute sarcopenia.
A profound age-related structural change is the severe mitochondrial degradation in old flies, indicated by a significant loss of cristae and enlarged mitochondria. Median-aged flies occasionally show signs of mitochondrial deterioration, with no deterioration found in young flies. Notably, the percent myofibril area decreases from young to median-aged to old due to mitochondrial enlargement and not myofibril removal, because chemically skinned young and median-aged fibers (in which interfibrillar space increases due to mitochondria solubilization) and intact old fibers (in which mitochondria are enlarged due to aging) have a similar percent myofibril area. These changes suggest a major role for mitochondria in initiating the age-related changes in performance of Drosophila IFM and are in agreement with previous Drosophila studies that found age-related damage to the mitochondrial structure (11–13).
Skinned muscle fiber mechanics
Myosin kinetics are unaffected by age because myosin attachment time and the oscillatory frequencies at which maximum power and work occur are similar between young and median-aged fibers. In addition, thin filament regulation and cooperativity are unaffected by age because pCa50 and the Hill coefficient remain unchanged. However, aging increases isometric tension as well as elastic and viscous modulus values regardless of whether cross-bridges are strongly attached (active and rigor conditions) or weakly attached (passive condition). The increase in fiber force production and oscillatory power output without a change in myosin kinetics suggests either an increase in the number of myosin heads interacting with actin or that the individual cross-bridge force production increases through an increase in cross-bridge stiffness (i.e., more force production per cross-bridge displacement).
The most likely candidates for driving the age-related changes in skinned muscle fiber mechanics are the connecting filaments (C-filaments), which bridge the I-band, linking the thick filaments to the Z-band. The high passive tension and stiffness in Drosophila IFM is primarily attributed to the short C-filaments, consisting of the large proteins Sls(700), projectin, and kettin (32,37). The age-related changes in projectin and Sls protein abundance suggest an isoform shift and/or protein modification. Based on these results, we hypothesize that aging results in a shorter, stiffer C-filament due to an isoform shift and/or protein modification, which would explain the dramatic age-related increase in passive isometric tension and slightly decreased sarcomere lengths. We further hypothesize that the increase in passive tension increases thick filament strain, thereby inducing the movement of myosin heads toward the thin filament. The resulting increase in the number of heads interacting with actin would explain the observed increase in passive moduli (more weakly attached heads) and increases in active moduli, force production, and oscillatory power output (more strongly attached heads) without a change in myosin kinetics. This proposed movement of myosin heads agrees with our x-ray diffraction results and explains the age-related increase in the fiber's radial stiffness. A similar movement of myosin heads in water bug (Lethocerus) IFM has been suggested to explain increases in passive modulus, active modulus, and active tension after increasing the passive tension (and presumably thick filament strain) by increasing the sarcomere length (38).
Our results are consistent with other, less likely possibilities. Miniparamyosin (36) and obscurin (32) are M-line proteins, and their abundance changes may affect M-line stiffness, potentially explaining the age-related increase in fiber radial stiffness. However, these changes would not cause the age-related increases in passive and active properties. More likely, the miniparamyosin changes are a response to the projectin and Sls changes because these C-filament proteins are anchored in the thick filament toward the end of the A-band, another location of miniparamyosin (36). We cannot rule out the possibility that passive stiffness increases also increase the ability of individual myosin heads to transmit force. However, this is unlikely to be the sole cause of the age-related changes because cross-linking experiments in young fibers, which should increase thick filament stiffness, did not mimic the results of aged fibers.
Flight performance
Decreases in flight performance are evident around middle-age and decline further with advancing age until old flies are unable to beat their wings, a finding that is in agreement with previous studies (15–17). We hypothesize that the decreases in wingbeat frequency and flight index that occur during aging, before complete loss of flight ability, are due to reduced availability of ATP from the age-related mitochondrial structural damage observed in this and other Drosophila studies (11–13), based upon previous correlations between wingbeat frequency and ATP availability. For example, wingbeat frequency tracks with rate of oxygen consumption, which should be proportional to the rate of ATP production (39), and wingbeat frequency is lower in flies with enzyme decrements in the Drosophila glycolytic pathway that reduce ATP production and availability (40). Additionally, reduced ATP availability reduces myosin kinetics in isolated fibers (41), and reduced myosin kinetics leads to decreases in wingbeat frequency (42). In further support of this hypothesis, microarray studies show that genes involved in energy metabolism are downregulated with age in flies (43), and biochemical studies show reduced mitochondrial enzyme activity (8) and respiration (9) with age in flies.
