Abstract
Fluid intelligence (gf) influences performance across many cognitive domains. It is affected by both genetic and environmental factors. Tasks tapping gf activate a network of brain regions including the lateral prefrontal cortex (LPFC), the presupplementary motor area/anterior cingulate cortex (pre-SMA/ACC), and the intraparietal sulcus (IPS). In line with the “intermediate phenotype” approach, we assessed effects of a polymorphism (val158met) in the catechol-O-methyltransferase (COMT) gene on activity within this network and on actual task performance during spatial and verbal gf tasks. COMT regulates catecholaminergic signaling in prefrontal cortex. The val158 allele is associated with higher COMT activity than the met158 allele. Twenty-two volunteers genotyped for the COMT val158met polymorphism completed high and low gf versions of spatial and verbal problem-solving tasks. Our results showed a positive effect of COMT val allele load upon the blood oxygen level–dependent response in LPFC, pre-SMA/ACC, and IPS during high gf versus low gf task performance in both spatial and verbal domains. These results indicate an influence of the COMT val158met polymorphism upon the neural circuitry supporting gf. The behavioral effects of val allele load differed inside and outside the scanner, consistent with contextual modulation of the relation between COMT val158met genotype and gf task performance.
Keywords: COMT, fMRI, g, genotype, intelligence, prefrontal cortex
Introduction
Fluid intelligence (gf) is a major dimension of individual differences in cognitive function. Commonly measured by tests of reasoning and novel problem solving (Cattell 1971), gf is conceptually distinct from crystallized intelligence (gc), which represents acquired knowledge and skill, and highly related to the psychometrically defined construct “g” (Spearman 1927), the primary or “general” cognitive factor underlying the observation that the same individuals tend to do well across many different cognitive tasks. A strong predictor of achievement in educational and other domains, at a mechanistic level, gf has been linked to processes ranging from cognitive flexibility and strategy development to manipulation of stored mental representations and attentional control, in particular, the inhibition of interference (Kane et al. 2005).
There has been much interest in the extent to which genetic factors influence gf. Heritability studies suggest that gf is strongly influenced by genetic as well as environmental factors, with genetic influences accounting for 40% or more of the variance in gf scores (Gray and Thompson 2004). Despite its high heritability, attempts to identify specific genetic contributions to gf have made relatively little progress. Advances in functional genomics and neuroimaging now enable us to adopt an “intermediate phenotype” approach, examining genetic contributions to variability not in behavior itself but in the underlying neural mechanisms. It has been argued that genetic influences may be clearer at the neurophysiological level as measured by the blood oxygen level–dependent (BOLD) signal in functional magnetic resonance imaging (fMRI) than at the level of behavior. The former is held to be closer to the neurobiological effects of the gene and less susceptible to the sources of noise that can influence behavioral performance, increasing the likelihood of detecting the effects of single genetic polymorphisms (Hariri and Weinberger 2003; Goldberg and Weinberger 2004). In addition to increasing sensitivity to gene effects, this intermediate phenotype approach has the potential to provide new insights into the neurochemical mechanisms that support gf, and may open a pathway to investigating how environmental factors also modulate the operation of these mechanisms.
During performance of tasks with high gf loadings, conspicuous activity is seen in the lateral prefrontal cortex (LPFC) (Prabhakaran et al. 1997; Duncan et al. 2000). Given this, a val158met polymorphism in the catechol-O-methyltransferase (COMT) gene is a striking candidate for influencing gf-related neural function. COMT metabolizes released dopamine (DA). It is thought to be particularly critical to regulating DA signaling in the prefrontal cortex due to the scarcity of DA transporter in this region (Sesack et al. 1998). The COMT val158 allele is associated with higher enzymatic activity than the less stable met allele, with heterozygous individuals showing intermediate enzyme activity (Lotta et al. 1995; Weinshilboum et al. 1999; Chen et al. 2004). Though a number of other single nucleotide polymorphisms (SNPs) in the COMT gene have also been identified (Bray et al. 2003; Chen et al. 2004), the val158met polymorphism is the only one to have been reliably shown to impact significantly upon COMT activity across both postmortem human dorsolateral prefrontal tissue samples and lymphoblast cultures (Chen et al. 2004). Haplotype analyses conducted within the context of these studies have also reported no effects on COMT activity other than those attributable to the val158met polymorphism (Chen et al. 2004). Although recent findings suggest that it may be premature to rule out more complex effects of genetic variation in COMT upon human LPFC function (Meyer-Lindenberg et al. 2006; Tunbridge et al. 2006), the val158met polymorphism is the strongest single candidate variant in the COMT gene for modulating LPFC function, providing a focus for genomic imaging studies where sample sizes may not easily allow for haplotype analyses.
In line with this, a number of neuroimaging studies have examined the impact of the COMT val158met polymorphism upon LPFC activity, reporting that the number of val alleles possessed correlates positively with LPFC activity during performance of cognitive tasks including measures of working memory (WM), encoding, and retrieval (Egan et al. 2001; Bertolino, Blasi, et al. 2006, Bertolino, Rubino, et al. 2006; Schott et al. 2006). In contrast to the consistency of these results, findings from investigations of the impact of the COMT val158met polymorphism upon behavioral indices of cognition have been more variable (Egan et al. 2001; Bilder et al. 2004; Diamond et al. 2004; Nolan et al. 2004; Tunbridge et al. 2006; Barnett et al. 2007). It is possible that both state factors, such as the stressfulness of the current environment and the nature of the task performed might influence whether a met or val behavioral performance advantage is observed (Bilder et al. 2004; Tunbridge et al. 2006). Alternatively, the variability in behavioral results could simply reflect difficulty in reliably detecting effects of single genetic polymorphisms at the behavioral level of analysis.
