Abstract
Background
The subtypes of γ-aminobutyric acid (GABA)A receptors mediating the discriminative stimulus effects of ethanol in nonhuman primates are not completely identified. The GABAA receptor positive modulator zolpidem has high, intermediate, and low activity at receptors containing α1, α2/3, and α5 subunits, respectively, and partially generalizes from ethanol in several species. The partial inverse agonist Ro15-4513 has the greatest affinity for α4/6-containing receptors, higher affinity for α5- and lower, but equal, affinity for α1- and α2/3-, containing GABAA receptors, and antagonizes the discriminative stimulus effects of ethanol.
Methods
This study assessed Ro15-4513 antagonism of the generalization of zolpidem from ethanol in male (n = 9) and female (n = 8) cynomolgus monkeys (Macaca fascicularis) trained to discriminate 1.0 g/kg (n = 10) or 2.0 g/kg (n = 7) ethanol (i.g.) from water with a 30-minute pretreatment interval.
Results
Zolpidem (0.017 to 5.6 mg/kg, i.m.) completely generalized from ethanol (≥80% of total session responses on the ethanol-appropriate lever) for 6/7 monkeys trained to discriminate 2.0 g/kg and 4/10 monkeys trained to discriminate 1.0 g/kg ethanol. Zolpidem partially generalized from 1.0 or 2.0 g/kg ethanol in 6/7 remaining monkeys. Ro15-4513 (0.003 to 0.30 mg/kg, i.m., 5-minute pretreatment) shifted the zolpidem dose-response curve to the right in all monkeys showing generalization. Analysis of apparent pKB from antagonism tests suggested that the discriminative stimulus effects of ethanol common with zolpidem are mediated by low-affinity Ro15-4513 binding sites. Main effects of sex and training dose indicated greater potency of Ro15-4513 in males and in monkeys trained to discriminate 1.0 g/kg ethanol.
Conclusions
Ethanol and zolpidem share similar discriminative stimulus effects most likely through GABAA receptors that contain α1 subunits, however, antagonism by Ro15-4513 of zolpidem generalization from the lower training dose of ethanol (1.0 g/kg) may involve additional zolpidem-sensitive GABAA receptor subtypes (e.g., α2/3 and α5).
Keywords: Ethanol, Ro15-4513, Zolpidem, Cynomolgus Monkeys, Drug Discrimination
DRUG DISCRIMINATION HAS been used extensively to identify the receptors mediating the discriminative stimulus effects of drugs of abuse including ethanol (Colpaert, 1986; Solinas et al., 2006). Drug discrimination is pharmacologically specific. For example, in rats, mice, gerbils, pigeons, and monkeys, systemically administered positive modulators of γ-aminobutyric acid (GABA)A receptors including benzodiazepines, barbiturates, and neuroactive steroids, generalize from ethanol in drug discrimination protocols, whereas direct GABAA agonists (e.g., muscimol) and μ-opioid receptor agonists (e.g., morphine) do not produce ethanol-appropriate responding (for a review, see Grant, 1999; Grant et al., 1996, 2000). Identification of the types of receptors mediating the discriminative stimulus effects of ethanol could contribute to the development of novel alcoholism pharmacotherapies.
The GABAA receptors are pentameric assemblies of subunits forming a Cl- channel, typically including 2 α,2 β, and 1 γ subunit (McKernan and Whiting, 1996). Subunit composition influences the pharmacological effect of receptor ligands (Sieghart, 1995). Responses to GABA at GABAA receptors that include α1, α2, α3, or α5 subunits are positively modulated by benzodiazepines, resulting in sedation, anxiolysis, anterograde amnesia, and anticonvulsant effects (Möhler et al., 2002).
The GABAA receptor subtypes that mediate the discriminative stimulus effects of ethanol, particularly in nonhuman primates, require further characterization. Zolpidem is a positive modulator with 5- to 10-fold selectivity for GABAA receptors containing α1 subunits compared with receptors containing α2 or α3 subunits (Lüddens et al., 1994; Sieghart, 1995; Smith et al., 2001). The activity of zolpidem at receptors containing α5 subunits is in general lower than at α1-3 subunits but appears to vary depending on the receptor subunit composition. In 1 study, zolpidem slightly enhanced (∼15%) responses to GABA at α5-containing receptors coexpressed with β2γ2 subunits (Sanna et al., 2002). Zolpidem is reported to be inactive at receptors containing α5 subunits coupled with β3 subunits (Lüddens et al., 1994), or slightly efficacious (19%) at α5β3γ2S receptors (Smith et al., 2001). Zolpidem appears to be inactive at α4-(Sanna etal., 2002) and α6-(Hadingham et al., 1996) containing receptors. Studies in which squirrel monkeys are trained to discriminate zolpidem from saline suggest that the GABAA receptor subtypes mediating the discriminative stimulus effects of zolpidem depend on the training dose (Rowlett et al., 1999, 2000, 2003), similar to ethanol (Grant, 1999). Benzodiazepines that are not selective among the α subunit types, such as diazepam, generalize from the discriminative stimulus effects of low (1.0 mg/kg, i.v.), but not intermediate or high (3.0 or 5.6 mg/kg, i.v.), doses of zolpidem (Rowlett et al., 1999, 2000). The discriminative stimulus effects of higher doses of zolpidem, in contrast, may be mediated by selective and high-efficacy positive modulation of GABAA receptors containing α1 subunits (Rowlett et al., 2003).
In general, zolpidem partially generalizes from the discriminative stimulus effects of ethanol. For example, zolpidem partially generalized from 1.0 g/kg ethanol (i.p.) in rats (Bienkowski et al., 1997; Sanger, 1997) and from 1.0 g/kg ethanol (i.v.) in squirrel monkeys (Platt et al., 2005), but did not generalize from 1.5 g/kg ethanol (i.p.) in mice (Shannon et al., 2004) or 1.0 g/kg ethanol (i.p.) in rats (Jankowska et al., 1996). Likewise, only 47% of the human participants in a study by Evans et al. (1990) indicated that the subjective effects of zolpidem resembled benzodiazepines, barbiturates, or ethanol.
The GABAA receptor subtypes by which zolpidem produces ethanol-like discriminative stimulus effects are not entirely clear. According to in vitro studies, zolpidem may generalize from ethanol by acting at GABAA receptors containing α1, α2, α3, or possibly α5 subunits. In vivo studies of squirrel monkeys found that the α1-preferring antagonist β-carboline-t-butyl ester (β-CCt) did not block the generalization of 1 mg/kg zolpidem (i.v.) from 1.0 g/kg ethanol (i.v.) (Platt et al., 2005). These findings suggest that zolpidem generalizes from 1.0 g/kg ethanol not by acting at α1-containing receptors, but at receptors containing α2, α3, or perhaps α5 subunits. The latter may be discounted because α5-preferring inverse agonists (L-655,708, RY-23) did not block the partial generalization of zolpidem from ethanol in squirrel monkeys (Platt et al., 2005).