Notably, a recent study found that individual flies with low wingbeat frequency values, measured at 5 days of age, tended to live longer than their cohorts with higher wingbeat frequencies (14). Thus, the lower wingbeat frequency of the median-aged flies in our study could potentially be due to the simple fact that flies with the highest wingbeat frequencies were otherwise dead or, if alive, unable to beat their wings. However, we found that the range of wingbeat frequency values for the median-aged flies (∼140 to 230 Hz) was much larger than in young flies (∼190 to 235 Hz), with similar maximum values. These results indicate that the reduced wingbeat frequency of median-aged flies was due to an overall population decrease in ability and not simply a loss of flies with high wingbeat frequency capabilities.
Another, less likely possibility for the decrease in flight performance is the pronounced increase in muscle fiber stiffness. Although an increase in muscle stiffness theoretically increases the flight system resonance frequency and, therefore, wingbeat frequency (20), the decoupling of fiber stiffness and wingbeat frequency that is evident in our study has been noted previously. Paramyosin phosphorylation site disruption increased wingbeat frequency (44), yet mechanics of IFM skinned fibers exhibited decreased passive, active, and rigor stiffness without affecting kinetics (22). Additional studies at various ages throughout the Drosophila lifespan are required to uncover the detailed relationship between flight ability, protein expression level, mitochondrial performance, and IFM fiber characteristics.
Relevance to human aging
In humans, various single fiber studies have been performed; all but one (45) of these studies found an age-related decrease in isometric tension and/or unloaded shortening velocity in which both parameters were measured (46–52). These age-related changes should result in a decrease in muscle power production (power = force × velocity) and may be due to changes in myosin kinetics (51,52). This finding is in agreement with kinetic changes observed in other animal studies (53–55). In flies, we observed the opposite: an increase in single fiber isometric tension and power output that is independent of myosin kinetics. An important consideration is that the middle-aged flies examined in our study were comparatively elderly (within 1 week of complete loss of flight ability); thus, the age-related changes in the muscle performance of younger flies may more closely approximate those in humans.
The age-related differences between humans and flies raise interesting questions about the abilities of the different muscles to respond to age-related challenges, which comparative approaches may answer. For instance, the lack of an age-related change in myosin kinetics in flies may be due to the fact that IFM, one of the most metabolically active muscles known (56), depends almost exclusively upon aerobic metabolism and high respiration rates. Thus, mechanisms may have evolved to protect the contractile machinery from these types of physiologic stress. A better understanding of the protection mechanisms in flies could aid in the understanding and treatment of age-related changes in human muscle. The increase in passive properties in flies, which drives their increase in power output, may also occur in aging humans, but it may not increase active muscle properties above values from young subjects because human passive properties are significantly smaller compared to flies. Although the effects of age on the passive properties of single human muscle fibers have not yet been reported, the notion that their passive properties can be increased was recently demonstrated in patients with spinal cord injury and muscle spasticity (57). In humans, as in flies, the increased fiber stiffness may be a compensatory mechanism for the reduction in ATP availability, i.e., a mechanism for economizing energy, and should be examined in future studies. Additionally, the MHC fragmentation found in older flies suggests an increase in MHC breakdown, which may occur in humans because an age-related decrease in MHC concentration has been observed (47). Comparative studies between species in single fiber muscle performance, as well as functional senescence, mitochondrial function, and gene expression, are likely to provide a wealth of information that will help advance the field of research on aging—from identifying biomarkers of aging to identifying potential strategies for treating sarcopenia in humans.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (R01 HL68034 to D.W.M.) and the National Science Foundation (MCB 0315865 and IOS 0718417 to J.O.V.). Mass spectrometry for this project was supported by the Vermont Genetics Network through grant P20 RR16462 from the IDeA Network of Biomedical Research Excellence (INBRE) program of the National Center for Research Resources (NCRR), a component of the NIH. Advanced Photon Source use for this project was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science under contract No. W-31-109-ENG-38. The Biophysics Collaborative Access Team (BioCAT) is an NIH-supported Research Center RR-08630. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NCRR or the NIH.
