Abstract
The Deese/Roediger–McDermott (DRM) false-memory effect has been extensively documented in psychological research. People falsely recognize critical lures or nonstudied items that are semantically associated with studied items. Behavioral research has provided evidence for age-related increases in the DRM false-recognition effect. The present event-related functional magnetic resonance imaging study was aimed at investigating neurodevelopmental changes in brain regions associated with true- and false-memory recognition in 8-year olds, 12-year olds, and adults. Relative to 8-year olds, adults correctly endorsed more studied items as “old” but also mistakenly endorsed more critical lures. Age-related increases in recollection were associated with changes in the medial temporal lobe (MTL) activation profile. Additionally, age-related increases in false alarms (FAs) to semantically related lures were associated with changes in the activation profile of left ventrolateral prefrontal cortex, a region associated with semantic processing. Additional regions exhibiting age-related changes include posterior parietal and anterior prefrontal cortices. In summary, concomitant changes in the MTL, prefrontal cortex, and parietal cortex underlie developmental increases in true and false recognition during childhood and adolescence.
Keywords: children, cognitive development, fMRI, frontoparietal, hippocampus, long-term memory
Introduction
Memory distortions have attracted the interest of psychologists and cognitive neuroscientists because of their potential to elucidate the principles of human memory functioning (Schacter et al. 1998). The Deese/Roediger–McDermott (DRM) paradigm is frequently used to examine false memory because it reliably induces a robust false-recognition effect (Roediger and McDermott 1995). After studying several word lists converging on a semantic theme captured in a word that is never presented during study (i.e., critical lure), participants perform an old/new recognition test that includes studied words (i.e., targets), critical lures, and other lures that are nonsemantically associated with the studied materials (i.e., unrelated lures). When tested under these conditions, adult participants are typically as likely to falsely recognize critical lures as they are to correctly recognize studied words (McDermott and Roediger 1998).
Most behavioral studies have provided evidence for age-related increases in the DRM false-recognition effect (e.g., Brainerd et al. 2002; Howe et al. 2004; Brainerd et al. 2006). However, several studies have not shown an identical pattern (Ghetti et al. 2002; Sugrue and Hayne 2006; Carneiro et al. 2007), suggesting that under certain conditions this effect may be limited by processes that counter false-memory formation. Whereas the development of recollection and memory monitoring should promote age-related decreases in the DRM effect (Brainerd et al. 2004; Ghetti and Angelini, forthcoming), the development of the ability to process the semantic theme of the lists should lead to age-related increases in the effect. Given that these processes may work against each other in producing the memory output, it is important to examine their development separately. Neuroimaging research holds much promise for characterizing the concomitant developmental changes in the various cognitive processes that are likely to play a role in age-related changes in the DRM effect.
Neuroimaging studies involving adult participants have implicated the medial temporal lobe (MTL), parietal cortex, and prefrontal cortex (PFC) in episodic memory retrieval and have provided evidence that these areas support recollection, semantic processing, and memory monitoring during the recovery of episodic information (Eldridge et al. 2000; Rugg et al. 2003; Dobbins et al. 2004; Henson 2005). The MTL is critically involved in the recollection of semantic and sensory properties of episodic information (Eldridge et al. 2000; Stark and Squire 2000; Cabeza et al. 2001; Eichenbaum et al. 2007) and novelty detection (Daselaar et al. 2006). For instance, using a DRM task with adults, a study of Cabeza et al. (2001) showed a dissociation between anterior and posterior MTL, suggesting that the former is involved in the recovery of semantic information and the latter is implicated in the recovery of perceptual features. Research in amnesic patients involving the DRM task and other paradigms indicates that the MTL is critical for the extraction, maintenance, and retrieval of gist information (Schacter, Verfaellie, et al. 1996; Verfaellie et al. 2002). As for the development of MTL region, a recent magnetic resonance imaging (MRI) study using the technique of cortical pattern matching (CPM) to assess cortical thickness at each point in the brain revealed that maturational changes in hippocampal structure are evident between ages 4 and 25 (Gogtay et al. 2006). This CPM study provided evidence of a reduction in cortical thickness in anterior hippocampus over development, with a concomitant increase in posterior hippocampus, supporting the idea that the MTLs do not mature as early as was previously thought.
Like MTL, increased activation in left posterior parietal cortex (PPC) has been associated with successful episodic retrieval (Cabeza et al. 2001; Shannon and Buckner 2004; Slotnick and Schacter 2004; Yonelinas et al. 2005; but see Wheeler and Buckner 2003). A graded pattern of responses is observed in this region, as a function of the amount of contextual information retrieved (Henson, Rugg, et al. 1999; Konishi et al. 2000; McDermott et al. 2000).
Within PFC, left ventrolateral prefrontal cortex (VLPFC) has been linked to “controlled” aspects of semantic processing, associated with the encoding of subsequent true memory and with episodic retrieval (Prince et al. 2005; Kim and Cabeza 2007). In associative false-memory paradigms, controlled semantic processing enhances memory for studied words as well as for nonstudied semantic associates (Gallo et al. 2001). Additionally, left dorsolateral prefrontal cortex (DLPFC) and right anterior prefrontal cortex (aPFC) have been generally found to be recruited more strongly for old than for new items (Buckner et al. 1998; Henson, Shallice, et al. 1999; Henson 2005), but this effect is only observed under certain task demands (Herron et al. 2004). DLPFC and aPFC activation during memory retrieval is thought to reflect monitoring demands and decision-related processes (Wagner, Desmond, et al. 1998; Dobbins et al. 2004). Of special interest, maturational changes in the structure and function of these PFC regions during childhood and adolescence may, in part, underlie developmental improvements in various cognitive processes (see Bunge and Wright 2007).