Given these considerations, we were interested in investigating the impact of the COMT val158met polymorphism upon gf task performance and associated neural activity. In particular, we were interested in whether, as proponents of the intermediate phenotype approach have argued (Hariri and Weinberger 2003; Goldberg and Weinberger 2004), the effects of single genetic variants such as the COMT val158met polymorphism might be more apparent upon gf-related neural activity than upon gf task performance. A number of studies have suggested that performance of tasks with high gf loadings does not activate LPFC alone but recruits a circumscribed cortical circuit including LPFC, presupplementary motor area/anterior cingulate cortex (pre-SMA/ACC), and the intraparietal sulcus (IPS) (Prabhakaran et al. 1997; Esposito et al. 1999; Gray et al. 2003). Consequently, we aimed to address the following questions: 1) whether COMT val158met genotype modulates LPFC activity during gf-related task performance, with COMT val allele load being associated with increased LPFC activation, 2) whether COMT val158met genotype selectively influences LPFC recruitment or modulates activity throughout the extended gf cortical network, 3) whether COMT val158met genotype modulation of gf-related neural activity is similar across different domains of processing (spatial and verbal), in keeping with COMT val158met genotype impacting upon a single common mechanism underlying both verbal and spatial forms of gf, and 4) whether COMT val158met genotype modulates gf-related neural activity more robustly than gf-related task performance.
Materials and Methods
fMRI Study
Participants and Procedure
Twenty-two participants (10 males and 12 females, all Caucasian of European descent, all right-handed, age 19–39 years) completed verbal and spatial problem-solving tasks while both behavioral and fMRI data were collected. Details of participant sex and age are given by COMT val158met genotype in Supplementary Table S1. The 3 COMT val158met genotype groups did not differ significantly on these characteristics (P values >0.1). Informed written consent was obtained from all volunteers, and the study was approved by the Cambridgeshire Local Research Ethics committee and performed in compliance with their guidelines. The standard Cambridge exclusion criteria for fMRI studies were followed (no metal and no history of neurological disease or head injury). In addition, all individuals with current or past history of inpatient psychiatric care, those currently on medication for anxiety, depression or sleeping problems, and those on any other form of medication that might influence neurotransmitter function were excluded. All participants completed a standard test of gf, the Cattell Culture Fair, Scale 2 Form B, in a separate behavioral testing session, conducted in a quiet environment at either the Department of Experimental Psychology, Cambridge University or the Medical Research Council (MRC) Cognition and Brain Sciences Unit, Cambridge.
Task Design
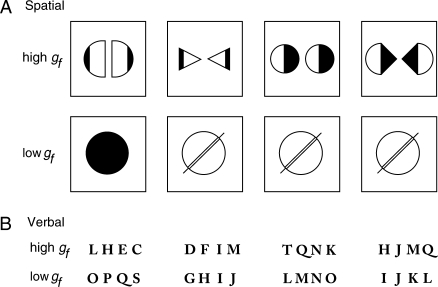
The verbal and spatial problem-solving tasks were taken from Duncan et al. (2000). In both tasks, each item consisted of 4 display elements—either drawings (spatial task) or letter sets (verbal task), see Figure 1. Participants were instructed to identify the “odd one out”—the element that in some sense differed from the others. In each task, items were split into high gf and low gf blocks, which lasted for 33 s (3 s for the block type to be specified and 30 s for completion of trials).
Figure 1.
Example test items for each task. Each item consisted of 4 display elements (drawings or letter sets), and the task was to identify the element that in some sense mismatched or differed from the others. Materials for the high gf tasks were adapted with permission from a standard nonverbal test of gf, Cattell Culture Fair, Scale 2 Form A and Scale 3 (Institute for Personality and Ability Testing 1973) and a standard letter-based problem-solving task, Letter Sets from the ETS kit of factor-referenced tests (Ekstrom et al. 1976). The high gf loading of these tasks was previously established (Wothke et al. 1991; Duncan et al. 2000). The low gf items were structurally similar but with a minimal problem-solving component. Participants were asked to select the only nonidentical item for the low gf spatial task and to select the string not in alphabetic order for the low gf verbal task.
Items in the high gf spatial blocks were adapted with permission from a standard nonverbal test of gf, Cattell Culture Fair, Scale 2 Form A and Scale 3 (Institute for Personality and Ability Testing 1973). Display elements were 4 panels, each containing one or more shapes, symbols, or drawings. One panel differed in some respect from the others; extensive problem solving was required to identify this panel because the difference could concern any property, often abstract and/or complex. In the example shown in Figure 1A, the relevant property is symmetry; the mismatching panel is the 3rd in the row. In the low gf spatial blocks, in contrast, there was minimal problem solving. In each display, the 4 panels each contained a single geometrical shape, 3 of which were physically identical, whereas the 4th differed in visually obvious features, such as shape, texture, size or orientation.
