Abstract
High-functioning older adults can exhibit normal recollection when measured subjectively, via “remember” judgments, but not when measured objectively, via source judgments, whereas low-functioning older adults exhibit impairments for both measures. A potential explanation for this is that typical subjective and objective tests of recollection necessitate different processing demands, supported by distinct brain regions, and that deficits in these tests are observed according to the degree of age-related changes in these regions. Here, we used event-related functional magnetic resonance imaging to measure the effects of aging on neural correlates of subjective and objective measures of recollection, in young, high-functioning (Old-High) and low-functioning (Old-Low) older adults. Behaviorally, the Old-High group showed intact subjective (“remember” judgments) but impaired objective recollection (for 1 of 2 spatial or temporal sources), whereas the Old-Low group was impaired on both measures. Imaging data showed changes in parietal subjective recollection effects in the Old-Low group and in lateral frontal objective recollection effects in both older adult groups. Our results highlight the importance of examining performance variability in older adults and suggest that differential effects of aging on brain regions are associated with different patterns of performance on tests of subjective and objective recollection.
Keywords: aging, familiarity, fMRI, frontal, recollection
Introduction
According to dual-process models of recognition memory, stimuli experienced previously can be recognized either by recollection of contextual details of the episode during which the stimuli were initially experienced or by familiarity for the stimuli in the absence of retrieval of contextual information (Mandler 1980; Yonelinas 2002). One way to assess these processes is to measure subjective reports of an individual's memory experience, such as by the “remember-know” procedure (Tulving 1985). Participants are instructed to respond “remember” when they recollect details associated with a previously encountered item and “know” when the item seems familiar but no contextual details are recollected. Another approach is to measure these processes objectively, as in a source memory task, in which participants are asked to determine which experimentally manipulated context (e.g. list membership, spatial location, color, size) was associated with an item during its prior exposure. The subjective method is more inclusive in that any detail, including the experimentally manipulated context, may be recollected. Both methods have been used to measure the effects of aging on recollection and familiarity, resulting in the general view that age-related memory loss is primarily restricted to recollection (Spencer and Raz 1995; Yonelinas 2002). Nonetheless, some studies depart from this view, finding intact subjective (Perfect et al. 1995; Mark and Rugg 1998; Duarte et al. 2006) and objective (Cabeza et al. 2002; Davidson and Glisky 2002) estimates of recollection.
There are several potential explanations for this apparent discrepancy. One possibility may relate to the high degree of interindividual variability in older adults that is evident in both underlying brain structure (Raz 2005; Van Petten et al. 2004) and cognitive performance (Rypma and D'Esposito 2000; Cabeza et al. 2002; Davidson and Glisky 2002; Daselaar et al. 2003; Duarte et al. 2006). The majority of previous aging studies have not examined this variability, leaving open the possibility that different subgroups of older individuals might exhibit different patterns of memory loss. One approach to addressing this issue is to separate older individuals into “high-functioning” and “low-functioning” groups. For example, some high-functioning older adults, as determined by neuropsychological tests or overall recognition memory performance, exhibit intact recollection, whereas low-functioning older adults exhibit impairments (Cabeza et al. 2002; Davidson and Glisky 2002; Duarte et al. 2006).
In addition to the potential contribution of interindividual variability, some evidence suggests that subjective and objective measures of recollection may be dissociable, though few aging studies have compared these measures directly. In a recent study for example, we found that high-functioning older adults, with overall recognition performance equivalent to that of the young, demonstrated intact recollection when measured by “remember” judgments, despite impairments when measured objectively by source judgments for the conceptual context implemented during study (Duarte et al. 2006). A similar pattern was observed in an earlier study of healthy older adults, although performance variability was not assessed (Mark and Rugg 1998). This evidence suggests that some older adults may endorse items as being phenomenologically recollected as often as young adults, despite not being able to retrieve the specific experimentally manipulated context as readily. Importantly, in our previous study, this “intact” performance was reflected in measures of discriminability, suggesting that the older adults did not simply exhibit a greater tendency (bias) to respond “remember.” One explanation for these findings is that these older adults base their remember judgments on recollection of information that is not necessarily relevant to the source memory judgment, so-called “noncriterial” information (Yonelinas and Jacoby 1996), which they are able to retrieve at least as effectively as the young.
Finally, although interindividual variability and noncriterial recollection may both contribute to the effects of aging on measures of recollection, it is additionally possible that the subjective and objective tests of recollection that are typically used necessitate somewhat different processing demands. For example, objective tests of source memory may require greater executive demands, such as the initiation of repeated episodic memory searches until the requisite source information is retrieved, compared with typical subjective tests, in which the first of any contextual details retrieved is sufficient to warrant a “remember” judgment. Consequently, the differences in performance on these tests may reflect the differential effects of aging on the brain regions that support these demands. For example, activity in the prefrontal cortex (PFC) measured during memory retrieval has been associated with the cueing and monitoring of episodic information retrieved from medial temporal (MTL) structures (Fletcher and Henson 2001; Simons and Spiers 2003; Dobbins and Han 2006) and selection of a subset of retrieved details relevant to one's current goal in the face of competition from irrelevant information (reviewed in Badre and Wagner 2007). Although such executive processes likely contribute to both subjective and objective measures of recollection, it is conceivable that they may be engaged to a greater extent for tests of objective than subjective recollection, as the latter is less restrictive in that it can be subserved by any number of details. Furthermore, there is substantial evidence suggesting that damage to the PFC impairs performance on tests of source memory (Shimamura and Squire 1987; Janowsky et al. 1989; Kopelman et al. 1997; Duarte et al. 2005; Swick et al. 2006) and, in some cases, may spare performance for subjective measures of recollection (Duarte et al. 2005; Ciaramelli and Ghetti 2007). Given that the PFC, both structurally and functionally, is believed by some to be disproportionately affected by normal aging—the so-called “frontal-aging hypothesis” (West 1996; Raz 2000; Greenwood 2000)—one might predict that tests of objective recollection would be disproportionately affected in older adults relative to tests of subjective recollection.