In the present study, we sought to examine whether MTL immaturity would account for age-related differences in the DRM false-recognition effect. Additionally or alternatively, developmental changes in this effect could be associated with changes in frontoparietal regions involved in semantic elaboration and controlled aspects of episodic retrieval as shown in previous DRM (McDermott et al. 2000; Slotnick and Schacter 2004) and memory recognition studies (Wagner, Schacter, et al. 1998; Prince et al. 2005). The objective of the present event-related functional magnetic resonance imaging (fMRI) study was to test these possible accounts. As such, we investigated age-related changes in the activation profile of MTL, parietal cortex, and PFC regions during true- and false-memory retrieval.
To date, no published study has investigated the neurodevelopmental correlates of true- and false-memory retrieval with fMRI. However, 2 recent studies (Menon et al. 2005; Chiu et al. 2006) have examined the neural correlates of memory encoding processes during childhood and adolescence. These studies show that, in some cases, associations between critical brain areas and subsequent memory were found in a larger number of areas in younger children (ages 7–8) compared with older children and adults (between the ages of 10 and 18 years) (i.e., VLPFC and aPFC in younger children but not in the older age group; Chiu et al. 2006). In other cases, these associations were evident in older but not younger children (e.g., hippocampus; Chiu et al. 2006). Further, functional connectivity analyses indicate that connectivity between MTL areas and PFC areas increases between 11 and 19 years of age (Menon et al. 2005). Overall, these results indicate that neurodevelopmental changes occur over the course of childhood and adolescence and that these changes may be reflected in the failure of younger children to recruit the areas that adults engage during memory retrieval. In the current study, we sought to characterize age-related changes in the engagement of brain systems underlying developmental differences in the DRM false-recognition effect on 3 age groups: 8-year olds, 12-year olds, and young adults.
Materials and Methods
Participants
Our sample consisted of 48 right-handed, native English-speaking participants distributed equally across gender and 3 age groups: 8-year olds (M = 8.55 years, range = 8.08–9), 12-year olds (M = 12.45 years, range = 12–12.92), and young adults (M = 21.2 years, range = 19.69–22.97). Data from 11 additional participants were excluded from analyses due to excessive head motion during imaging (i.e., ≥6 mm across the entire scan session, where 6 mm corresponds to twice the in-plane voxel dimensions), technical difficulties during fMRI data acquisition, scores in the clinical range on the behavioral checklist screenings (i.e., Child Behavior Checklist, Achenbach and Rescorla 2001; Symptom Checklist-90-Revised, Derogatis and Savitz 1999), or failure to understand the task. Participants received either monetary compensation or course credit for their participation. Prior to taking part in the experiment, all participants gave informed consent based on procedures approved by the Internal Review Board of the University of California (UC), Davis.
Task and Procedure
Twenty-three lists of 12 words each were adapted from materials used previously in the DRM experimental paradigm (Roediger and McDermott 1995). These lists were selected on the basis of adult norms for DRM lists (Stadler et al. 1999), in such a way that they produced variable intrusion rates of the critical lure. During the study phase, which occurred outside the scanner, participants studied the 23 word lists. Words within each list were presented in order of decreasing strength of association. Participants were instructed to do their best to remember each word. Lists were presented auditorily at a rate of 1 word every 2500 ms, and presentation order of the lists was random. After listening to each list, participants were asked to perform a 30-s filler task, to prevent rehearsal during this interval. In this filler task participants were required to count backwards by 2 (8-year olds) or by 6 (12-year olds and adults), starting from different numbers each time. Initial piloting on 12 behavioral participants indicated that 8-year olds could count backwards by 2 at a similar rate (M = 2857 ms, SD = 2328) as 12-year olds and adults could count backwards by 6 (M = 3136 ms, SD = 1413 and M = 2913, SD = 1586, respectively). Reminders of the counting instructions were provided throughout the session.
In preparation for the test phase, participants were instructed to respond “yes” if they remembered the word from the study session or “no” if they did not. The fMRI data acquisition occurred during this retrieval phase. The recognition memory test included 138 words: 46 studied items (i.e., targets), 46 nonstudied semantic associates (i.e., critical lures), and 46 new unrelated items (i.e., unrelated lures). As in the DRM study of Cabeza et al. (2001) in adults, targets consisted of 2 items from each studied list (always those in serial positions 1 and 8) and critical lures were the 1st and 3rd associate for each list (which were not presented during the study session). Unrelated lures were selected from nonsemantically related words on the basis of the Medical Research Council Psycholinguistic Database (Coltheart 1981). These unrelated lures matched critical lures in frequency, familiarity, concreteness, and age of acquisition norms (Kucera and Francis 1967; Gilhooly and Logie 1980).
The 138 trials were presented in 2 functional runs, with 16 randomized orders. First, participants viewed a drawing depicting an ear for 1500 ms, which signaled that a word was being presented auditorily. Next, the words “Yes” and “No” were projected on the presentation screen for 2000 ms, instructing participants to respond by making left-handed keypresses on a 2-button fiber-optic box. Finally, a fixation crossbar was displayed for 500 ms prior to the start of the next trial.