Materials for the high gf verbal blocks were adapted with permission from a standard letter-based problem-solving task, Letter Sets from the Educational Testing Service (ETS) kit of factor-referenced tests (Ekstrom et al. 1976). The high gf loading of the original test was established by analysis of a large preexisting data set (Wothke et al. 1991). Display elements were 4 sets of 4 letters each. One set differed in some respect from the others; again, the task required extensive problem solving because a variety of alphabetic and other rules could distinguish the mismatching letter set in any given test item. In the example given in Figure 1B, the mismatching set is the 3rd, whose letters are equally spaced in the alphabet. In the low gf verbal blocks, the task was simply to find the one set in each display whose letters were not in strict alphabetical order.
For both tasks, the position of the mismatching element was indicated by pressing the corresponding key on a 4-choice keyboard, operated with middle and index fingers of the 2 hands. The screen cleared when a response was made, and a new test item was presented immediately. Participants were instructed not to guess but to continue thinking about each problem until they were confident of their answer or until the block terminated. With this constraint, participants were asked to complete as many items as possible during each block. These arrangements ensured that participants worked continuously, despite long solution times for high gf items but much shorter times for low gf items.
The spatial task comprised 4 high gf and 4 low gf blocks. The verbal task comprised 5 high gf and 5 low gf blocks. Prior to each task, participants were given full instructions and practice items from both the high gf and low gf conditions. Stimuli were projected onto a translucent screen positioned behind the head of the participant visible via an angled mirror within the scanner coil. The visual angle subtended by the 4 stimuli presented on each trial was approximately 12°.
Image Acquisition
BOLD contrast functional images were acquired with echo-planar T2*-weighted (EPI) imaging using a 3-T Bruker Medspec scanner based at the Wolfson Brain Imaging Centre, Addenbrooke's Hospital, Cambridge, UK. Image volumes were acquired in 23 interleaved 5-mm-thick axial oblique slices giving whole-brain coverage with an in-plane resolution of 3.75 × 3.75 mm (repetition time = 1,200 ms; echo time = 30 ms, flip angle = 67.5°). For each participant, data for the spatial task were acquired in a single scanning run of 4 min and data for the verbal task in a single scanning run of 5 min. The first 11 volumes of each run were discarded to allow for T1 equilibration effects.
Image Analysis
SPM software was used (http://www.fil.ion.ucl.ac.uk/spm). Standard preprocessing was conducted comprising slice-timing correction, realignment, undistortion (Cusack et al. 2003), and skull-stripped normalization of each participant's EPI data to the Montreal Neurological Institute's MNI/ICBM template. Images were resampled into this space with 3-mm isotropic voxels and smoothed with a Gaussian kernel of 10-mm full-width at half-maximum. Blocks were modeled with step functions of 33-s duration, convolved with the canonical hemodynamic response function to form regressors. A high-pass filter of 75 s was used to remove low-frequency noise. A voxelwise random effects analysis was used to analyze data at a group level. Across-participant whole-brain analyses were conducted separately for the spatial and verbal problem-solving tasks. Neural regions showing increased activity during performance of high gf versus low gf conditions were reported if activity passed a whole-brain false-detection rate (fdr) threshold of P < 0.05 (Genovese et al. 2002).
Our key analyses examined the effect of COMT val158met genotype upon high gf − low gf task-related activity across a network of cortical regions previously implicated in high gf task performance (Prabhakaran et al. 1997; Esposito et al. 1999; Duncan et al. 2000; Gray et al. 2003; Haier and Jung 2007). This network includes bilateral dorsal and ventral regions of LPFC, the former corresponding to the dorsal LPFC (DLPFC) region focused upon in genomic imaging studies of WM (Egan et al. 2001; Bertolino, Blasi, et al. 2006), the latter being centered on the frontal operculum/anterior insula (FO/AI). It also encompasses bilateral regions of parietal cortex centered on the IPS and a cortical region extending from the anterior cingulate cortex (ACC) to the presupplementary motor area (pre-SMA). Regions of interest (ROIs) for these regions were derived from a meta-analysis of brain areas coactivated across tasks posing diverse cognitive demands (Duncan and Owen 2000; Duncan 2006). The cortical activation foci from the studies reviewed in those papers were transposed onto 1 hemisphere, smoothed (15-mm Gaussian kernel), and added. The resulting sum map was thresholded to show regions of maximum clustering. This produced peaks in DLPFC ±42, 24, 24; FO/AI ±36, 18, 0; IPS ±36, −54, 39; pre-SMA 0, 21, 45; and ACC 0, 30, 21. It should be noted that the ACC and pre-SMA clusters were not clearly distinct. Our ROIs comprised 10-mm radius spheres centered on these peak coordinates.
The ROIs described above were used to examine the influence of COMT val158met genotype upon gf-related neural activity. Specifically, a regressor was created to represent the number of COMT val alleles possessed (0: met/met, 1: val/met, and 2: val/val). This effectively divided up the volunteer sample into 3 groups, according to COMT val allele load. Two sets of between-group correlational analyses were conducted. First, in line with the analytic approach adopted in previous studies (e.g., Smolka et al. 2005), neural activity during high gf versus low gf task performance was regressed against the number of COMT val alleles possessed. This analysis was conducted on a voxelwise basis within each ROI, with small volume corrections for multiple comparisons being applied (Worsley et al. 1996). A linear regressor was used, given evidence that val/met heterozygotes show COMT activity that is intermediate between that of met/met and val/val homozygotes. This enabled us to test, for each voxel within our ROIs, whether the magnitude of the BOLD response associated with high gf − low gf performance varied as a function of the number of val alleles possessed.