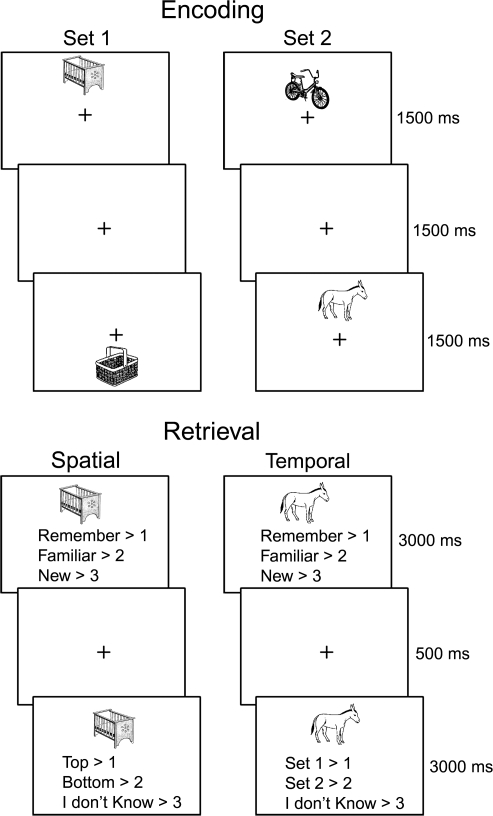
The current study, schematically depicted in Figure 1, was designed to address the above issues. We used event-related functional magnetic resonance imaging (fMRI) to separate neural activity associated with cognitive processes contributing to subjective and objective measures of recollection in groups of young adults, high-functioning and low-functioning older adults. During the study phase, pictures of objects were embedded in a specific spatial and temporal context. During the subsequent test phase, participants first made “remember,” “familiar,” or “new” judgments to intermixed studied and unstudied items. After each such judgment, they made a second judgment about either the spatial or the temporal source of the item (which included a “don't know” option, particularly appropriate for previous “new” judgments). We hypothesized that 1) the high-functioning older adult group, who were matched on overall recognition discrimination to the young group, would demonstrate intact performance on the subjective measure and impaired performance on the objective measure, whereas the low-functioning older adult group would demonstrate impairments on both; 2) MTL and medial/lateral parietal activity, which has previously been associated with tests of both subjective and objective recollection (Henson 2005; Wagner et al. 2005), would be most affected in the low-functioning older group. This prediction is also consistent with previous findings of reduced MTL memory-related activity, specifically, in low-functioning older adults with reduced recognition performance relative to the young (Daselaar et al. 2003). Similarly, in a previous event-related potential study, we found that older adults with reduced recognition performance exhibited reduced “parietal old–new effects,” which are sensitive to both subjective and objective recollection (Rugg 1995) and may be generated by the MTL (Duzel et al. 2001) and/or the parietal cortex (Rugg and Curran 2007). Furthermore, 1 recent study found that older adults with impaired subjective recollection estimates demonstrated reduced recollection-related activity in hippocampal and lateral parietal regions (Daselaar, Fleck, Dobbins, et al. 2006). Finally, 3) PFC activity associated with the measure of objective recollection would be affected in both high and low-functioning older adults, consistent with the “frontal-aging hypothesis.”
Figure 1.
Example stimuli and task requirements during study and test blocks.
Method
Participants
Seventeen young adults between 18 and 30 years of age and 27 older adults between 60 and 70 years of age were recruited from local universities, science fairs and the Medical Research Council Cognition and Brain Sciences Unit volunteer panel. Participants were paid for their time and signed consent forms approved by the Cambridge Local Research Ethics Committee. Participants were right-handed, fluent English speakers with normal or corrected-to-normal vision (using MRI-compatible glasses when necessary). None reported cognitive complaint, a history of psychiatric or neurological disorder (including depression and epilepsy), vascular disease (including diabetes) or psychoactive drug use. None of the participants were taking central nervous system–active medications or antihypertensive medications. All MRI scans were screened by a radiologist for abnormalities (excessive white matter lesions, stroke, hydrocephalus, etc.). Group characteristics are shown in Table 1. Groups did not differ for years of education or gender (p values > 0.9).
Table 1.
Group characteristics
| Measure | Young (n = 17) | Old-High (n = 13) | Old-Low (n = 14) |
| Age | 23.6 (2.8) | 62.6 (2.9) | 62.7 (2.8) |
| Gender | 11/17 female | 6/13 female | 7/14 female |
| Education | 15.2 (1.8) | 14.4 (2.5) | 14.7 (2.3) |
| Corrected recognition (Pr) | 0.73 (0.09) | 0.68 (0.08) | 0.53 (0.05) |
| Rey complex figure delayed recall* | — | 0.54 (1.13) | −0.17 (0.62) |
| WCST (errors) | — | 2.25 (0.16) | 2.21 (0.23) |
| WMS-R digit span forward | — | 1.78 (0.65) | 1.61 (0.71) |
| WMS-R digit span backward | — | 1.33 (0.83) | 1.51 (1.0) |
| WMS-III logical memory immediate | — | 0.62 (0.86) | 0.83 (1.13) |
| WMS-III logical memory delayed | — | 0.88 (0.73) | 0.97 (0.99) |
| RMT face recognition* | — | 0.96 (0.59) | 0.29 (0.94) |
| Warrington scene recognition | — | 1.05 (0.61) | 0.56 (1.38) |
Note: Standard deviations in parentheses. All neuropsychological tests are reported as z-scores according to the age-adjusted published norms for these tests. *Significant difference between Old-High and Old-Low at P < 0.05 for 2-tailed t-tests.
The older adults were divided into 2 groups based on the Pr measure of recognition discrimination: probability of Hits minus probability of False Alarms (FA), collapsing across “remember” (R) and “familiar” (F) judgments (i.e., (p(R, Hits) + p(F, Hits)) − (p(R, FA) + p(F, FA))). Older participants whose Pr scores were within 1 standard deviation of the mean of that of the Young group were classified as high functioning (the “Old-High” group, n = 13), whereas older participants that did not meet this criterion were classified as low functioning (“Old-Low” group, n = 14). (Note that this procedure does not bias the reaction time (RT) or fMRI results, at least under most standard models, given that these measures reflect the mean of each response category, which is unbiased by group differences in the number of responses in each category [e.g., R Hits]. Neither does it necessarily bias the subjective estimates of recollection in these 2 groups. In support of this assertion, there was no significant correlation between overall Pr and Pr(R) in any of the 3 groups [r values < 0.3, p values > 0.16]. Although we expect, and find, that Pr(R) > Pr(F) and Pr(Old-High) > Pr(Old-Low), this does not necessarily imply that Pr(R, Old-High) > Pr(R, Old-Low). This is because the Old-High group could have a greater proportion of R relative to F judgments than the Low group, without any group difference in the discriminability (i.e., Pr) associated with those R judgments.) As expected, this manipulation produced an Old-High group that did not differ significantly from the Young on this Pr measure (P = 0.2), whereas the Old-Low group performed reliably worse than the other 2 groups (p values < 0.001). These mean corrected recognition scores are shown for each group in Table 1.
Neuropsychological Testing
In order to screen for cognitive deficits below the age-associated norms, all older participants were administered a battery of standardized neuropsychological tests in a separate testing session within 2 months of the MRI scanning session. Tests sensitive to prodromal cognitive deficits were used to ensure that the older adults in this study, although varying in degree of recollection and familiarity impairments, were not obviously in the early stages of dementia. The battery included tests of working and long-term memory, executive function, and visuospatial ability: Wechsler Memory Scale-Revised (WMS-R) Digit Span Forward and Backward (Wechsler 1997), Warrington Recognition Memory Test (RMT) face recognition (Warrington 1984), a topographical scene recognition memory test (Warrington and Whitley 1978), the Logical Memory test (Wechsler 1997), the Wisconsin Card Sorting Test (Lezak 1995), and the Rey Complex Figure Test (Rey 1941). The primary objective in performing these tests was to rule out the possibility that any older adults included in our sample were affected by Mild Cognitive Impairment, rather than to assess differences between young and older groups. For this reason, we did not obtain neuropsychological test scores for the young participants. Although it remains an open question whether the young would be matched more closely on these tests to the Old-High than to the Old-Low adults, our assumption is that this would be the case, given that the Old-High group exhibited generally higher scores on these tests than the Old-Low group (Table 1).