The main dependent measures of behavior were hits (proportion of recognized studied items), FAs to critical lures (proportion of falsely recognized critical items), and FAs to unrelated lures (proportion of recognized unrelated items). When examining the fMRI results, we were also interested in examining the neural correlates of correct rejections (CRs) of critical and unrelated lures.
fMRI Data Acquisition
Whole-brain fMRI was conducted on a 3-T Siemens TRIO whole-body MRI scanner (Siemens Medical Solutions, Erlangen, Germany) at the UC Davis Imaging Research Center using a standard whole-head coil. Functional images were acquired using a gradient-echo echo-planar pulse sequence (time repetition = 2000 ms, time echo = 25 ms, 34 axial slides, no inter-slice gap, flip angle = 90°, field of view = 220 mm, 189 volumes per run). Coplanar T2-weighted and high-resolution T1-weighted anatomical images were collected. To limit head movement, the area between participants' heads and the head coil was padded with foam, and participants were asked to remain as still as possible. Snugly fitting headphones (MR Confon, Magdeburg, Germany) dampened background scanner noise and enabled auditory stimulus presentation and communication with experimenters while in the scanner.
fMRI Data Analysis
Data were preprocessed with SPM2 (Wellcome Department of Cognitive Neurology, London). Images were corrected for differences in timing of slice acquisition followed by rigid body motion correction. The motion parameters for translation (i.e., x, y, z) and rotation (i.e., yaw, pitch, roll) were included as covariates of noninterest in the general linear model (GLM). Structural and functional volumes were spatially normalized to T1 and echo-planar imaging templates, respectively. The normalization algorithm used a 12-parameter affine transformation together with a nonlinear transformation involving cosine basis functions. During normalization, the volumes were resampled to 3-mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al. 1997), an approximation of Talairach space (Talairach and Tourneaux 1988). These procedures have been validated for use in children aged 6 and above (e.g., Burgund et al. 2002; Kang et al. 2003). Functional volumes were spatially smoothed with an 8-mm full width at half maximum isotropic Gaussian kernel.
Statistical analyses were performed on individual participants' data by using the GLM in SPM. The fMRI time series data were modeled by a series of events convolved with a canonical hemodynamic response function (HRF). The least squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pairwise contrasts. Contrast images, computed on a participant-by-participant basis, were submitted to group analyses. At the group level, whole-brain exploratory contrasts between conditions were computed by performing 1-tailed t-tests on these images, treating participants as a random effect (Supplementary Table 1). Task-related responses were considered significant if they consisted of at least 5 contiguous voxels that exceeded an uncorrected threshold of 0.001 for adults and 12-year olds and of 0.005 for 8-year olds. All brain coordinates are reported in Montreal Neurological Institute atlas space (Cocosco et al. 1997).
Multiple regression analyses were performed across all participants on the average images for 3 contrasts of interest (Supplementary Table 2). The contrasts were selected to reveal activation related to true recognition, hits > unrelated lure CRs; semantic processing, critical lure FAs > unrelated lure CRs; and memory monitoring, critical lure CRs > unrelated lure CRs. These analyses allowed us to examine regions that exhibited changes across the 3 age groups and/or performance-related increases or decreases in brain activation during true and false recognition, while controlling for the influence of other variables. Regression analyses were performed at a statistical threshold of P < 0.005, uncorrected for multiple comparisons, with an extent threshold of 5 contiguous voxels.
Region of interest (ROI) analyses were performed for MTL, PPC, VLPFC, and aPFC, and additionally for lateral temporal cortex and DLPFC (Supplementary Fig. 6), with the MARSBAR toolbox for use with SPM2 (Brett et al. 2002). ROIs consisted of active voxels for contrasts identified from multiple regressions across all participants within a specific MARSBAR anatomical ROI. For ROI analyses, effects were considered significant at an alpha equal to 0.005. The center of mass of each ROI is reported in figures. Blood oxygenation level–dependent activity time series, averaged across all voxels in an ROI, were extracted for each experimental session by using MARSBAR.
Results
Behavioral Results
Recognition Memory
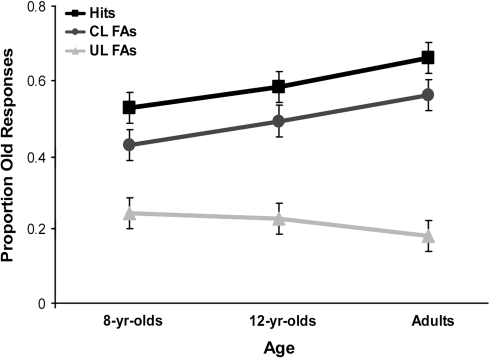
A 3 (age: 8-year olds, 12-year olds, adults) × 3 (item type: targets, critical lures, unrelated lures) mixed model analysis of variance (ANOVA) revealed that a significant main effect of item type, F2,90 = 181.91, P < 0.001, was qualified by a significant age × item type interaction, F4,90 = 5.24, P < 0.01, (Fig. 1).
Figure 1.
Behavioral results. Mean proportion of endorsements as a function of age and item type (hits, critical lure FAs, unrelated lure FAs).
Simple-effects analyses indicated that adults produced a higher proportion of hits than did 8-year olds, P < 0.01. Hits in 12-year olds were intermediate to the other 2 groups and did not statistically differ from those observed in either 8-year olds, P = 0.15, or adults, P = 0.45. The same pattern of results was found for FAs to critical lures; that is, adults were more likely to falsely recognize critical lures than were 8-year olds, P < 0.05. As was the case for hits, false recognition of critical lures in 12-year olds did not statistically differ from either that of 8-year olds or adults, P values >0.50. In contrast, no age differences were found in FAs to unrelated lures, P = 0.43. Thus, adults not only exhibited higher true recognition for targets than 8-year olds but also exhibited a larger DRM false-recognition effect for critical lures. Twelve-year olds' performance was intermediate to that of the oldest and youngest age groups. There were no age-related-differences among age groups in terms of performance on unrelated lures (see Supplementary Material for response time analysis).
Signal-Detection Measures
Performance was also analyzed using A′ and BD″ signal-detection measures, which provide estimates of sensitivity and response bias, respectively (Macmillan and Creelman 2005). These analyses were intended to examine whether the developmental differences in performance discussed thus far can be attributed specifically to changes in 1) “item-specific recollection” or the ability to discriminate between correctly endorsed studied items from FAs to critical lures (CL) and unrelated lures (UL) (i.e., hits-CL FAs, hits-UL FAs) and/or 2) “gist memory” or the ability to distinguish between FAs to critical lures, treating them as a form of gist-like memory, and FAs to unrelated lures (i.e., CL FAs-UL FAs).