In addition, confirmatory analyses of the influence of COMT val158met genotype upon neural activation during high gf − low gf task performance were conducted using the MARSBAR ROI toolbox for SPM99 (Brett et al. 2002). This enables the researcher to extract and average the activation associated with a given contrast across all voxels within an ROI. Using this method, the correlation between the number of COMT val alleles possessed by each individual (0–2) and their high gf − low gf neural activity was calculated for each ROI for both the spatial and verbal tasks.
DNA Isolation and Genotyping Analyses
All volunteers gave informed consent for a buccal swab to be obtained using a buccal brush. DNA was isolated using the MasterAMP Buccal Swab DNA Extraction Kit (Epicentre Technologies, Madison, WI). This provides yields of 0.5 to 3 μg of DNA from each buccal sample. Polymerase chain reaction (PCR) restriction fragment length polymorphism analysis with horizontal gel electrophoresis was used to determine COMT val158met genotype (following Daniels et al. 1996; Egan et al. 2001; Bertolino et al. 2004; Bertolino, Blasi, et al. 2006; Schott et al. 2006) Taq polymerase, PCR buffer, and deoxyribonucleotide triphosphates were obtained from Qiagen (www.qiagen.com) and used at recommended concentrations for a 20-ul PCR. A “touchdown” PCR cycling regimen and the addition of dimethyl sulfoxide (10% final v:v) was used in order to optimize the hybridization stringency. Forward: 5′-ACTGTGGCTACTCAGCTGTG-3′ and reverse 5′-CCTTTTTCCAGGTCTGACAA-3′ primers were used. PCR conditions were as follows: 94 °C for 3 min initial heating, then 12 cycles of 94 °C for 30 s, 58 °C for 45 s, and 72 °C for 30 s and then 28 cycles of 94 °C for 30 s, 50 °C for 45 s, and 72 °C for 30 s. This was followed by restriction digestion with NlaIII. Gel electrophoresis in Metaphor agarose followed by staining in ethidium bromide was used to resolve and visualize DNA fragments. See Supplementary Materials for further details.
Additional Behavioral Study
A total of 146 volunteers (63 males, 83 females; mean age = 25.9 years, all Caucasian of European descent) came into either the Department of Experimental Psychology at Cambridge University or the MRC Cognition and Brain Sciences Unit, Cambridge, for a 1-h behavioral testing session. Informed written consent was obtained from all volunteers and the study was approved by the Cambridgeshire Local Research Ethics committee and performed in compliance with their guidelines. During the behavioral session, participants completed the Cattell Culture Fair, Scale 2 Form B. It was administered in a quiet environment, according to the manual guidelines. In addition, participants completed a number of questionnaires and a medical screening form (for past neurological injury, medication, and psychiatric history) and provided a buccal swab for DNA analysis (as described under DNA Isolation and Genotyping Analyses above). Participant sex and age are given by COMT val158met genotype in Supplementary Table S1. The 3 COMT genotype groups did not differ significantly on these characteristics (P values >0.1).
Results
Across Participants: High gf Conditions Activate LPFC, IPS, and Pre-SMA/ACC
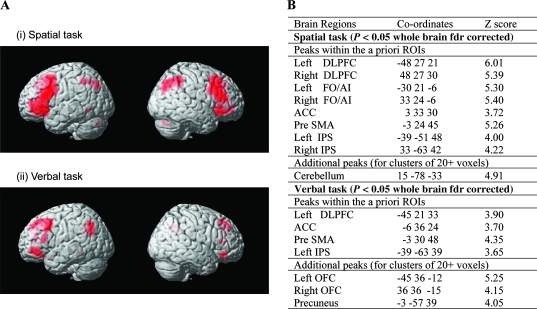
Across participants, whole-brain analyses conducted separately on data from the spatial and verbal tasks revealed significant increases in activity in LPFC, pre-SMA/ACC, and IPS during high gf versus low gf conditions. These results held for both tasks (see Fig. 2). The high gf versus low gf subtraction gave extensive bilateral activations in the spatial task and predominantly left lateralized activation in the verbal task, in line with our prior results (Duncan et al. 2000). This subtraction also produced activation in the cerebellum for the spatial task and in the precuneus and orbitofrontal cortex for the verbal task, but no regions outside of the predicted network showed enhanced activity across both high gf conditions.
Figure 2.
Neural activation associated with high gf versus low gf task performance. (A) Significant activations at a whole-brain fdr threshold of P < 0.05, rendered onto the canonical T1-weighted brain image of SPM99. (i) Spatial high gf − spatial low gf. (ii) Verbal high gf − verbal low gf. (B) Activation peaks. For significant (fdr, P < 0.05) clusters of any size that overlap the a priori specified ROIs, the table gives peak voxel within the ROI. For other brain areas, peaks are reported only for clusters of 20 or more significant voxels. OFC: orbitofrontal cortex.
