Abstract
Exogenous gangliosides affect the angiogenic activity of fibroblast growth factor-2 (FGF-2), but their mechanism of action has not been elucidated. Here, a possible direct interaction of sialo-glycolipids with FGF-2 has been investigated. Size exclusion chromatography demonstrates that native, but not heat-denatured, 125I-FGF-2 binds to micelles formed by gangliosides GT1b, GD1b, or GM1. Also, gangliosides protect native FGF-2 from trypsin digestion at micromolar concentrations, the order of relative potency being GT1b > GD1b > GM1 = GM2 = sulfatide > GM3 = galactosyl-ceramide, whereas asialo-GM1, neuraminic acid, and N-acetylneuramin-lactose were ineffective. Scatchard plot analysis of the binding data of fluorochrome-labeled GM1 to immobilized FGF-2 indicates that FGF–2/GM1 interaction occurs with a Kd equal to 6 μM. This interaction is inhibited by the sialic acid-binding peptide mastoparan and by the synthetic fragments FGF-2(112–129) and, to a lesser extent, FGF-2(130–155), whereas peptides FGF-2(10–33), FGF-2(39–59), FGF-2(86–96), and the basic peptide HIV-1 Tat(41–60) were ineffective. These data identify the COOH terminus of FGF-2 as a putative ganglioside-binding region. Exogenous gangliosides inhibit the binding of 125I-FGF-2 to high-affinity tyrosine-kinase FGF-receptors (FGFRs) of endothelial GM 7373 cells at micromolar concentrations. The order of relative potency was GT1b > GD1b > GM1 > sulfatide a = sialo-GM1. Accordingly, GT1b,GD1b, GM1, and GM2, but not GM3 and asialo-GM1, prevent the binding of 125I-FGF-2 to a soluble, recombinant form of extracellular FGFR-1. Conversely, the soluble receptor and free heparin inhibit the interaction of fluorochrome-labeled GM1 to immobilized FGF-2. In agreement with their FGFR antagonist activity, free gangliosides inhibit the mitogenic activity exerted by FGF-2 on endothelial cells in the same range of concentrations. Also in this case, GT1b was the most effective among the gangliosides tested while asialo-GM1, neuraminic acid, N-acetylneuramin-lactose, galactosyl-ceramide, and sulfatide were ineffective. In conclusion, the data demonstrate the capacity of exogenous gangliosides to interact with FGF-2. This interaction involves the COOH terminus of the FGF-2 molecule and depends on the structure of the oligosaccharide chain and on the presence of sialic acid residue(s) in the ganglioside molecule. Exogenous gangliosides act as FGF-2 antagonists when added to endothelial cell cultures. Since gangliosides are extensively shed by tumor cells and reach elevated levels in the serum of tumor-bearing patients, our data suggest that exogenous gangliosides may affect endothelial cell function by a direct interaction with FGF-2, thus modulating tumor neovascularization.
INTRODUCTION
Gangliosides are neuraminic acid (NeuAc)1-containing glycosphingolipids. Under physiological conditions, gangliosides are mainly associated to the cell membranes where they play different roles in controlling cell growth, cell adhesion, and cell–cell interaction (Hakomori, 1990; Zeller and Marchase, 1992). Gangliosides shed in the microenvironment during tumor growth and metastasis (Merritt et al., 1994; Chang et al., 1997) possibly as a consequence of their aberrant overproduction by tumor cells induced by various cytokines. Indeed, IL-1 (Kjaer et al., 1992), interferon-γ (IFN-γ), IL-2, IL-4 (Hoons et al., 1991; Ando et al., 1996), tumor necrosis factor-α (Furukawa et al., 1990), PDGF (Pilkington et al., 1993), fibroblast growth factor-2 (FGF-2), and EGF (Drago et al., 1989) affect the synthesis and surface expression of different gangliosides. Conversely, both free and cell-associated gangliosides can modulate the expression of cytokines. For instance, gangliosides inhibit the production of IL-1 β, tumor necrosis factor-α, and IL-6 (Ziegler-Heitbrock et al., 1992; Dumontet et al., 1994) while GD3 stimulates the production of vascular endothelial growth factor in human glioma cells (Koochekpour et al., 1996).
Gangliosides modulate the biological activity of growth factors and cytokines. Exogenous GM1, GM3, and GT1b inhibit neurite outgrowth induced by PDGF, insulin, nerve growth factor, and insulin-like growth factor-1 (Hynds et al., 1997). They also inhibit neuroblastoma cell proliferation induced by PDGF (Hynds et al., 1995; Zhang et al., 1995). GM1 and GM3 affect EGF- and PDGF-dependent fibroblast proliferation (Bremer et al., 1986). Moreover, gangliosides modulate IL-2- and IL-3-dependent proliferation of different cell types of the immune system (Sharom et al., 1991; Nakamura et al., 1996). Also, GM2 and GT1 are able to modulate the antiviral activity of human IFN (Besancon and Ankel, 1974; Vengris et al., 1976). Finally, glucosylceramide synthesis has been demonstrated to be required for FGF-2 to stimulate axonal growth (Boldin and Futerman, 1997). Accordingly, gangliosides influence FGF-2–dependent mitogenesis and migration of glial cells (Meuillet et al., 1996a,b), and GM3 inhibits the proliferation of fibroblasts exposed to FGF (Bremer and Hakomori,1982).
The mechanisms by which gangliosides modulate the biological activity of growth factors and cytokines are not fully elucidated. Experimental evidence indicates that exogenous gangliosides are incorporated into the plasma membrane and may affect the activity of tyrosine kinase receptors and intracellular signaling. For instance, membrane-incorporated GM3 inhibits ligand-induced autophosphorylation of EGF receptor. This occurs in the absence of a direct interaction of the ganglioside with the growth factor or modifications of the binding of EGF to its receptor (Bremer et al., 1986; Hanai et al., 1988a,b; Weis and Davis, 1990; Song et al., 1991). Gangliosides inhibit ligand-induced dimerization and autophosphorylation of PDGF receptor (Nojiri et al., 1991; Van Brocklyn et al., 1993; Hynds et al., 1995) and prevent the activation of down-stream second messengers (Saqr et al., 1995; Sachinidis et al., 1996). On the other hand, the incorporation of GM1 and GM3 into the cell membrane of 3T3 fibroblasts increases the affinity of PDGF binding in the absence of a direct interaction with PDGF (Bremer et al., 1984), whereas exogenous GM1 and GM2 inhibit PDGF binding to its receptors, suggesting an interaction of free gangliosides with the growth factor and/or the receptor (Sachinidis et al., 1996). Indeed, exogenous gangliosides have been shown to bind directly to IFN (Besancon and Ankel, 1974), IL-2 (Chu and Sharom, 1990), IL-4 (Chu and Sharom, 1995), and to the nerve growth factor receptor Trk (Mutoh et al., 1995). In conclusion, gangliosides play an important role in regulating the biological activity of growth factors and cytokines by different mechanisms of action. In turn, growth factors regulate the ganglioside composition of the plasma membranes and of the extracellular environment.
Angiogenesis is the process of generating new capillary blood vessels. Uncontrolled endothelial cell proliferation is observed in tumor neovascularization. Several growth factors and cytokines have been shown to stimulate endothelial cell proliferation in vitro and in vivo, and FGF-2 was one of the first among them to be characterized (Moscatelli et al., 1986). FGF-2 is a Mr 18,000 heparin-binding cationic polypeptide that induces proliferation, migration, and protease production in endothelial cells in culture and neovascularization in vivo (Basilico and Moscatelli, 1992). FGF-2 interacts with endothelial cells through two distinct classes of receptors, the high-affinity tyrosine-kinase receptors (FGFRs) and low-affinity heparan sulfate proteoglycans (HSPGs) present on the cell surface and in the extracellular matrix (Jonhson and Williams, 1993). Both classes of receptors are necessary for the transduction of the signal generated by the growth factor (Yayon et al., 1991) and for its internalization inside the cell (Roghani and Moscatelli, 1992; Rusnati et al., 1993).
Gangliosides are highly expressed in the hypervascularized areas of gliomas (Koochekpour and Pilkington, 1996), and they regulate the neovascularization process in vivo (Ziche et al., 1989, 1992; Gullino et al., 1990; Cockerill et al., 1995; Gullino, 1995). Interestingly, GM2 and GM3 inhibit FGF-2–mediated endothelial cell proliferation, and the addition of GD3 restores optimal levels of cell growth (Alessandri et al., 1992; Ziche et al., 1992). In contrast, GD3 enhances the chemotactic activity exerted by FGF-2 on endothelial cells, which is counteracted by GM3 (Ziche et al., 1992). Moreover, GM1, GD1b, and GT1b act synergistically with FGF-2 in favoring survival, growth, and motility of capillary endothelial cells (De Cristian et al., 1990). Finally, angiogenesis induced by FGF-2 in the rabbit cornea assay can be stimulated or repressed by modulating the GM3:GD3 molar ratio (Ziche et al., 1992). An increase of the angiogenic activity of FGF-2 can also be obtained by increasing the local concentration of GM1 and GT1b (Ziche et al., 1989).
Little is known about the mechanism(s) by which gangliosides affect the angiogenic activity of FGF-2 during tumor growth. The shedding of gangliosides by tumor cells can be so extensive as to alter the ganglioside composition of the extracellular environment of the tumor and to cause an increase of their serum levels (Kloppel et al., 1977). Different observations have shown the capacity of gangliosides to bind directly to certain growth factors (see above). Moreover, the heparin-binding properties of FGF-2 and its capacity to interact with various polysulfated/polysulfonated compounds point to the possibility that anionic NeuAc groups of sialo-gangliosides may mimic sulfated/sulfonated groups of glycosaminoglycans in FGF-2 interaction. In the present article we investigated the capacity of exogenous free gangliosides to interact directly with FGF-2 and to affect the biological activity of the growth factor in endothelial cells.
MATERIALS AND METHODS
Chemicals
Human recombinant FGF-2 was expressed and purified from transformed Escherichia coli cells by heparin-Sepharose chromatography (Isacchi et al., 1991). Recombinant FGF-1 and FGF-4 were gifts from C. Basilico (New York University Medical Center, New York, NY). The recombinant, soluble form of the extracellular domain of FGFR-1/flg (xcFGFR-1) (Bergonzoni et al., 1992) was provided by A. Isacchi (Pharmacia-Upjohn, Nerviano, Italy). Synthetic peptides representing different fragments of human FGF-2 (Schubert et al., 1987) were kindly donated by A. Baird (Prizm Pharmaceuticals, San Diego, CA). The synthetic peptide representing the basic domain of HIV-1 Tat protein was from the Medical Research Council AIDS Reagent Project (Potters Bar, Herts, United Kingdom). 4,4-Di- fluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoil acid (BODIPY-dodecanoil acid) was obtained from Molecular Probes (Eugene, OR). Gangliosides, N-acetylneuramin-lactose, and mastoparan were from Sigma (St. Louis, MO). Sulfatide was prepared from pig brain by the method of Hara and Radin (1979), and its chromatographic purity and conversion to the sodium salt were determined as described (Cestaro et al., 1982). Details about the structure of the gangliosides and ganglioside-related molecules utilized in this study are detailed in Table 1.
