Abstract
Neural activity fluctuates dynamically with time, and these changes have been reported to be of behavioral significance, despite occurring spontaneously. Through electroencephalography (EEG), fluctuations in α-band (8–14 Hz) activity have been identified over posterior sites that covary on a trial-by-trial basis with whether an upcoming visual stimulus will be detected or not. These fluctuations are thought to index the momentary state of visual cortex excitability. Here, we tested this hypothesis by directly exciting human visual cortex via transcranial magnetic stimulation (TMS) to induce illusory visual percepts (phosphenes) in blindfolded participants, while simultaneously recording EEG. We found that identical TMS-stimuli evoked a percept (P-yes) or not (P-no) depending on prestimulus α-activity. Low prestimulus α-band power resulted in TMS reliably inducing phosphenes (P-yes trials), whereas high prestimulus α-values led the same TMS-stimuli failing to evoke a visual percept (P-no trials). Additional analyses indicated that the perceptually relevant fluctuations in α-activity/visual cortex excitability were spatially specific and occurred on a subsecond time scale in a recurrent pattern. Our data directly link momentary levels of posterior α-band activity to distinct states of visual cortex excitability, and suggest that their spontaneous fluctuation constitutes a visual operation mode that is activated automatically even without retinal input.
Keywords: electroencephalography (EEG), phosphene, state dependency, transcranial magnetic stimulation (TMS), visual perception
Introduction
Visual perception does not only depend on the physical properties of the sensory stimulus but also on the state of visual areas at time of sensory input. This state can be voluntarily modulated to influence perception by deployment of visual attention. For example, covertly directing attention to a specific position in space increases activity in visual areas tuned to the attended position, as revealed by single-cell recordings (Luck et al. 1997) and functional magnetic resonance imaging (fMRI) (Kastner et al. 1999; Müller et al. 2003). These changes have also been termed shifts in visual baseline activity, because they occur in the absence of visual stimulation and most likely originate through top-down control from higher-order attention areas (Luck et al. 1997; Kastner et al. 1999; Hopfinger et al. 2000; Giesbrecht et al. 2006). In electro- and magnetoencephalography (EEG/MEG) studies on humans, prestimulus baseline shifts during attention deployment occur in the α-frequency band (∼8–14 Hz), which is thought to reflect the state of cortical excitability (Pfurtscheller 2001). Part of these attention-related changes in oscillatory α-band activity appear to be generated in areas of the visual network, as suggested by their retinotopic specificity (Worden et al. 2000; Rihs et al. 2007) and source distribution (Yamagishi et al. 2005). The shifts consist of sustained α-decreases (α-desynchronization) or α-increases (α-synchronization) over posterior recording sites that occur in accordance to the direction of attention either contralaterally to the attended position (α-desynchronization; Sauseng et al. 2005; Yamagishi et al. 2005; Thut et al. 2006) or ipsilaterally (α-synchronization; Worden et al. 2000; Kelly et al. 2006; Rihs et al. 2007), and that predict imminent visual processing (Thut et al. 2006).
Fluctuations in visual baseline activity appear to also occur spontaneously, that is, without experimentally manipulating the attention focus. This moment-by-moment variability in neuronal activity may be considered “physiological” noise. Yet, a growing body of evidence suggests otherwise. Spontaneous fluctuations in brain activity occur along well-defined neural networks (Goldman et al. 2002; Leopold and Logothetis 2003; Laufs et al. 2003, 2006; Beckmann et al. 2005; Damoiseaux et al. 2006) suggesting that fluctuations in neuronal activity must have a functional significance, and may account for the variability in neuronal or behavioral responses to physically identical stimuli. In the visual system, animal studies have revealed a link between spontaneously varying patterns of early visual cortex activity at baseline, and the size or latency of the neuronal response to the forthcoming stimulus (Arieli et al. 1996; Azouz and Gray 1999; Fries et al. 2001; van der Togt et al. 2005), as well as whether the stimulus reaches awareness (Super et al. 2003). In humans, trial-by-trial variability in the fMRI base response relates to variability in visual perception (Ress et al. 2000). Finally, using EEG, distinct oscillatory signatures have been identified in the α frequency band at prestimulus baseline that relate to processing of an upcoming visual stimulus. Momentary states of decreased versus increased occipito-parietal α-band activity predict whether a visual threshold stimulus will be detected or not, as well as the size of the evoked brain response (Ergenoglu et al. 2004). Likewise, low versus high resting α-band activity differentiates between good versus bad performers in visual discrimination tasks (Hanslmayr et al. 2005, 2007).
Taken together, these findings provide ample evidence for neuronal state dependency of visual perception, and ample, although indirect arguments for baseline α-band power over posterior sites to reveal the momentary state of visual cortex excitability, conditioning future visual processing performance (e.g., Worden et al. 2000; Ergenoglu et al. 2004; Sauseng et al. 2005; Thut et al. 2006). The findings also indicate that perceptually relevant shifts in posterior α-band activity can be voluntarily driven (e.g., Worden et al. 2000; Sauseng et al. 2005; Thut et al. 2006) but can also occur spontaneously without experimental modulation of the attention focus (Ergenoglu et al. 2004), yet the source of these latter changes in visual state is unknown. The origin of these changes can range from occasional drowsiness and general inattention (Super et al. 2003) to trial-by-trial variability in visual spatial attention (Ress et al. 2000). Whereas the first mechanism would be overall detrimental to task performance, fluctuations in focused attention might play a functional role, for example, through periodically reallocating attention to different positions (visual scanning).