The imidazobenzodiazepine Ro15-4513 has been called a specific ethanol antagonist because it antagonizes a range of ethanol effects including discriminative stimulus effects of 1.0 and 1.5, but not 2.0 g/kg ethanol (i.g.) in rats (Gatto and Grant, 1997), ethanol self-administration in rats (Petry, 1995; but see Shelton and Grant, 2001; cynomolgus monkeys), ethanol-induced incoordination in mice (Hoffman et al., 1987), sedation in rats (Suzdak et al., 1986), and anticonvulsant effects in mice (Nutt and Lister, 1987). Ro15-4513 has affinity for GABAA receptor subtypes with α4 or α6 subunits that are insensitive to classical benzodiazepines (Hanchar et al., 2006). In addition, Ro15-4513 has approximately equal affinity for GABAA receptors containing α1, α2, or α3 subunits. At α5-containing receptors, however, Ro15-4513 has approximately 10-fold greater affinity compared with α1, α2 or α3-containing receptors (coexpressed with β1γ2 subunits; Hadingham et al., 1993).
The current study assessed antagonism of the generalization of zolpidem from ethanol by Ro15-4513 to gain information about the GABAA receptors mediating the discriminative stimulus effects of ethanol. Zolpidem and Ro15-4513 are useful for this purpose because they vary in selectivity for GABAA receptors depending on the α subunit. Whereas both drugs act at receptors containing α1, α2, α3, and α5 subunits, zolpidem is most potent at α1-containing receptors (Sanna et al., 2002) and Ro15-4513 has the greater affinity for α5-containing receptors (Lüddens et al., 1994). The potency with which zolpidem generalizes from the discriminative stimulus effects of ethanol, and the potency with which Ro15-4513 antagonizes this generalization, may suggest hypotheses about the receptor population at which zolpidem acts to produce ethanol-like discriminative stimulus effects.
MATERIALS AND METHODS
Subjects
Male (n = 9, 4.9 to 7.1 kg) and female (n = 8, 2.9 to 5.1 kg) adult cynomolgus monkeys (Macaca fascicularis) served as subjects. The monkeys were housed in a colony room in 76 × 60 × 70 cm stainless steel cages modified to allow social housing. The colony room was temperature- (23 ± 1°C) and humidity- (40% to 60%) controlled with a 12-hour light dark cycle (lights on at 06:00 am). The monkeys were housed individually for training and feeding (3 to 4 hours), but in groups of 2, 3, or 4 at all other times. The monkeys’ diet included the food obtained in the experimental sessions, nutritionally complete monkey chow (Purina Mills, St Louis, MO) and daily fruit. Water was always available except while the monkeys were being trained or tested. Additional information about these monkeys’ origin, housing, and ethanol discrimination training have been published (Grant et al., 1996, 2000). These studies were conducted to comply with the Wake Forest University Animal Care and Use Committee and the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (NRC 1996).
Apparatus
Training and test sessions occurred in chambers (1.50 × 0.74 × 0.76 m) that were ventilated and sound-attenuating (Med Associates Inc., St Albans, VT). The chambers accommodated a primate chair (1.17 × 0.61 × 0.61 m; Plas Labs, Lansing, MI). A panel (0.48 × 0.69 m) with 2 retractable levers, 3 stimulus lights (amber, green, and red) above each lever, and a central white stimulus light was located within arm’s reach of a monkey sitting in a primate chair, 0.72 m from the bottom of the chamber. The chamber was illuminated by 2 rear house lights. A feeder containing 1-g banana-flavored pellets (P.J. Noyes, Lancaster, NH) was set outside of the chamber. Vinyl tubing connected the feeder to a food tray that was attached to the primate chair in a position accessible to a monkey sitting in the chair. The scheduling of events and data acquisition was accomplished with a PC- or Macintosh-compatible computer connected to an interface (Med Associates Inc.) programmed with LabView software (National Instruments, Austin, TX).
Procedure
Experimental Design
The effects of sex and ethanol training dose were evaluated in a 2 × 2 experimental design with 4 groups of monkeys. Six male (4864, 4865, 4867, 4892, 4889, and 4995) and 4 female (2830, 2852, 2913, and 3123) monkeys were trained to discriminate 1.0 g/kg ethanol (20% w/v, 5 ml/kg) from water. Three male (4890, 4891, and 5496) and 4 female (2835, 5400, 3220, and 5999) monkeys were trained to discriminate 2.0 g/kg ethanol (20% w/v, 10 ml/kg) from water. The monkeys and training conditions for the current study are similar to those reported by Grant et al. (2000) because collection of zolpidem generalization data overlapped temporally.
Discrimination Training
For each session, the monkey was seated in a primate chair and wheeled into the chamber. First, the monkeys were trained to respond on the levers. Next, the response requirement was increased from fixed ratio-1 (FR-1) to a terminal schedule of FR-15 to FR-100. The terminal FR schedule varied across monkeys so that all the pellets were delivered in approximately 5 minutes for each subject as individuals differed in response rate. Males received 20 to 25 pellets and females received 10 to 15 pellets per session to control for the percentage of each monkey’s diet obtained during the session. The monkeys therefore received up to 25 pellets per session. At the start of each session, the house and center stimulus lights illuminated. Upon completion of the response requirement, the center light shut off, a banana pellet was immediately delivered, and then the center light was reilluminated (<1 seconds). A session ended either after all the pellets were delivered or 30 minutes elapsed, whichever occurred first.
After achieving the terminal FR schedule, the monkeys were habituated to nasogastric (i.g.) gavage. For gavage, an infant feeding tube (5 French, 1.7 × 381 mm) was inserted through 1 nostril to pass through the esophagus and into the stomach. For 5 consecutive sessions, the monkeys were administered tap water (5 ml/kg, i.g.) and 30 minutes later the “water-appropriate” lever extended into the chamber. The monkeys were reinforced for responding on this lever according to their terminal FR schedule. For each of the next 5 sessions, the monkeys were administered a training dose of ethanol (1.0 or 2.0 g/kg, i.g.) and 30 minutes later the “ethanol-appropriate” lever extended into the chamber. The monkeys were reinforced for responding on this lever according to their terminal FR schedule. After these 10 sessions, either ethanol or water was administered, and following the 30-minute pretreatment period both levers extended into the chamber. As drug effects are influenced by drug concentration in the brain and whether these concentrations are increasing or decreasing over time, all behavior was measured in approximately 5 minutes, and always after a 30-minute pretreatment. Each monkey was reinforced for responding on the lever appropriate to the substance administered according to that individual’s FR schedule. Responding on the incorrect lever reset the FR for the correct lever to zero.