Table 1.
Structural characteristics of gangliosides and related compounds utilized in this study
| Moleculea | Schematic structure |
|---|---|
| N-Acetylneuramin-lactose | NeuAc 2→ 3Gal 1→ 4Glc 1 |
| Galactosyl-ceramide | 3Gal 1→ 1′Cer |
| Sulfatide | SO3−→ 3Gal 1→ 1′Cer |
| asialo-GM1 | Gal 1→ 3GalNAc 1→ 4Gal 1→ 4Glc 1→ 1′Cer |
| GM1 | Gal 1→ 3GalNAc 1→ 4Gal(3← 2NeuAc) 1→ 4Glc 1→ 1′Cer |
| GM2 | GalNAc 1→ 4Gal(3← 2NeuAc) 1→ 4Glc 1→ 1′Cer |
| GM3 | NeuAc 2→ 3Gal 1→ 4Glc 1→ 1′Cer |
| GD1b | Gal 1→ 3GalNAc 1→ 4Gal (3← 2NeuAc8← 2NeuAc) 1→ 4Glc 1→ 1′Cer |
| GT1b | NeuAc 2→ 3Gal 1→ 3GalNAc 1→ 4Gal(3← 2NeuAc8← 2NeuAc) 1→ 4Glc 1→ 1′Cer |
Glc, glucose; Gal, galactose; GalNAc, N-acetyl-galactosamine; NeuAc, neuraminic-acid; Cer, ceramide.
Ganglioside nomenclature according to Svennerholm (1964).
Cell Cultures
Transformed fetal bovine aortic endothelial GM 7373 cells were obtained from the N.I.G.M.S. Human Genetic Mutant Cell Repository (Institute for Medical Research, Camden, NJ). They correspond to the BFA-1c multilayered transformed clone described by Grinspan et al. (1983). GM 7373 cells were grown in Eagle’s minimal essential medium containing 10% FCS, vitamins, and essential and nonessential amino acids. Spontaneously immortalized BALB/c mouse aortic endothelial 22106 cells (MAE cells) were grown in DMEM containing 10% FCS (Bastaki et al., 1996).
125I-FGF-2 Cell Binding and Internalization
FGF-2 was iodinated as described (Neufeld and Gospodarowicz, 1985) at a specific radioactivity equal to 800 cpm/fmol. GM 7373 were incubated at 4°C in serum-free medium containing 10 ng/ml 125I-FGF-2, 0.15% gelatin, 20 mM HEPES buffer (pH 7.5), and the indicated concentrations of the ganglioside under test. After 2 h, the amount of 125I-FGF-2 bound to low- and high-affinity binding sites was evaluated as described (Moscatelli, 1987). Briefly, after a PBS wash, cells were rinsed twice with 2 M NaCl in 20 mM HEPES buffer (pH 7.5) to remove 125I-FGF-2 bound to low-affinity binding sites and twice with 2 M NaCl in 20 mM sodium acetate (pH 4.0) to remove 125I-FGF-2 bound to high-affinity binding sites. Nonspecific binding was measured in the presence of a 100-fold molar excess of unlabeled FGF-2 and subtracted from all the values.
In some experiments, GM 7373 cells were preloaded with GM1 before the 125I-FGF-2 cell-binding assay. To this purpose, cells were seeded at 70,000 cells/cm2 in 24-well dishes. After 16 h, cells were incubated for an additional 72 h in fresh medium containing 0.4% FCS in the absence or in the presence of 100 μM GM1. At the end of incubation, cells were extensively washed with PBS and incubated at 4°C in serum-free medium containing 10 ng of 125I-FGF-2 per ml in the absence of free ganglioside. After 2 h, the amount of 125I-FGF-2 bound to low- and high-affinity binding sites was evaluated as described above. To assess the amount of ganglioside incorporated during the preloading incubation period, parallel cultures were trypsinized and cells were sonicated at 50 W for 2 min at 4°C. Then, samples were centrifuged for 20 min at 40,000 × g, and the amount of NeuAc was evaluated in the cell membrane and cytosolic fractions as described previously (Svennerholm, 1956).
For cell internalization assays, GM 7373 cells were incubated with 125I-FGF-2 exactly as described above. After 2 h, cell cultures were shifted at 37°C and incubated for an additional 6 or 24 h. At the end of incubation, surface-bound 125I-FGF-2 was removed as described above, and cell-internalized 125I-FGF-2 was recovered by lysing the cells with 0.1 mM Tris-HCl (pH 8.1) containing 0.5% Triton X-100.
Cell Proliferation and DNA Synthesis Assays
Cell proliferation assay on GM 7373 cells was performed as described (Presta et al., 1989). Briefly, GM 7373 cells were seeded at 70,000 cells/cm2 in 24-well dishes. Plating efficiency was higher than 90%. After overnight incubation, cells were incubated for 24 h in fresh medium containing 0.4% FCS in the absence or in the presence of 10 ng/ml FGF-2 and the indicated concentrations of gangliosides. At the end of incubation, cells were trypsinized and counted in a Burker chamber. For DNA synthesis assay, MAE cells were seeded at 25,000 cells/cm2 in 24-well dishes and incubated for 2 d with 0.5% FCS. Quiescent cell cultures were then supplemented with the different mitogens in the absence or in the presence of GT1b and incubated for 16 h at 37 C°. At the end of incubation, cells were pulse labeled with [3H]thymidine (1 μCi/ml) for 6 h. The amount of radioactivity incorporated into the trichloroacetic acid-precipitable material was then measured.
Size Exclusion Chromatography
To assess the association between FGF-2 and micellar gangliosides, 100-μl samples containing 3 pmol of 125I-FGF-2 were incubated for 10 min at 4°C with 125 nmol of the different gangliosides. Then, samples were chromatographed on a size-exclusion fast protein liquid chromatography Superose-12 column (Pharmacia, Piscataway, NJ) in PBS with a flow rate equal to 1.0 ml/min. Elution profiles of FGF-2 and of the ganglioside were obtained by quantification of the radioactivity and of the NeuAc content of the different fractions, respectively. Ferritin (Mr 440,000), immunoglobulin G (Mr 150,000), ovalbumin (Mr 45,000), soybean trypsin inhibitor (Mr 20,100), and cytochrome C (Mr 12,000) were chromatographed under the same experimental conditions as molecular size standards.
Proteolytic Digestion and SDS-PAGE
The protective effect of gangliosides on tryptic digestion of FGF-2 was evaluated as described (Coltrini et al., 1993). Briefly, FGF-2 aliquots (55 pmol) were equilibrated at 37°C for 5 min in 50 mM Tris-HCl (pH 7.5) in the presence of increasing amounts of the ganglioside under test. Then, 60 ng of trypsin (Sigma, St. Louis, MO) were added in a final volume of 100 μl, and digestion was allowed to proceed at 37°C for 3 h. At the end of trypsin digestion, samples were added with an equal volume of SDS-reducing sample buffer, boiled at 100°C for 2 min, and subjected to 15% SDS-PAGE. Gels were stained with the silver staining procedure. The amount of undigested protein in a given line was estimated by soft-laser scanning of the gel.
Preparation of BODIPY-12-labeled GM1
BODIPY-12-GM1 was synthesized and purified according to previously described procedures (Marchesini et al., 1994) by acylation of lyso-GM1 with the N-hydroxy succinimide ester of BODIPY-dodecanoic acid.
Coating of FGF-2 to Plastic and Binding Assay
Aliquots (100 μl) of 100 mM NaHCO3 (pH 9.6) (carbonate buffer), containing 20 μg/ml native or heat-denatured FGF-2, were added to polystyrene nontissue culture microtiter plates. After 16 h of incubation at 4°C, the solution was removed and wells were washed three times with PBS. Experiments using 125I-FGF-2 as a tracer revealed that up to 10% of the protein binds to plastic under these experimental conditions (Rusnati et al., 1997a).
For competition binding assays, the indicated amounts of BODIPY-12-GM1 were incubated for 10 min at room temperature into wells coated with 20 μg/ml native or heat-denatured FGF-2 in the absence or in the presence of the indicated concentrations of unlabeled GM1, heparin, xcFGFR-1, or synthetic FGF-2 fragments. At the end of incubation, wells were washed three times with PBS, and FGF-2-associated GM1 was eluted from the wells with 100 μl of methanol-chloroform solution (40:60, vol/vol) and measured with a FCT-150 spectrofluorimeter (Jasco Spectroscopic, Tokyo, Japan) at its optimal excitation and emission wavelengths. Nonspecific binding was measured in wells incubated with carbonate buffer and was subtracted from all the data.
For the determination of the Kd of the interaction of BODIPY-12-GM1 with FGF-2, 100-μl aliquots of PBS containing different concentrations of labeled GM1 were added into wells coated with 20 μg of FGF-2 per ml. Then samples were processed exactly as described above. Nonspecific binding was subtracted from all the data, which were then analyzed by the Scatchard plot procedure (Scatchard, 1949).
Cross-Linking of 125I-FGF-2 to xcFGFR-1
125I-FGF-2 (0.3 pmol) was incubated in PBS for 2 h at 37°C with 3 pmol of the soluble extracellular form of FGFR-1/flg (xcFGFR-1) in the absence or in the presence of 1.5 nmol of the ganglioside under test. At the end of the incubation, the complexes between xcFGFR-1 and 125I-FGF-2 were cross-linked by adding 1 mM bis[2-(succinimidoxy-carbonyloxy)ethyl] sulfone (BSOCOES, Pierce Chemical, Rockford, IL). After 30 min of incubation at room temperature, the reaction was stopped by the addition of reducing SDS-PAGE sample buffer. Samples were boiled and analyzed by 10% SDS-PAGE. Gels were dried and exposed to Kodak X-OMAT AR film (Eastman Kodak, Rochester, NY) at −70°C for 1 wk.