The main goal of the present experiment was to specifically probe for a link between spontaneous fluctuations in α-band activity, visual cortex excitability, and perception without experimental modulation of the attention focus; and to gain further insight into the origin of these changes. To address this issue, and unlike previous EEG studies on the link between α-band activity and perception (e.g., Ergenoglu et al. 2004; Hanslmayr et al. 2005, 2007; Thut et al. 2006), we stimulated visual cortex via transcranial magnetic stimulation (TMS) inducing illusory visual percepts (phosphenes) in the absence of retinal input (Cowey and Walsh 2000; Kammer et al. 2005). This allowed to directly assess visual cortex excitability at discrete time points (Silvanto et al. 2006; Bestmann et al. 2007; Ramos-Estebanez et al. 2007), while simultaneously recording EEG activity prior to TMS. We tested whether the same, physically identical TMS-stimulus (applied at perceptual threshold intensity) was more likely to excite visual cortex (i.e., to evoke phosphenes) in trials with low than high prestimulus α-band activity. In addition, to gain further insight into the origin of the α-band changes and the associated visual excitability states, we studied their temporal and spatial profiles prior to the stimulus and their evolution over the experimental session. If mechanisms akin to fluctuations in the spatial attention focus were at the origin of the fluctuations, α-band changes and excitability states would be expected to fluctuate rapidly, that is, on a subsecond time scale. If occasional drowsiness with lapses in vigilance and periods of general inattention were at their origin, changes in α-band activity would last over several seconds to minutes, as would the state of low cortical excitability. In terms of spatial characteristics, variations in focused spatial attention would be expected to lead to spatially specific, retinotopically organized changes of α-band activity over posterior sites covarying with fluctuations in visual cortex excitability of the same area (spatially specific α/excitability link). Drowsiness, on the other hand, would more likely be associated with changes in the general state of the visual cortex and therefore result in global changes of visual cortex excitability and a location unspecific α/excitability link. We found spontaneous fluctuations in posterior α-activity (without experimental modulation of the attention focus) to be linked to variability in visual cortex excitability. In addition, these fluctuations were akin to the changes of α-activity observed during active covert attention shifts (in terms of subsecond time scale and location specificity). We thus observed rapid, spontaneous fluctuations in α-band activity that seemed to constitute a visual operation mode rather than being coupled to drowsiness.
Materials and Methods
Participants
Fifteen healthy, right-handed volunteers were screened to identify those who were able to see TMS-induced phosphenes in the first test session. Consistent with data in the literature (Kammer et al. 2005), 60% of the screened volunteers readily perceived TMS-induced phosphenes without extensive training and were included in the study. All participants (5 women, 4 men, mean age: 30 years, range: 24–39 years) gave written informed consent to the study, which had been approved by the Ethical Committee of the Geneva University Hospital.
Training Sessions
Participants first underwent several perceptual training sessions (n = 3–5) at separate days to accurately define in each individual the optimal scalp position over which TMS induced a visual percept, to precisely identify the required stimulation intensity, and to establish its reproducibility. Each of these sessions included careful determination of the site from which a single TMS pulse induced the strongest phosphene, documentation of the phosphenes' shape, size and position in the visual field through drawings by the participant, and the assessment of input–output curves, quantifying perceptual output (percentage of visual percepts) as a function of different input-intensities (in this study 80%, 85%, 90%, 95%, 98%, 100%, 102%, 105%, 110%, 115%, vs. 120% of phosphene threshold). This served to evaluate the consistency of phosphene perception through stimulation above and below perceptual threshold and for individual psychophysical titration of stimulus intensity. During training, participants were blindfolded, except for documentation of the TMS-induced phosphene's shape, size, and position, during which the blindfold mask was removed, the participants fixated a central white spot on black background while stimulated (in the darkened room), and sketched on a sheet the TMS-induced phosphene relative to fixation. The optimal TMS coil position and intensity, the orientation of the coil handle for evoking the strongest phosphenes and the shape, size, and position of the perceived phosphenes varied across participants but was constant for each participant across the various training sessions. The same individual TMS-parameters (site, coil orientation, intensity) that proved to be most effective for evoking phosphenes during the training sessions were used for stimulation during the experiment.
Experimental Session
Single TMS pulses were applied in the blindfolded subjects at variable intervals (see below) over each individual's optimal stimulation site (occipital pole, predetermined during training) at constant, pretitrated intensity to evoke phosphenes in approximately 50% of all trials (individual phosphene threshold; PT). Participants indicated after each pulse via a right-hand button press whether they perceived a phosphene (P-yes: right index) or not (P-no: right middle finger). The interval between button press and the subsequent TMS pulse varied from 3 to 5 s (n = 5 intervals of 3-, 3.5-, 4-, 4.5-, or 5-s duration, randomly intermixed). In total, there were 7 experimental runs of approximately 6-min duration (consisting of n = 75 single pulses each) leading to a total of 525 trials per participant (n ∼ 260 P-yes and 260 P-no trials). After each run, we computed the number of P-yes versus P-no trials and slightly readjusted TMS intensity if there were more P-yes than P-no trials (or vice versa) in the preceding run (which would suggest a change in PT). To prevent systematic drifts in PT due to adaptation to darkness or drowsiness, however, lights were turned on and participants were asked to take off the blindfold mask as well as to open the eyes in the breaks between the experimental runs. Concurrently with TMS, EEG was continuously recorded using a TMS-compatible device (see below). A PC computer running E-prime (E-Prime 1.1, Psychology Software Tools, Pittsburgh, PA) controlled TMS pulse delivery and response data collection.