A 3 (age: 8-year olds, 12-year olds, adults) × 3 (type of discrimination: hits-CL, hits-UL, CL-UL) mixed model ANOVA revealed that the significant main effect of age, F1,45 = 4.11, P < 0.05, and type of discrimination, F2,90 = 77.60, P < 0.001, were subsumed by an age × type of discrimination interaction, F4,90 = 2.74, P < 0.05, (Supplementary Fig. 4). Simple-effects analyses revealed that, compared with 8-year olds, adults exhibited a higher discrimination between hits and FAs to unrelated lures and between FAs to critical lures and unrelated lures, P values <0.05. Thus, in their recognition judgments adults relied on item-specific recollection, discriminating between studied and unrelated nonstudied items, and also on gist-based memory, discriminating between FAs to critical lures and unrelated lures, to a greater extent than younger children.
In contrast, the A′ values for the discrimination between hits and FAs to critical lures did not differ significantly between the examined age groups, P = 0.95. Thus, the DRM false-recognition effect appears to be similar across age groups when memory for studied items is taken into consideration because of the simultaneous increase with age in the proportion of hits and FAs to critical lures (Brainerd et al. 2002). Despite the apparent proximity of A′ hits-CL values to chance performance levels (i.e., 0.5), additional comparisons confirmed that these A′ values significantly differed from chance overall, P < 0.001, as well as within each age group, P values <0.01 (see Supplementary Material for response bias analyses).
fMRI Results
Our analytical approach for the fMRI data consisted of 3 types of analyses: Whole-brain contrasts, multiple regression analyses, and ROI analyses. Analyses of whole-brain contrasts were conducted on separate age groups so as to gauge the extent to which the different groups engaged the same or different regions in the recognition task (Supplementary Table 1 and Fig. 5). Overall, relatively few activation foci were identified for 8-year olds in these whole-brain contrasts, likely as a result of higher interindividual variability.
Whole-brain multiple regression analyses were conducted to examine how age and behavioral performance affected brain activity for contrasts related to true recognition, semantic processing, and memory monitoring (Supplementary Table 2 online). Both positive and negative associations were examined. Finally, we employed ROI analyses for 2 main reasons. First, these analyses allowed us to test for differences in the pattern of activation among the 3 age groups for contrasts of theoretical interest within regions identified in the regression analyses. Second, they allowed us to examine the activation within each region across all conditions included in this study.
Multiple Regression Analyses
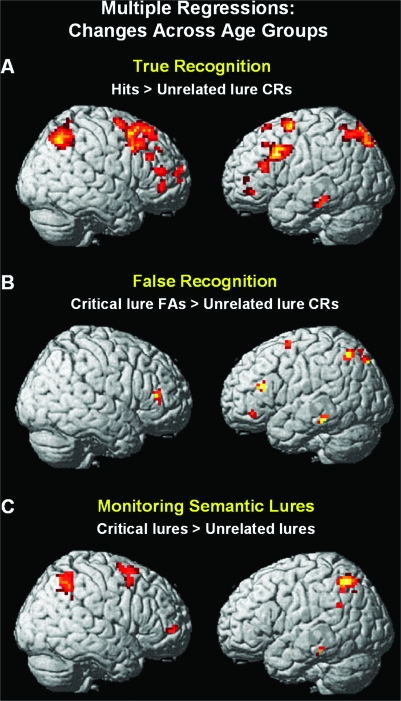
Multiple regression analyses were conducted across all participants to examine whether age and/or behavioral performance would predict brain activity related to true recognition, false recognition or semantic processing of the theme of the lists, and memory monitoring (Supplementary Table 2). We 1st used the contrast target hits > unrelated lure CRs as the outcome variable of true recognition. This contrast was selected because it reflects the general ability to correctly discriminate old from new items. Age and hit rates were entered as the predictors. This analysis revealed that age was positively associated with activation in the left hippocampus, bilateral parietal cortex (Brodmann's area [BA] 7), bilateral DLPFC (BA 9/46), right aPFC (BA 10), and left VLPFC (BA 47) (Fig. 2A). No additional significant association with performance was found when age was taken into account.
Figure 2.
Multiple regressions reflecting changes across age groups for (A) discriminating correctly studied versus new items or true recognition (i.e., hits > unrelated lure CRs), (B) endorsing false versus rejecting new items or false recognition (i.e., critical lure FAs > unrelated lure CRs), and (C) monitoring semantic lures (i.e., critical lures > unrelated lures).
The 2nd regression analysis concerned false recognition. Specifically, we examined whether brain activity observed in the contrast of critical lure FAs > unrelated lure CRs was predicted by age and false-alarm rates to critical lures. Brain activity in left middle temporal gyrus, left parietal cortex (BA 7), left VLPFC (BA 47), and bilateral DLPFC (BA 46) showed a positive correlation with age (Fig. 2B). No additional significant positive association with performance was found when age was taken into account.
A 3rd regression analysis was conducted to examine whether brain activity related to monitoring semantic lures (i.e., critical lures > unrelated lures) was predicted by age group and proportion of CRs of critical lures. This analysis revealed a positive relation between age group and activity in bilateral parietal cortex (BA 40), right aPFC (BA 10), and left VLPFC (BA 47) (Fig. 2C). No additional significant association with performance was found when age was taken into account.
ROI Analyses
Based on the predictions described in the introduction, we conducted hypothesis-driven analyses using an ROI approach in selected areas of MTL, PPC, VLPFC, and aPFC.