RESULTS
Size Exclusion Chromatography of 125I-FGF-2–Ganglioside Complexes
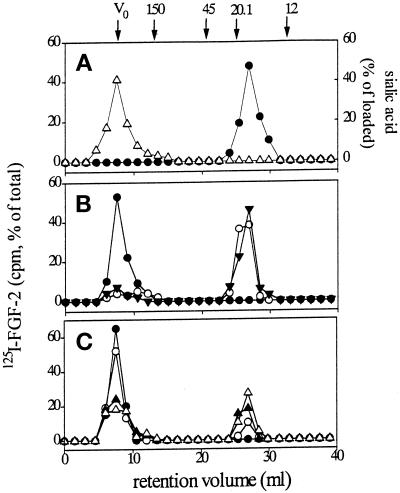
Gangliosides form high-molecular-weight micelles when dissolved in aqueous solutions at concentrations higher than the critical micellar concentration (which usually ranges from 10−8 to 10−5 M) (Formisano et al., 1979; Ulrich-Bott and Wiegandt, 1984; Saqr et al., 1993). Accordingly, gel filtration chromatography performed on a Superose-12 size-exclusion fast protein liquid chromatography column (Pharmacia) demonstrates that gangliosides dissolved in PBS at 1.25 × 10−3 M form high-molecular-weight micelles that elute with the void volume of the column (7 ml) (Figure 1A). Conversely, low-molecular-weight 125I-FGF-2 (Mr 18,000) elutes with a retention volume equal to 27 ml. On this basis, to assess a possible interaction of FGF-2 with gangliosides, 3 pmol of 125I-FGF-2 were preincubated for 10 min at room temperature with 125 nmol of GM1 (final concentration of the ganglioside equal to 1.25 × 10−3 M) and then loaded onto the Superose-12 column. Under these experimental conditions, 125I-FGF-2 preincubated with GM1 dramatically changes its chromatographic behavior and coelutes with the ganglioside in the void volume of the column, thus indicating the formation of 125I-FGF-2–GM1 complexes (Figure 1B). Similar results were obtained when 125I-FGF-2 was preincubated with the same doses of GM2, GM3, GD1b, or GT1b (our unpublished results), whereas asialo-GM1 was unable to complex the growth factor (Figure 1B). Also, no 125I-bFGF–GM1 complexes were observed when the growth factor was heat denatured before incubation with the ganglioside (Figure 1B), thus indicating that NeuAc and a correct three-dimensional structure of FGF-2 are required for ganglioside interaction.
Figure 1.
Size-exclusion chromatography of FGF-2–ganglioside complexes. (A) 100 μl samples containing 3 pmol of 125I-FGF-2 (•) or 125 nmol of GM1 (▵) in PBS were incubated for 10 min at room temperature, loaded separately onto size-exclusion fast protein liquid chromatography Superose-12 column, and eluted in PBS at 1 ml/min flow rate. Radioactivity or NeuAc concentration was measured in each fraction for the evaluation of 125I-FGF-2 or ganglioside content, respectively. (B) 3 pmol of native (•, ○) or of heat-denatured 125I-FGF-2 (▾) were incubated for 10 min at room temperature with 125 nmol of GM1 (closed symbols) or of asialo-GM1 (○). Then, samples were subjected to size-exclusion chromatography as in panel A, and radioactivity was measured in each fraction. (C) 3 pmol of 125I-FGF-2 were incubated for 10 min at room temperature with decreasing concentrations of GM1 [12.5 nmol (•), 5 nmol (○), 1.25 nmol (▴), or 0.125 nmol (▵)]. Then, samples were subjected to size-exclusion chromatography as in panel A, and radioactivity was measured in each fraction. Molecular size standards (in thousands) were ferritin (Mr 440,000), that eluted with the void volume of the column (V0), IgG (Mr 150,000), ovalbumin (Mr 45,000), soybean trypsin inhibitor (Mr 20,100), and cytochrome C (Mr 12,000).
The capacity of gangliosides to complex with FGF-2 is dose dependent, as shown by the progressive reduction of the high-molecular-weight peak corresponding to the 125I-bFGF–GM1 complex paralleled by the appearance of a retained peak of free 125I-bFGF when the growth factor was preincubated with decreasing doses of GM1 (Figure 1C).
Gangliosides Protect FGF-2 from Trypsin Digestion
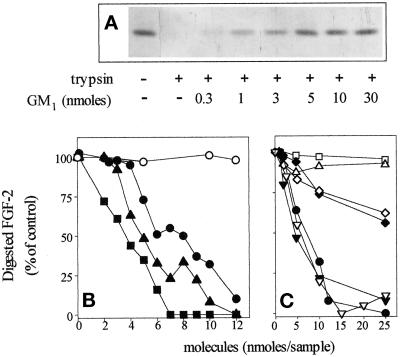
Sulfated glycosaminoglycans bind to FGF-2 and protect it from tryptic digestion (Coltrini et al., 1993). This capacity has been utilized to study the structural features of FGF-2–binding polysulfated/polysulfonated compounds (Coltrini et al., 1993). On this basis, the possibility that ganglioside interaction can prevent FGF-2 proteolysis was investigated. To this purpose, aliquots of FGF-2 (55 pmol) were equilibrated at 37°C for 5 min in the presence of increasing amounts of the different gangliosides. Then, 60 ng of trypsin were added, and proteolytic digestion was allowed to proceed at 37°C for 3 h. At the end of incubation, samples were analyzed by SDS-PAGE followed by silver staining of the gel (Figure 2A), and the amount of undigested FGF-2 was quantified by soft laser scanning. As shown in Figure 2, A and B, gangliosides protect FGF-2 from tryptic digestion in a dose-dependent manner as a function of the number of NeuAc residues of the molecule, the order of relative potency of the gangliosides tested being GT1b > GD1b > GM1. It should be pointed out that GT1b was unable to protect heat-denatured FGF-2 from trypsin digestion (Figure 3), thus confirming that the protective effect of gangliosides depends on the interaction with FGF-2, and not with the proteolytic enzyme, and that this interaction occurs only when the growth factor is present in the proper native conformation.
Figure 2.
Protection of FGF-2 from tryptic digestion by gangliosides and related molecules. (A) Representative experiment in which 1 μg aliquots of FGF-2 were incubated at 37° C for 3 h with 60 ng of trypsin in the absence or in the presence of the indicated amounts of GM1. Then samples were analyzed by 15% SDS-PAGE followed by silver staining of the gel. (B and C) Aliquots (1 μg) of FGF-2 were incubated with trypsin in the presence of the indicated amounts of asialo-GM1 (○), GM1 (•), GD1b (▴), GT1b (▪) (panel B) or GM1 (•), NeuAc (□), N-acetylneuramin-lactose (▵), GM2 (▾), GM3 (♦), sulfatide (▿), galactosyl-ceramide (⋄) (panel C). Then samples were analyzed by 15% SDS-PAGE followed by silver staining of the gel. The amount of nondigested FGF-2 was evaluated by soft-laser scanning of the gel, and data are expressed as percentage of digested FGF-2 in respect to samples in which trypsin was omitted. Each point is the mean of two to five determinations in duplicate. SEM never exceeded 13% of the mean value.
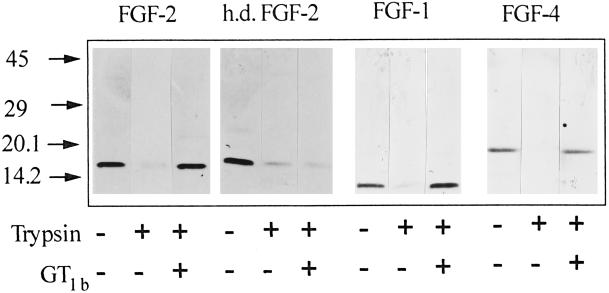
Figure 3.
Ganglioside-mediated protection of FGF-1 and FGF-4 from tryptic digestion. Aliquots (1 μg) of native or heat-denatured (h.d.) FGF-2, FGF-1, or FGF-4 were incubated at 37°C for 3 h with 60 ng of trypsin in the absence or in the presence of 8 nmol of GT1b. Then samples were analyzed by 15% SDS-PAGE followed by silver staining of the gel.
The above data suggest that NeuAc residue(s) are of importance in gangliosides–FGF-2 interaction. Accordingly, asialo-GM1 does not prevent tryptic digestion of FGF-2 (Figure 2B). In addition, however, free NeuAc and N-acetylneuramin-lactose, a disaccharide bearing one NeuAc group, do not protect FGF-2 from tryptic digestion at doses up to 250 μM (Figure 2C), thus suggesting that NeuAc residue(s) associated with defined glycosphingolipidic structures are required for optimal FGF-2 interaction. Relevant to this point is the observation that GM3 shows a reduced capacity to bind and protect FGF-2 from trypsin digestion when compared with GM2 and GM1 (Figure 2C). Since these monosialo-gangliosides differ in the length of their oligosaccharide chain (see Table 1), the data point to the importance of the saccharide structure in presenting NeuAc to FGF-2.
Anionic groups as sulfates can equal the protein-recognition properties of sialic acids (Rosen and Bertozzi, 1994), and specific sulfated glycolipids have been demonstrated to bind to hepatocyte growth factor (Kobayashi et al., 1994). Accordingly, sulfatide was able to protect FGF-2 from proteolytic cleavage with a potency similar to GM1 and GM2, whereas galactosyl-ceramide exerted a limited effect (Figure 2C). Taken together, the data indicate that NeuAc residue(s), the oligosaccharide chain, and, to a limited extent, the ceramide moiety of the ganglioside play a role in FGF-2 interaction.
FGF-2 belongs to a family of heparin-binding growth factors (Basilico and Moscatelli, 1992). To assess whether the ganglioside-binding capacity is limited to FGF-2, trypsin digestion experiments were also performed with FGF-1 and FGF-4. As shown in Figure 3, all FGFs tested are protected from trypsin digestion by GT1b, suggesting that various members of the FGF family share structural features responsible for ganglioside interaction.
Gangliosides Bind to Immobilized FGF-2
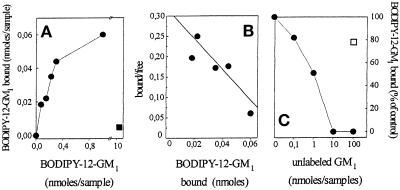
FGF-2 immobilized onto nontissue culture plastic retains its cell-binding capacity and biological activity (Presta et al., 1992; Rusnati et al., 1997a). On this basis, FGF-2 was adsorbed to plastic and evaluated for its capacity to bind to BODIPY-12-labeled GM1. As shown in Figure 4A, fluorochrome-labeled GM1 binds to immobilized FGF-2. The binding was dose dependent and saturable, specificity being demonstrated by the incapacity of BODIPY-12-GM1 to interact with immobilized heat-denatured FGF-2. Scatchard plot analysis of the binding data indicates that BODIPY-12-GM1 binds to immobilized FGF-2 with a Kd equal to 6.3 ± 2 μM (Figure 4B). Unlabeled GM1 competed for the binding of BODIPY-12-GM1 to FGF-2 in a dose-dependent manner, half-maximal inhibition being observed at equimolar concentrations of the two compounds (Figure 4C). Asialo-GM1 did not exert any inhibitory effect on the binding of the labeled ganglioside to the growth factor. These data demonstrate that the BODIPY fluorochrome group does not interfere with FGF-2–GM1 interaction. On this basis, BODIPY-12-GM1 was utilized for further studies.
Figure 4.