TMS Apparatus and EEG Acquisition
Participants wore a lycra swimming cap on which the optimal position of the coil for evoking strongest phosphenes was marked. TMS was delivered via a 70-mm figure-of-eight coil (maximum field strength: 2.2 T) and a Magstim Rapid Transcranial Magnetic Stimulator (Magstim Company, Whitland Wales, UK).
EEG was recorded with a TMS-compatible unit (Ives EEG solutions, Inc., Burlington, Ontario, Canada) at 200 Hz sampling rate from 45 scalp electrodes, glued manually according to the international 10–10 electrode system. Conductive plastic-body electrodes coated with a thin layer of silver epoxy were used to prevent overheating of the electrodes during TMS. EEG-signals were recorded using a bipolar montage and were recalculated off-line against the average reference. Eye movements were monitored by 2 additional bipolar horizontal and vertical electrooculographic (EOG) derivations. Impedance was kept below 10 kΩ. For more details of the TMS-compatible unit see Thut et al. (2005) and Ives et al. (2006). Continuous single-epochs of 1000-ms duration (−1000 to 0 ms prior to TMS) were included in the analysis if not contaminated by muscle artifacts.
EEG Analysis
The time course and spatial (scalp) distribution of oscillatory activity preceding a visual percept (P-yes) or no percept (P-no) were calculated using a modified temporal spectral evolution (TSE) algorithm (Salmelin and Hari 1994), applied also in (Worden et al. 2000; Kelly et al. 2006; Thut et al. 2006; Rihs et al. 2007), and related to the event-related desynchronization/synchronization method (Pfurtscheller and Lopes da Silva 1999). TSE was computed for the theta (3–7 Hz), alpha (8–14), beta (15–30 Hz), and gamma (30–50 Hz) frequency bands for the 1000 ms preceding the TMS pulse, and for each participant, electrode and condition (P-yes vs. P-no) using the following calculation steps:
1. Data of each artifact-free single-epoch were bandpass-filtered in each of the 4 frequency bands;
2. Rectified (negative potentials become positive);
3. Smoothed by averaging over 10 consecutive 5-ms time points (200 Hz recording rate) leading to 20 × 50-ms time bins; and finally
4. Averaged across all single trials.
For the statistical analysis, data were collapsed within time windows of 250-ms duration, in analogy to previous studies on TSE-waveforms in the α-frequency band (Foxe et al. 1998; Worden et al. 2000; Sauseng et al. 2005; Rihs et al. 2007). This resulted in 4 consecutive 250-ms windows ranging from −1000 to −750 ms (w1), −750 to −500 ms (w2), −500 to −250 ms (w3), and −250 to 0 ms pre-TMS (w4). Statistics consisted of analysis of variance (ANOVA) on TSE-waveforms over posterior recording sites (PO7-PO3-O1-O2-PO4-PO8) with the within-subject factors Perceptual Outcome (P-yes vs. P-no), Time (w1, w2, w3, vs. w4), Hemisphere (contra- vs. ipsilateral to induced phosphene), and interstimulus interval (ISI) (3, 3.5, 4, 4.5, vs. 5 s), followed by Bonferroni-corrected post hoc tests (where appropriate).
Results
Phosphene Perception, Stimulation Site, and Stimulation Intensity
Induced percepts consisted of brief flashes of light (phosphenes) in the lower visual field quadrant opposite to the stimulated occipital cortex (Fig. 1A,B) corresponding to stimulation of the dorsal part of the occipital pole representing the lower visual field, and in accordance with previous reports (Cowey and Walsh 2000; Kammer et al. 2005). During training, all participants showed a steady input–output curve, with stronger phosphene induction (percentage of induced visual percepts) as TMS intensity increased (main effect of input-intensity: F10,80 = 71.6, P < 0.0001; repeated-measure ANOVA), further ensuring the presence of reliable phosphenes. During the experiment, TMS was applied at constant, pretitrated stimulation intensity (individual PT; mean: 67.5% of maximum stimulator output; range: 59–76%) always targeting the same, optimal stimulation site localized on average (±SE) 2.95 ± 0.27 cm above the Inion and 2.0 ± 0.84 cm lateral to it. Overall, phosphenes were evoked in 48 ± 2% of all trials (P-yes trials, mean ± SE) and no phosphene in the remaining 52 ± 2% of trials (P-no trials). Critically, the design thus gave rise to 2 types of trials that were identical regarding all aspects of stimulation within individuals (site, intensity), but differed by the subjective experience of whether a phosphene was evoked or not (reported by button press), so that neither variability in stimulation site, stimulation intensity, nor in the click or the tapping sensation associated with TMS can account for the differential behavioral consequences of the TMS pulses (P-yes or P-no).
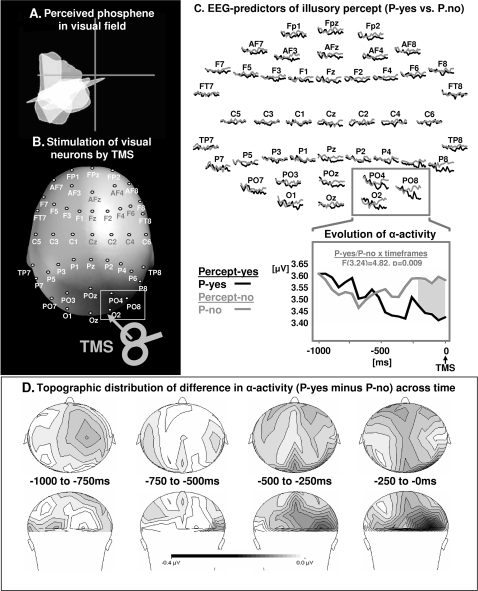
Figure 1.