Left Hippocampus
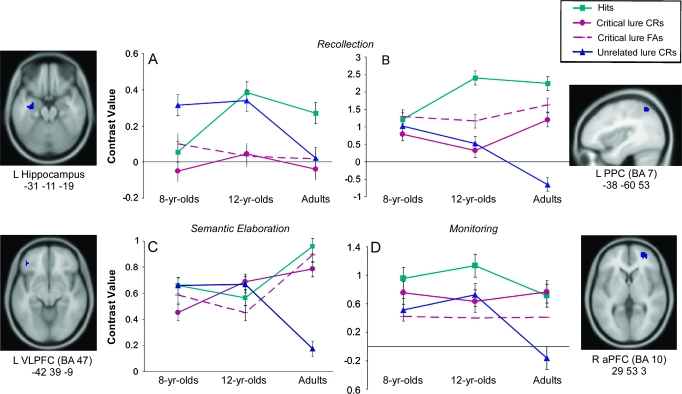
An ROI analysis was conducted to test whether left hippocampus was engaged in distinguishing targets from the other item types. Activation levels for each participant were extracted from the left anterior hippocampus region (−31, −11, −19) previously described in the multiple regression analysis predicting activation in the contrast hits > unrelated lure CRs. A 3 (age) × 3 (item type: hits vs. critical lure FAs vs. unrelated lure CRs) mixed ANOVA showed a significant main effect of item type, F2,90 = 3.18, P < 0.05, such that the left hippocampus was significantly more engaged for recognition of studied items compared with FAs and CRs of critical lures, P values <0.05 (Fig. 3A).
Figure 3.
Average contrast values for 8-, 12-year olds, and adults for ROI analyses functionally identified from regression analyses predicting changes across age groups in (A) Left anterior hippocampus (−31, −11, −19), identified from hits > unrelated lure CRs; (B) left PPC (−38, −60, 53; BA 7), identified from hits > unrelated lure CRs; (C) left VLPFC (−42, 39, −9; BA 47), identified from critical lure FAs > unrelated lure CRs; and (D) right aPFC (29, 53, 3; BA 10), identified from critical lures > unrelated lures. Hypotheses-driven age × item type mixed model ANOVAs conducted per each of these regions including 3 item type conditions (i.e., hits, critical lure FAs or critical CRs, and unrelated lure CRs). The 4th line in the ROIs graphs is included to illustrate additional t-test comparisons.
Further, there was a tendency for an age by item type interaction, F4,90 = 2.09, P = 0.09, . Simple-effects analyses revealed that the results concerning item type appear to follow a different pattern depending on the age group. Adults exhibited greater left hippocampal activation for hits compared with both FAs to critical lures and CRs of unrelated lures, P values <0.05. Twelve-year-olds exhibited marginally greater activation in this region for hits compared with FAs to critical lures, P = 0.07, but not for hits relative to CRs of unrelated lures, P = 0.67. Finally, 8-year olds showed a markedly different pattern from the other 2 groups, engaging left hippocampus marginally more for CRs of unrelated lures than for hits, P = 0.06, but no difference in activation for hits compared with FAs to critical lures, P = 0.81.
PPC
We conducted a 3 (age) × 3 (item type: hits vs. critical lure CRs vs. unrelated lure CRs) mixed ANOVA on the ROI extracted from the left superior parietal region (−38, −60, 53; BA 7) identified as predicting activation in the contrast hits > unrelated lure CRs. Our goal was to test the hypothesis that the PPC would be involved in the recollection of episodic memories, as indicated by greater activation for hits compared with CRs of unrelated lures. There was a significant main effect of item type, F2,90 = 18.35, P < 0.001, that was qualified by a significant age × item type interaction, F4,90 = 5.64, P < 0.001, (Fig. 3B). Activation in this area was greater for hits compared with CRs of critical and unrelated lures among 12-year olds and adults, P values <0.05 but not for 8-year olds, P values ≥0.52. Of interest, adults showed a graded response to recovery effect, engaging left superior PPC more for hits than for correct responses to critical lures and more for correct responses to critical lures than for correct responses to unrelated lures (P values <0.05, for both comparisons). However, engagement of this region in adults did not differentiate between hits and FAs to critical lures, P = 0.19.
The same pattern of results was obtained when participants' brain activation levels were extracted from a contiguous (slightly inferior) left PPC region (i.e., −41, −60, 52; BA 7/40) that was positively associated with age group in the multiple regression analyses conducted for the contrast critical lures > unrelated lures. Thus, this pattern of results in PPC is observed regardless of the contrast used for ROI extraction.
Left VLPFC
To test the hypothesis that left VLPFC is involved in controlled semantic processing associated with true memory and episodic retrieval, a 3 (age) × 3 (item type: hits vs. critical lures FAs vs. unrelated lures CRs) mixed ANOVA was conducted on the ROI extracted from left VLPFC (−42, 39, −9; BA 47) whose activity was positively correlated with age group for the contrast critical lures FAs > unrelated lures CRs. This analysis revealed a significant interaction between age and item type, F4,90 = 3.01, P < 0.05, . In adults, this region was more active for hits and for FAs to critical lures compared with CRs of unrelated lures, P values <0.001; this pattern was not reliable in 8-year olds or 12-year olds, P values >0.05 (Fig. 3C). Additional comparisons showed that in adults, left VLPFC did not discriminate between targets and critical lures, P ≥ 0.46.