Binding of fluorochrome-labeled GM1 to immobilized FGF-2. (A) Increasing concentrations of BODIPY-12-GM1 were incubated for 5 min at room temperature into wells coated with 20 μg/ml native (•) or heat-denatured (▪) FGF-2. At the end of incubation, the BODIPY-12-GM1 bound to the immobilized growth factor was extracted and measured with a spectrofluorimeter. In panel B, binding data were analyzed by the Scatchard plot procedure. They are representative of three independent experiments that gave similar results. (C) Aliquots (50 μl) containing 1 nmol of BODIPY-12-GM1 were incubated for 5 min at room temperature into FGF-2-coated wells in the presence of 100 nmol of asialo-GM1 (□) or of increasing concentrations of unlabeled GM1 (•). At the end of incubation, the amount of BODIPY-12-GM1 bound to the immobilized growth factor was extracted, measured, and compared with the amount of BODIPY-12-GM1 bound to immobilized FGF-2 in the absence of any competitor. Each point is the mean of two determinations in duplicate. SEM never exceeded 5% of the mean value.
Gangliosides Interact with the COOH Terminus of FGF-2
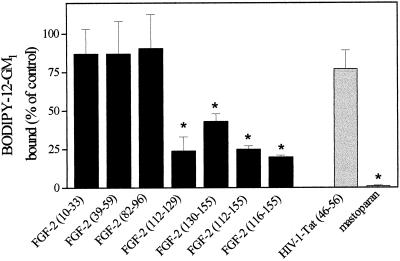
To identify the region(s) of the FGF-2 molecule responsible for ganglioside interaction, 2.4 nmol of BODIPY-12-GM1 were incubated for 10 min at room temperature with equimolar concentrations of synthetic peptides representing different regions of the FGF-2 molecule (in the present article, amino acid numbering 1–155 was utilized for FGF-2). Then, the mixtures were added to FGF-2–coated wells, and the capacity of the different FGF-2 fragments to prevent the binding of BODIPY-12-GM1 to the immobilized growth factor was evaluated. An irrelevant basic peptide, represented by the basic domain (amino acid residues 41–60) of HIV-1 Tat, a protein able to bind heparin and other polyanionic compounds (Rusnati et al., 1997b), and mastoparan, a peptide from wasp venom that binds to sialic acid residue(s) of gangliosides (Bueb et al., 1990), were used as negative and positive controls, respectively. Among the FGF-2 peptides tested, only FGF-2(112–129) and, to a lesser extent, FGF-2(130–155) inhibit the binding of BODIPY-12-GM1 to immobilized FGF-2 (Figure 5). Accordingly, two synthetic peptides containing both FGF-2 fragments and corresponding to amino acid sequences FGF-2(112–155) and FGF-2(116–155) inhibited the binding of BODIPY-12-GM1 (Figure 5). Under the same experimental conditions, mastoparan abolished FGF-2–GM1 interaction while the basic peptide HIV-1 Tat(41–60) was ineffective. Thus, the data implicate the COOH terminus of the FGF-2 molecule in ganglioside interaction.
Figure 5.
Mapping of the ganglioside-binding region of FGF-2. Aliquots (50 μl) containing 2.4 nmol of BODIPY-12-GM1 were incubated for 5 min at room temperature into FGF-2–coated wells in the presence of 2.5 nmol of various synthetic peptides representing different fragments of FGF-2, of the basic domain of HIV-1-Tat, or of mastoparan. At the end of incubation, the amount of BODIPY-12-GM1 bound to the immobilized growth factor was extracted, measured, and compared with the amount of fluorescent GM1 bound to immobilized FGF-2 in the absence of any peptide. Each point is the mean ± SEM of two to three determinations in duplicate. *, Statistically different from control (p < 0.05).
Gangliosides Inhibit FGF-2 Interaction with Tyrosine-Kinase FGF Receptor
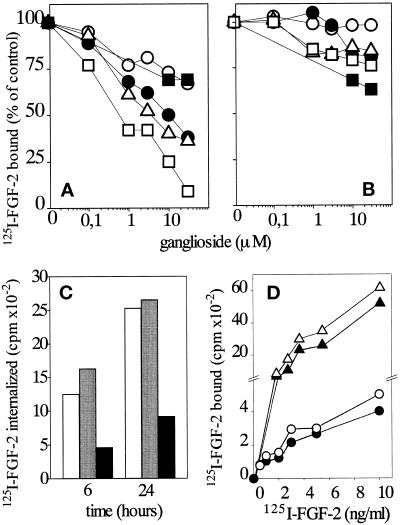
The above data prompted us to investigate whether the interaction of free gangliosides with FGF-2 is able to modulate the ability of the growth factor to bind to high-affinity tyrosine-kinase FGFRs and/or to low-affinity HSPGs in endothelial cells. To this purpose, experimental conditions (i.e., low temperature and short time of incubation) were adopted to minimize possible alterations of ligand-receptor interaction due to ganglioside uptake and incorporation into the cell membrane (Saqr et al., 1993). On this basis, subconfluent cultures of endothelial GM 7373 cells were incubated at 4°C with 10 ng/ml 125I-FGF-2 in the absence or in the presence of increasing concentrations of the different gangliosides. After 2 h, the amount of 125I-FGF-2 associated with FGFRs and HSPGs was evaluated. As shown in Figure 6A, gangliosides inhibit the binding of 125I-FGF-2 to FGFR in a dose-dependent manner. Among the gangliosides tested, GT1b showed the strongest antagonist activity and fully inhibited 125I-FGF-2 binding to FGFRs at the dose of 30 μM. GM1 and GD1b showed intermediate inhibitory capacity, whereas asialo-GM1 and sulfatide were the least effective. At variance with the FGFR binding data, the gangliosides tested did not inhibit significantly the binding of 125I-FGF-2 to endothelial HSPGs, a limited effect being exerted by sulfatide only (Figure 6B). Finally, free NeuAc did not affect the binding of 125I-FGF-2 to FGFRs nor to HSPGs, even when tested at doses as high as 300 μM (our unpublished results).
Figure 6.
Effect of exogenous gangliosides on the binding of 125I-FGF-2 to FGFRs and HSPGs and its internalization in endothelial cells. (A and B) Subconfluent cultures of GM 7373 cells were incubated for 2 h at 4°C with 10 ng/ml 125I-FGF-2 in the presence of the indicated concentrations of asialo-GM1(○), GM1 (•), GD1b (▵), GT1b (□), and sulfatide (▪). At the end of incubation, 125I-FGF-2 bound to FGFRs (A) and to HSPGs (B) was evaluated as described in MATERIALS AND METHODS and expressed as percentage of the radioactivity measured in cell cultures incubated in the absence of ganglioside. Each point is the mean of three determinations in duplicate. SEM never exceeded 9% of the mean value. (C) Subconfluent cultures of GM 7373 cells were incubated for 2 h at 4° C with 10 ng/ml 125I-FGF-2 with no addition (open bars) or in the presence of asialo-GM1 (gray bars) or of GT1b (black bars), both at 30 μM. At the end of incubation, cell cultures were shifted at 37 °C and incubated at this temperature for the indicated periods of time. At the end of incubation, cell surface-associated 125I-FGF-2 was removed, and the amount of internalized 125I-FGF-2 was measured. The data are representative of two independent experiments in duplicate. (D) GM 7373 cells were incubated for 72 h at 37°C in the absence (•, ▴) or in the presence (○, ▵) of GM1 (100 μM). At the end of the incubation, cell cultures were washed extensively and incubated for 2 h at 4° C with increasing concentrations of 125I-FGF-2 in the absence of free ganglioside. At the end of incubation, 125I-FGF-2 bound to FGFRs (circles) and to HSPGs (triangles) was evaluated as described in MATERIALS AND METHODS. The data are representative of four independent experiments in duplicate.
FGF-2 interaction with the endothelial cell surface leads to its internalization (Roghani and Moscatelli, 1992; Rusnati et al., 1993). On this basis, we investigated the effect of free gangliosides on FGF-2 internalization in GM 7373 cells. As shown in Figure 6C, GT1b inhibits both early and late internalization of 125I-FGF-2 into GM 7373 cells, while the control ganglioside asialo-GM1 was ineffective. It must be pointed out that, because of the contribution of HSPGs to FGF-2 cell entry (Roghani and Moscatelli, 1992; Rusnati et al., 1993), a limited internalization of FGF-2 occurs also in the presence of concentrations of GT1b (30 μM) sufficient to cause a complete inhibition of FGF-2 binding to FGFRs (see Figure 6A).
To rule out the possibility that the observed effects of gangliosides on FGF-2–FGFR interaction were due to plasma membrane alterations consequent to a limited incorporation of the ganglioside during the short-term binding assay, GM 7373 cells were exposed for 72 h to fresh medium containing 0.4% FCS in the absence or in the presence of 100 μM GM1. Under these conditions, gangliosides are efficiently incorporated into the cell (Saqr et al., 1993). Accordingly, a significant increase of the content of plasma membrane-associated NeuAc (2.7 vs. 1.2 nmol of NeuAc/106 cells) and of cytosolic NeuAc (6.0 vs. 4.0 nmol of NeuAc/106 cells) was observed in GM1-treated cells in respect to control cells. This corresponds to an incorporation into the cells of ∼2% of the originally added exogenous GM1. After loading with the ganglioside, control and GM1-loaded cells were washed with ganglioside-free medium and incubated for 2 h at 4°C with increasing concentrations of 125I-FGF-2. As shown in Figure 6D, no significant differences were observed between control and GM1-loaded cells in the capacity of 125I-FGF-2 to bind to low-affinity HSPGs and high-affinity FGFRs. Taken together, the data demonstrate that exogenous free ganglioside, but not membrane-incorporated GM1, affects FGF-2–FGFR interaction in intact cells.