Procedure
Stimuli consisted of 384 grayscale line drawings of nameable concrete objects. Objects were taken from the International Picture Naming Project Database (http://crl.ucsd.edu/∼aszekely/ipnp/) and were chosen from the database if they had greater than 70% naming agreement. Objects subtended a maximum vertical and horizontal visual angle of up to 4.16°. A short practice version of the experiment was administered to participants outside of the scanner immediately prior to scanning. Both study and test periods were scanned, but only the data from the test periods are reported here. Participants responded using buttons on a box placed under their right hand.
There were 128 trials in each of 2 study/encoding sessions that were separated by a 5-min magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan. This separation was to make the study sessions, or “sets,” more temporally distinct. Half of the objects were presented above a central fixation cross and half were presented below. Objects were presented in 1 of 16 possible vertical positions along the midline, with 8 above and 8 below fixation, (Fig. 1), given that piloting showed this was effective in reducing spatial source accuracy performance from ceiling (and producing a close match to temporal source accuracy). In order to encourage incidental encoding of the spatial/temporal context, participants performed a semantic judgment task on each object, responding whether it would, or would not, fit inside a shoebox.
Study was followed by 4 test/retrieval sessions of 64 studied objects (32 from each study set, half of which previously presented above fixation and half previously presented below) plus 32 unstudied items, presented in a pseudorandom order. For blocks of trials within each test session, participants were cued to either perform the spatial or temporal retrieval task (Fig. 1). The spatial and temporal task blocks consisted of 24 trials each. Instructions for the test phase included a description of the appropriate use of the subjective “remember,” “know,” and “new” response categories, modeled after previous studies (Gardiner and Java 1991; Rajaram 1993), though we replaced the term “know” for the term “familiar” to ease exposition. Objects were all centrally presented above a response cue stating these 3 choices. After a 500-ms fixation screen, a new response cue appeared in place of the previous asking the participants to make objective source decisions. In the spatial blocks, participants decided where the object was presented on the screen during the study phase (“top” or “bottom”) and in the temporal blocks, which study set the object was presented in (“set 1” or “set 2”). A third response option of “don't know” was offered when the relevant context could not be recollected. For all “new” judgments, participants were instructed to respond “don't know” to the second response cue, in order to balance the number of responses across all conditions. For the purposes of the present manuscript, where our main interests were in comparisons between measures of subjective and objective recollection, we collapsed all objective (source) decisions across the spatial and temporal tasks. Although it would be of interest to investigate age-related changes in subjective and objective estimates of recollection for spatial and temporal tasks separately, there were insufficient numbers of trials for each of the groups, particularly the older adult groups, to investigate neural activity associated with subjective and objective recollection for spatial and temporal tasks separately. The Huynh–Feldt correction, reflected in the p values, was used in the behavioral analyses, where appropriate. Two-tailed t-tests were used for pairwise comparisons of the neuropsychological and behavioral data.
fMRI Acquisition
Scanning was performed on a 3-T Siemens TIM Trio system. Functional data were acquired using a gradient-echo pulse sequence (32 transverse slices oriented along the anterior–posterior commissural axis, repetition time 2 s, echo time 30 ms, 3 × 3 × 3.5 mm voxels, 0.8 mm interslice gap). Each encoding session (n = 2) included 193 volumes and each retrieval session (n = 4) included 356 volumes. The first 5 volumes per session were discarded to allow for equilibration effects. A high-resolution T1-weighted MPRAGE image was collected for anatomical localization.
fMRI Analysis
Data were analyzed using Statistical parametric mapping (SPM)2. Images were realigned and the resulting mean EPI image was used to estimate normalization parameters to the standard Montreal Neurological Institute echo planar image (MNI EPI) template, which were then applied to all EPI volumes. Normalized images were resliced to 3 × 3 × 3 mm and smoothed with an 8-mm full-width half-maximum isotropic Gaussian kernel. The data were high-pass filtered to a maximum of 1/128 Hz and grand mean scaled to 100.
Statistical analysis was performed in 2 stages. In the first stage, neural activity was modeled by a sequence of delta functions at onset of the various event types and convolved with a canonical hemodynamic response function. The time courses were downsampled to the middle slice to form the covariates for the General Linear Model. Temporal autocorrelations within a session were corrected using an AR(1) model. For each participant and session, 6 covariates representing residual movement-related artifacts, determined by the spatial realignment step, were included in the first level model to capture residual (linear) movement artifacts.
Contrasts of the parameter estimates for each participant were submitted to the second stage of analysis (treating participants as a random effect). Incorrect responses to unstudied items (“false alarms”) were not considered further because of insufficient numbers for all participants. A mixed analysis of variance (ANOVA) model was created for the retrieval period that allowed us to examine both within-group effects as well as condition-by-group interactions. The 4 conditions were contrasts of: 1) remember + correct source, 2) remember + incorrect source + don't know, 3) familiar, and 4) missed trials, all contrasted against correct rejections (CR) of unstudied items. Correction rejections served as our baseline, and allowed us to confirm basic “old-new” effects (data not shown) as a validation of our data and analysis. For both models, the between-group factor referred to Young, Old-High, and Old-Low groups. A weighted least squares estimation procedure was used to correct for inhomogeneity of covariance across within-group conditions and inhomogeneity of variance across groups. All main effects of condition (across groups) and group-by-condition interaction SPMs were evaluated under an uncorrected alpha level of 0.001 and a minimum cluster size of 5 contiguous voxels. The SPM for the main effect of condition was masked exclusively with the SPM for the group-by-condition interaction, using a liberal uncorrected threshold of P < 0.05 for the mask in order to restrict effects to those “common” (i.e., similar size) across groups. (Note that a liberal threshold for an exclusive mask is more conservative in excluding regions from the masked SPM.) All T-contrasts were 1-tailed. Simple effect SPMs (for within-group comparisons) were similarly evaluated under an uncorrected threshold of P < 0.001 and a minimum cluster size of 5. Maxima of significant clusters were localized on individual normalized structural images.
Results
Neuropsychological Test Results
Group characteristics and standardized Z-scores for neuropsychological tests, according to the published age-adjusted norms, are shown for the older groups in Table 1. As can be seen in the table, both groups were within (and numerically above) the age-adjusted norms for all tests, further supporting our assertion that the older adults were not obviously clinically impaired. Although the Old-High group tended to exhibit numerically better performance for most of the tests than the Old-Low group, performance on the complex figure recall and face recognition were the only tests that significantly differed between groups [t(25) values > 2.0, p values < 0.05].