COMT val158met Genotype Modulates Activity across the Whole Frontoparietal High gf Network during Performance of High gf versus Low gf Tasks
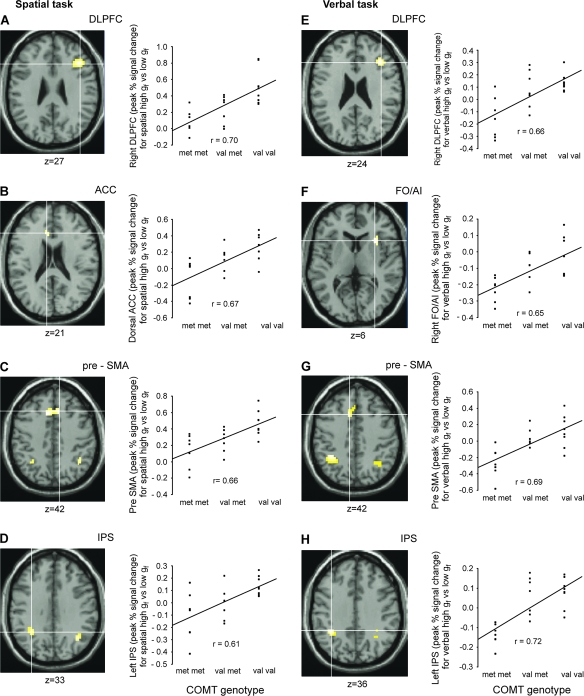
As predicted, between-group regression analyses showed a significant positive correlation between COMT val allele load (number of val alleles possessed, 0–2) and high gf − low gf neural activity in LPFC. Parallel effects were observed in other regions across the extended high gf network including pre-SMA, ACC, and IPS (see Fig. 3 and Table 1). The results obtained with the independent measures of gf-related brain activity from the spatial and verbal tasks were strikingly similar, with right DLPFC, right and left IPS, and pre-SMA showing a significant correlation between val allele load and high gf − low gf neural activity in both tasks.
Figure 3.
Correlation between number of COMT val alleles possessed (0: met/met, 1: val/met, and 2: val/val) and activation for high gf versus low gf conditions for the spatial problem-solving task (A: right DLPFC, B: dorsal ACC, C: pre-SMA, and D: left IPS) and the verbal problem-solving task (E: right DLPFC, F: right FO/AI, G: pre-SMA, and H: left IPS). Left: Activations thresholded at P < 0.05 svc are overlaid on the canonical T1 SPM99 brain. Right: Individual BOLD responses were extracted from the voxel with the highest Z value inside the ROI and plotted against number of COMT val alleles possessed.
Table 1.
Voxelwise correlational analyses of the effect of COMT val158met genotype upon high gf − low gf neural activity
| Brain regions | Coordinatesa | Z score | Pb | rc | rpd |
| Spatial task | |||||
| Right DLPFC | 42, 24, 27 | 3.62 | P < 0.005 | 0.70 | 0.60 |
| ACC | −9, 27, 21 | 3.43 | P < 0.01 | 0.67 | 0.65 |
| Pre-SMA | 9, 24, 42 | 3.32 | P < 0.02 | 0.66 | 0.59 |
| Left IPS | −33, −51, 33 | 3.04 | P < 0.05 | 0.61 | 0.54 |
| Right IPS | 39, −60, 33 | 3.27 | P < 0.02 | 0.65 | 0.63 |
| Verbal task | |||||
| Left DLPFC | −36, 24, 18 | 2.70 | P = 0.065 | 0.56 | 0.52 |
| Right DLPFC | 39, 27, 24 | 3.37 | P < 0.02 | 0.66 | 0.64 |
| Left FO/AI | −27, 15, 3 | 2.96 | P < 0.05 | 0.60 | 0.55 |
| Right FO/AI | 30, 18, 6 | 3.28 | P < 0.02 | 0.65 | 0.69 |
| Pre-SMA | −9, 18, 42 | 3.52 | P < 0.01 | 0.69 | 0.37 |
| Left IPS | −36, −45, 36 | 3.77 | P < 0.005 | 0.72 | 0.69 |
| Right IPS | 36, −57, 48 | 3.10 | P < 0.05 | 0.62 | 0.59 |
Note: The number of COMT val alleles possessed (0–2) was correlated against high gf − low gf neural activity on a voxel by voxel basis within each ROI.
Correlation peak coordinates. Peaks are reported for each ROI where one or more voxels showed a significant or near significant (P < 0.1) correlation between number of COMT val alleles possessed and high gf − low gf neural activity after small volume correction for multiple comparisons.
P value after small volume correction.
Correlation at peak voxel.
Partial correlation at peak voxel after controlling for high gf behavioral performance (all reported partial correlations are significant at least at P < 0.05 uncorrected).
In order to confirm these results, additional whole-ROI analyses were conducted using the MARSBAR ROI toolbox for SPM (Brett et al. 2002; see Materials and Methods). In these analyses, activation was averaged across all the voxels in each ROI, the resulting composite value for each ROI being regressed, across volunteers, against the number of COMT val alleles possessed (0–2). These analyses also revealed a significant positive relationship between val allele load and high gf − low gf neural activity in right DLPFC, right and left IPS, and pre-SMA across both tasks, with a similar but weaker pattern in ACC (see Table 2).
Table 2.