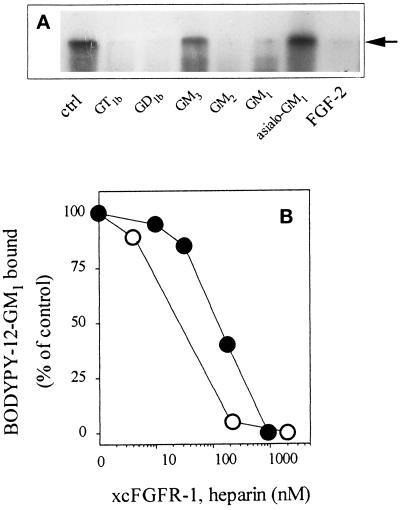
The capacity of gangliosides to prevent the binding of 125I-FGF-2 to cell-associated FGFRs prompted us to assess their ability to affect FGF-2 interaction with the extracellular domain of FGFR in a cell-free system. To this purpose, 0.3 pmol of 125I-FGF-2 was incubated for 2 h at 37°C with 3 pmol of a soluble extracellular form of FGFR-1/flg (xcFGFR-1) (Bergonzoni et al., 1992) in the absence or in the presence of 1.5 nmol of the different gangliosides. At the end of incubation 125I-FGF-2–xcFGFR-1 complexes were chemically cross-linked and analyzed by SDS-PAGE followed by autoradiography of the gel. GM1, GM2, GD1b, and GT1b, but not asialo-GM1 and GM3, were able to prevent the binding of 125I-FGF-2 to xcFGFR-1, as demonstrated by the lack of appearance on the gel of the Mr 68,000 radiolabeled band corresponding to the 125I-FGF-2–xcFGFR-1 complex (Figure 7A). Conversely, xcFGFR-1 is able to prevent the binding of 1 nmol of BODIPY-12-GM1 to immobilized FGF-2 in a dose-dependent manner, a complete inhibition being observed at ∼1 μM (corresponding to 50 pmol of soluble receptor per sample, Figure 7B). Under the same experimental conditions, free heparin prevents the interaction of BODIPY-12-GM1 with FGF-2 at ∼200 nM (corresponding to 10 pmol per sample, Figure 7B). The fivefold weaker potency of xcFGFR-1, when compared with free heparin, in preventing the binding of BODIPY-12-GM1 to immobilized FGF-2 is in keeping with the relative affinity of the two molecules for the growth factor (Kd equal to 5–10 and 1 nM for FGF-2–xcFGFR-1 and FGF-2–heparin interaction, respectively) (Bergonzoni et al., 1992; Li and Seddon, 1994).
Figure 7.
Effect of gangliosides on the binding of FGF-2 to soluble xcFGFR-1. (A) 125I-FGF-2 (0.3 pmol) was incubated for 2 h at 37°C with 3 pmol of recombinant, soluble xcFGFR-1 in the absence (ctrl) or in the presence of 1.5 nmol of the indicated gangliosides. Then, 125I-FGF-2–xcFGFR-1 complexes were chemically cross-linked with BSOCOES and analyzed by 10% SDS-PAGE followed by autoradiography of the gel. Arrow points to the cross-linked Mr 69,000 kDa 125I-FGF-2–xcFGFR-1 complex (Rusnati et al., 1994), which is abrogated by incubation with a 100-fold excess of unlabeled growth factor (FGF-2). (B) Aliquots (50 μl) containing 1 nmol of BODIPY-12-GM1 were incubated into plastic dishes coated with 20 μg/ml FGF-2 in the presence of increasing concentrations of xcFGFR-1 (•) or of heparin (○). At the end of incubation, fluorescent GM1 bound to the immobilized growth factor was extracted, measured with a spectrofluorimeter, and compared with the amount of fluorescent GM1 bound to immobilized FGF-2 in the absence of the competitor.
Gangliosides Affect the Biological Activity of FGF-2 in Endothelial Cells
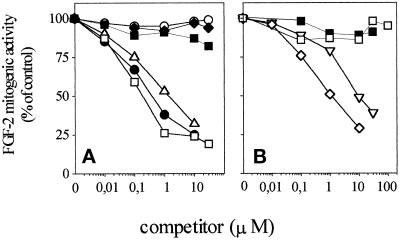
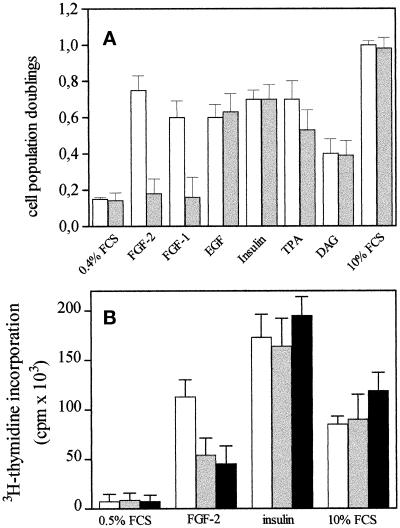
To assess the biological consequences of FGF-2–ganglioside interaction, we evaluated the effects of free gangliosides onto the mitogenic activity exerted by FGF-2 on cultured endothelial cells in a short-term cell proliferation assay (Presta et al., 1989). Confluent cultures of GM 7373 cells were incubated for 24 h with 10 ng/ml FGF-2 in the absence or in the presence of increasing concentrations of different gangliosides. At the end of incubation, cells were trypsinized and counted. As shown in Figure 8, sialo-gangliosides inhibit FGF-2 mitogenic activity in a dose-dependent manner. In contrast, asialo-GM1, sulfatide, and galactosyl-ceramide do not inhibit FGF-2–mediated cell proliferation, in keeping with their inability to inhibit FGF-2–FGFR interaction (see Figure 6A). As observed for FGF-2–ganglioside interaction (see above), the inhibitory potency of the various gangliosides depends, at least in part, on the number of NeuAc residues (GT1b being the most potent inhibitor, Figure 8A), to the length of the oligosaccharide chain (GM1 being more potent than GM2 and GM3, Figure 8B), and to the presence of a ceramide portion (free NeuAc and N-acetylneuramin-lactose being inactive, Figure 8B). The inhibitory effect exerted by gangliosides on the mitogenic activity of FGF-2 appears to be specific and restricted to the members of the FGF family. Indeed, 10 μM GM1 inhibits the mitogenic activity of FGF-2 and FGF-1 without affecting cell proliferation induced by the phorbol ester 12-O-tetradecanoyl phorbol 13-acetate, 1,2-dioctanoyl-sn-glycerol, 10% FCS, EGF, or insulin (Figure 9). It must be pointed out that the lack of inhibitory activity of GM1 on FGF-independent stimuli does not reflect the relative potency of the mitogen under test, the ganglioside being equally ineffective when cells were stimulated by a potent inducer (e.g., 10% FCS) or by a much weaker mitogen (e.g., 1,2-dioctanoyl-sn-glycerol).
Figure 8.
Effect of exogenous gangliosides and related compounds on the mitogenic activity of FGF-2 in endothelial cells. Subconfluent cultures of GM 7373 cells were incubated for 24 h at 37°C with 10 ng/ml FGF-2 in the presence of increasing concentrations of asialo-GM1 (○), GM1 (•), GD1b (▵), GT1b (□), galactosyl-ceramide (▪), or sulfatide (♦) (panel A) or of GM2 (⋄), GM3 (▿), NeuAc (□), N-acetylneuramin-lactose (▪) (panel B). At the end of incubation, cells were trypsinized and counted in a Burker chamber. For the different experimental conditions, data have been calculated as cell population doublings during the 24-h incubation period and expressed as percentage of the mitogenic activity exerted by FGF-2 in the absence of any competitor (equal to 0.8 cell population doublings, see also Figure 9A). Each point is the mean of two to six determinations in duplicate. SEM never exceeded 16% of the mean value.
Figure 9.
Effect of GM1 on the mitogenic activity of different endothelial cell mitogens. (A) Subconfluent cultures of GM 7373 cells were incubated for 24 h at 37°C in 0.4% FCS with no addition (control) or with FGF-2 (10 ng/ml), FGF-1 (30 ng/ml), EGF (30 ng/ml), insulin (10 μg/ml), 10% FCS, 12-O-tetradecanoyl phorbol 13-acetate (TPA, 30 ng/ml), or 1,2-dioctanoyl-sn-glycerol (DAG, 5 μg/ml) in the absence (white bars) or in the presence (gray bars) of 10 μM GM1. At the end of incubation, cells were trypsinized and counted in a Burker chamber. Data are expressed as cell population doublings during the 24-h incubation period. Each point is the mean ± SEM of two to six determinations in duplicate. All mitogens induce a statistically significant increase of cell proliferation rate (Student’s t test, p < 0.05). (B) MAE cells were incubated for 2 d with 0.5% FCS. Quiescent cell cultures were then treated with vehicle (0.5% FCS), FGF-2 (30 ng/ml), insulin (10 μg/ml), or 10% FCS in the absence (white bars) or in the presence of 30 μM (gray bars) or 100 μM (black bars) of GT1b. After 16 h, cells were pulse labeled with [3H]thymidine (1 μCi/ml) for 6 h. The amount of radioactivity incorporated into the trichloroacetic acid-precipitable material was measured. Each point is the mean ± SEM of three determinations in triplicate. At both concentrations, GT1b causes a statistically significant decrease of DNA synthesis induced by FGF-2 (Student’s t test, p < 0.05).
The FGF-2-antagonist activity of gangliosides is not restricted to GM 7373 cells. Indeed, GT1b inhibits DNA synthesis induced by FGF-2 in MAE cells in culture (Figure 9B). Also, in this case the inhibitory effect appears to be specific since GT1b does not affect [3H]thymidine incorporation stimulated by insulin or 10% FCS.
DISCUSSION
Previous observations had shown that gangliosides can modulate the biological activity of FGF-2 in vitro (Bremer and Hakomori, 1982; De Cristian et al., 1990) and in vivo (Ziche et al., 1989, 1992). Here we demonstrate that FGF-2 binds to gangliosides in solution. This interaction is able to prevent the binding of FGF-2 to tyrosine-kinase FGFRs with a consequent inhibition of the mitogenic activity exerted by the growth factor on endothelial cells.
FGF-2–ganglioside interaction depends upon defined structural features of both molecules. Binding to FGF-2 and consequent inhibition of receptor binding and mitogenic activity of the growth factor occur in the micromolar range of concentrations of ganglioside, above its critical micellar concentration (Formisano et al., 1979; Ulrich-Bott and Wiegandt, 1984). Under these experimental conditions the oligosaccharide chain of the glycosphingolipid is exposed to the aqueous environment and available for FGF-2 interaction. Several observations point to the importance of NeuAc residues of the oligosaccharide chain in this interaction. Indeed, the relative potency of the ganglioside in protecting FGF-2 from trypsin digestion, in inhibiting its interaction with cell surface FGFRs, and in preventing its mitogenic action appears to be related, at least in part, to the number of sialic acid residues present on the glycosphingolipid, GT1b being usually the most effective. Moreover, the lack of NeuAc groups in the oligosaccharide chain impairs the capacity of the ganglioside to interact with FGF-2, as observed for asialo-GM1. However, sialic acid alone fails to bind to the growth factor. Also, the presence of one sialic acid residue in N-acetylneuramin-lactose is not sufficient to confer to this molecule the capacity to interact with FGF-2. Taken together, these data indicate that NeuAc must be presented to FGF-2 in the contest of a defined glycolipidic structure to exert its FGF-2 binding capacity.
In N-acetylneuramin-lactose, which is unable to bind FGF-2, NeuAc is linked to a short glucose-galactose disaccharide. This structure is comparable to that of the oligosaccharide chain of GM3 (see Table 1) that, among the monosialo-gangliosides tested, has the shortest oligosaccharide chain and the poorest FGF-2 antagonist activity. This suggests that the length and structure of the oligosaccharide chain are also of importance in determining the FGF-2-binding activity of the ganglioside. The lack of FGF-2–binding activity of N-acetylneuramin-lactose, when compared with GM3, may also suggest that the ceramide portion of the ganglioside is involved in FGF-2 interaction, as supported by the observation that galactosyl-ceramide protects FGF-2 from trypsin digestion with a potency similar to that of GM3. Taken together, the results indicate that FGF-2–ganglioside interaction occurs via NeuAc residue(s) and is strictly regulated by other components of the glycolipidic structure. Similar conclusions have been drawn from sialic acid recognition studies of selectins to sialylated Lewis blood group epitopes (McEver et al., 1995). Also in this case, selectin interaction depends not only on the presence and structure of the sialic acid residue but also on that of other saccharide residues (i.e., fucose and galactose) in the oligosaccharide chain.