Behavioral Results
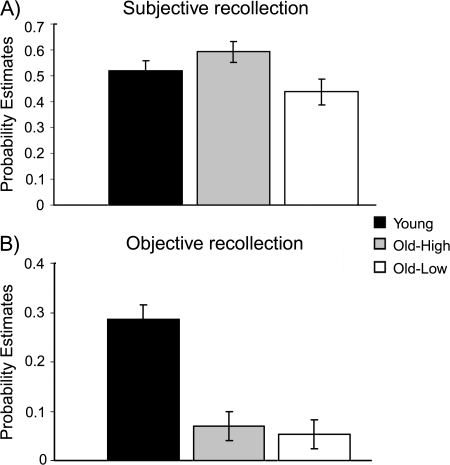
The mean proportions of “remember” (R), “familiar” (F), and “new” responses made to studied (i.e., misses [M]) and unstudied items (i.e., CR) and corresponding RTs are shown for all 3 groups in Table 2. Although the Old-High group gave numerically, though not statistically (t values < 1.6, p values > 0.11), more R responses to studied items than either the Young or Old-Low groups, their R responses to unstudied items were also elevated, relative to the Young adults [t(28) = 3.2, P = 0.003]. In an attempt to accommodate this apparent bias, the accuracy of R judgments was estimated by the Pr measure of discriminability, that is, subtracting the probability of FA from the probability of hits. The corrected estimates of subjective recollection, (p(R, Hit) − p(R, FA)), are shown for all groups in Figure 2A. The estimate of subjective recollection was lower in the Old-Low group than in each of the Young and Old-High groups (t values > 2.0, p values < 0.05), with no reliable difference between the latter 2 groups [t(28) = 1.1, P = 0.27]. Thus, the greater estimate of subjective recollection in the Old-High than Old-Low group does not appear to reflect simply a greater propensity to give R judgments in the Old-High group.
Table 2.
(A) Proportions of studied and unstudied items given remember, familiar or new judgments and (B) proportions of remember judgments associated with correct, incorrect, or “don't know” judgments about objective source
| Judgment | Young |
Old-High |
Old-Low |
|||
| (A) | ||||||
| Studied items | ||||||
| Remember (R) | 0.54 (0.21) | 1435 (281) | 0.66 (0.17) | 1490 (264) | 0.52 (0.25) | 1721 (303) |
| Familiar (F) | 0.32 (0.19) | 1755 (358) | 0.20 (0.18) | 2104 (315) | 0.31 (0.22) | 2042 (343) |
| New (M) | 0.14 (0.08) | 1599 (365) | 0.14 (0.08) | 1906 (271) | 0.17 (0.11) | 1966 (345) |
| Unstudied items | ||||||
| Remember (FA) | 0.02 (0.02) | 1611 (397) | 0.07 (0.06) | 1733 (492) | 0.08 (0.09) | 1914 (378) |
| Familiar (FA) | 0.11 (0.07) | 1858 (360) | 0.11 (0.10) | 2112 (251) | 0.23 (0.13) | 2146 (395) |
| New (CR) | 0.87 (0.08) | 1370 (258) | 0.82 (0.09) | 1651 (245) | 0.69 (0.12) | 1763 (343) |
| (B) | ||||||
| Remember + correct source | 0.56 (0.10) | 1404 (280) | 0.40 (0.15) | 1476 (262) | 0.48 (0.10) | 1703 (309) |
| 0.30 (0.12) | 0.26 (0.11) | 0.25 (0.10) | ||||
| Remember + incorrect source | 0.27 (0.07) | 1452 (270) | 0.32 (0.13) | 1451 (281) | 0.42 (0.08) | 1738 (325) |
| 0.15 (0.06) | 0.21 (0.10) | 0.22 (0.09) | ||||
| Remember + don't know source | 0.17 (0.13) | 1554 (329) | 0.28 (0.24) | 1501 (202) | 0.10 (0.12) | 1675 (322) |
| 0.09 (0.09) | 0.19 (0.16) | 0.05 (0.09) | ||||
Note: RTs for each such condition also shown. SD in parentheses. Nonitalicized values in (B) represent proportions calculated out of the total number of R responses to studied items, whereas italicized values represent proportions calculated out of the total number of studied items.
Figure 2.
Behavioral results for each group. (A) Subjective recollection estimates and (B) Objective recollection estimates for Young, Old-High, and Old-Low groups. Error bars depict the standard error of the mean across participants.
The proportion of correct, incorrect and “don't know” source judgments for R responses are shown in Table 2. As seen in the table, the proportion of remember responses associated with “don't know” source judgments was greater in the Old-High than the Old-Low group [t(25) = 2.4, P = 0.02], with no reliable differences between the Young and either of the 2 older groups (t values < 1.4, p values > 0.12). This suggests a potential difference in response bias between the older adult groups. In order to investigate this hypothesis directly, we calculated Br estimates of bias for objective decisions for R responses, calculated as R + incorrect source/(1 − ((R + correct source) – (R + incorrect source)) (Snodgrass and Corwin 1988), for each group, producing Br estimates of 0.38 in the young, 0.35 in the Old-High and 0.44 in the Old-Low group. Although the Old-Low adults exhibited a more liberal bias than either the Young or Old-High groups [t values > 2.3, p values < 0.025], there was no difference between the latter 2 groups [t < 1]. Thus, a more conservative bias for the source decision following “remember” judgments may account for the elevated proportion of “don't know” responses in the Old-High relative to the Old-Low group.
For the analysis of objective source recollection, data were collapsed across spatial and temporal source tasks (as noted above). We also performed analyses for subjective and objective measures of recollection for the spatial and temporal retrieval tasks separately. The group differences reported for these measures did not differ from those presented here when collapsed across the retrieval task. As with the above analysis of R and F judgments, accuracy was measured by Pr, in order to allow for potential group differences in the propensity to guess the source, where Pr = p(Correct) − p(Incorrect), that is, excluding “don't knows.” Given that many participants did not attempt source attributions after giving F judgments, as would be expected, the Pr measure was restricted to R judgments. These estimates are shown in Figure 2B. Pairwise group comparisons showed that, whereas the estimate of objective recollection was higher in the Young than older groups (t values > 4.3, p values < 0.001), there was no reliable difference between the older groups [t(25) < 1].
These results support the predicted discrepancy between estimates of subjective and objective recollection in the Old-High group. In support of this, an ANOVA employing factors of Recollection (Subjective, Objective) and Group (Young, Old-High) yielded a significant interaction (F1,28 = 7.1, P = 0.012) but no main effect of Group (F1,28 = 1.2, P = 0.27), whereas the opposite pattern was observed for the ANOVA comparing the Young and the Old-Low groups (interaction: [F1,29 < 1], Group: [F1,29 = 7.7, P = 0.01]). Thus, although the estimate of objective but not subjective recollection was impaired in the Old-High group, both estimates were impaired in the Old-Low group.
An ANOVA employing factors of Response (R + correct source, R + incorrect + don't know source, F hits, M, CR) and Group (Young, Old-High, Old-Low) for the RTs shown in Table 2 yielded main effects of Response (F4,164 = 32.2, P < 0.00001) and Group (F2,51 = 7.7, p < 0.001). As shown in the table, the main effect of Group indicates that both groups of older participants were slower to respond to test items than young adults, with no significant difference between the older groups (F1,25 = 2.2, P = 0.15). Pairwise comparisons confirmed that RTs were longer for F than all other judgments (t values > 3.3, p values < 0.02) and for M than CR judgments (t values > 4.6, p values < 0.001) for all groups, with no significant differences between the other response categories (t values < 1.7, p values > 0.11).
fMRI Results
Contrasts
To identify regions associated with subjective recollection, we used the contrast between R and F hits (collapsed across objective decisions). In order to identify regions related to objective recollection, we examined the contrast between R items associated with correct source judgments (R + source) and R items associated with incorrect source or “don't know” judgments (R − source). In all cases, neural activity was examined that was 1) common to and 2) different between the groups, where common activity was defined using exclusive masking with the interaction (see Methods).
Effects Common to Groups
Subjective recollection.