Ethanol and water discrimination training sessions occurred in a double-alternating pattern (e.g., ethanol, ethanol, water, water). If ≥70% of the responses in the first FR and ≥90% in the entire session were to the substance-appropriate lever, the drug condition was changed for the next session. Discrimination acquisition was considered complete when, for 5 consecutive sessions, ≥70% of the responses in the first FR, and ≥90% in the entire session, were to the substance-appropriate lever.
Generalization and Antagonism Testing
Once the monkeys acquired the discrimination between ethanol and water, test sessions were conducted twice a week. A single test session occurred per day. For test sessions, a single dose of zolpidem (0, 0.017, 0.03, 0.056, 0.1, 0.17, 0.30, 0.56, 1.0, or 1.7 mg/kg, i.m.) was administered, and 30 minutes later the monkeys were allowed to choose between the water- and ethanol-appropriate levers. During test sessions, completion of the FR on either lever resulted in pellet delivery. As in past studies in this lab, in other labs (e.g., Platt et al., 2005) and in comprehensive reviews of drug discrimination (Kelly et al., 2003), complete generalization was defined as ≥80% of responses on the ethanol-appropriate lever after zolpidem was administered. Partial generalization was defined as ≥20% responding on the ethanol-appropriate lever that did not reach complete generalization. Discrimination training sessions occurred between test sessions. If responding during an intervening training session failed to meet the discrimination criteria, the training conditions were continued until the criteria were met for 3 consecutive sessions. In 90% of cases, percentage ethanol-appropriate responding was double-determined such that each zolpidem dose was tested both after an ethanol training session and after a water training session. The range of doses tested was selected individually for each monkey and included a minimal dose that neither altered rates of responding nor generalized from ethanol to a maximal dose that decreased response rates by ≥50% from the preceding training session. For some monkeys, zolpidem did not decrease rates of responding perhaps because zolpidem is only a weak myorelaxant, in contrast to typical benzodiazepines (Depoortere et al., 1986). The order of doses tested began with the middle of the dose range, followed by an equal distribution of higher and lower doses. The maximum dose of zolpidem tested was limited by its solubility in β-cyclodextrin, the volume of i.m. injection tolerated by the monkey, and sedative effects. If a monkey showed signs of pain during an injection (e.g., grimace), or if an injection was followed by the monkey failing to consume a complete meal the following day, higher doses were not tested.
Antagonism of generalization was tested with single doses of Ro15-4513 (0.003 to 0.56 mg/kg, i.m.) administered 5-minute before the session. In 90% of tests, each Ro15-4513 dose was administered once per monkey, with 41% of tests occurring after a training session during which water was administered. In the remaining 10% of cases, each Ro15-4513 dose tested was administered twice per monkey, once after a training session with water, and once after a training session with ethanol. As Ro15-4513 vehicle tests (i.e., 0 mg/kg) did not disrupt performance in the other monkeys, these tests were not conducted for monkeys 3123, 2830, and 5496. The range of Ro 15-4513 doses tested was selected individually for each monkey, and typically included a minimum dose that did not affect response rates or generalize from ethanol and a maximum dose that decreased response rates by ≥50% from the monkey’s average baseline response rate. A mid-range dose was usually tested first, followed by higher and lower doses equally distributed among the dose range.
Drugs
Anhydrous ethanol (1.0 or 2.0 g/kg; Warner-Graham Co., Cockeysville, MD) was diluted to 20% w/v with tap water and was administered in a 5 to 10 ml/kg (i.g.) volume followed by a 5 ml flush of water 30 minutes before each training session. Zolpidem (0.017 to 1.7 mg/kg, i.m.; Sigma, St Louis, MO) was dissolved in β-cyclodextrin (45% w/v; Cerestar, Hammond, IN) in concentrations ≤ 1 mg/ml and injected 30 minutes before each test session. Ro15-4513 (ethyl 8-azido-6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a]-[1,4]benzodiazepine-3-carboxylate, 0.003 to 0.30 mg/kg, i.m.) was also dissolved in β-cyclodextrin (45%) at a concentration of ≤1 mg/ml and injected 5 minutes before the start of the session. Zolpidem and Ro15-4513 were administered in multiple injection volumes of 1 ml/kg. Large volumes required multiple injections, limiting the maximum dose tested due to tolerability issues. Equivalent volumes were administered during vehicle tests which included matched injection sites. All drugs were prepared fresh daily.
Data Analysis
The percentage of total responding on the ethanol-appropriate lever (total responses on the ethanol-appropriate lever/total responses) and response rate (total responses/session time) were calculated for each monkey and test session. The data were included in percentage ethanol-appropriate responding if the monkey completed at least 1 FR requirement in the session. For double-determinations, the average percentage ethanol-appropriate post ethanol training and post water training was calculated. Baseline response rate was calculated by averaging the response rates from all the ethanol and water training sessions immediately before each test session, and therefore reflected behavior during all sessions preceding each test session. The ED50 dose at which ethanol-appropriate responding was 50% or response rate decreased by 50% from baseline was computed via linear interpolation between the 2 doses that encompassed the 50% effect.
When the ED50 for zolpidem generalization with Ro15-4513 was greater than the ED50 without Ro15-4513, a dose ratio was computed: ED50 with Ro15-4513/ED50 without Ro15-4513. For all monkeys tested, the potency of Ro15-4513 to increase the ED50 twofold was quantified with apparent pKB values: -log[B/(dose ratio - 1)], where B is the dose of Ro15-4513 in mol/kg. Apparent pKB values provided a quantitative measure of shifts in the zolpidem dose-response function after Ro15-4513 administration (e.g., Rowlett and Woolverton, 1996). For 4 monkeys, ≥3 shifts in the zolpidem dose-response function were obtained, permitting a more accurate calculation of Ro15-4513 potency with apparent pA2 values. To calculate apparent pA2, for each monkey, nonlinear regression was conducted with GraphPad Prism software (GraphPad Prism Software, Inc., San Diego, CA) with log (dose ratio - 1) as the dependent variable and the antagonist dose [-log(mol/kg)] as the independent variable (i.e., Schild plots). When the slope of the Schild plot is -1, the antagonist dose at which log (dose ratio - 1) is 0 indicates apparent pA2 (Arunlakshana and Schild, 1959). For each monkey, regression slopes for which the 95% confidence intervals included -1 and excluded 0 were constrained to -1 (Paronis and Bergman, 1999; Tallarida et al., 1979) and the antagonist potency was referred to as pKB (Jenkinson et al., 1995). To estimate antagonist potency, mol/kg was calculated from apparent pKB, which is expressed in units of -log(mol/kg).