Sulfation of sialyl Lewis X is of importance for selectin interaction (Rosen and Bertozzi, 1994), and anionic groups as sulfates can equal the protein-recognition properties of sialic acids, as shown by the capacity of L- and P-selectins to bind to sulfatides and subsets of heparin fragments (Rosen and Bertozzi, 1994). Accordingly, our data demonstrate that sulfatide can bind FGF-2 and protect it from proteolytic cleavage with a potency similar to mono-sialo gangliosides. These observations are of particular relevance when the heparin-binding properties of FGF-2 and its capacity to interact with various polysulfated/polysulfonated compounds are considered (Coltrini et al., 1993). On this basis, the possibility that negatively charged NeuAc groups might interact with the strongly cationic FGF-2 molecule could be anticipated. However, our observations indicate that the binding of sialo-gangliosides to FGF-2 is not the consequence of a mere electrostatic interaction but depends upon specific structural features of the growth factor. Indeed, FGF-2 must be present in a proper three-dimensional conformation to interact with gangliosides. Also, a ganglioside-binding region has been identified in the COOH terminus of the FGF-2 molecule by synthetic peptide-binding experiments. Peptide FGF-2(112–129) and to a lesser extent peptide FGF-2(130–155), as well as peptides FGF-2(112–155) and FGF-2(116–155), were able to prevent the binding of BODIPY-12-GM1 to the immobilized growth factor, whereas peptides FGF-2(10–34), FGF-2(39–59), and FGF-2(82–96) were ineffective (note that in the present work amino acid numbering 1–155 has been used for FGF-2). The specificity of these observations is confirmed by the inability of the highly charged basic peptide HIV-1 Tat(41–60) to bind GM1 under the same experimental conditions.
Peptide FGF-2(112–129) and larger FGF-2 fragments containing this amino acid sequence had been shown to bind heparin and to prevent the binding of FGF-2 to its high-affinity FGFRs, suggesting that this region (formerly known as the putative receptor-binding loop) is involved in receptor recognition and binding (Baird et al., 1988). More recent observations, based on site-directed mutagenesis of the FGF-2 molecule, x-ray crystallography data, isothermal titrating calorimetry, and computer modeling, have indicated that two separate receptor-binding sites adjacent to a discontinuous heparin-binding domain exist in FGF-2 (Pantoliano et al., 1994; Springer et al., 1994; Thompson et al., 1994). This allows the formation of heparin–FGF-2–FGFR ternary complexes (Pantoliano et al., 1994). The primary, high-affinity receptor-binding site is comprised of six discontinuous residues that are located on the same face of the FGF-2 molecule. The second, low-affinity receptor-binding site is a surface-exposed type I β-turn within the putative receptor-binding loop and is composed of residues FGF-2(120–124). This region is required for receptor dimerization in vitro, and mitogenic signal transduction in cultured cells (Springer et al., 1994). We have observed that gangliosides hamper the capacity of 125I-FGF-2 to complex in solution with the recombinant form of xcFGFR-1 and prevent its interaction with FGFRs present on the endothelial cell surface. However, none of the gangliosides tested prevent the binding of 125I-FGF-2 to GM 7373 cell surface HSPGs, even though this interaction occurs with a much lower affinity than FGF-2–FGFR interaction (Kd equal to 300 and 20 pM for the two interactions, respectively). The higher abundance of cell-surface HSPGs in respect to FGFRs (4.4 × 105 vs. 1.6 × 104 binding sites/cell, respectively) may explain this apparent discrepancy (Rusnati et al., 1993). Indeed, under appropriate experimental conditions, both heparin and soluble xcFGFR-1 can prevent the binding of fluorochrome-labeled GM1 to immobilized FGF-2 (see Figure 7B). Thus, gangliosides bind to COOH-terminal region(s) of FGF-2 overlapping or adjacent to those involved in heparin–heparan sulfate and FGFR interactions.
These observations raise the possibility that exogenous gangliosides may exert a FGF-2 antagonist activity by a direct interaction with the growth factor, thus preventing its binding to tyrosine-kinase FGFRs. This hypothesis is supported by experimental evidence indicating that the structural features of the ganglioside required to bind FGF-2 and protect it from trypsin digestion are similar to those required to prevent FGFR binding and mitogenic activity. Moreover, both GM 7373 cells and MAE cells proliferate when exposed to different tyrosine-kinase- and protein kinase C-dependent mitogens, including phorbol ester, diacyl-glycerol, serum, EGF, or insulin, in the presence of concentrations of ganglioside sufficient to inhibit the mitogenic activity exerted by FGF-2. This demonstrates that the inhibitory activity exerted by gangliosides is specific for FGF-2 and is not the consequence of a general impairment of the capacity of endothelial cells to respond to mitogenic stimuli. Interestingly, gangliosides are also able to inhibit the mitogenic activity of FGF-1, another member of the FGF family that shares with FGF-2 various structural and biological features, including FGFR- and HSPG-binding capacity (Jonhson and Williams, 1993). These findings, together with the observation that FGF-1, FGF-2, and FGF-4 are protected from trypsin digestion by GT1b, suggest that different members of the FGF family share structural features responsible for ganglioside interaction.
As stated above, sulfatide binds FGF-2 and protects it from proteolytic cleavage. Nevertheless, free sulfatide is unable to prevent FGF-2–FGFR interaction and to inhibit FGF-2–mediated cell proliferation. These observations indicate that the capacity of a molecule to interact with FGF-2 in vitro does not necessarily reflect its FGF-2 antagonist potential. Similar conclusions had been drawn for FGF-2–binding heparin derivatives (Ishihara et al., 1993; Coltrini et al., 1994). For instance, N-desulfated/N-acetylated beef lung heparin is as potent as unmodified heparin in preventing the proteolytic digestion of FGF-2, but it is highly inefficient in inhibiting the receptor-binding and mitogenic activity of the growth factor (Coltrini et al., 1994). Thus, the capacity of a molecule to bind FGF-2 in a cell-free system and to modulate its biological activity can be dissociated at the structural level. This appears to be of importance for the development of synthetic FGF-2 inhibitors.
Here we have shown that a short-term incubation of GM 7373 cells with FGF-2 in the presence of free gangliosides causes an inhibition of the receptor-binding and mitogenic activity of the growth factor. In apparent contrast with these observations, De Cristian et al. (1990) demonstrated that a 6-h preincubation of endothelial cells with GT1b followed by a further 72-h incubation in the presence of both GT1b and FGF-2 increases the mitogenic activity of the growth factor. The addition of exogenous gangliosides to cell cultures is a widely used approach to investigate their effects on cell behavior. However, the diversified conditions under which they are added to cultured cells cause different degrees of ganglioside incorporation into cell membrane, making comparison among experiments difficult (Saqr et al., 1993). Conflicting results may therefore depend on the free or cell-associated status of the ganglioside and reflect different mechanisms of action of these glycolipids. Our data demonstrate that free gangliosides present in their micellar form in the cell culture medium bind and sequester FGF-2, preventing its interaction with cell-surface FGFRs. In contrast, cell membrane-incorporated glycolipids may regulate the biological activity of FGF-2 in the absence of free gangliosides by different mechanisms of action, possibly by affecting the activity of tyrosine kinase receptors and intracellular signaling, as already demonstrated for various growth factors, including PDGF and EGF (see INTRODUCTION). Accordingly, we have observed that a 72-h incubation of GM 7373 cells with GM1 leads to a significant incorporation of the glycolipid into the cell membrane. Even though this does not result in a significant modification of the capacity of the cells to bind FGF-2 (see above), GM1 preloaded cells proliferate more efficiently than control cells in response to FGF-2 with a consequent 10-fold increase of the potency of the growth factor (ED50 equal to 1.0 and 10 ng/ml FGF-2 for GM1 preloaded and control cells, respectively) (Rusnati and Urbinati, unpublished data). In agreement with this hypothesis is the observation that membrane-associated gangliosides modulate the biological activity of FGF-2 in fibroblasts and glial cells in the absence of a direct interaction with the growth factor (Bremer and Hakomori, 1982; Meuillet et al., 1996a,b), probably by regulating tyrosine autophosphorylation of FGFR (Meuillet et al., 1996a,b).
Taken together, the data suggest that exogenous free gangliosides and membrane-incorporated glycolipids can modulate the activity of FGF-2 by different mechanisms of action. It is interesting to note that soluble and cell-associated sulfated glycosaminoglycans have also been demonstrated to play contrasting roles in modulating the biological activity of FGF-2 (Rusnati and Presta, 1996). As observed for exogenous gangliosides, soluble glycosaminoglycans protect FGF-2 from proteolytic cleavage and inhibit FGF-2–FGFR interaction and FGF-2–dependent cell proliferation (Coltrini et al., 1994; Rusnati et al., 1994). In contrast, cell-associated HSPGs increase the local concentration of FGF-2 and modulate FGFR binding, dimerization, and signaling, thus promoting the biological activity of the growth factor (Rusnati and Presta, 1996).
In conclusion, we have demonstrated that exogenous free gangliosides 1) protect FGF-2 from proteolytic degradation; 2) modulate the binding of the growth factor to tyrosine kinase FGFRs; 3) modulate cell internalization of FGF-2; and 4) inhibit FGF-2–dependent endothelial cell proliferation. All these effects occur at concentrations of free ganglioside between 0.3 and 30 μM. During tumor growth and metastasis, gangliosides shed in the microenvironment (Kloppel et al., 1977; Merritt et al., 1994; Chang et al., 1997). This process can be so extensive as to alter the ganglioside composition of the extracellular environment of the tumor (Kloppel et al., 1977). It has been demonstrated that tumor cells can shed up to 0.5% of their membrane ganglioside content per hour (Li and Ladish, 1991) and that gangliosides are present at concentrations as high as 10 μM in the serum of tumor-bearing patients (Valentino and Ladisch, 1992). On this basis, because of the possible role of FGF-2 in tumor angiogenesis (Rak and Kerbel, 1997), gangliosides shed by tumor cells may affect endothelial cell function by interacting with FGF-2, thus modulating tumor neovascularization.