Gerrie P. Farman's present address is the Dept. of Physiology and Biophysics, University of Illinois, Chicago, IL 60612.
Editor: Leepo C. Yu.
References
- 1.Janssen, I., S. B. Heymsfield, and R. Ross. 2002. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 50:889–896. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner, R. N., K. M. Koehler, D. Gallagher, L. Romero, S. B. Heymsfield, R. R. Ross, P. J. Garry, and R. D. Lindeman. 1998. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 147:755–763. [DOI] [PubMed] [Google Scholar]
- 3.Metter, E. J., L. A. Talbot, M. Schrager, and R. A. Conwit. 2004. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J. Appl. Physiol. 96:814–821. [DOI] [PubMed] [Google Scholar]
- 4.Degens, H. 2007. Age-related skeletal muscle dysfunction: causes and mechanisms. J. Musculoskelet. Neuronal Interact. 7:246–252. [PubMed] [Google Scholar]
- 5.Zahn, J. M., R. Sonu, H. Vogel, E. Crane, K. Mazan-Mamczarz, R. Rabkin, R. W. Davis, K. G. Becker, A. B. Owen, and S. K. Kim. 2006. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grotewiel, M. S., I. Martin, P. Bhandari, and E. Cook-Wiens. 2005. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 4:372–397. [DOI] [PubMed] [Google Scholar]
- 7.Fleming, J. E., E. Quattrocki, G. Latter, J. Miquel, R. Marcuson, E. Zuckerkandi, and K. G. Bensch. 1986. Age-dependent changes in proteins of Drosophila melanogaster. Science. 231:1157–1159. [DOI] [PubMed] [Google Scholar]
- 8.Das, N., R. L. Levine, W. C. Orr, and R. S. Sohal. 2001. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J. 360:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, M., R. J. Mockett, Y. Shen, W. C. Orr, and R. S. Sohal. 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahn, M., H. Yamaza, Y. Sun, J. Sinclair, H. Li, and S. Zou. 2007. Temporal and spatial transcriptional profiles of aging in Drosophila melanogaster. Genome Res. 17:1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb, S., and M. A. Tribe. 1974. Are there major degenerative changes in the flight muscle of ageing diptera? Exp. Gerontol. 9:43–49. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi, A., D. E. Philpott, and J. Miquel. 1970. Electron microscope studies on aging Drosophila melanogaster III. Flight muscle. J. Gerontol. 25:222–228. [DOI] [PubMed] [Google Scholar]
- 13.Walker, D. W., and S. Benzer. 2004. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc. Natl. Acad. Sci. USA. 101:10290–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrosyan, A., I. H. Hsieh, and K. Saberi. 2007. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant Methuselah. Behav. Genet. 37:585–594. [DOI] [PubMed] [Google Scholar]
- 15.Graves, J. L., L. S. Luckinbill, and A. Nichols. 1988. Flight duration and wing beat frequency in long- and short-lived Drosophila melanogaster. J. Insect Physiol. 34:1021–1026. [Google Scholar]
- 16.Carey, J. R., N. Papadopoulos, N. Kouloussis, B. Katsoyannos, H. G. Muller, J. L. Wang, and Y. K. Tseng. 2006. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol. 41:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magwere, T., R. Pamplona, S. Miwa, P. Martinez-Diaz, M. Portero-Otin, M. D. Brand, and L. Partridge. 2006. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 61:136–145. [DOI] [PubMed] [Google Scholar]
- 18.Drummond, D. R., E. S. Hennessey, and J. C. Sparrow. 1991. Characterisation of missense mutations in the Act88F gene of Drosophila melanogaster. Mol. Gen. Genet. 226:70–80. [DOI] [PubMed] [Google Scholar]
- 19.Tohtong, R., H. Yamashita, M. Graham, J. Haeberle, A. Simcox, and D. Maughan. 1995. Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature. 374:650–653. [DOI] [PubMed] [Google Scholar]
- 20.Hyatt, C. J., and D. W. Maughan. 1994. Fourier analysis of wing beat signals: assessing the effects of genetic alterations of flight muscle structure in Diptera. Biophys. J. 67:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton, B., G. Ayer, N. Heymann, D. W. Maughan, F. O. Lehmann, and J. O. Vigoreaux. 2005. Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J. Exp. Biol. 208:549–560. [DOI] [PubMed] [Google Scholar]