(A) Visual sensations (phosphenes) in the 4 visual field quadrants (delimited by a cross-hair) induced by (B). TMS over the occipital pole, online to simultaneous EEG-recordings from 45 scalp electrodes. (C) Time course of oscillatory α-band (8–14 Hz) activity (TSE) prior to TMS as a function of visual cortex excitability. (D) Scalp distribution of differences in α-band activity across the 4 windows of analysis. The TSE-waveforms are shown over a 1000-ms time window for all 45 scalp sensors (C: upper panel), and for the parieto-occipital (PO) and occipital (O) electrodes contralateral to the induced visual experience (data collapsed over electrodes, C: lower panel), that is, ipsilateral to the site of magnetic stimulation (see also B). Black lines correspond to those trials in which the participants reported perception of a phosphene induced by TMS (P-yes, high visual cortex excitability), gray lines represent those trials without perception of a TMS-induced phosphene (P-no, low visual cortex excitability). The gray area illustrates the time window of significant differences between trials (post hoc tests, Bonferroni corrected). Note that the most effective site for inducing phosphenes was localized for most individuals over the right hemisphere (n = 7, both left and right occipital poles tested). In only 2 participants it was easier to elicit stable phosphenes over the left hemisphere. The data of these 2 participants were flipped left–right for analysis and representation in this and all other figure.
α-Band Activity in P-yes versus P-no Trials
We found a differential time course of oscillatory α-band (8–14 Hz) activity prior to TMS-delivery over posterior recording sites (PO7-PO3-O1-O2-PO4-PO8) between trials where a phosphene was evoked (P-yes) (Fig. 1C, black line) and those where a phosphene was not evoked (P-no) (Fig. 1C, gray line), and that depended on the side of recording relative to phosphene induction (3-way ANOVA interaction of Perceptual Outcome [P-yes vs. P-no] × Time × Hemisphere [contra- vs. ipsilateral to phosphene]: F3,24 = 3.13, P = 0.044). The difference in time course was restricted to posterior electrodes contralateral to the induced visual experience (i.e., ipsilateral to magnetic stimulation, Fig. 1A,B, Fig. 1C, gray frame), as shown by a significant 2-way interaction of Perceptual Outcome × Time (F3,24 = 4.82, P = 0.009, Fig. 1C, lower panel) that was not observed over the corresponding electrodes of the opposite hemisphere (F3,24 = 0.49, P = 0.69). A continuous decrease in pre-TMS α-band activity was observed in those trials in which a visual percept was induced, indexing high visual cortex excitability at TMS-delivery (Fig. 1C: lower panel, black line). Increases in α-band activity prior to TMS, on the other hand, were associated with no TMS-induced percept, indicating low visual cortex excitability at TMS discharge (Fig. 1C: lower panel, gray line). These differential time courses led to significant differences in α-band activity between P-yes and P-no trials opposite to the induced phosphene immediately prior to TMS (last 250-ms window: P = 0.033, Bonferroni-corrected post hoc comparisons; Fig. 1C, lower panel and Fig. 1D). The post hoc analysis further revealed that the α-decrease preceding P-yes trials was significant (first 250-ms vs. last 250-ms window: P = 0.029), indicating that the perceptually relevant changes occurred on a subsecond time scale. The increase of α-activity in P-no trials did not reach significance over time bins, and there were also no significant differences between P-yes and P-no trials in terms of pre-TMS activity in other frequency bands (other tested bands: theta, 3–7 Hz; beta, 15–30 Hz; gamma, 30–50 Hz).
Spontaneous Fluctuations in α-Band Activity Indexing State of Visual Excitability: Relation to Voluntary Attention Control and Eye Movements
To address the question to what extend the prestimulus fluctuations in α-band activity/visual cortex excitability were driven by attentional mechanisms under voluntary control (spatial attention shifts) or arose spontaneously, we subjected our data to a number of additional analyses. We looked for spatially specific modulations of α-band activity (independently of perceptual outcome) shown to index systematic attention deployment to a lateralized position, namely differential α-modulation over contra- versus ipsilateral posterior recording sites (e.g., Worden et al. 2000; Sauseng et al. 2005; Thut et al. 2006). In addition, we broke down the trials according to ISI (n = 5 intervals of 3, 3.5, 4, 4.5, or 5-s duration) to take into account potentially increasing anticipatory attention deployment due to growing stimulus expectancy over longer ISIs. Finally, we also looked at eye movements prior to TMS to rule out overt orienting.