ACKNOWLEDGMENTS
We thank Prof. P. Gullino for having inspired this work, Miss G. Benaglia, Miss M. Fazio, and Dr. M. D’Adda for their expert technical assistance, and the Medical Research Council AIDS Reagent Project (Potters Bar, Herts, United Kingdom) for the synthetic HIV-1 Tat(41–60) peptide. This work was supported by grants from Consiglio Nazionale Ricerche (Progetto Finalizzato Biotecnologie n°. 97.01186.PF49), Associazione Italiana Ricerca sul Cancro (Special Project Angiogenesis), Ministero Università Ricerca Scientifica e Tecnologica (“Cofinanziamento 1997 Infiammazione: biologia e clinica” to M.P., Cofinanziamento 1998 Meccanismi mole colari di comunicazione intercellulare to M.R., and “60%” to M.P. and M.R.), and from Istituto Superiore della Sanità (AIDS Project) to M.P.
Footnotes
Abbreviations: FGF, fibroblast growth factor; FGFR, tyrosine-kinase FGF receptor; HSPGs, heparan sulfate proteoglycans; IFN, interferons; MAE cells, mouse aortic endothelial cells; NeuAc, neuraminic acid; xcFGFR-1, soluble extracellular form of FGFR-1/flg. Gangliosides are named according to the nomenclature of Svennerholm (1964).
REFERENCES
- Alessandri G, De Cristian G, Ziche M, Cappa APM, Gullino PM. Growth and motility of microvascular endothelium are modulated by the relative concentration of gangliosides in the medium. J Cell Physiol. 1992;151:23–28. doi: 10.1002/jcp.1041510105. [DOI] [PubMed] [Google Scholar]
- Ando I, Komine M, Otsuka F, Kukita A. Alteration of human melanoma gangliosides by IFN-gamma, IL-2, and IL-4. J Dermatol. 1996;23:225–229. doi: 10.1111/j.1346-8138.1996.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Baird A, Schubert D, Ling N, Guillemin R. Receptor and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci USA. 1988;85:2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Bastaki M, Nelli EE, Dell’Era P, Rusnati M, Molinari-Tosatti MP, Parolini S, Auerbach R, Ruco LP, Possati L, Presta M. Basic fibroblast growth factor-induced angiogenic phenotype in mouse endothelium: a study on aortic and microvascular endothelial cell lines. Arterioscler Thromb Vasc Res. 1996;17:454–464. doi: 10.1161/01.atv.17.3.454. [DOI] [PubMed] [Google Scholar]
- Bergonzoni L, Caccia P, Cletini O, Sarmientos P, Isacchi A. Characterization of a biologically active extracellular domain of the FGF receptor-1 expressed in Escherichia coli. Eur J Biochem. 1992;210:823–829. doi: 10.1111/j.1432-1033.1992.tb17485.x. [DOI] [PubMed] [Google Scholar]
- Besancon F, Ankel H. Binding of interferon to gangliosides. Nature. 1974;252:478–480. doi: 10.1038/252478a0. [DOI] [PubMed] [Google Scholar]
- Boldin S, Futerman AH. Glucosylceramide synthesis is required for basic fibroblast growth factor and laminin to stimulate axonal growth. J Neurochem. 1997;68:882–885. doi: 10.1046/j.1471-4159.1997.68020882.x. [DOI] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S-I. GM3 ganglioside induces hamster fibroblast growth factor inhibition in chemically-defined medium: ganglioside may regulate growth factor receptor function. Biochem Biophys Res Commun. 1982;106:711–718. doi: 10.1016/0006-291x(82)91769-7. [DOI] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S-I, Bowen-Pope DF, Raines E, Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984;259:6818–6825. [PubMed] [Google Scholar]
- Bremer EG, Schlessinger J, Hakomori S-I. Gangliosides-mediated modulation of cell growth. J Biol Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- Bueb JL, Mousli M, Bronner C, Rouot B, Landry Y. Activation of Gi-like proteins, a receptor-independent effect of kinins in mast cells. Mol Pharmacol. 1990;38:816–822. [PubMed] [Google Scholar]
- Cestaro B, Pistolesi E, Hershkowitz N, Gatt S. Preparation of asymmetric, cerebroside sulfate-containing phospholipid vesicles. Biochim Biophys Acta. 1982;8:13–20. doi: 10.1016/0005-2736(82)90028-1. [DOI] [PubMed] [Google Scholar]
- Chang F, Li R, Ladisch S. Shedding of gangliosides by human medulloblastoma cells. Exp Cell Res. 1997;234:341–346. doi: 10.1006/excr.1997.3619. [DOI] [PubMed] [Google Scholar]
- Chu JW, Sharom FJ. Interleukin-2 binds to gangliosides in micelles and lipid bilayers. Biochim Biophys Acta. 1990;1028:205–214. doi: 10.1016/0005-2736(90)90168-n. [DOI] [PubMed] [Google Scholar]
- Chu JW, Sharom FJ. Gangliosides interact with interleukin-4 and inhibit interleukin-4-stimulated helper T-cell proliferation. Immunology. 1995;84:396–403. [PMC free article] [PubMed] [Google Scholar]
- Cockerill GW, Gamble JR, Vadas MA. Angiogenesis: models and modulators. Int Rev Cytol. 1995;159:113–160. doi: 10.1016/s0074-7696(08)62106-3. [DOI] [PubMed] [Google Scholar]
- Coltrini D, Rusnati M, Zoppetti G, Oreste P, Grazioli G, Naggi A, Presta M. Different effects of mucosal, bovine lung and chemically modified heparin on selected biological properties of basic fibroblast growth factor. Biochem J. 1994;303:583–590. doi: 10.1042/bj3030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltrini D, Rusnati M, Zoppetti G, Oreste P, Isacchi A, Caccia P, Bergonzoni L, Presta M. Biochemical bases of the interaction of human basic fibroblast growth factor with glycosaminoglycans. Eur J Biochem. 1993;214:51–58. doi: 10.1111/j.1432-1033.1993.tb17895.x. [DOI] [PubMed] [Google Scholar]
- De Cristian G, Morbidelli L, Alessandri G, Ziche M, Cappa APM, Gullino PM. Synergism between gangliosides and basic fibroblast growth factor in favouring survival, growth, and motility of capillary endothelium. J Cell Physiol. 1990;144:505–510. doi: 10.1002/jcp.1041440319. [DOI] [PubMed] [Google Scholar]
- Drago J, Reid KL, Bartlett PF. Induction of the ganglioside marker A2B5 on cultured cerebellar neural cells by growth factors. Neurosci Lett. 1989;107:245–250. doi: 10.1016/0304-3940(89)90825-2. [DOI] [PubMed] [Google Scholar]
- Dumontet C, Rebbaa A, Bienvenu J, Portoukalian J. Inhibition of immune cell proliferation and cytokine production by lipoprotein-bound gangliosides. Cancer Immunol Immunother. 1994;38:311–316. doi: 10.1007/BF01525509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano S, Johnson ML, Lee G, Aloj SM, Edelhoch H. Critical concentrations of gangliosides. Biochemistry. 1979;18:1119–1124. doi: 10.1021/bi00573a028. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Arita Y, Satomi N, Eisinger M, Lloyd KO. Tumor necrosis factor enhances ganglioside expression in cultured human melanocytes. Arch Biochem Biophys. 1990;281:70–75. doi: 10.1016/0003-9861(90)90414-t. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Stephen NM, Levine EM. Bovine endothelial cell transformed in vitro by benzo(a)pyrene. J Cell Physiol. 1983;114:328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- Gullino PM. Prostaglandins and gangliosides of tumor microenvironment: their role in angiogenesis. Acta Oncol. 1995;34:439–441. doi: 10.3109/02841869509094005. [DOI] [PubMed] [Google Scholar]
- Gullino PM, Ziche M, Alessandri G. Gangliosides, copper ions and angiogenic capacity of adult tissues. Cancer Metastasis Rev. 1990;9:239–251. doi: 10.1007/BF00046363. [DOI] [PubMed] [Google Scholar]
- Hakomori S-I. Bifunctional role of glycosphingolipids. J Biol Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- Hanai N, Dohi T, Nores GA, Hakomori S-I. A novel ganglioside, de-N-acetyl-GM3 (II3NeuNH2LacCer), acting as a strong promoter for epidermal growth factor receptor kinase and as a stimulator for cell growth. J Biol Chem. 1988a;263:6296–6301. [PubMed] [Google Scholar]
- Hanai N, Nores GA, MacLeod C, Torres-Mendez C-R, Hakomori S-I. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 and lyso-GM3 in tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1988b;263:10915–10921. [PubMed] [Google Scholar]
- Hara A, Radin NS. Simple procedures for the rapid cleavage of ester lipids and for the large-scale isolation from brain of cerebroside sulfate. Anal Biochem. 1979;100:364–370. doi: 10.1016/0003-2697(79)90242-2. [DOI] [PubMed] [Google Scholar]
- Hoons DS, Okun E, Banez M, Irie RF, Morton DL. Interleukin 4 alone and with γ-interferon or α-tumor necrosis factor inhibits cell growth and modulates cell surface antigens on human renal cell carcinomas. Cancer Res. 1991;51:5687–5693. [PubMed] [Google Scholar]
- Hynds DL, Burry RW, Yates AJ. Gangliosides inhibit growth factor-stimulated neurite outgrowth in SH-SY5Y human neuroblastoma cells. J Neurosci Res. 1997;47:617–625. doi: 10.1002/(sici)1097-4547(19970315)47:6<617::aid-jnr7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hynds DL, Summers M, Van Brocklyn J, O’Dorisio MS, Yates AJ. Gangliosides inhibit platelet-derived growth factor-stimulated growth, receptor phosphorylation, and dimerization in neuroblastoma SH-SY5Y cells. J Neurochem. 1995;65:2251–2258. doi: 10.1046/j.1471-4159.1995.65052251.x. [DOI] [PubMed] [Google Scholar]
- Isacchi A, Statuto M, Chiesa R, Bergonzoni L, Rusnati M, Sarmientos P, Ragnotti G, Presta M. A six amino acid deletion in basic fibroblast growth factor dissociates its mitogenic activity from its plasminogen activator-inducing capacity. Proc Natl Acad Sci USA. 1991;88:2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Tyrrell DJ, Stauber GB, Brown S, Cousens LS, Stack RJ. Preparation of affinity-fractionated, heparin-derived oligosaccharides and their effect on selected biological activities mediated by basic fibroblast growth factor. J Biol Chem. 1993;268:4675–4683. [PubMed] [Google Scholar]
- Jonhson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Rygaard J, Bendtzen K, Josefsen K, Bock T, Buschard K. Interleukins increase surface gangliosides expression of pancreatic islet cells in vitro. APMIS. 1992;100:509–514. doi: 10.1111/j.1699-0463.1992.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Kloppel TM, Keenan TW, Freeman MJ, Morre DJ. Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc Natl Acad Sci USA. 1977;74:3011–3013. doi: 10.1073/pnas.74.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Honke K, Miyazaki T, Matsumoto K, Nakamura T, Ishizuka I, Makita A. Hepatocyte growth factor specifically binds to sulfoglycolipids. J Biol Chem. 1994;269:9817–9821. [PubMed] [Google Scholar]