Percentage ethanol-appropriate responding and percentage base-line response rate were analyzed with 2 (sex) × 2 (training dose) ANOVA that also included zolpidem dose and Ro15-4513 dose as between-subjects factors, when appropriate. Drug dose could not be included as a within-subjects factor because the same doses were not always tested in each monkey. The pKB values were analyzed with a 2 (sex) × 2 (training dose) ANOVA. For all tests, α was 0.05.
RESULTS
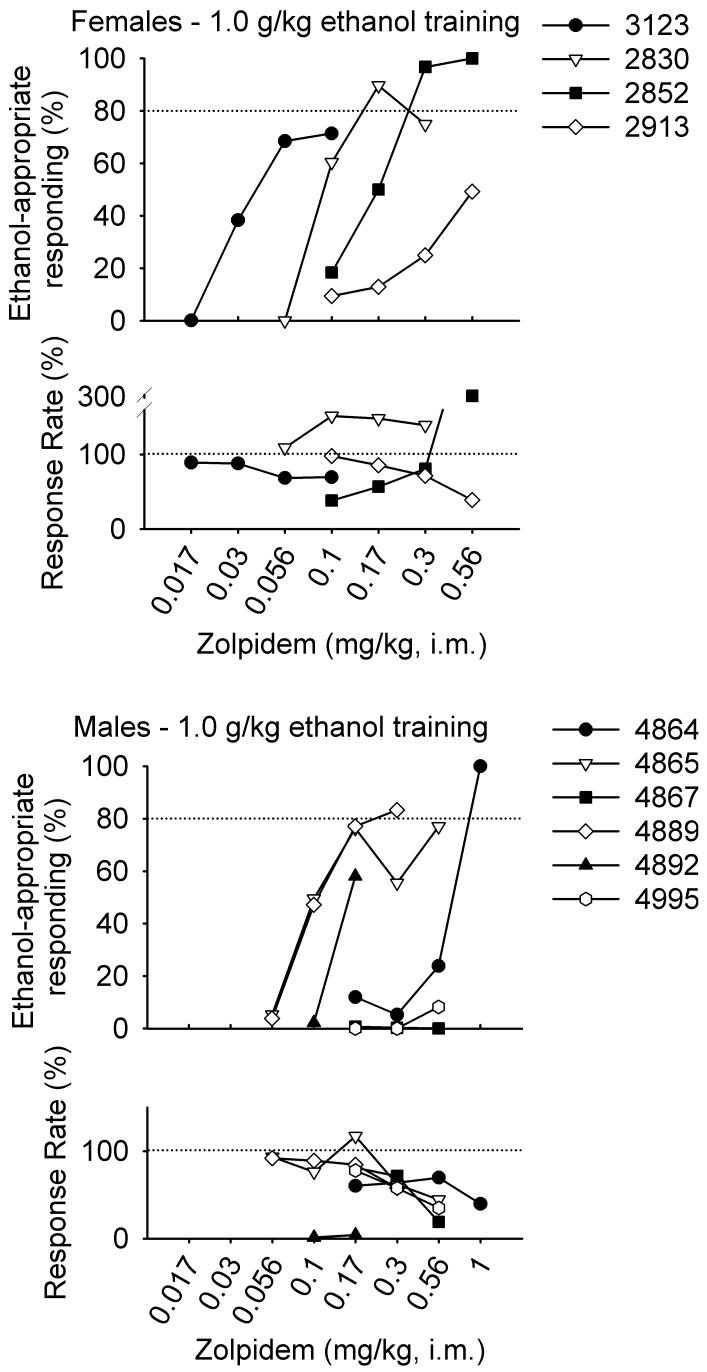
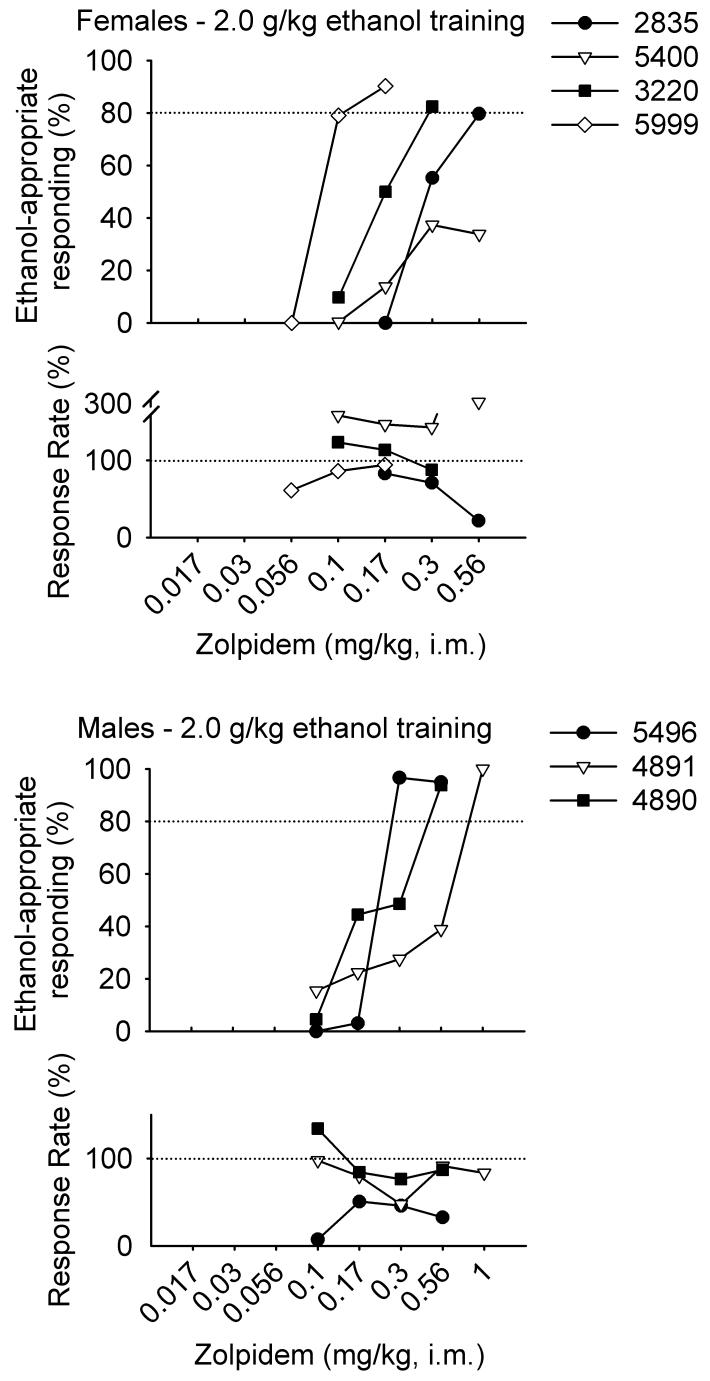
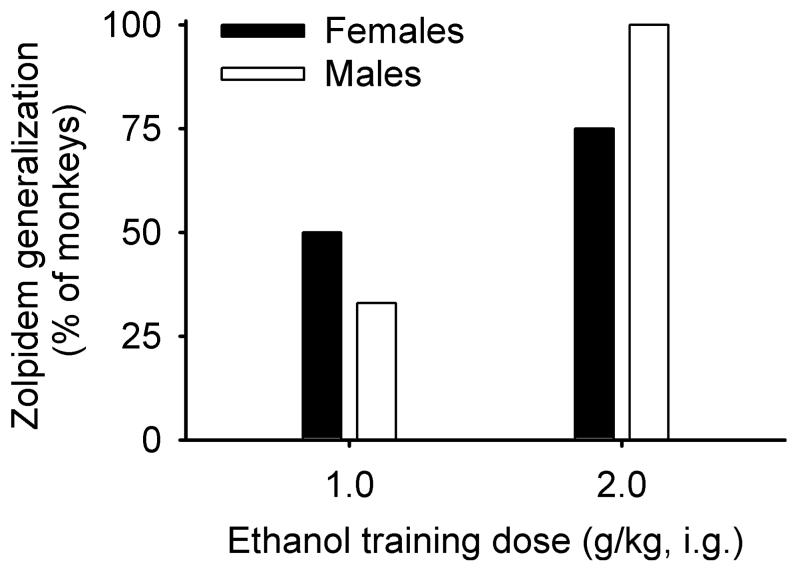
Figures 1 and 2 show the percentage of ethanol-appropriate responding following each dose of zolpidem for individual female and male monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively. A 2 (sex) × 2 (training dose) × 12 (zolpidem dose) ANOVA revealed only a main effect of zolpidem dose, F(11, 45) = 2.63, p = 0.01. Ethanol-appropriate responding increased with zolpidem dose. Zolpidem completely generalized from 1.0 g/kg in 2/4 female monkeys and from 2.0 g/kg ethanol in 3/4 female monkeys. Zolpidem completely generalized from 1.0 g/kg ethanol 2/6 male monkeys and from 2.0 g/kg ethanol in 3/3 male monkeys. The only group showing complete generalization of zolpidem from ethanol in every subject was the males trained to discriminate 2.0 g/kg ethanol (Fig. 3).
Fig. 1.
Percentage of total session responding on the ethanol-appropriate lever and percentage of training session response rate 30 minutes after administration of zolpidem (i.m.) in female and male monkeys trained to discriminate 1.0 g/kg ethanol (i.g.) from water.
Fig. 2.
Percentage of total session responding on the ethanol-appropriate lever and percentage of training session response rate 30 minutes after administration of zolpidem (i.m.) in female and male monkeys trained to discriminate 2.0 g/kg ethanol (i.g.) from water.
Fig. 3.
Percentage of monkeys in which zolpidem completely generalized from ethanol.
The ED50 values for zolpidem generalization from ethanol varied widely, with a two- to sevenfold difference in ED50 values even within the same sex and training dose group. The limited number of subjects showing full generalization prevented a statistical comparison of ED50 values between the groups, but the ED50 values were on average lower for female compared with male monkeys (Table 1).