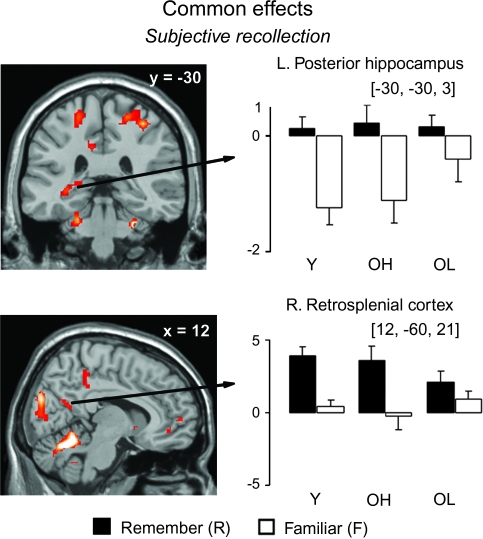
Activity associated with subjective recollection was found in several regions, including the posterior hippocampus, retrosplenial cortex and posterior cingulate, which are shown in Figure 3. As can be seen in the plots, these regions exhibited greater activity for R than F items. Although F items appeared to show less activity than CR (corresponding to the zero level in the plots) in the posterior hippocampus, a pattern which might be taken to reflect familiarity, simple effects (within group) analyses determined that this difference was not reliable in any group. Other notable regions, listed in Table 3, included the anterior PFC (Broadmann's area [BA] 10) and the right angular gyrus (BA 39). No regions demonstrated greater activity for F than R items.
Figure 3.
Memory effects exhibiting common activity across groups, shown in selected regions, displayed on the MNI reference brain. Plots depict size of activation for each of the trial types versus baseline (CR trials) for each group. Error bars depict standard error of the mean across participants, for each group. Y = Young, OH = Old-High, and OL = Old-Low groups.
Table 3.
Regions showing effects common to both young and older age groups
| Contrast | Region | L/R | MNI coordinates (x, y, z) | BA | T score | Cluster size |
| Subjective recollection (R > F) | Posterior hippocampus | L | −30, −30, −3 | 20 | 3.70 | 33 |
| Retrosplenial cortex | R | 12, −60, 21 | 23/30 | 3.64 | 13 | |
| Posterior cingulate | R | 3, −39, 39 | 23 | 3.57 | 15 | |
| Angular gyrus | R | 45, −69, 27 | 39 | 4.68 | 36 | |
| Superior medial frontal gyrus | R | 15, 51, 9 | 10 | 3.76 | 15 | |
| Superior frontal gyrus | L | −24, 57, 9 | 10 | 3.60 | 11 | |
| Middle orbital frontal gyrus | R | 6, 45, −9 | 11 | 3.30 | 6 | |
| Middle frontal gyrus | L | −30, 15, 42 | 46 | 3.89 | 11 | |
| Cuneus | R | 9, −84, 24 | 18 | 3.95 | 35 | |
| Inferior temporal gyrus | R | 39, −51, −6 | 37 | 3.62 | 8 | |
| Postcentral gyrus | L | −21, −30, 60 | 4 | 3.81 | 26 | |
| L | −48, −15, 51 | 4 | 3.72 | 14 | ||
| Postcentral gyrus | R | 36, −30, 57 | 3 | 3.66 | 16 | |
| Precentral gyrus | R | 24, −30, 63 | 3 | 3.60 | 12 | |
| Thalamus | R | 30, −12, −6 | 48 | 3.75 | 22 | |
| Cerebellum | B | 3, −66, −24 | 5.08 | 721 |
Note: L = Left; R = Right; B = Bilateral; F = studied items judged “familiar”; R = studied items judged “remember.”
Objective recollection.
Somewhat surprisingly, no regions demonstrated reliable effects of objective recollection common to all 3 groups.
Differences between Groups
Subjective recollection.
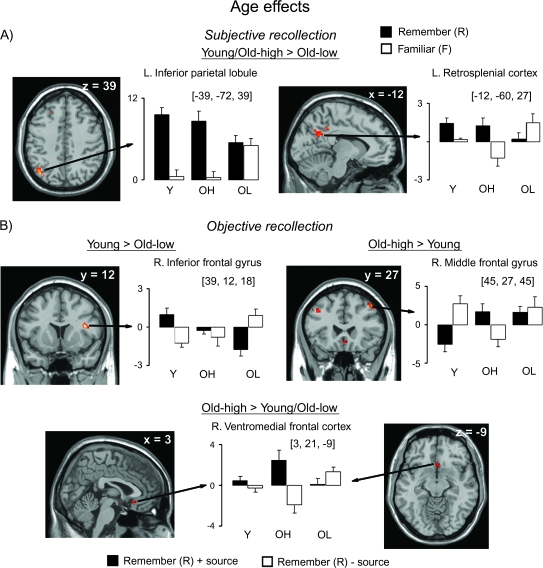
The comparison of subjective recollection between groups yielded significant differences between Young and Old-Low, and between Old-High and Old-Low groups, as shown in Table 4. As shown in Figure 4A, activity in the left inferior parietal cortex was greater for R than F items in the Young and the Old-High groups, with no reliable effect in the Old-Low group. Interestingly, although activity in this region seemed to be specific to recollection in the Young and Old-High groups (i.e., R > F = CR), both R and F items, exhibited greater activity than CR items (corresponding to the zero level in the plots) in the Old-Low group (i.e., R = F > CR). In the left retrosplenial cortex, homologous to the right-lateralized region implicated in subjective recollection for all groups, activity was greater for R than F responses in the Young and Old-High groups, with a somewhat opposite pattern in the Old-Low group, though within-group analyses showed that the difference between R and F trials was reliable in the Young and Old-High groups only. There were no significant differences between the Young and Old-High groups.
Table 4.
Regions showing significant differences between groups for subjective and objective recollection
| Group | Region | L/R | MNI coordinates (x, y, z) | BA | T score | Cluster size |
| Subjective recollection (R > F) | ||||||
| Young > Old-Low | Inferior parietal lobule | L | −39, −72, 39 | 39 | 3.78 | 27 |
| Retrosplenial cortex | L | −12, −60, 27 | 23 | 3.15 | 16 | |
| Old-High > Old-Low | Inferior parietal lobule | L | −39, −72, 39 | 39 | 3.18 | 15 |
| Posterior cingulate/Retrosplenial cortex | L | −9, −45, 24 | 23/26 | 3.41 | 8 | |
| R | 12, −42, 27 | 26 | 3.40 | 6 | ||
| Middle occipital gyrus | L | −15, −93, 15 | 18 | 3.54 | 6 | |
| Superior occipital gyrus | R | 24, −87, 18 | 18 | 3.71 | 11 | |
| Inferior occipital gyrus | R | 36, −81, 0 | 19 | 3.57 | 11 | |
| Objective recollection (R + source > R − source) | ||||||
| Young > Old-Low | Inferior frontal gyrus | R | 39, 12, 18 | 48 | 4.25 | 15 |
| Old-High > Young | Middle frontal gyrus | R | 45, 27, 45 | 9 | 4.27 | 15 |
| L | −39, 21, 39 | 46 | 3.58 | 6 | ||
| Superior frontal gyrus | L | −9, 12, 51 | 8 | 4.23 | 24 | |
| R | 15, 9, 57 | 8 | 3.69 | 6 | ||
| Gyrus rectus (ventromedial PFC) | R | 3, 21, −9 | 11 | 3.10 | 12 | |
| Inferior temporal gyrus | L | −51, −24, −18 | 20 | 3.99 | 13 | |
| Angular gyrus | L | 42, −45, 30 | 40 | 3.92 | 9 | |
| Rolandic operculum | R | 39, −21, 27 | 48 | 3.90 | 19 | |
| Old-High > Old-Low | Gyrus rectus (ventromedial PFC) | R | 15, 21, −12 | 11 | 3.81 | 24 |
| Inferior temporal gyrus | L | −60, −15, −18 | 20 | 3.73 | 19 | |
| Supplementary motor | L | −9, 18, 69 | 6 | 4.08 | 19 | |
| Rolandic operculum | R | 39, −15, 24 | 48 | 3.92 | 10 | |
Note: L = Left; R = Right; F = studied items judged “familiar”; R = studied items judged “remember.”