- 22.Liu, H., M. S. Miller, D. M. Swank, W. A. Kronert, D. W. Maughan, and S. I. Bernstein. 2005. Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 102:10522–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irving, T. C., and D. W. Maughan. 2000. In vivo x-ray diffraction of indirect flight muscle from Drosophila melanogaster. Biophys. J. 78:2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson, M. H., C. J. Hyatt, F. O. Lehmann, J. R. Moore, M. C. Reedy, A. Simcox, R. Tohtong, J. O. Vigoreaux, H. Yamashita, and D. W. Maughan. 1997. Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys. J. 73:3122–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer, B. M., T. Suzuki, Y. Wang, W. D. Barnes, M. S. Miller, and D. W. Maughan. 2007. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys. J. 93:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawada, K., and M. Kawai. 1990. Covalent cross-linking of single fibers from rabbit psoas increases oscillatory power. Biophys. J. 57:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reedy, M. C., B. Bullard, and J. O. Vigoreaux. 2000. Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscles. J. Cell Biol. 151:1483–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zappaterra, M. D., S. N. Lisgo, S. Lindsay, S. P. Gygi, C. A. Walsh, and B. A. Ballif. 2007. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J. Proteome Res. 6:3537–3548. [DOI] [PubMed] [Google Scholar]
- 29.Yu, L. C., A. C. Steven, G. R. Naylor, R. C. Gamble, and R. J. Podolsky. 1985. Distribution of mass in relaxed frog skeletal muscle and its redistribution upon activation. Biophys. J. 47:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irving, T. C. 2006. X-ray diffraction of indirect flight muscle from Drosophila in vivo. In Nature's Versatile Engine: Insect Flight Muscle Inside and Out. J. O. Vigoreaux, editor. Landes Bioscience, Georgetown, TX. 197–213.
- 31.Asif, M., J. Egan, S. Vasan, G. N. Jyothirmayi, M. R. Masurekar, S. Lopez, C. Williams, R. L. Torres, D. Wagle, P. Ulrich, A. Cerami, M. Brines, and T. J. Regan. 2000. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc. Natl. Acad. Sci. USA. 97:2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkart, C., F. Qiu, S. Brendel, V. Benes, P. Haag, S. Labeit, K. Leonard, and B. Bullard. 2007. Modular proteins from the Drosophila sallimus (sls) gene and their expression in muscles with different extensibility. J. Mol. Biol. 367:953–969. [DOI] [PubMed] [Google Scholar]
- 33.Bagnato, P., V. Barone, E. Giacomello, D. Rossi, and V. Sorrentino. 2003. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 160:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronert, W. A., P. T. O'Donnell, A. Fieck, A. Lawn, J. O. Vigoreaux, J. C. Sparrow, and S. I. Bernstein. 1995. Defects in the Drosophila myosin rod permit sarcomere assembly but cause flight muscle degeneration. J. Mol. Biol. 249:111–125. [DOI] [PubMed] [Google Scholar]
- 35.Arredondo, J. J., M. Mardahl-Dumesnil, R. M. Cripps, M. Cervera, and S. I. Bernstein. 2001. Overexpression of miniparamyosin causes muscle dysfunction and age-dependant myofibril degeneration in the indirect flight muscles of Drosophila melanogaster. J. Muscle Res. Cell Motil. 22:287–299. [DOI] [PubMed] [Google Scholar]