Table 1.
Individual and Mean (±SEM) ED50 (mg/kg, i.m.) Values for Zolpidem Generalization From Ethanol
| 1.0 g/kg Ethanol training | 2.0 g/kg Ethanol training | ||
|---|---|---|---|
| Females | |||
| 3123a | 0.04 | 2835 | 0.27 |
| 2830 | 0.09 | 5400 | NC (0.66) |
| 2852 | 0.17 (0.13) | 3220 | 0.17 |
| 2913 | NC | 5999 | 0.08 (0.14) |
| Mean | 0.10 ± 0.04 | Mean | 0.17 ± 0.05 |
| Males | |||
| 4864 | 0.71 | 5496 | 0.26 |
| 4865a | 0.10 (0.08) | 4891 | 0.64 |
| 4867 | NC | 4890 | 0.31 (0.09) |
| 4889 | 0.11 | ||
| 4892a | 0.16 | ||
| 4995 | NC | ||
| Mean | 0.27 ± 0.15 | Mean | 0.40 ± 0.12 |
Values from sessions with vehicle (i.m.) pretreatment are shown in parentheses but not included in calculation of the mean. NC, not calculable because maximum ethanol-appropriate responding <50%.
Maximum ethanol-appropriate responding ≤79% (i.e., partial generalization).
For monkeys trained to discriminate 1.0 and 2.0 g/kg ethanol, respectively, mean (±SEM) response rates during non-test sessions preceding the generalization tests were 3.17 ± 0.19 and 1.30 ± 0.17 responses/s for females, and 1.75 ± 0.12 and 1.57 ± 0.14 responses/s for males. A 2 (sex) × 2 (training dose) × 12 (zolpidem dose) ANOVA revealed only a main effect of sex, F(1, 45) = 4.88, p = 0.03. Rates of responding were greater for females compared with males. Zolpidem did not significantly decrease response rates from baseline, consistent with the low potency of zolpidem to produce myorelaxation (Depoortere et al., 1986). Nonetheless, Figures 1 and 2 show decreased response rates for some monkeys. Zolpidem was tested up to doses that decreased response rate to 50% of baseline in 2/4 female and 5/6 male monkeys trained to discriminate 1.0 g/kg ethanol. The response rate of monkey 4889 decreased to a maximum of 58% of baseline (Fig. 1). For monkeys trained to discriminate 2.0 g/kg ethanol, zolpidem decreased rates of responding in 1/4 female and 2/3 male monkeys. Overall, the effects of zolpidem on response rate were not particularly systematic.