ROI-composite correlational analyses of the effect of COMT val158met genotype upon high gf − low gf neural activity
| Neural ROI (region, ROI centre point)a | Z score | Significance (P) |
| Spatial task | ||
| Left DLPFC (−42, 24, 24) | 0.94 | P > 0.1 |
| Right DLPFC (42, 24, 24) | 3.11 | P < 0.001b |
| Left FO/AI (−36, 18, 0) | 0.97 | P > 0.1 |
| Right FO/AI (36, 18, 0) | 0.88 | P > 0.1 |
| ACC (0, 30, 21) | 2.22 | P < 0.02 |
| Pre-SMA (0, 21, 45) | 2.66 | P < 0.005c |
| Left IPS (−36, −54, 39) | 2.68 | P < 0.005c |
| Right IPS (36, −54, 39) | 2.69 | P < 0.005c |
| Verbal task | ||
| Left DLPFC (−42, 24, 24) | 2.46 | P < 0.01 |
| Right DLPFC (42, 24, 24) | 2.60 | P < 0.005c |
| Left FO/AI (−36, 18, 0) | 2.31 | P < 0.01 |
| Right FO/AI (36, 18, 0) | 2.75 | P < 0.003c |
| ACC (0, 30, 21) | 1.39 | P = 0.08 |
| Pre-SMA (0, 21, 45) | 2.65 | P < 0.005c |
| Left IPS (−36, −54, 39) | 2.83 | P < 0.005c |
| Right IPS (36, −54, 39) | 2.75 | P < 0.005c |
Note: For each ROI, the number of COMT val alleles possessed (0–2) was correlated against high gf − low gf neural activity using a composite measure of activation extracted from and averaged across all voxels within the ROI.
All ROIs were 10-mm radius spheres.
Significant at P < 0.01 when corrected for number of ROIs examined.
Significant at P < 0.05 when corrected for number of ROIs examined.
Behavioral Results and Behavior/Brain Regression Analyses
We were interested in whether the relationship between COMT val158met genotype and activity across the high gf network would be linked to differences in gf-related task performance. As noted in the introduction, proponents of the intermediate phenotype approach have suggested that gene–behavior effects may be less robust and harder to reliably detect than genetic influences upon neural activity. Given this and in the light of null results from previous studies investigating influences of specific genetic markers upon intelligence test scores (Plomin et al. 2001), we did not have strong predictions as to whether we would observe COMT val158met genotype effects upon performance of our gf measures.
Our behavioral results were as follows. In the scanner, a positive correlation was observed between COMT val allele load and performance (number of correct responses) for the spatial high gf task; r = 0.47, P < 0.03 2 tailed, with a nonsignificant trend in the same direction for the verbal high gf task; r = 0.31, P = 0.15 2 tailed. We conducted additional regression analyses in order to examine the relationship between performance in the spatial and verbal high gf tasks and task-related neural activity across the entire group of volunteers. These revealed a positive relationship between performance and recruitment of right DLPFC during high versus low gf conditions of the spatial task, x, y, z = 42, 24, 27, Z = 3.10, P = 0.02 small volume corrected (svc), whereas for the verbal task, there was a trend towards a similar result for left FO/AI, x, y, z = −36, 27, −3, Z = 2.68, P = 0.06 svc. There was no significant relationship between high gf task performance and activity in any of the other ROIs (P values >0.1).
Notably, with high gf task performance entered as a covariate, all the reported associations between COMT val158met genotype and regional fMRI activity remained significant (see Table 1). However, entering high gf versus low gf right DLPFC activity as a covariate for the spatial task and high gf versus low gf left FO/AI activity for the verbal task removed any relationship between COMT val158met genotype and high gf task performance; r(19) = 0.19, P > 0.4; r(19) = 0.11, P > 0.5, respectively.
Effect of COMT val158met Genotype on High gf Task Performance Outside the Scanner
As mentioned above, previous studies have found no significant effect of COMT val158met genotype on behavioral performance of other standardized tests of intelligence such as the Wechsler Intelligence Scale for Children—Revised (Plomin et al. 2001). While these measures arguably have greater gc loadings than the measures used in the current study, we were interested in whether the behavioral effect we observed for the spatial gf task performed within the scanner would be replicated with a parallel measure administered outside of the scanner environment.
All participants in the fMRI study were additionally asked to complete scale 2B of Cattell Culture Fair (Institute for Personality and Ability Testing 1973) as part of a separate behavioral testing session. In contrast to the results obtained with the spatial high gf task in the scanner, no significant association was observed between COMT val allele load and scale 2B performance outside of the scanner environment, r(22) = 0.10, P > 0.1. We confirmed this result with a larger sample of 146 volunteers who also completed scale 2B of Cattell Culture Fair outside the fMRI environment (see Materials and Methods and Supplementary Table S1). Here again, we found no significant association between COMT val allele load and scale 2B scores, r(145) = −0.11, P > 0.1.
Discussion
Across participants, whole-brain analyses conducted separately on data from the spatial and verbal gf tasks revealed significant increases in activity in LPFC, pre-SMA/ACC, and IPS during high gf versus low gf conditions. No other neural regions showed enhanced activity across both high gf conditions. This supports the contention that a fairly constrained network of regions including LPFC, pre-SMA/ACC, and IPS comprises the neural substrate that supports gf. This falls in line with findings by Prabhakaran et al. (1997), Esposito et al. (1999), and Gray et al. (2003) and extends the results from our earlier PET study (Duncan et al. 2000), which primarily indicated a role for LPFC.
Regression analyses showed a significant positive correlation between COMT val allele load and high gf − low gf neural activity in LPFC, pre-SMA/ACC, and IPS. This held for both verbal and spatial measures of gf. It is of note that COMT val158met genotype modulated activity associated with high gf versus low gf task performance across the whole frontoparietal extended high gf network and not just in LPFC. There are a number of potential explanations for this finding. First, the COMT val158met polymorphism may have a direct impact on DA metabolism in all the regions concerned. LPFC, ACC, and IPS all receive dopaminergic projections (Bentivoglio and Morelli 2005). Furthermore, in addition to the established influence of the COMT val158met polymorphism upon LPFC function (Winterer and Goldman 2003), recent studies have also reported COMT val158met genotype modulation of ACC and IPS function (Blasi et al. 2005; Bertolino, Blasi, et al. 2006; Williams-Gray et al. 2007). Second, it is possible that the COMT val158met polymorphism affects activity in one or more regions of the high gf network through its influence upon metabolism of norepinephrine (Mannisto and Kaakkola 1999, though see Tunbridge et al. 2006); the ascending catecholamine neuromodulatory systems operating both separately and conjointly to influence cortical function (Robbins and Everitt 1995). Finally, an alternative account would be that modulation of LPFC function by COMT val158met genotype in turn up- or downregulates activation in other key regions, accounting for the association between COMT val158met genotype and activation in these additional areas.