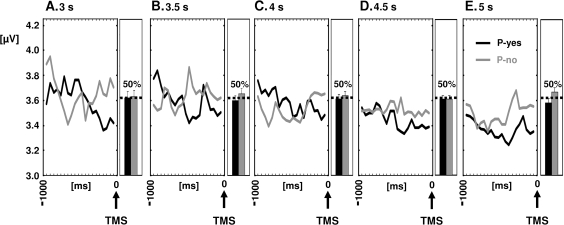
We found no evidence for differential α-band changes over time between posterior recordings sites located contra- versus ipsilaterally to the evoked phosphene (no 2-way interaction of Time × Hemisphere: F3,24 = 0.06, P = 0.99, data collapsed over P-yes/P-no trials). In addition, this interaction term was independent of ISI and thus stimulus expectancy (no 3-way interaction: F12,96 = 1.00, P = 0.45), speaking against the presence of voluntary covert attention shifts toward predictable phosphene positions as a strategy for task execution. Moreover, stimulus expectancy did not influence our key (location specific) effect, namely the differential time course of oscillatory α-band activity preceding P-yes versus P-no trials contralateral to induced phosphenes (Fig. 2, line plots, no 3-way interaction ISI × Perceptual Outcome × Time Frame: F12,96 = 1.09, P = 0.38). Likewise, the probability of perceiving phosphenes or not (P-yes vs. P-no-trials) was about 50% (1:1 ratio) irrespective of expectancy (Fig. 2, bar plots, no 2-way interaction ISI × Perceptual Outcome: F4,32 = 0.38, P = 0.81), further suggesting that voluntary attention modulation/control was absent.
Figure 2.
Time course of oscillatory α-band (8–14 Hz) activity prior to TMS over posterior recording sites contralateral to induced phosphenes, that is, ipsilateral to stimulation (line plots, data collapsed over same parieto-occipital (PO) and occipital (O) derivations as in Figure 1C gray frame) and proportion of P-yes and P-no trials (bar plots), as a function of ISI (A–E). The α-band changes leading to P-yes versus P-no percepts (associated with low vs. high visual cortex excitability) were not influenced by ISI, nor was the proportion of P-yes versus P-no trials, indicating independence from stimulus expectancy.
In regards to eye movements, there was no difference in prestimulus EOG-activity between P-yes and P-no trials as revealed by the analysis over the 1000-ms, pre-TMS time window. The EOG-signal prior to TMS did not change over time (no main effect of Time: F3,24 = 2.15/2.54, P = 0.12/0.08, for vertical/horizontal EOG derivations), was identical between P-yes versus P-no trials (no main effect of Perceptual Outcome: F1,8 = 1.73/0.00, P = 0.22/0.99), and there was no interaction between the 2 factors (Perceptual Outcome × Time: F3,24 = 0.44/0.47, P = 0.73/0.71). This revealed stable eye-positions prior to TMS independently of whether a phosphene was perceived or not, and speaks against overt orienting toward the eventual phosphene position for task execution.
Note that there was a main effect of expectancy (ISI) on posterior α-band activity (F4,32 = 3.59, P = 0.016) which was due to a α-decrease with increasing ISI (Fig. 2A–E, line plots). Because this effect was independent of Hemisphere (interaction ISI × Hemisphere: F4,32 = 0.45, P = 0.77), that is, equally observed over the occipito-parietal recording sites located contra- and ipsilaterally to phosphene induction (main effect of ISIs: contra- vs. ipsilateral sites: F4,32 = 3.53 vs. 2.94, P = 0.017 vs. 0.035), it cannot be assigned to spatial attention shifts and most probably reflects spatially unspecific processes (Klimesch et al. 1998; Bastiaansen et al. 2001). Note also that this effect was primarily driven by ISI differences in the first time window (−1000 to −750 ms prior to TMS, F4,32 = 4.05, P = 0.009) but absent in the last window (−250 to 0 ms prior to TMS, F4,32 = 1.6, P = 0.20) and is thus unlikely to have influenced perception, in line with the equal proportion of TMS-induced P-yes versus P-no trials across ISIs (Fig. 2A–E, box plots, see above for statistics).
Sequence of P-yes and P-no Trials Across Time
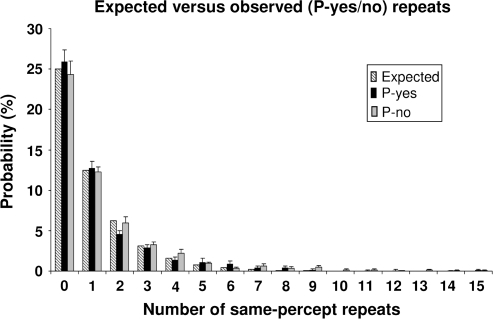
To provide further information on the origin of the fluctuations in visual excitability states, we analyzed the sequence of P-yes and P-no trials across time over the experimental session. Although transient states of absent-mindedness, general inattention or vigilance decrease might have led to a decrease in cortical excitability, to an increase in α-band activity, and caused α-activity and perceptual performance to covary, these processes would be expected to occur over several trials leading to disproportionately long series of P-no repeats. Yet, we found the probability to encounter long series of consecutive same-percepts (either P-yes or P-no) to be negligibly low (Fig. 3). In accordance with a random, binomial distribution of P-yes and P-no responses, the frequency of these same-percept repeats exponentially decreased over number of repeats (Fig. 3, no significant differences between expected and observed frequencies; P-yes vs. expected: χ2 = 3.7, P = 0.998, P-no vs. expected: χ2 = 3.0, P = 0.999, df = 15). In other words, whether in any given trial a percept was induced or not was random and independent of previous trial history.
Figure 3.
Observed probability of same-percept repeats over all sessions (P-yes vs. P-no; black vs. gray bars) as a function of number of repeats (n = 0–15) and what would be expected based on a random, binomial distribution of P-yes and P-no responses (dashed bars). There was a high amount of single percepts (P-yes followed by a P-no report, or vice versa; i.e., n = 0 repeats) and much fewer same-percept repeats (e.g., n = 1 repeat: P-yes/P-no followed by another P-yes/P-no report). This provides evidence for yes/no-percepts and therefore perceptive states to alternate randomly on a subtrial-by-trial time scale (≤5 s). Whiskers represent SEs.