Pretreatment with Ro15-4513 shifted the zolpidem dose-response functions for ethanol-appropriate responding rightward in all 8 monkeys tested (Fig. 4). For each shift in the dose-response curve, 1 apparent pKB value was calculated. The data from monkey 3123, for example, provides 2 pKB values. Table 2 lists individual monkeys’ mean pKB for antagonism of zolpidem generalization from ethanol by Ro15-4513. Shifts in the dose-response curves were generally parallel (Fig. 4). For all 4 monkeys showing 3 or more shifts in the zolpidem dose-response curve, Schild analyses were conducted. In 3/4 cases, the confidence interval of the slope included both -1 and 0, so apparent pA2 was not calculated [slope (95% confidence interval): 2852, -0.98 (-3.68, 1.72); 5999, -0.63 (-1.20, -0.06); 4890, -0.57 (-4.59, 3.45); 5496, -0.59 (-5.34, 4.17)]. For 5999, pA2 [6.82 (6.66, 7.07)] closely corresponded to the pKB values calculated from individual antagonist doses. A 2 (sex) × 2 (training dose) ANOVA revealed main effects of sex, F(1, 15) = 4.79, p = 0.045, and training dose, F(1, 15) = 7.28, p = 0.017, on apparent pKB values. There was no interaction between sex and training dose, F(1, 15) = 0.05, but assessment of the interaction was underpowered (observed power = 0.06) as only 2 values from a single monkey contributed to the mean pKB for the 1.0 g/kg male group. Males (mean ± SEM pKB, 7.29 ± 0.14) were slightly, but significantly, more sensitive than females (pKB, 7.02 ± 0.81) to Ro15-4513 antagonism of zolpidem generalization from ethanol. The variability in potency among female monkeys (range pKB: 6.34 to 7.89) was slightly greater than among males (range pKB: 6.73 to 7.96), with substantial overlap among males and females. Monkeys trained to discriminate 1.0 g/kg ethanol (pKB, 7.36 ± 0.18) were significantly more sensitive to Ro15-4513 antagonism compared with monkeys trained to discriminate 2.0 g/kg ethanol (pKB, 7.00 ± 0.10).
Fig. 4.
Ro15-4513 shifts the dose-response curve for zolpidem (i.m.) generalization from ethanol in female and male monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) from water. Ro15-4513 was administered (mg/kg, i.m., symbols) 5 minutes before the session.
Table 2.
Individual and Mean Apparent pKB (95% confidence interval) for Antagonism of Zolpidem Generalization From 1.0 and 2.0 g/kg Ethanol by Ro15-4513 in Cynomolgus Monkeys
| pKB |
Mean pKB |
|
|---|---|---|
| 1.0 g/kg Ethanol training | ||
| Females | ||
| 3123 | 7.89, 7.42 | 7.66 (7.20, 8.11) |
| 2830 | 7.76 | |
| 2852 | 6.76, 6.89, 6.74 | 6.80 (6.70, 6.89) |
| Mean | 7.24 (6.83, 7.66) | |
| Males | ||
| 4865 | 7.96, 7.47 | 7.72 (7.23, 8.19) |
| 2.0 g/kg Ethanol training | ||
| Females | ||
| 5400 | 6.34 | |
| 5999 | 7.05, 6.82, 6.82, 6.75 | 6.86 (6.73, 6.99) |
| Mean | 6.76 (6.53, 6.98) | |
| Males | ||
| 5496 | 7.16, 6.73, 6.90 | 6.93 (6.68, 7.18) |
| 4890 | 7.40, 7.61, 7.06 | 7.36 (7.04, 7.67) |
| Mean | 7.14 (6.89, 7.40) | |
Percentage of baseline response rate was not affected by Ro15-4513 dose, F(4, 29) = 2.13, p = 0.10. There were no interactions involving Ro15-4513 on percentage of baseline response rate.
DISCUSSION
The results showed that the discriminative stimulus effects of ethanol include zolpidem-sensitive GABAA receptors, particularly in monkeys trained to discriminate 2.0 g/kg ethanol. The ethanol-like effects of zolpidem were antagonized by Ro15-4513 with individuals’ potency in the 11 to 457 nM range. The receptor population that is common to Ro15-4513 and zolpidem activity should therefore contribute to the discriminative stimulus effects of ethanol. Zolpidem competes with [11C]Ro15-4513 binding with differential affinity and efficacy across brain areas. The greatest displacement of Ro15-4513 by zolpidem (10 mg/kg, i.v.) occurs in the rhesus monkey cerebellum and occipital cortex, with full displacement occurring at low-affinity (Kd, 50 nM) sites (Maeda et al., 2003). We estimated that 11 to 457 nM Ro15-4513 antagonizes the ethanol-like effects of zolpidem, consistent with in vitro binding data at low-affinity sites. High-affinity (Kd, 3.8 nM) binding sites exist for Ro15-4513 in the frontal and temporal cortices and limbic system, but zolpidem only displaces 20% to 40% of [11C]Ro15-4513 at these sites (Maeda et al., 2003). As noted by Maeda et al. (2003), the approximately 14-fold difference in affinity between high- and low-affinity binding sites resembles the 10- to 20-fold greater affinity of Ro15-4513 for GABAA receptors containing α5 compared with α1, α2, or α3 subunits (Hadingham et al., 1993). The ethanol-like effects of zolpidem that were antagonized by Ro15-4513 in the current study may therefore be mediated by low-affinity cerebellar and cortical GABAA receptors primarily composed of α1, α2, or α3 subunits. This suggestion will remain speculative until studies are conducted using subtype-selective, rather than subunit-preferring, drugs.
In the current study, the potency of Ro15-4513 to antagonize zolpidem generalization from ethanol varied widely across individuals (11 to 457 nM; pKB, 7.96 to 6.34). In the 4 monkeys with ≥3 rightward shifts of the dose-response function, the slopes of the Schild plots were not significantly different from -1, but 3/4 confidence intervals included 0, precluding calculation of pA2 (Paronis and Bergman, 1999). For these 3 monkeys, Schild analyses suggested that antagonism of zolpidem generalization by Ro15-4513 is not a competitive interaction and could involve multiple receptor subtypes. In contrast, for monkey 5999, apparent pA2 suggests that Ro15-4513 competitively antagonized zolpidem binding at receptors mediating ethanol-like discriminative stimulus effects. As the apparent pA2 value calculated from 5999 was very similar to the mean pKB calculated from individual antagonist doses (Table 2), all the data are consistent with competitive antagonism of zolpidem generalization from ethanol by Ro15-4513.
The affinity estimates in the current study resemble the potency of Ro15-4513 to antagonize the discriminative stimulus effects of benzodiazepines. For example, Ro15-4513 antagonized the generalization of triazolam in rhesus monkeys trained to discriminate 0.56 mg/kg midazolam with a pA2 range of 7.53 to 6.88 (Lelas et al., 2000), suggesting that zolpidem may generalize from the discriminative stimulus effects of ethanol by acting at benzodiazepine-sensitive GABAA receptors. Together, the data using Ro15-4513 to antagonize either triazolam generalization from midazolam or zolpidem generalization from ethanol further strengthen the hypothesis that the discriminative stimulus effects of ethanol in primates are mediated by benzodiazepine-sensitive GABAA receptors.