Another question of interest concerns the relationship of the current findings to previous reports of COMT val158met genotype modulation of prefrontal activity during tasks tapping executive function, in particular, WM as assessed by the “n-back” task (Egan et al. 2001; Bertolino, Blasi, et al. 2006). Here, work on the relationship between gf and WM is particularly pertinent (Ackerman et al. 2005; Kane et al. 2005). The observation of relatively low zero-order correlations between performance on WM tasks and tests of gf (Engle et al. 1999; Ackerman et al. 2005) has led to a relative consensus that WM capacity is not isomorphic with g or gf (Kane et al. 2005; Birney et al. 2006; Heitz et al. 2006). Latent variable analyses of short-term memory tasks (which primarily involve temporary storage of information) and WM tasks (which involve online manipulation as well as temporary storage of information, e.g., the n-back task) have suggested that it is the WM residual (the component not shared with short-term memory tasks) which loads strongly onto gf (Engle et al. 1999, though see Colom et al. 2006). This has been taken as evidence that the component of WM variance explained by gf is related to the executive or attentional aspects of WM (Engle et al. 1999; Gray et al. 2003). Given this, an interesting prediction for future work is that no significant effect of COMT val158met genotype on n-back–related LPFC activity will be observed after controlling for this attentional or executive component. More generally, continued application of the latent variable approach to tests of gf, attentional control, WM, and other cognitive processes, combined with analysis of genomic imaging data from multiple tests of higher order cognition, should enable us to achieve a clearer picture of the component processes central to gf and their individual or collective modulation by both the COMT val158met SNP and other functional genetic polymorphisms.
We turn now to consideration of COMT val158met genotype effects upon gf-related task performance. Within the scanner, a positive correlation was observed between COMT val allele load and performance on the spatial high gf task, with a trend in the same direction for the verbal high gf task. Regression analyses revealed that entering high gf versus low gf LPFC activity as a covariate eliminated the relationship between COMT val158met genotype and high gf task performance. In contrast, with high gf task performance entered as a covariate, all the reported associations between COMT val158met genotype and regional fMRI activity remained significant. These findings provide some support for the claim put forward by proponents of the “candidate gene intermediate phenotype” approach that a more direct and robust relationship exists between genetic markers and neural activity than between genetic markers and behavioral performance (Hariri and Weinberger 2003; Goldberg and Weinberger 2004). This suggests that attempts to advance understanding of genetic and environmental influences upon gf beyond assessment of heritability may be facilitated not only by the consideration of specific genetic markers but also by complementing behavioral indices of performance with consideration of intermediate neural mechanisms.
The positive correlation observed between COMT val allele load and the spatial measure of gf derived from the Cattell Culture Fair for use within the scanner is consistent with the prediction of a val advantage on tasks requiring flexible cognition (Bilder et al. 2004). However, it is of note that, outside the scanner, we observed no significant relationship between COMT val158met genotype and Cattell Culture Fair Scale 2B scores. This is in line with previous studies which have failed to find a replicable effect of single genetic polymorphisms, including the COMT val158met polymorphism, upon behavioral performance of standardized intelligence tests (e.g., Plomin et al. 2001) though, as mentioned earlier, those studies used less pure indices of gf. The difference in our behavioral findings inside and outside the scanner might simply reflect the low power of gene-behavior analyses, leading to difficulties in the replication of behavioral results with relatively small sample sizes. Indeed, it has been suggested that sample sizes in the 100s to 1,000s may be needed to reliably detect effects of single genes upon cognitive performance measures, whereas far smaller samples may suffice for detecting effects of the same genes upon neural activation indices (Hariri and Weinberger 2003). An alternative, potentially more interesting possibility is that the scanner environment itself may have a moderating influence. Like ours, other studies have reported differing behavioral effects of the COMT val158met polymorphism inside and outside the fMRI environment (Egan et al. 2001; Goldberg et al. 2003). It has also been speculated that state or environmental factors impacting on arousal levels may modulate the effect of COMT val158met genotype upon prefrontal function (Tunbridge et al. 2006). Scanner noise could be one such factor. It has been demonstrated that noise can act similarly to DA D1 agonists in moving subjects along the inverted U–shaped response function that characterizes the relationship between DA D1 receptor stimulation and prefrontal function (Arnsten and Goldman-Rakic 1998). It is conceivable that scanner noise could alter positioning on the D1-PFC response function such that increased DA metabolism associated with the COMT val allele optimizes high gf task performance. Other factors associated with task performance within the scanner—for example partial immobilization leading to mild claustrophobia or “restraint stress” in some individuals—could also contribute to a potential interaction of COMT genotype by context (task performed inside vs. outside the scanner). The possibility that cognitive function in general and indices of gf in particular may be influenced by aspects of the current environment that alter arousal levels or affective state is receiving renewed attention (Gray et al. 2002; Blair 2006). Together with increasing awareness of the potential impact of immediate environmental context and longer term environmental factors upon gene–brain and gene–behavior associations (Caspi et al. 2003; Canli et al. 2006; Tunbridge et al. 2006), this highlights the need for future work directly addressing these issues.