In addition and to further rule out that fluctuations in perception were dependent on drowsiness or absent-mindedness, which might have increased as the experiment evolved, we analyzed phosphene perception as a function of the 7 consecutive experimental runs (using repeated-measure 1-way ANOVAs with the factor ExpRun). There was no significant differences between runs, neither regarding the TMS intensity needed to evoke phosphenes in approximately half of the trials (F6,48 = 0.76, P = 0.61) nor regarding the percentage of P-yes responses per run (F6,48 = 0.51, P = 0.80). This altogether excludes long-lasting absent-mindedness or other general mechanisms as an explanation for our result, which additionally would be expected to lead to global, tonic α-band changes and not to the local, phasic (subsecond) changes in areas coding for the visual field where the phosphene was induced (Fig. 1).
Discussion
In this article, we show a direct link between fluctuations in oscillatory α-band (8–14 Hz) activity over posterior recording sites and visual cortex excitability, measured through EEG and online stimulation of the visual cortex via TMS, respectively. Visual cortex was more excitable by TMS at moments when α-band activity was low, and less excitable when α-band activity was high, leading to TMS-induced visual percepts (phosphenes) or no percepts, respectively. In addition of being perceptually relevant, the fluctuations in α-band activity over posterior sites seemed to be spontaneously generated, to occur on a relatively rapid (subsecond) time scale and to be location specific, that is, fluctuations that were relevant for lateralized phosphene perception occurred over occipital cortex contralaterally to the induced percept. This suggests that the distribution of α-band activity over posterior sites signals momentary excitability of visual areas tuned to the contralateral visual field. It should be noted that although our finding applies by experimental design to the visual system, α-band activity is not a stand-alone feature of visual areas in the occipital lobe as combined fMRI–EEG studies have mapped spontaneous fluctuations in this activity also to frontal, insular, and parietal sites (Goldman et al. 2002; Laufs et al. 2003, 2006; Moosmann et al. 2003; Martinez-Montes et al. 2004; de Munck et al. 2007).
Our results provide direct support to the common view that periods of decreased α-activity (α-desynchronization) reflect a state of enhanced cortical excitability (Pfurtscheller 2001) and increased α-activity (α-synchronization) a state of cortical idling (Pfurtscheller 2001) or even active inhibition (Foxe et al. 1998; Fu et al. 2001; Hummel et al. 2002) in which cortical excitability is reduced (see also Klimesch et al. 2007). In line with an association between posterior α-band activity and excitability of visual cortex, there is a growing body of evidence that these measures undergo spatially specific modulations when subjects direct their attention to a lateralized position in space, in accordance with the perceptual benefit and cost for visual stimulus processing at attended versus unattended locations (Posner et al. 1980). Voluntary, lateralized attention deployment leads to α-decreases or increases over areas representing the attended versus unattended visual field (Worden et al. 2000; Sauseng et al. 2005; Yamagishi et al. 2005; Kelly et al. 2006; Thut et al. 2006; Rihs et al. 2007), and to an enhancement or reduction of cortical excitability in visual areas tuned to the attended versus unattended visual space (Bestmann et al. 2007), as revealed by EEG and TMS, respectively. These data accord with work not involving spatial attention shifts (foveal stimuli) showing that visual detection/discrimination performance covaries inversely with prestimulus α-band activity over posterior sites (Ergenoglu et al. 2004; Hanslmayr et al. 2005, 2007). Note that 1 previous study has found high prestimulus α-activity to facilitate subsequent visual stimulus detection (Babiloni et al. 2006), in apparent disagreement with an inverse relationship between α-activity and visual perception. However, as has been put forward previously (Hanslmayr et al. 2007), the detection task of this study had a memory component due to delayed responses so that it might have tested memory rather than perceptual performance, former shown to be positively related to prestimulus α-activity (Hanslmayr et al. 2005).
In comparison with these previous studies, we show a direct link between posterior α-band activity and visual cortex excitability. We further confirm the existence of trial-by-trial fluctuations with perceptual relevance without experimental modulation of the attention focus (Ergenoglu et al. 2004), and provide novel information on the mechanisms that lie at the origin of these spontaneous α-band changes. Spontaneous fluctuations may simply be generated by periods of general inattention or absent-mindedness, that is, reflect occasional disengagement from the task by drops in vigilance (as suggested by Super et al. 2003); or may alternatively represent a functional component similar to variations in focused spatial attention (akin to the observation by Ress et al. 2000). Our findings speak against transient states of general inattention as an account for the observed fluctuations, given that the trial-by-trial changes in visual cortex excitability (phosphene induction) were indicative of rapid, random alternation between receptive versus unreceptive states, and not of prolonged persistence in states of visual non-excitability such as expected with reduced vigilance. We can further discard transient states of general inattention as a possible explanation, because in terms of α-band activity this process would be associated with global changes over the entire occipital pole, that is, would co-occur over both the left and right hemisphere and hence show location unspecific links to perceptual performance. Instead, the α-band changes exhibited several characteristics of attention modulation, including location specificity of the α/excitability link and a subsecond timing. The α-band fluctuations are thus akin to what has been observed during active covert attention shifts in terms of temporal and spatial profiles (e.g., Worden et al. 2000; Thut et al. 2006; Rihs et al. 2007), although in the present study the attention focus was not experimentally modulated (invariant phosphene location).