The current study, however, provides less definitive data regarding the subunit composition of GABAA receptors mediating the ethanol-like discriminative stimulus effects of zolpidem. For example, the α1-preferring antagonist, β-CCt, does not block the generalization of 1 mg/kg zolpidem (i.v.) from 1.0 g/kg ethanol (i.v.) (Platt et al., 2005). The selectivity of β-CCt for α1-containing GABAA receptors is >10-fold greater than α2- or α3-containing GABAA receptors, and >100-fold more selective than α5 (Cox et al., 1995). These data indicate that zolpidem produces an ethanol-like discriminative stimulus effect independent from, or in addition to, α1 subunits. Indeed, confidence intervals for the slopes of the Schild analyses deviated from unity in 3/4 cases, implying that a complex mechanism possibly involving multiple receptor subtypes mediates the ethanol-like effects of zolpidem.
The current data show that antagonism of the ethanol-like discriminative stimulus effects of zolpidem by Ro15-4513 was slightly more potent in monkeys trained to discriminate 1.0 g/kg compared with 2.0 g/kg ethanol. Thus, compared with 2.0 g/kg ethanol, the receptor subtypes mediating the effects of zolpidem that generalize from 1.0 g/kg ethanol may include more Ro15-4513-sensitive receptors. The GABAA receptor subtypes mediating the discriminative stimulus effects of high (3.0 or 5.6 mg/kg, i.v.) doses of zolpidem appear to have greater specificity (Rowlett et al., 2003) compared with the receptors mediating low doses of zolpidem (1.0 mg/kg, i.v.) (Rowlett et al., 1999, 2000). That is, nonselective benzodiazepines completely generalized in monkeys trained to discriminate low but not high doses of zolpidem, from which α1-preferring agonists completely generalized. Of course, the zolpidem-sensitive GABAA receptors that mediate the effects of ethanol may not include all receptor subtypes that mediate the discriminative stimulus effects of zolpidem itself. It is possible, however, that the dose-dependency for the receptor population mediating the discriminative stimulus effects of zolpidem also occurs with respect to the subset of zolpidem-sensitive receptors that mediate ethanol-like effects. Thus, the greater potency of Ro15-4513 to antagonize zolpidem generalization from 1.0 g/kg compared with 2.0 g/kg ethanol in the current study may indicate a contribution of receptor subtypes other than α1 to the discriminative stimulus effects of 1.0 g/kg ethanol. In contrast, the receptors mediating the discriminative stimulus effects of zolpidem that are similar to 2.0 g/kg ethanol may involve subtypes with lower affinity for Ro15-4513, including α1-containing GABAA receptors. What remains unclear, however, is what other GABAA receptor subtypes mediate the generalization of zolpidem from 1.0 g/kg ethanol. Additional studies must be conducted to identify the role of α2, α3, and α5 subunits in the discriminative stimulus effects of ethanol. The role of α2 subunits is of particular interest as alleles of the α2 subunit in humans have been shown to moderate the subjective effects of alcohol (Pierucci-Lagha et al., 2005) and drinking behavior (Bauer et al., 2007; Covault et al., 2008).
On average, antagonism of the ethanol-like effects of zolpidem by Ro15-4513 was slightly more potent in male compared with female monkeys. Some females (3123 and 2830) were equally sensitive to Ro15-4513 antagonism of zolpidem generalization from ethanol compared with males. The monkeys used in the current study were the subjects for previous discrimination studies (Grant et al., 2000; Vivian et al., 2002). For each of the monkeys in the current study included in these past studies, Table 3 lists the drugs that generalize from ethanol by sex and training dose. Some monkeys included in the current study are excluded from Table 3 because they were not included in past studies. As shown in Table 3, there are more cases of complete generalization of GABAA positive modulators from ethanol in females compared with males, indicating less specificity in the receptor population that mediates the discriminative stimulus effects of ethanol in females. Ovarian-derived steroids and ethanol appear to have additive discriminative stimulus effects (Grant et al., 1997; Green et al., 1999). Training monkeys to discriminate ethanol across all menstrual cycle phases may train them to respond on the ethanol-appropriate lever when a range of GABAA receptor subtypes are stimulated. Individual differences in ovarian steroids could therefore contribute to variability in the receptor subtypes mediating the discriminative stimulus effects of ethanol in females. Such an explanation could account for the wider range of potencies for Ro15-4513 antagonism of zolpidem generalization from ethanol in females compared with males.
Table 3.
Test Drugs Generalizing From Ethanol in Cynomolgus Monkeys From Published Reports and the Current Data Set (Zolpidem)
| Training Dose | 1.0 g/kg Ethanol (i.g.) |
2.0 g/kg Ethanol (i.g.) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Female |
Male |
Female |
Male |
|||||||||||
| Monkey | 3123 | 2830 | 2852 | 2913 | 4864 | 4995 | 4865 | 4867 | 5400 | 2835 | 3220 | 5999 | 4890 | 4891 | 5496 |
| Muscimol (i.m.)a | □ |
|
□ |
|
□ | NC | □ | □ | NC | □ | □ | NC |
|
□ | NC |
| Zolpidem (i.m.) |
|
■ | ■ |
|
■ | □ |
|
□ |
|
■ | ■ | ■ | ■ | ■ | ■ |
| Midazolam (i.g.)a | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
|
|
■ |
|
■ | ■ |
|
|
| Pentobarbital (i.g.)a | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
|
■ |
| Dizocilpine (i.m.)b | ■ | ■ | ■ | ■ | ■ |
|
□ | ■ |
|
■ | ■ | ■ | □ | □ | □ |
| Phencyclidine (i.m.)b | □ | ■ | ■ | ■ | ■ |
|
■ | ■ |
|
■ | ■ |
|
□ |
|
|
| Ketamine (i.m.)b |
|
■ | ■ |
|
■ |
|
|
|
■ |
|
■ | ■ | □ | □ |
|
■, complete generalization (≥80% ethanol-appropriate responding for the entire session);  partial generalization (≥20 and ≤79% ethanol-appropriate responding for the entire session); □, no generalization (<20% ethanol-appropriate responding for the entire session); NC, data not collected.
partial generalization (≥20 and ≤79% ethanol-appropriate responding for the entire session); □, no generalization (<20% ethanol-appropriate responding for the entire session); NC, data not collected.
Table 3 also shows that zolpidem is the only drug tested that completely generalized from 2.0 g/kg ethanol in all the male monkeys. These same monkeys were noticeably insensitive to N-methyl-d-aspartate receptor-mediated discriminative stimulus effects (Vivian et al., 2002). An acute dose of 1.0 g/kg ethanol contains approximately the same amount of ethanol as 4 standard drinks. As ethanol training sessions were conducted 1 to 2 times/wk, the 1.0 and 2.0 g/kg ethanol training conditions approximate 4 to 8 and 8 to 16 drink-equivalents per week, respectively. The current data suggest that pharmacotherapeutic development for heavier drinking males could target GABAA receptors that are especially sensitive to zolpidem, such as α1-containing receptors.