Figure 4.
Memory effects exhibiting group differences for (A) subjective recollection and (B) objective recollection. Group differences are shown in selected regions, displayed on the MNI reference brain. Plots depict size of activation for each of the trial types versus baseline (CR trials) for each group. Error bars and abbreviations as in Figure 3.
Objective recollection.
Regions showing significant group differences for objective recollection are listed in Table 4. Notable among these are right lateral frontal regions showing differences between the Young and each older group (Fig. 4B). Activity in the right inferior frontal gyrus was greater for R + source than R − source items in the Young, whereas the opposite pattern was observed in the Old-Low group (i.e., R − source > R + source). A similar “cross-over” effect was observed in bilateral middle frontal gyri between Young and Old-High groups, although in the opposite direction (i.e., R + source > R − source in the Old-High and R − source > R + source in the Young). Within-group analyses determined that each of these frontal effects was reliable for each of the respective groups. Finally, only the Old-High group exhibited activity associated with objective recollection in the ventromedial frontal and inferior temporal cortices. As can be seen in the figure (for the ventromedial PFC), activity was greater for R + source than R − source items in the Old-High group only.
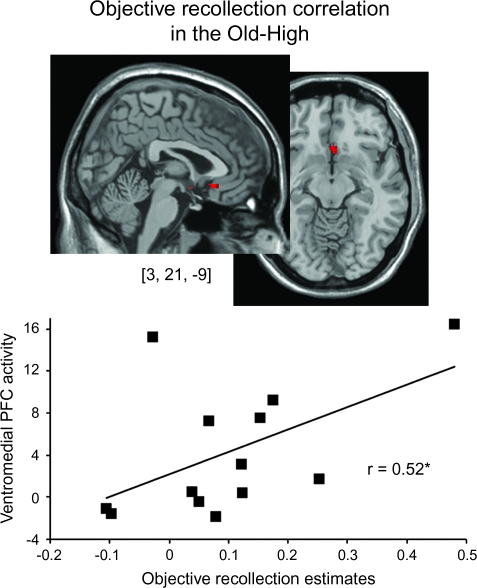
Given the “over-recruitment” of the ventromedial (gyrus rectus) and inferior temporal regions in the high-functioning older adults and lack of recruitment of these regions in the young adults, we wanted to determine whether a more direct relationship existed between performance on the objective recollection test and the size of the objective recollection effect in these regions, consistent with theories of functional compensation in older adults (Rajah and D'Esposito 2005). For the peak voxel coordinates from these 2 regions (listed in Table 4), activity in the ventromedial region was positively correlated with objective recollection performance in the Old-High group (r = 0.522, P = 0.03), as shown in Figure 5, but not in either of the other 2 groups (p values> 0.9). This same correlation with the inferior temporal region was not significant for any group (p values > 0.3).
Figure 5.
Correlation between objective recollection activity in the ventromedial PFC and objective recollection performance in the Old-High group.
Discussion
In the present experiment, we examined the effects of aging on the neural correlates of subjective and objective measures of recollection in older adults with relatively high or low overall recognition memory performance. The results yielded several interesting findings regarding the relationship between performance variability in healthy aging, different measures of recollection and brain regions supporting the performance on these tests. In relation to our 3 predictions, we replicated our previous findings (Duarte et al. 2006) that high-functioning older adults can show intact performance, relative to the young, on tests of subjective but not objective recollection, whereas low-functioning older adults are impaired according to both measures. Secondly, we found activations in medial and lateral parietal cortex that have previously been associated with recollection, but which were reduced or absent in the Old-Low group. Finally, we found differences between age groups in the PFC in association with estimates of objective recollection, generally consistent with the frontal-aging hypothesis. These results and their implications are discussed in more detail below.
Dissociations between Measures of Subjective and Objective Recollection
Consistent with our previous study (Duarte et al. 2006), we found that high-functioning older adults demonstrated intact performance on a measure of subjective recollection, but impaired performance on a measure of objective recollection compared with young adults. One potential reason for this dissociation was that these older adults recollected as much contextual information as did the young but this information was not necessarily relevant to the source memory judgment (i.e., “noncriterial recollection”). Indeed, this may be 1 potential explanation, in addition to a more conservative response bias for source decisions, for the elevated number of “don't know” responses subsequent to “remember” judgments in the high-functioning relative to the low-functioning older adults, and to a lesser extent relative to the young. High-functioning older adults may have been more likely to encode and subsequently retrieve internally generated contextual associations than the low-functioning older adults. Some support for this hypothesis comes from findings suggesting that older adults may attend more to their own thoughts and feelings than do young adults when making memory judgments (Johnson 2006). Though not criterial, such associations would have been sufficient to endorse items as being subjectively recollected according to the Remember/Know procedure.
In addition to the contribution of noncriterial recollection to intact subjective estimates in the Old-High, we hypothesized that dissociable brain regions may support measures of subjective and objective recollection and that older adults may exhibit deficits in these measures according to the degree of underlying impairment in these corresponding regions. The imaging results were largely consistent with this hypothesis.
Activity in the posterior hippocampus, retrosplenial, and posterior parietal cortex contributed to measures of subjective recollection in each group. Similar areas have been associated with recollection in several previous imaging studies, measured subjectively (Henson et al. 1999; Eldridge et al. 2000; Woodruff et al. 2005; Yonelinas et al. 2005; Daselaar, Fleck, Cabeza 2006; Johnson and Rugg 2007) but also objectively (Cansino et al. 2002; Davachi et al. 2003; Ranganath et al. 2004). One possible reason for the lack of association of these areas to estimates of objective recollection in the present study is that this contrast was collapsed across the spatial and temporal retrieval tasks, potentially obscuring the contribution of these regions to objective recollection. However, this was not supported by separate analyses of temporal versus spatial source in the Young group. More specifically, given the known role of the MTL in spatial processing, we hypothesized that objective recollection effects may have been present in this area particularly for the spatial retrieval task. Our present contrast for objective recollection, which was collapsed across spatial and temporal source retrieval tasks, may have obscured any such effects. Thus, we examined neural activity associated with objective recollection separately for the spatial and temporal tasks. This analysis was restricted to the young participants, as the reduced objective recollection performance in the older adults resulted in too few R + source trials to obtain reliable estimates of activity in the majority of the participants. Whole brain analysis revealed minimal differences in objective recollection effects between the spatial and temporal conditions. Moreover, region of interest analyses of both hippocampal and parahippocampal regions (using masks defined by Automatic Anatomical Labeling of the MNI brain), failed to show significant objective recollection effects for either spatial or temporal conditions (all corrected p values > 0.3). Another possibility reflects the fact that the items involved in the contrast used to estimate activity specific to objective recollection processes were all associated with “remember” judgments (i.e., R + source vs. R − source). Thus, the recollection of information not specifically relevant to the objective decision may have swamped any activity specific to the retrieval of spatial and/or temporal details. A related possibility is that the hippocampus and potentially other regions (e.g., retrosplenial cortex) are particularly sensitive to the retrieval of conceptual details of a previously encountered event (i.e., internal source rather than external source). Support for this idea comes from imaging studies revealing equivalent hippocampal contributions to the retrieval of veridical and illusory memories, which are more likely to contain overlapping conceptual than perceptual associations (Cabeza et al. 2001). Conceptual associations, such as internally generated thoughts and feelings, would have been more likely to support tests of subjective than objective recollection in the current study.