In summary, our findings indicate that the COMT val158met polymorphism has a significant influence upon neural activity across a network of cortical regions, including LPFC, pre-SMA/ACC, and IPS, during performance of high gf versus low gf tasks. We obtained 2 measures of gf-related neural activation using tasks from very different domains (1 spatial and 1 verbal). In both cases, the number of the more metabolically active COMT val alleles possessed significantly predicted the strength of the neural response in right DLPFC, pre-SMA, and bilateral IPS during performance of high gf versus low gf items. The strong influence of the COMT val158met polymorphism upon the extended cortical circuitry underlying gf-related task performance is in line with the suggestion that the effects of single genes may be observed relatively clearly at the neurophysiological level of analysis achieved by fMRI. It also suggests that genetic influences upon catecholamine modulation of this circuitry might account for a significant proportion of individual variability in the neural response to increases in higher cognitive demands across differing domains of processing. These genetic influences are inevitably complex and likely to extend beyond the val158met SNP studied here. Additional studies of other common genetic variants impacting upon catecholamine function, together with larger scale haplotype studies and analysis of imaging data acquired using multiple measures of higher cognitive function, should aid in further clarifying genetic contributions to catecholamine modulation of higher cognitive function.
Funding
UK Medical Research Council (U.1055.01.001.00001.01 and G120/919); Betty Behrens Research Fellowship (Clare Hall, Cambridge University); Marie Curie Outgoing International Fellowship (MOIF-CT-2005-8884).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Supplementary Material
Acknowledgments
Our thanks go B. Cox and S. Strangeways for graphical assistance, M. Brett and R. Henson for advice on imaging analysis, and V. Lupson, L. Chamberlain, L. Germine, H. Lloyd and R. Nowotarski for assistance with fMRI data collection. Conflict of Interest: None declared.
References
- Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: the same or different constructs? Psychol Bull. 2005;131:30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Morelli M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunnett SB, Bentivoglio M, Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy. Vol. 21. Dopamine. Amsterdam (NL): Elsevier; 2005. pp. 1–107. [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Birney DP, Bowman DB, Pallier G. Prior to paradigm integration, the task is to resolve construct definitions of gf and WM. Behav Brain Sci. 2006;29:127–129. [Google Scholar]
- Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behav Brain Sci. 2006;29:109–125. doi: 10.1017/S0140525X06009034. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:2. [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP. Neural correlates of epigenesis. Proc Natl Acad Sci USA. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Abilities: their structure, growth and action. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Rebollo I, Abad FJ, Shih PC. Complex span tasks, simple span tasks, and cognitive abilities: a reanalysis of key studies. Mem Cognit. 2006;34:158–171. doi: 10.3758/bf03193395. [DOI] [PubMed] [Google Scholar]
- Cusack R, Brett M, Osswald K. An evaluation of the use of magnetic field maps to undistort echo-planar images. Neuroimage. 2003;18:127–142. doi: 10.1006/nimg.2002.1281. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry. 1996;153:268–270. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Q J Exp Psychol. 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;10:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Goldman D, Weinberger DR. Effect of COMT val 108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harmon HH, Derman D. ETS kit of factor-referenced cognitive tests. Princeton (NJ): Educational Testing Service; 1976. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122:963–979. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase val158met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldberg T, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE. Beautiful minds (i.e., brains) and the neural basis of intelligence. Behav Brain Sci. 2007;30:174–178. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Redick TS, Hambrick DZ, Kane MJ, Conway AR, Engle RW. Working memory, executive function, and general fluid intelligence are not the same. Behav Brain Sci. 2006;29:135–136. [Google Scholar]
- Institute for Personality and Ability Testing. Measuring intelligence with the culture fair tests. Champaign (IL): The Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Kane MJ, Hambrick DZ, Conway AR. Working memory capacity and fluid intelligence are strongly related constructs: comment on Ackerman, Beier, and Boyle 2005. Psychol Bull. 2005;131:66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol-O methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, Mattay VS, Egan M, Weinberger DR. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Plomin R, Hill L, Craig IW, McGuffin P, Purcell S, Sham P, Lubinski D, Thompson LA, Fisher PJ, Turic D, et al. A genome-wide scan of 1842 DNA markers for allelic associations with general cognitive ability: a five-stage design using DNA pooling and extreme selected groups. Behav Genet. 2001;31:497–509. doi: 10.1023/a:1013385125887. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga M, et al., editors. The cognitive neurosciences. Cambridge (MA): MIT Press; 1995. pp. 703–720. [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG Tischmeyer W, Gundelfinger ED, Heinze HJ, Duzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The abilities of man. New York: Macmillan; 1927. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. COMT val158met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Goldman D. Genetics of human prefrontal function. Brain Res Rev. 2003;43:134–163. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wothke W, Curran LT, Fairbank BA, Augustin JW, Gillet AH, Guerrero C., Jr . A Factor analytic examination of the armed services vocational aptitude battery (ASVAB) and the kit of factor-referenced tests Rep No AFHRL-TR-90-67. Brooks Air Force Base (TX): Air Force Human Resources Laboratory; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.