Right aPFC
Finally, we conducted a 3 (age) × 3 (item type: hits vs. critical lure CRs vs. unrelated lure CRs) mixed ANOVA on the ROI extracted from the region in right aPFC (29, 53, 3; BA 10) identified as being positively associated with age for the contrast critical lures > unrelated lures. This analysis revealed a significant main effect of item type, F2,90 = 3.34, P < 0.05, such that right aPFC was more active overall for hits and CRs of critical lures compared with CRs of unrelated lures, P values <0.05 (Fig. 3D). Of special interest, activation in this region did not differ between hits and critical lure CRs, P = 0.45. When conducting additional comparisons, we found that right aPFC was also more active for hits than for FAs to critical lures, P values <0.01.This pattern of results was reliably observed in adults, P < 0.05, but not in 12- or 8-year-old children, P ≥ 0.15. In summary, adults but not children exhibited strongest right aPFC activation for hits and critical lure CRs—that is, conditions in which monitoring was both required (due to the presentation of semantically relevant stimuli) and successful (leading to a correct response).
Discussion
The present study was aimed at examining age-related differences in the neural correlates of true and false memory associated with developmental changes in recollection, semantic elaboration, and memory monitoring processes. Informed by prior studies in adults, we focused on regions in MTL, parietal cortex, and lateral PFC. These activations were observed primarily in left-lateralized regions, consistent with previous neuroimaging studies of true and false recognition in adults (Konishi et al. 2000; Cabeza et al. 2001; Slotnick and Schacter 2004). Although it has been suggested that common neural activations between studied and nonstudied items in the DRM paradigm may be due to the retrieval of studied items when trying to decide whether a nonstudied item had been presented previously (Gallo 2006), our data do not show evidence for this recall-to-reject mechanism. Brain regions typically involved in recollection (MTL, PPC) were significantly more active for correctly identifying studied items than for correctly rejecting critical lures. The age-related differences observed in the examined ROIs indicate that the neural substrates of item-specific recollection and gist-based memory are both changing during childhood and adolescence.
Our analyses revealed 4 main results, discussed in greater detail below: 1) Anterior MTL, which was engaged in the processing of novel items in 8-year olds, was increasingly associated with item-specific recollection in older groups; 2) PPC, which failed to discriminate between new versus perceived old information (i.e., endorsed studied items and critical lures) in 8-year olds, showed a graded response as a function of contextual information retrieved or perceived as old in older groups; 3) Left VLPFC, which failed to discriminate between semantically versus nonsemantically related conditions in 8- and 12-year olds, was engaged by semantically related information in adults; 4) Additional anterior and dorsal prefrontal regions were recruited in adults, but not in children, for monitoring and/or decision-related processes in adults.
MTL-Dependent Recollection
We 1st examined the possibility that age-related differences in the DRM false-recognition effect were associated with the development of the MTL. In adults and 12-year olds, but not in 8-year olds, left anterior hippocampus distinguished between conceptual- and item-specific sensory features of episodic information such that it was principally involved when recovering sensory, but not semantic, properties of the episodic information (Vargha-Khadem et al. 1997). These results are consistent with evidence from neuropsychological and neuroimaging studies suggesting a specific role for the hippocampus in recollection (Eichenbaum et al. 2007) and with evidence indicating that the processing of associative information may occur in the hippocampus as well as in other MTL cortices (Squire et al. 2004; Henson 2005). Given that age-related increases in recollection have been documented in the behavioral literature (Brainerd et al. 2004; Ghetti and Angelini, forthcoming), this result suggests a specific developmental progression in the recruitment of the anterior MTL in the service of recollection. The dissociation between anterior and posterior MTL regions found in the DRM neuroimaging study of Cabeza et al's (2001) with adults was not found in the present study (see also Squire et al. 2004; Henson 2005). In contrast to our study, Cabeza et al. instructed their subjects to remember the words as well as the source who presented the word list (a man's or a woman's voice) to enhance the perceptual encoding of studied items.
Critically, in the present study, it is not simply the case that younger children failed to engage the hippocampus. Eight-year-olds showed strong novelty effects recruiting anterior hippocampus for correct identification of new unrelated items. In contrast, whereas adults engaged this region for distinguishing true from false, 12-year olds showed an intermediate pattern of results relative to the other age groups. The differential role of the left anterior hippocampus across the age groups studied here may be explained by maturational changes in its structure (left anterior hippocampus shows volume loss between 4 and 25 years of age; Gogtay et al. 2006) and/or in its anatomical and functional connections. The anterior, but not posterior, part of the hippocampus projects to the PFC (Cavada et al. 2000), and these projections, as well as the strength of functional connectivity between MTL and PFC, increase with age (Menon et al. 2005). Based on evidence for lower true and false recognition in amnesic patients with known MTL dysfunction relative to other control groups (Schacter, Verfaellie, et al. 1996), our results are consistent with the hypothesis that MTL immaturity in younger children may determine the age-related differences usually found in the DRM false-recognition effect. However, we argue that the development of MTL regions is not the only source of age-related differences in true and false recognition, as discussed in the next section.
Frontoparietal Network: Recollection/Oldness Perception and Semantic Elaboration
In light of recent mounting evidence of the contribution of frontoparietal regions to episodic retrieval, we sought to examine the possibility that developmental changes in illusory memory may be associated with changes in frontoparietal regions involved in semantic elaboration and controlled aspects of episodic memory retrieval (Wagner, Schacter, et al. 1998; McDermott et al. 2000; Slotnick and Schacter 2004; Prince et al. 2005).