- Koochekpour S, Merzak A, Pilkington GJ. Vascular endothelial growth factor production is stimulated by gangliosides and TGF-β isoforms in human glioma cells in vitro. Cancer Lett. 1996;102:209–215. doi: 10.1016/0304-3835(96)04161-4. [DOI] [PubMed] [Google Scholar]
- Koochekpour S, Pilkington GJ. Vascular and perivascular GD3 expression in human glioma. Cancer Lett. 1996;104:97–102. doi: 10.1016/0304-3835(96)04231-0. [DOI] [PubMed] [Google Scholar]
- Li R, Ladisch S. Abrogation of shedding of immunosuppressive neuroblastoma gangliosides. Biochim Biophys Acta. 1991;1083:57–64. doi: 10.1016/0005-2760(91)90124-z. [DOI] [PubMed] [Google Scholar]
- Li LY, Seddon AP. Fluorospectrometric analysis of heparin interaction with fibroblast growth factors. Growth Factors. 1994;11:1–7. doi: 10.3109/08977199409015046. [DOI] [PubMed] [Google Scholar]
- Marchesini S, Demasi L, Cestone P, Preti A, Agmon V, Dagan A, Navon R, Gatt S. Sulforhodamine GM1-ganglioside: synthesis and physicochemical properties. Chem Phys Lipids. 1994;72:143–152. doi: 10.1016/0009-3084(94)90098-1. [DOI] [PubMed] [Google Scholar]
- McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Merritt WD, Der-Minassian V, Reaman GH. Increased GD3 ganglioside in plasma of children with T-cell acute lymphoblastic leukemia. Leukemia. 1994;8:816. , 822. [PubMed] [Google Scholar]
- Meuillet E, Cremel G, Dreyfus H, Hicks D. Differential modulation of basic fibroblast growth factor and epidermal growth factor receptor activation by ganglioside GM3 in cultured retinal Muller glia. Glia. 1996a;17:206–216. doi: 10.1002/(SICI)1098-1136(199607)17:3<206::AID-GLIA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Meuillet E, Cremel G, Hicks D, Dreyfus H. Ganglioside effects on basic fibroblast growth factor and epidermal growth factor receptor in retinal glial cells. J Lipid Mediat Cell Signal. 1996b;14:277–288. doi: 10.1016/0929-7855(96)00536-6. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987;131:123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Moscatelli D, Presta M, Rifkin DB. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci USA. 1986;83:2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor fucntion. Proc Natl Acad Sci USA. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Kirito K, Tsunoda A, Hara K, Furukawa Y, Saito M. Interleukin-3-associated ganglioside GD1a is induced independently of normal interleukin-3 receptor in murine myelogenous leukemia NFS60 cells transfected with the interleukin-3 gene. Glycoconjugate J. 1996;13:255–261. doi: 10.1007/BF00731500. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985;260:13860–13868. [PubMed] [Google Scholar]
- Nojiri H, Stroud M, Hakomori S-I. A specific type of ganglioside as a modulator of insulin-dependent cell growth and insulin receptor tyrosine kinase activity. J Biol Chem. 1991;266:4531–4537. [PubMed] [Google Scholar]
- Pantoliano MW, Horlick RA, Springer BA, Van Dyk DE, Tobery T, Wetmore DR, Lear JD, Nahapetian AT, Bradley JD, Sisk WP. Multivalent ligand-receptor binding interactions in the fibroblast growth factor system produce a cooperative growth factor and heparin mechanism for receptor dimerization. Biochemistry. 1994;33:10229–10247. doi: 10.1021/bi00200a003. [DOI] [PubMed] [Google Scholar]
- Pilkington GJ, Dunan JR, Rogers JP, Clarke TM, Knott JC. Growth factor modulation on surface ganglioside expression in cloned neoplastic glia. Neurosci Lett. 1993;149:1–5. doi: 10.1016/0304-3940(93)90332-f. [DOI] [PubMed] [Google Scholar]
- Presta M, Maier JAM, Rusnati M, Ragnotti G. The mitogenic signaling pathway but not the plasminogen activator-inducing pathway of basic fibroblast growth factor is mediated through protein kinase C in fetal bovine aortic endothelial cells. J Cell Biol. 1989;109:1877–1884. doi: 10.1083/jcb.109.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M, Rusnati M, Urbinati C, Tanghetti E, Statuto M, Pozzi A, Gualandris A, Ragnotti G. Basic fibroblast growth factor bound to cell substrate promotes cell adhesion, proliferation, and protease production in cultured endothelial cells. Experientia. 1992;61:205–209. doi: 10.1007/978-3-0348-7001-6_31. [DOI] [PubMed] [Google Scholar]
- Rak J, Kerbel RS. bFGF and tumor angiogenesis … back in the limelight? Nat Med. 1997;3:1083–1084. doi: 10.1038/nm1097-1083. [DOI] [PubMed] [Google Scholar]
- Roghani M, Moscatelli D. Basic fibroblast growth factor is internalized through both receptor-mediated and heparan sulfate-mediated mechanisms. J Biol Chem. 1992;267:22156–22162. [PubMed] [Google Scholar]
- Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Coltrini D, Caccia P, Dell’Era P, Zoppetti G, Oreste P, Valsasina B, Presta M. Distinct role of 2-O-, N-, and 6-O-sulfate groups of heparin in the formation of the ternary complex with basic fibroblast growth factor and soluble FGF receptor-1. Biochem Biophys Res Commun. 1994;203:450–458. doi: 10.1006/bbrc.1994.2203. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Int J Clin Lab Res. 1996;26:15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Tanghetti E, Dell’Era P, Gualandris A, Presta M. αvβ3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997a;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M, Tulipano G, Urbinati C, Tanghetti E, Giuliani R, Giacca M, Ciomei M, Corallini A, Presta M. The basic domain in HIV-1 Tat as a target for polysulfated heparin-mimicking extracellular Tat antagonist. J Biol Chem. 1997b;273:16027–16037. doi: 10.1074/jbc.273.26.16027. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Urbinati C, Presta M. Internalization of basic fibroblast growth factor (bFGF) in cultured endothelial cells: role of the low affinity heparin-like bFGF receptors. J Cell Physiol. 1993;154:152–161. doi: 10.1002/jcp.1041540119. [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Kraus R, Seul C, Meyer zu Brickweddle MK, Schulte K, Ko Y, Hoppe J, Vetter H. Gangliosides GM1, GM2, and GM3 inhibit the platelet-derived growth factor-induced signaling transduction pathway in vascular smooth muscle cells by different mechanisms. Eur J Cell Biol. 1996;71:79–88. [PubMed] [Google Scholar]
- Saqr HE, Pearl DK, Yates AJ. A review and predictive models of ganglioside uptake by biological membranes. J Neurochem. 1993;61:395–411. doi: 10.1111/j.1471-4159.1993.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Saqr HE, Walters JD, Guan Z, Stokes BT, Yates AJ. Gangliosides inhibit PDGF-induced signal transduction events in U-1242 MG human glioma cells. Neurochem Res. 1995;20:1389–1395. doi: 10.1007/BF00992515. [DOI] [PubMed] [Google Scholar]
- Scatchard G. The attraction of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- Schubert D, Ling N, Baird A. Multiple influences of a heparin-binding growth factor on neuronal development. J Cell Biol. 1987;104:635–643. doi: 10.1083/jcb.104.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom FJ, Chiu AL, Chu JW. Membrane gangliosides modulate interleukin-2-stimulated T-lymphocyte proliferation. Biochim Biophys Acta. 1991;1094:35–42. doi: 10.1016/0167-4889(91)90023-q. [DOI] [PubMed] [Google Scholar]
- Song W, Vacca MF, Welti R, Rintoul DA. Effects of gangliosides GM3 and de-N-acetyl GM3 on epidermal growth factor receptor kinase activity and cell growth. J Biol Chem. 1991;266:10174–10181. [PubMed] [Google Scholar]
- Springer BA, Pantoliano MW, Barbera FA, Gunyuzlu PL, Thompson LD, Herblin WF, Rosenfeld SA, Book GW. Identification and concerted function of two receptor binding surfaces on basic fibroblast growth factor required for mitogenesis. J Biol Chem. 1994;269:26879–26884. [PubMed] [Google Scholar]
- Svennerholm L. On the isolation and characterization of N-acetyl-sialic acid. Nature. 1956;177:524–527. [PubMed] [Google Scholar]
- Thompson LD, Pantoliano MW, Springer BA. Energetic characterization of the basic fibroblast growth factor-heparin interaction: identification of the heparin binding domain. Biochemistry. 1994;33:3831–3840. doi: 10.1021/bi00179a006. [DOI] [PubMed] [Google Scholar]
- Ulrich-Bott B, Wiegandt H. Micellar properties of glycosphingolipids in aqueous media. J Lipid Res. 1984;25:1233–1245. [PubMed] [Google Scholar]
- Valentino LA, Ladisch S. Localization of shed human tumor gangliosides: association with serum lipoproteins. Cancer Res. 1992;52:810–814. [PubMed] [Google Scholar]
- Van Brocklyn JR, Bremer EG, Yates AJ. Gangliosides inhibit platelet-derived growth factor-stimulated receptor dimerization in human glioma U-1242MG and Swiss 3T3 cells. J Neurochem. 1993;61:371–374. doi: 10.1111/j.1471-4159.1993.tb03581.x. [DOI] [PubMed] [Google Scholar]
- Vengris VE, Reynolds FH, Jr, Hollenberg MD, Pitha PM. Interferon action: role of membrane gangliosides. Virology. 1976;72:486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Weis FMB, Davis RJ. Regulation of epidermal growth factor receptor signal transduction. J Biol Chem. 1990;265:12059–12066. [PubMed] [Google Scholar]
- Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zeller CB, Marchase RB. Gangliosides as modulators of cell function. Am J Physiol. 1992;262:C1341–C1355. doi: 10.1152/ajpcell.1992.262.6.C1341. [DOI] [PubMed] [Google Scholar]
- Zhang C, Paller AS, Mirkin BL. Inhibitory action of ganglioside GM3 on murine neuroblastoma cell proliferation: modulating effect of fetal calf serum. Anticancer Res. 1995;15:661–666. [PubMed] [Google Scholar]
- Ziche M, Alessandri G, Gullino PM. Gangliosides promote the angiogenic response. Lab Invest. 1989;61:629–634. [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Alessandri G, Gullino PM. Angiogenesis can be stimulated or repressed in vivo by a change in GM3:GD3 ganglioside ratio. Lab Invest. 1992;67:711–715. [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Kafferlein E, Haas JG, Meyer N, Strobel M, Weber C, Flieger D. Gangliosides suppress tumor necrosis factor production in human monocytes. J Immunol. 1992;148:1753–1758. [PubMed] [Google Scholar]