This raises the question to what extend the fluctuations we observed occurred in the absence of voluntary visual spatial attention control and were thus generated automatically or could represent variability in voluntary control per se. We argue that our data favor the former mechanism for the following reasons. We did not find any evidence of differential modulations of α-activity over the 2 hemispheres (when P-yes and P-no trials were collapsed) which would signal the presence of systematic voluntary attention shifts directed to a lateralized position, as shown previously (e.g., Worden et al. 2000; Sauseng et al. 2005; Thut et al. 2006). To this adds that prolonging ISI did not enhance perception (same proportion of visual percepts or no-percepts independently of ISI). This suggests that participants did not make use of the spatial predictability of phosphene appearance to covertly direct attention to the relevant position (no lateralized α-modulation when P-yes/no trials collapsed, no enhancement of perception with increasing expectancy) and hence that α-activity fluctuations occurred in the absence of voluntary spatial attention control. In line with this interpretation is that participants were unfamiliar with the concept of covert attention shifts and that there was also no evidence for systematic eye movements toward the eventual phosphene's position prior to TMS, that is, for overt orienting. Alternatively, participants might have made use of the predictability of phosphene position to shift attention but prolonging ISIs beyond 3 s might not further enhance perception. Variability in voluntary control per se with occasionally less effective attention shifts might then have played a role in the generation of the location-specific α-band and excitability fluctuations. Yet, even if there were spatial attention control, the fact that the same, subsecond pattern of α-band fluctuations (covarying with visual cortex excitability) was observed across all ISIs (Fig. 2A–E) is indicative of a recurrent and thus automatically generated pattern playing a functional role. Fluctuations due to intermittently ineffective control would be expected to resemble the temporal profile of vigilance drops with no visual function and not those of continuous, periodic attention shifts.
Regarding function, spontaneous fluctuations in excitability of visual areas, and thus in the sensitivity of neurons coding for different parts of the visual field, would increase the probability of detecting spatially unpredictable events in the visual world. Similar to the dynamics of visual processing at a given spatial position that involve temporary inhibition of visual processing at previously examined locations (inhibition of return) as revealed through spatial priming paradigms (Tanaka and Shimojo 1996, 2000; Klein 2000), spontaneous fluctuations of visual network activity in the absence of visual input may govern the prospective gathering of visual information across space in an ecological manner by periodically changing sensitivity for a given location. This would indicate that the mechanisms of inhibition of return take place even without any phenomenal experience as previously suggested (Lamme 2003). In keeping with this notion, there is evidence that coding of spatial information might be privileged in its tendency to occur automatically relative to other information, even if task-irrelevant (Tsal and Lavie 1993; Tsal and Lamy 2000; Meegan and Honsberger 2005).
Spontaneous fluctuations in brain activity are likely to play an important role in cortical function. Fluctuations within areas of the visual network have been shown to account for the trial-by-trial variability in behavioral or neuronal responses to identical stimuli using in vivo recordings in animals (Arieli et al. 1996; Azouz and Gray 1999; Fries et al. 2001; Super et al. 2003; van der Togt et al. 2005, 2006; Womelsdorf et al. 2006), and EEG/MEG (Rahn and Basar 1993; Ergenoglu et al. 2004; Babiloni et al. 2006) or fMRI techniques in humans (Ress et al. 2000). Spontaneous fluctuations also occur in well-defined cerebral networks when one is at rest without any external input or behavioral output (eyes closed or simple visual fixation) as revealed by fMRI (e.g., Goldman et al. 2002; Beckmann et al. 2005; Damoiseaux et al. 2006; Fox et al. 2006). The resting-state fluctuations in fMRI-signals involve striate and adjacent extrastriate cortex (Goldman et al. 2002; Beckmann et al. 2005; Damoiseaux et al. 2006) and covary with spontaneous changes in EEG α-band activity (e.g., Goldman et al. 2002; Moosmann et al. 2003; Martinez-Montes et al. 2004), although at a slower rate (fluctuation of 0.1 Hz) than in the present study, due to the temporal resolution of fMRI. Brain areas typically activated at “rest” as compared with the execution of experimental tasks have been implicated in implicit monitoring of extrapersonal space and the self, emotional processing, episodic memory retrieval, self-generated thought, and other mental operations (Gusnard and Raichle 2001). Our results further confirm the state dependency of visual perception and suggest that the spontaneous fluctuations of neuronal activity in areas of the visual network occurring here in the presence of a task do not only modulate perception (e.g., by being coupled to drowsiness) but may underlie a functional role.
In conclusion, our findings demonstrate a direct link between α-band activity and visual cortex excitability, and suggest that perceptually relevant changes in these measures can arise spontaneously. We argue that this mechanism is likely to sustain covert, automatic scanning of the visual space as part of a visual/attentional operation mode that becomes activated even in the absence of retinal input.
Funding
Swiss National Science Foundation grant (3200B0-105867); the Leenaards Foundation and the Ernst and Luice Schmidheiny Foundation (to G.T.); National Institutes of Health grants (K24 RR018875, RO1 EY12091, R21 EY0116168) to A.P.-L.; the International Human Frontiers Science Program Organization to A.A.; and by the “Centre d'Imagerie BioMédicale” (CIBM).