PPC has been shown in adults to contribute to successful episodic retrieval (Henson, Rugg, et al. 1999; McDermott et al. 2000; Shannon and Buckner 2004). As predicted, our results showed that 12-year olds and adults recruited left superior parietal cortex (BA 7) when they correctly recognized studied items compared with when they correctly rejected critical or unrelated lures. In contrast, this result was not found for 8-year olds. The progressively increasing levels of activity observed from unrelated lures to critical lures and from critical lures to studied items is also consistent with the left parietal event-related potential effect corresponding to a graded response in the engagement of this region as a function of the source and the amount of contextual information retrieved (Henson, Rugg, et al. 1999) or believed to be retrieved (Okado and Stark 2003; Wheeler and Buckner 2003). Furthermore, the parietal region identified here (i.e., −38, −60, 53) is located close to foci associated with the graded response effect in previous functional neuroimaging studies of recognition memory (Henson, Rugg, et al. 1999; Konishi et al. 2000). Wheeler and Buckner (2003) found that as long as participants perceived an item as old, left parietal cortex was active regardless of accuracy (see also Wagner et al. 2005). Consistent with this claim, the engagement of this area did not differentiate hits from FAs to critical lures in adults and 12-year olds, replicating the findings from several studies in true and false memories (Cabeza et al. 2001; Okado and Stark 2003; Slotnick and Schacter 2004). The absence of a graded PPC response in 8-year olds, and the presence in 12-year olds of an activation profile that approximates that observed in adults, is consistent with the notion that during the course of childhood, regions associated with item-specific recollection, or perception of “oldness,” become more specialized. Indeed, a recent neuroimaging study showed positive correlations with age in children between 8 and 15 years of age in the ability to engage left parietal lobe for semantic judgment tasks (Chou et al. 2006).
To account for the effects of the development of the ability to elaborate information semantically, we examined age differences in left VLPFC, which has been implicated in tasks involving online manipulation of semantic representations and semantic elaboration (such as verb generation, category decision, semantic judgment tasks, semantic matching, semantic encoding, and semantic priming [Wagner et al. 1997]). Left VLPFC is also usually engaged during episodic retrieval operations involving accessing and screening available information about studied items (Prince et al. 2005; Kim and Cabeza 2007). Activation in left anterior VLPFC has been interpreted as evidence of the difficulty of retrieving and/or selecting between appropriate semantic features (Fletcher et al. 2000). In fact, lower performance on several semantic tasks across children and adults has been associated with higher activation in this region (Fletcher et al. 2000; Wagner et al. 2001; Blumenfeld et al. 2006).
In the present study, ROI analysis for left VLPFC (BA 47) revealed that adults recruited this area to a similar extent for semantically related information, including true and false recognition (i.e., hits and critical lures). These results are also supported by fMRI, transcranial magnetic stimulation, and neuropsychological evidence indicating that this region is involved in processing the actual semantic relation among items irrespective of whether or not individuals accurately detect such relation (Devlin et al. 2002; Blumenfeld et al. 2006). The fact that this pattern of results is only observed in adults converges with the wealth of evidence for late maturation of PFC function. Of interest, given that anterior MTL distinguishes between true and false memories in adults, but the left VLPFC does not, it seems that activity in this latter area may be particularly critical to observe a more robust DRM effect in adults than children.
In sum, developmental differences in the DRM false-recognition effect appear to depend not only on MTL immaturity (or reduced MTL involvement in item-specific recollection) but also on changes in frontal and parietal function. Given that the patterns of activations observed in adults for these regions seem to extend to 12-year olds for PPC, but not for VLPFC, we suggest that PPC may reach maturity with respect to its contribution to episodic retrieval earlier than VLPFC. Future research should further characterize this developmental lag.
Contribution of Additional PFC Regions in Adults: Decision-Related Processes
We examined the activation profiles of aPFC and DLPFC because of their purported role in strategic decision-related processes that pertain to episodic retrieval (Henson, Shallice, et al. 1999). Here, we focus on results concerning aPFC, in which more pronounced age-related differences were observed. For a discussion of results concerning DLPFC, see the Supplementary Material. We predicted that activity in aPFC would not reflect recognition success per se (Dobbins et al. 2003) but rather the operation of item-specific decision-related process put into place when a high degree of monitoring is required (Wagner, Desmond, et al. 1998; Dobbins et al. 2004; Dobbins and Han 2005; for additional accounts of the role of aPFC, see Tulving 1983; Schacter, Alpert, et al. 1996; Lepage et al. 2000; Velanova et al. 2003). Consistent with this prediction, the regression analysis showed that the right lateral aPFC was involved in monitoring differences between critical lures and unrelated distracters, replicating results from previous studies with adults on veridical and illusory recognition (McDermott et al. 2000; Slotnick and Schacter 2004).
Moreover, contrast values for right aPFC activation in relation to the different item type conditions and accuracy revealed that only adults appear to recruit this area to distinguish correctly between studied items and semantically related but unstudied items (critical lure CRs and hits)—the items for which monitoring demands were expected to be highest. In adults, right aPFC was recruited to a lesser extent for items for which monitoring was important but was not successful (i.e., critical lure FAs) and was least engaged for items that are easier to identify as nontargets (unrelated lures). Thus, this lateral right aPFC region appears to be more recruited with age for conditions that require higher monitoring demands and item-specific decision-related processes regarding information retrieved from episodic memory (Wagner, Desmond, et al. 1998; Dobbins et al. 2004; Dobbins and Han 2005), consistent with evidence suggesting that processes supported by this region are engaged flexibly according to the specific retrieval demands (Herron et al. 2004; Ranganath et al. 2007) and with findings indicating that neurodevelopmental changes in prefrontal regions are still improving considerably during the course of adolescence (Chiu et al. 2006).
In conclusion, our fMRI data provide evidence of several neurodevelopmental trends underlying age-related increases in the DRM false-recognition effect, including changes in the pattern of engagement of left anterior MTL, PPC, and VLPFC. Compared with younger children, 12-year olds showed more differentiation but did not yet show the adult pattern in these regions. Finally, unlike young and older children, adults recruited right aPFC and left DLPFC regions for monitoring and/or decision-related processes. Developmental changes in performance on memory tasks involving semantically related stimuli over childhood and adolescence appear to result from the concurrent refinement of neural systems for recollection and for semantic elaboration and controlled aspects of episodic memory retrieval.