In contrast to the MTL, bilateral dorsal frontal cortex contributed disproportionately to measures of objective recollection during retrieval. This is consistent with previous imaging (Nolde et al. 1998; Ranganath et al. 2000; Raye et al. 2000; Cansino et al. 2002; Dobbins et al. 2003; Mitchell et al. 2004) and human lesion studies (Janowsky et al. 1989; Kopelman et al. 1997; Duarte et al. 2005; Swick et al. 2006) that implicate lateral frontal regions in source memory processing. Activity in the PFC during source memory retrieval presumably reflects the engagement of executive control processes, such as the inhibition of irrelevant or intrusive information and the monitoring and repeated evaluation of information retrieved from MTL structures in the service of making a decision (Fletcher and Henson 2001; Simons and Spiers 2003; Dobbins and Han 2006). It is conceivable that such processes may have disproportionately contributed to the measure of objective recollection in the current study, which required the retrieval of highly specific spatial or temporal information, in comparison with the measure of subjective recollection, which could have been supported by any number of contextual associations.
Subjective Recollection Impairments in Low-Functioning Older Adults
Consistent with our prediction, low-functioning older adults exhibited impaired subjective estimates of recollection. The reduced subjective estimates in this group are consistent with numerous previous studies demonstrating similar impairments in older adults (Yonelinas 2002; Bastin and Van der Linden 2003; Daselaar, Fleck, Dobbins, et al. 2006; Howard et al. 2006; Prull et al. 2006), although performance variability was not examined in these studies. Our results suggest that the subjective recollection impairments in these previous studies may have been driven by low-functioning older adults.
The current imaging results demonstrated reduced subjective recollection effects in inferior parietal and retrosplenial regions in low-functioning older adults relative to the other 2 groups. This is consistent with our prediction that reductions in medial and lateral parietal activity contribute to subjective recollection impairments in older adults. Interestingly, although activity within the left inferior parietal lobule was specific to recollection in the Young and Old-High groups, activity in this region distinguished all recognized items (remember and familiar) from those that were not recognized (i.e., misses and CR) in the Old-Low group. The lack of specificity in this region for recollection in the low-functioning older adults is generally consistent with the dedifferentiation hypothesis, suggesting a breakdown in the functional specialization evident in young and, in this case, high-functioning older adults (Li and Lindenberger 1999).
Although some evidence from human lesion studies supports a necessary role for the retrosplenial cortex in recollection (e.g. Valenstein et al. 1987), there is little such evidence for the posterior parietal cortex (Simons et al. 2007). However, neuroanatomical evidence from nonhuman primates (Clower et al. 2001; Lavenex et al. 2002) and functional connectivity studies in humans (Vincent et al. 2006) suggest that the medial and lateral parietal regions identified here are highly connected with the MTL, including the hippocampus. Although we did not observe any direct MTL activity changes in the Old-Low group, these previous findings suggest that reductions within an MTL–parietal network may underlie subjective recollection deficits in older adults, when they are observed. This is supported by recent imaging findings showing impaired estimates of recollection in conjunction with reduced recollection-related activity in hippocampal, medial, and lateral parietal cortex in older adults relative to the young (Daselaar, Fleck, Dobbins, et al. 2006).
Objective Recollection Impairments in Older Adults
Consistent with numerous previous findings of age-associated source memory impairments (e.g. Spencer and Raz 1995; Mark and Rugg 1998; Mitchell et al. 2006; Dodson et al. 2007; Wegesin et al. 2002), both high and low-functioning older adults exhibited impaired estimates of objective recollection relative to the young. This is in contrast to some previous evidence suggesting that high-functioning older adults can exhibit intact performance on tests of objective recollection (Cabeza et al. 2002; Davidson and Glisky 2002). There are a few possible explanations that may account for this discrepancy. For example, 1 possibility is that older adults' performance on tests of objective recollection may be differentially impaired depending on the nature of the contextual associations to be retrieved. Perhaps the spatial and temporal contexts implemented here were more sensitive to age-associated impairment than, for example, the modality discrimination (auditory or visual) used by Cabeza et al. One further consideration is that the stimulus sets used here were larger than in the studies by Cabeza et al., and Davidson and Glisky. It is conceivable that source memory performance may be preserved in older adults for relatively small stimulus sets, as some previous evidence suggests (Olson et al. 2004). One final possibility is that the high-functioning older adults in the current study are not equivalent, and somewhat impaired, relative to the high-functioning older adults in these previous studies. Although it is difficult to rule out this possibility entirely, it is worth noting that neuropsychological test performance in the Old-High group, and to a lesser extent in the Old-Low group, indicated that they scored above the age-adjusted levels for most of the tests, similarly to the older adults in these previous studies.
Consistent with our prediction that frontal activity changes might contribute to objective recollection deficits in older adults, objective recollection effects in right lateral PFC were affected in both high and low-performers, with left lateral regions additionally affected in the Old-High adults during retrieval. This is consistent with 2 recent studies, which showed that source retrieval success effects were affected by aging in bilateral PFC (Duverne et al. 2007; Morcom et al. 2007). Generally, these data offer support for the “frontal hypothesis of aging” and the finding that right lateral frontal regions were affected in both groups of older adults is somewhat consistent with the “right-hemi aging” hypothesis, which states that the right hemisphere shows greater age-associated functional decline than the left hemisphere (Rajah and D'Esposito 2005). Indeed, it seems likely that processes that have been attributed disproportionately to the right lateral PFC, such as sustained attention, inhibition of irrelevant information (Aron et al. 2004) and postretrieval monitoring (Henson et al. 2000; Rugg et al. 2003), may directly support the successful recovery of source information and that age-associated changes in these processes may contribute to performance deficits.
What is somewhat unclear is the nature of many of the age-related differences in the objective recollection contrast, with seemingly opposite patterns of activity for correct and incorrect source retrieval across Young and Old-Low groups in the right inferior PFC, and across Young and Old-High groups in the right dorsal PFC. In 2 previous studies, the majority of age-related differences in source memory effects were observed as enhanced activity for new relative to successful source trials in the young, with the opposite pattern of activity in the old (Duverne et al. 2007; Morcom et al. 2007). Although it has yet to be determined whether these different patterns of recollection effects reflect different cognitive processes, either beneficial or detrimental to performance, the fact that they were observed in lateral frontal regions is consistent with the idea that executive functions mediated by the PFC contribute to source memory performance and are affected by aging (Johnson et al. 1993).