Acknowledgments
We thank Margitta Seeck and Theodor Landis for helpful comments and discussion, and Denis Brunet for development of analysis software (Cartool). Conflict of Interest: None declared.
References
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci. 1999;19:2209–2223. doi: 10.1523/JNEUROSCI.19-06-02209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Bultrini A, Luca Romani G, Rossini PM. Pre- and poststimulus alpha rhythms are related to conscious visual perception: a high-resolution EEG study. Cereb Cortex. 2006;16:1690–1700. doi: 10.1093/cercor/bhj104. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Bocker KB, Brunia CH, de Munck JC, Spekreijse H. Event-related desynchronization during anticipatory attention for an upcoming stimulus: a comparative EEG/MEG study. Clin Neurophysiol. 2001;112:393–403. doi: 10.1016/s1388-2457(00)00537-x. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blakemore C, Driver J, Thilo KV. Spatial attention changes excitability of human visual cortex to direct stimulation. Curr Biol. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Walsh V. Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport. 2000;11:3269–3273. doi: 10.1097/00001756-200009280-00044. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Huijboom L, Kuijer JP, Pouwels PJ, Heethaar RM, Lopes da Silva FH. The hemodynamic response of the alpha rhythm: an EEG/fMRI study. Neuroimage. 2007;35:1142–1151. doi: 10.1016/j.neuroimage.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Weissman DH, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Res. 2006;1080:63–72. doi: 10.1016/j.brainres.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, Pecherstorfer T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci Lett. 2005;375:64–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002;125:404–420. doi: 10.1093/brain/awf030. [DOI] [PubMed] [Google Scholar]
- Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electro-encephalographic recording during transcranial magnetic stimulation in humans and animals. Clin Neurophysiol. 2006;117:1870–1875. doi: 10.1016/j.clinph.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Kammer T, Puls K, Erb M, Grodd W. Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp Brain Res. 2005;160:129–140. doi: 10.1007/s00221-004-1992-0. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuo-spatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Why visual attention and awareness are different. Trends Cogn Sci. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, Kleinschmidt A. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31:1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Spatial patterns of spontaneous local field activity in the monkey visual cortex. Rev Neurosci. 2003;14:195–205. doi: 10.1515/revneuro.2003.14.1-2.195. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Martinez-Montes E, Valdes-Sosa PA, Miwakeichi F, Goldman RI Cohen MS. Concurrent EEG/fMRI analysis by multiway partial least squares. Neuroimage. 2004;22:1023–1034. doi: 10.1016/j.neuroimage.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Meegan DV, Honsberger MJ. Spatial information is processed even when it is task-irrelevant: implications for neuroimaging task design. Neuroimage. 2005;25:1043–1055. doi: 10.1016/j.neuroimage.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Müller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the “Zoom Lens” of visual attention. J Neurosci. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional brain imaging based on ERD/ERS. Vision Res. 2001;41:1257–1260. doi: 10.1016/s0042-6989(00)00235-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Rahn E, Basar E. Enhancement of visual evoked potentials by stimulation during low prestimulus EEG stages. Int J Neurosci. 1993;72:123–136. doi: 10.3109/00207459308991629. [DOI] [PubMed] [Google Scholar]
- Ramos-Estebanez C, Merabet LB, Machii K, Fregni F, Thut G, Wagner TA, Romei V, Amedi A, Pascual-Leone A. Visual phosphene perception modulated by sub-threshold cross-modal sensory stimulation. J Neurosci. 2007;27:4178–4181. doi: 10.1523/JNEUROSCI.5468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–955. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr Clin Neurophysiol. 1994;91:237–248. doi: 10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Super H, van der Togt C, Spekreijse H, Lamme VA. Internal state of monkey primary visual cortex (V1) predicts figure-ground perception. J Neurosci. 2003;23:3407–3414. doi: 10.1523/JNEUROSCI.23-08-03407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Shimojo S. Location vs feature: reaction time reveals dissociation between two visual functions. Vision Res. 1996;36:2125–2140. doi: 10.1016/0042-6989(95)00272-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Shimojo S. Repetition priming reveals sustained facilitation and transient inhibition in reaction time. J Exp Psychol Hum Percept Perform. 2000;26:1421–1435. doi: 10.1037//0096-1523.26.4.1421. [DOI] [PubMed] [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic (EEG) activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y, Lavie N. Location dominance in attending to color and shape. J Exp Psychol Hum Percept Perform. 1993;19:131–139. doi: 10.1037//0096-1523.19.1.131. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Lamy D. Attending to an object's color entails attending to its location: support for location-special views of visual attention. Percept Psychophys. 2000;62:960–968. doi: 10.3758/bf03212081. [DOI] [PubMed] [Google Scholar]
- van der Togt C, Kalitzin S, Spekreijse H, Lamme VA, Super H. Synchrony dynamics in monkey V1 predict success in visual detection. Cereb Cortex. 2006;16:136–148. doi: 10.1093/cercor/bhi093. [DOI] [PubMed] [Google Scholar]
- van der Togt C, Spekreijse H, Super H. Neural responses in cat visual cortex reflect state changes in correlated activity. Eur J Neurosci. 2005;22:465–475. doi: 10.1111/j.1460-9568.2005.04237.x. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Goda N, Callan DE, Anderson SJ, Kawato M. Attentional shifts towards an expected visual target alter the level of alpha-band oscillatory activity in the human calcarine cortex. Brain Res Cogn Brain Res. 2005;25:799–809. doi: 10.1016/j.cogbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]