Finally, individual differences in the GABAA receptor subtypes used to discriminate ethanol may also correlate with individual differences in the propensity to develop alcoholism (Holtzman, 1990) and suggest specific pharmacotherapeutic targets for alcoholism treatment. There were large individual differences in the potency of zolpidem to produce ethanol-appropriate responding (Figs 1 and 2), with a 2- to 17-fold range of ED50 values across individuals (Table 1). Factors that influence the expression of benzodiazepine-sensitive GABAA receptors, including sex (Wilson, 1992) and age (Ruano et al., 2000), may provide information about the receptor subtypes that mediate the discriminative stimulus effects of ethanol in specific populations and inform novel pharmacotherapies.
REFERENCES
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Iwinska K, Stefanski R, Kostowski W. Discriminative stimulus properties of ethanol in the rat: differential effects of selective and nonselective benzodiazepine receptor agonists. Pharmacol Biochem Behav. 1997;58:969–973. doi: 10.1016/s0091-3057(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination: behavioral, pharmacological, and molecular mechanisms of discriminative drug effects. In: Goldman SR, Stolerman IP, editors. Behavior Analysis of Drug Dependence. Academic Press; New York: 1986. pp. 161–193. [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ED, Hagen TJ, McKernan RM, Cook JM. BZ1 receptor specific ligands. Synthesis and biological properties of BCCt, a BZ1 receptor subtype specific antagonist. Med Chem Res. 1995;5:710–718. [Google Scholar]
- Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, Bartholini G. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. J Pharmacol Exp Ther. 1986;237:649–658. [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–1255. [PubMed] [Google Scholar]
- Gatto GJ, Grant KA. Attenuation of the discriminative stimulus effects of ethanol by the benzodiazepine partial inverse agonist Ro 15-4513. Behav Pharmacol. 1997;8:139–146. [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology. 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA. The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav. 1999;64:379–383. doi: 10.1016/s0091-3057(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji Dalip JS, Whiting PJ. Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α6 subunit and characterization of the pharmacology of recombinant α6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkin P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6βδ GABAA receptors. Proc Natl Acad Sci. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B, Szabo G, Suzdak PD, Paul SM. Effect of an imidazobenzodiazepine, Ro15-4513, on the incoordination and hypothermia produced by ethanol and pentobarbital. Life Sci. 1987;41:611–619. doi: 10.1016/0024-3205(87)90415-2. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of drugs: relationship to potential for abuse. In: Adler MW, Cowan A, editors. Modern Methods in Pharmacology. Vol. 6. Wiley-Liss; New York: 1990. pp. 193–210. [Google Scholar]
- Jankowska E, Iwiniska K, Stefanski R, Biekowski P, Kostowski W. The effects of zolpidem on motor activity, ethanol drinking and ethanol discriminative stimulus in rats. Behav Pharmacol. 1996;7:54. [Google Scholar]
- Jenkinson DH, Barnard EA, Hoyer D, Humphrey PPA, Leff P, Shankley NP. International union of pharmacology committee on receptor nomenclature and drug classification. IX. Recommendations on terms and symbols in quantitative pharmacology. Pharmacol Rev. 1995;47:255–266. [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cog Neurosci Rev. 2003;2:227–260. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. Antagonism of the discriminative stimulus effects of positive γ-aminobutyric acidA modulators in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther. 2000;294:902–908. [PubMed] [Google Scholar]
- Lüddens H, Seeburg PH, Korpi ER. Impact of β and γ variants on ligand-binding properties of γ-aminobutyric acid type A receptors. Mol Pharmacol. 1994;45:810–814. [PubMed] [Google Scholar]
- Maeda J, Suhara T, Kawabe K, Okauchi T, Obayashi S, Hojo J, Suzuki K. Visualization of α5 subunit of GABAA/benzodiazepine receptor by [11C]Ro15-4513 using positron emission tomography. Synapse. 2003;47:200–208. doi: 10.1002/syn.10169. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lister RG. The effect of the imidazodiazepine Ro 15-4513 on the anticonvulsant effects of diazepam, sodium pentobarbital and ethanol. Brain Res. 1987;413:193–196. doi: 10.1016/0006-8993(87)90170-3. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther. 1999;290:1222–1229. [PubMed] [Google Scholar]
- Petry NM. Ro 15-4513 selectively attenuates ethanol but not sucrose reinforced responding in a concurrent access procedure: comparison to other drugs. Psychopharmacology. 1995;121:192–203. doi: 10.1007/BF02245630. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Platt DM, Duggan A, Spealman RD, Cook JM, Li X, Yin W, Rowlett JK. Contribution of the α1GABAA and α5GABAA receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys. J Pharmacol Exp Ther. 2005;313:658–667. doi: 10.1124/jpet.104.080275. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Spealman RD. Transduction of the discriminative stimulus effects of zolpidem by GABAA/α1 receptors. Eur J Pharmacol. 2000;406:R9–R10. doi: 10.1016/s0014-2999(00)00669-5. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD, Lelas S. Discriminative stimulus effects of zolpidem in squirrel monkeys: comparison with conventional benzodiazepines and sedative-hypnotics. J Pharmacol Exp Ther. 1999;291:1233–1241. [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD, Lelas S, Cook JM, Yin W. Discriminative stimulus effects of zolpidem in squirrel monkeys: role of GABAA/α1 receptors. Psychopharmacology. 2003;165:209–215. doi: 10.1007/s00213-002-1275-z. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies. Psychopharmacology. 1996;128:1–16. doi: 10.1007/s002130050103. [DOI] [PubMed] [Google Scholar]
- Ruano D, Araujo F, Revilla E, Vela J, Bergis O, Vitorica J. GABAA and α-amino-3-hydroxy-5-methylsoxazole-4-propionate receptors are differentially affected by aging in the rat hippocampus. J Biol Chem. 2000;275:19585–19593. doi: 10.1074/jbc.M000700200. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. The effects of new hypnotic drugs in rats trained to discriminate ethanol. Behav Pharmacol. 1997;8:287–292. doi: 10.1097/00008877-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABAA receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Shelton KL, Vivian JA, Yount I, Morgan AR, Homanics GE, Grant KA. Discriminative stimulus effects of ethanol in mice lacking the γ-aminobutyric acid type A receptor δ subunit. Alcohol Clin Exp Res. 2004;28:906–913. doi: 10.1097/01.alc.0000128227.28794.42. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Effects of naltrexone and Ro 15-4513 on a multiple schedule of ethanol and tang self-administration. Alcohol Clin Exp Res. 2001;25:1576–1585. [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-amino-butyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR. Effect of α subunit on allosteric modulation of ion channel function in stably expressed human recombinant γ-aminobutyric acidA receptors determined using 36Cl ion flux. Mol Pharmacol. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg S. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Tallarida FJ, Cowan A, Adler MW. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979;25:637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology. 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–172. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]