We note that the high-functioning older group demonstrated enhanced activity in the ventromedial PFC (VMPFC), relative to the other 2 groups, in association with estimates of objective recollection. Some previous neuroimaging studies have identified increased memory-related activity in older adults in regions not seemingly recruited by young adults (e.g. Cabeza et al. 1997; Madden et al. 1999; Grady et al. 2005). One theory postulates that this enhanced recruitment reflects functional compensation for less efficiently recruited memory supportive brain regions, in the service of better memory performance (Dolcos et al. 2002; Rajah and D'Esposito 2005). Alternatively, this over-recruitment may represent a breakdown in functional specialization, resulting in impaired cognition (Logan et al. 2002). Although it is conceivable that compensatory over-recruitment might be evident in older adults with impaired memory performance (i.e., “insufficient compensation”), our results demonstrating that activity in the VMPFC was positively correlated with estimates of objective recollection exclusively in the high-functioning older adults offer support for the compensation hypothesis.
Previous imaging studies have implicated the ventromedial PFC in the processing of information related to the self, such as mentalizing about one's own internal thoughts and feelings (Amodio and Frith 2006) and patients with damage to this area can exhibit impairments in “metamemory” or making accurate judgments about their memory performance (Schnyer et al. 2004). One interesting possibility is that high-functioning older adults were able to recruit these VMPFC-mediated processes as a compensatory strategy (albeit not sufficiently) for deficits in other inefficiently recruited lateral frontal regions, whereas the low-functioning older were not. Although further study is necessary, activity in this region may reflect the retrieval of internally generated information when making objective decisions about externally derived details (Hashtroudi et al. 1989), in the service of better source memory performance in high-functioning older adults. This hypothesis is consistent with evidence suggesting that older adults report more internally generated thoughts and feelings than externally derived perceptual details when making memory judgments about previously encountered events (Johnson 2006). As we suggested earlier, the old-high adults may have recruited similar processes in order to encode and/or report upon noncriterial recollection.
Conclusion
In conclusion, results from the present data emphasize the importance of examining performance variability and different behavioral measures when investigating the effects of aging on recollection. Although high-functioning older adults exhibit intact recollection, when measured subjectively, both high and low-functioning older adults exhibit impairments in objective estimates of recollection. The imaging data support our proposal that the typically used subjective and objective tests of recollection necessitate somewhat dissociable cognitive processes and that age-associated deficits may be observed in accordance with the degree of underlying changes in the regions that support these processes. We propose that subjective recollection impairments may be observed in older adults in conjunction with altered neural activity with an MTL–parietal network, as with the low-functioning participants here, whereas objective recollection impairments may be more pervasive in aging, in part, due to altered executive control processes within the PFC, consistent with the frontal-aging hypothesis.
Funding
Medical Research Council and the Alzheimer's Research Trust.
Acknowledgments
Conflict of Interest: None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S. What are confabulators' memories made of? A study of subjective and objective measures of recollection in confabulation. Neuropsychologia. 2007;45:1489–1500. doi: 10.1016/j.neuropsychologia.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. J Cogn Neurosci. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Slotnick SD. Aging, source memory, and misrecollections. J Exp Psychol Learn Mem Cogn. 2007;33:169–181. doi: 10.1037/0278-7393.33.1.169. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.022. Forthcoming. [DOI] [PubMed] [Google Scholar]
- Duzel E, Vargha-Khadem F, Heinze HJ, Mishkin M. Brain activity evidence for recognition without recollection after early hippocampal damage. Proc Natl Acad Sci USA. 2001;98:8101–8106. doi: 10.1073/pnas.131205798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI. Forgetting in recognition memory with and without recollective experience. Mem Cognit. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and source monitoring. Psychol Aging. 1989;4:106–112. doi: 10.1037//0882-7974.4.1.106. [DOI] [PubMed] [Google Scholar]
- Henson RN. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. In: Graham KS, Gaffan D, editors. The role of the medial temporal lobe in memory and perception: evidence from rats, nonhuman primates and humans. East Sussex, UK: Psychology Press; 2005. pp. 340–360. [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Memory and reality. Am Psychol. 2006;61:760–771. doi: 10.1037/0003-066X.61.8.760. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Rev. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17(11):2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N, Kingsley D. Temporal and spatial context memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia. 1997;35:1533–1545. doi: 10.1016/s0028-3932(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J Comp Neurol. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 1995. New York: Oxford University Press. [Google Scholar]
- Li SC, Lindenberger U. Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson LG, Markowitsch HJ, editors. Cognitive neuroscience of memory. Toronto, Canada: Hogrefe and Huber; 1999. pp. 103–146. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognising: the judgment of previous occurrence. Pschol Rev. 1980;87:252–271. [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121(Pt 5):861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Greene EJ. Prefrontal cortex activity associated with source monitoring in a working memory task. J Cogn Neurosci. 2004;16:921–934. doi: 10.1162/0898929041502724. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17(11):2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. Neuroreport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Olson IR, Zhang JX, Mitchell KJ, Johnson MK, Bloise SM, Higgins JA. Preserved spatial memory over brief intervals in older adults. Psychol Aging. 2004;19:310–317. doi: 10.1037/0882-7974.19.2.310. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Williams RB, Anderton-Brown C. Age differences in reported recollective experience are due to encoding effects, not response bias. Memory. 1995;3:169–186. doi: 10.1080/09658219508258964. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: two means of access to the personal past. Mem Cognit. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Nolde SF, D'Esposito M. fMRI investigations of left and right PFC contributions to episodic remembering. Psychobiology. 2000;28:197–206. [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. 2000. [Google Scholar]
- Raz N. The aging brain observed in vivo: differential changes and their modifiers. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging. 2005. New York: Oxford University Press. p. 19–57. [Google Scholar]
- Rey A. Psychological examination of a case of post-traumatic encephalopathy. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rugg MD. ERP studies of memory. In: Rugg MD, editor. Electrophysiology of mind, event-related potentials and cognition. New York: Oxford University Press; 1995. pp. 132–170. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefrontal cortex in accurate feeling-of-knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42:957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. A neuropsychological study of fact memory and source amnesia. J Exp Psychol Learn Mem Cogn. 1987;13:464–473. doi: 10.1037//0278-7393.13.3.464. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.07.024. Forthcoming. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol. 1988;116:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Swick D, Senkfor AJ, Van Petten C. Source memory retrieval is affected by aging and prefrontal lesions: behavioral and ERP evidence. Brain Res. 2006;1107:161–176. doi: 10.1016/j.brainres.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Warrington E. Warrington recognition memory test. Windsor: NFER-Nelson; 1984. [Google Scholar]
- Warrington E, Whitley A. Selective impairment of topographical memory: a single case study. Neurol Neurosurg Psychiatry. 1978;41:575–578. doi: 10.1136/jnnp.41.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: an ERP replication and extension. Brain Res Cogn Brain Res. 2002;13:323–338. doi: 10.1016/s0926-6410(01)00126-4. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. Noncriterial recollection: familiarity as automatic, irrelevant recollection. Conscious Cogn. 1996;5:131–141. [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]