- 36.Maroto, M., J. Arredondo, D. Goulding, R. Marco, B. Bullard, and M. Cervera. 1996. Drosophila paramyosin/miniparamyosin gene products show a large diversity in quantity, localization, and isoform pattern: a possible role in muscle maturation and function. J. Cell Biol. 134:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulke, M., C. Neagoe, B. Kolmerer, A. Minajeva, H. Hinssen, B. Bullard, and W. A. Linke. 2001. Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J. Cell Biol. 154:1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granzier, H. L., and K. Wang. 1993. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J. Gen. Physiol. 101:235–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtsinger, J. W., and C. C. Laurie-Ahlberg. 1981. Genetic variability of flight metabolism in Drosophila melanogaster. I. Characterization of power output during tethered flight. Genetics. 98:549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eanes, W. F., T. J. Merritt, J. M. Flowers, S. Kumagai, E. Sezgin, and C. T. Zhu. 2006. Flux control and excess capacity in the enzymes of glycolysis and their relationship to flight metabolism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 103:19413–19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swank, D. M., V. K. Vishnudas, and D. W. Maughan. 2006. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc. Natl. Acad. Sci. USA. 103:17543–17547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molloy, J. E., V. Kyrtatas, J. C. Sparrow, and D. C. White. 1987. Kinetics of flight muscles from insects with different wingbeat frequencies. Nature. 328:449–451. [Google Scholar]
- 43.Landis, G. N., D. Abdueva, D. Skvortsov, J. Yang, B. E. Rabin, J. Carrick, S. Tavare, and J. Tower. 2004. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 101:7663–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maughan, D., and J. Vigoreaux. 2005. Nature's strategy for optimizing power generation in insect flight muscle. Adv. Exp. Med. Biol. 565:157–166. [DOI] [PubMed] [Google Scholar]
- 45.Trappe, S., P. Gallagher, M. Harber, J. Carrithers, J. Fluckey, and T. Trappe. 2003. Single muscle fibre contractile properties in young and old men and women. J. Physiol. 552:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Antona, G., M. A. Pellegrino, C. N. Carlizzi, and R. Bottinelli. 2007. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur. J. Appl. Physiol. 100:603–611. [DOI] [PubMed] [Google Scholar]
- 47.D'Antona, G., M. A. Pellegrino, R. Adami, R. Rossi, C. N. Carlizzi, M. Canepari, B. Saltin, and R. Bottinelli. 2003. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J. Physiol. 552:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson, L., X. Li, and W. R. Frontera. 1997. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am. J. Physiol. 272:C638–C649. [DOI] [PubMed] [Google Scholar]
- 49.Yu, F., M. Hedstrom, A. Cristea, N. Dalen, and L. Larsson. 2007. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol. (Oxf.). 190:229–241. [DOI] [PubMed] [Google Scholar]
- 50.Krivickas, L. S., D. Suh, J. Wilkins, V. A. Hughes, R. Roubenoff, and W. R. Frontera. 2001. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am. J. Phys. Med. Rehabil. 80:447–455 (quiz 456–457). [DOI] [PubMed] [Google Scholar]
- 51.Ochala, J., D. J. Dorer, W. R. Frontera, and L. S. Krivickas. 2006. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 452:464–470. [DOI] [PubMed] [Google Scholar]
- 52.Ochala, J., W. R. Frontera, D. J. Dorer, J. Van Hoecke, and L. S. Krivickas. 2007. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J. Gerontol. A Biol. Sci. Med. Sci. 62:375–381. [DOI] [PubMed] [Google Scholar]
- 53.Prochniewicz, E., D. D. Thomas, and L. V. Thompson. 2005. Age-related decline in actomyosin function. J. Gerontol. A Biol. Sci. Med. Sci. 60:425–431. [DOI] [PubMed] [Google Scholar]
- 54.Lowe, D. A., J. T. Surek, D. D. Thomas, and L. V. Thompson. 2001. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 280:C540–C547. [DOI] [PubMed] [Google Scholar]
- 55.Lowe, D. A., D. D. Thomas, and L. V. Thompson. 2002. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am. J. Physiol. Cell Physiol. 283:C187–C192. [DOI] [PubMed] [Google Scholar]
- 56.Sacktor, B. 1976. Biochemical adaptations for flight in the insect. Biochem. Soc. Symp. 41:111–131. [PubMed] [Google Scholar]
- 57.Olsson, M. C., M. Kruger, L. H. Meyer, L. Ahnlund, L. Gransberg, W. A. Linke, and L. Larsson. 2006. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J. Physiol. 577:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]