Funding
Imaging Research Center at UC Davis (to S.G.); National Science Foundation (0448844 to S.A.B.); Research Training Program of Basque Government (to P.M.P.); and UC Davis Faculty Research (to G.G.).
Supplementary Material
Supplementary materials can be found at: http://www.cercor.oxfordjournals.org/.
Supplementary Material
Acknowledgments
We thank C. Wendelken, D. Hunt, J. Britz, E. Olsen, C. Spitze, S. Teng, S. Riley, and J. Breck for assistance with the study and A.D. Wagner for helpful comments on a previous version of the manuscript.
Conflict of Interest: None declared.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. 2006;99:226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Forrest TJ, Karibian D, Reyna VF. Development of the false-memory illusion. Dev Psychol. 2006;42:662–679. doi: 10.1037/0012-1649.42.5.962. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Holliday RE, Reyna VF. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Dev. 2004;75:505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Forrest TJ. Are young children susceptible to the false-memory illusion? Child Dev. 2002;73:1363–1377. doi: 10.1111/1467-8624.00477. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Dale AM, Rotte M, Rosen BR. Functional-anatomic study of episodic retrieval: II. Selective averaging of event-related fMRI trials to test the retrieval success hypothesis. Neuroimage. 1998;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro P, Alburquerque P, Fernandez A, Esteves F. Analyzing false memories in children with associative lists specific for their age. Child Dev. 2007;78:1171–1185. doi: 10.1111/j.1467-8624.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Schmithorst VJ, Brown RD, Holland SK, Dunn S. Making memories: A cross-sectional investigation of episodic memory encoding in childhood using fMRI. Dev Neuropsychol. 2006;29:321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Lu D, Cao F. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum Brain Mapp. 2006;27:915–924. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RK-S, Evans AC. BrainWeb: online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:S425. [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quart J of Exp Psychol. 1981;33A:497–505. [Google Scholar]
- Daselaar SM, Fleck MS, Prince SE, Cabeza R. The medial temporal lobe distinguishes old from new independently of consciousness. J Neurosci. 2006;26:5835–5839. doi: 10.1523/JNEUROSCI.0258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Savitz KL. Brief symptom inventory and matching clinical rating scales. Mahwah (NJ): Lawrence Erlbaum; 1999. The SCL-90-R. [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, Tyler LK. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating rule- versus evidence-based prefrontal activity during episodic and lexical discrimination: a functional magnetic resonance imaging investigation of detection theory distinctions. Cereb Cortex. 2005;16:1614–1622. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16:908–920. doi: 10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. “Sculpting the response space”—an account of left prefrontal activation at encoding. Neuroimage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Gallo DA. Associative illusions of memory: false memory research in DRM and related tasks. New York: Psychology Press; 2006. [Google Scholar]
- Gallo DA, Roediger HL, McDermott KB. Associative false recognition occurs without strategic criterion shifts. Psychon Bull Rev. 2001;8:579–586. doi: 10.3758/bf03196194. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. Forthcoming doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Qin J, Goodman GS. False memories in children and adults: age, distinctiveness, and subjective experience. Dev Psychol. 2002;38:705–718. [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1944 words. Behav Res Methods Instrum. 1980;12:395–427. [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JE, Henson RN, Rugg MD. Probability effects on the neural correlates of retrieval success: an fMRI study. Neuroimage. 2004;21:302–310. doi: 10.1016/j.neuroimage.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Howe ML, Cicchetti D, Toth SL, Cerrito BM. True and false memories in maltreated children. Child Dev. 2004;75:1402–1417. doi: 10.1111/j.1467-8624.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cereb Cortex. 2007;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence (RI): Brown University Press; 1967. [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user's guide. 2nd ed. Mahwah (NJ): Lawrence Erlbaum Associates Publishers; 2005. [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Roediger HL. Attempting to avoid illusory memories: robust false recognition of associates persists under conditions of explicit warnings and immediate testing. J Mem and Lang. 1998;39:508–520. [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Res Cogn Brain Res. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Okado Y, Stark C. Neural processing associated with true and false memory retrieval. Cogn Affect Behav Neurosci. 2003;3:323–334. doi: 10.3758/cabn.3.4.323. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller AS, Wilding EL. Dissociable correlates of two classes of retrieval processing in prefrontal cortex. Neuroimage. 2007;35:1663–1673. doi: 10.1016/j.neuroimage.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21:803–814. [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission topography. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Pradere D. The neuropsychology of memory illusions: false recall and recognition in amnesic patients. J Mem and Lang. 1996;35:319–344. [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggests nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stadler MA, Roediger HL, 3rd, McDermott KB. Norms for word lists that create false memories. Mem Cogn. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Sugrue K, Hayne H. False memory produced by children and adults in the DRM paradigm. Appl Cogn Psychol. 2006;20:625–631. [Google Scholar]
- Talairach J, Tourneaux P. Co-planar stereotaxic atlas of the human brain. Stuttgart (Germany): Thieme; 1988. [Google Scholar]
- Tulving E. Elements of episodic memory. London: Oxford University Press; 1983. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Schacter DL, Cook SP. The effect of retrieval instructions on false recognition: exploring the nature of the gist memory impairment in amnesia. Neuropsychologia. 2002;40:2360–2368. doi: 10.1016/s0028-3932(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JD. Semantic repetition priming for verbal and pictorial knowledge: a functional MRI study of left inferior prefrontal cortex. J Cogn Neurosci. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JD. Prefrontal cortex and recognition memory. Functional-MRI evidence for context-dependent retrieval processes. Brain. 1998;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Left prefrontal and temporal activation during human encoding is associated with whether experiences are remembered or forgotten. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cog Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.