Abstract
Cocaine abusers show impaired performance on cognitive tasks that engage prefrontal cortex. These deficits may contribute to impaired control and relapse in abusers. Understanding the neuronal substrates that lead to these deficits requires animal models that are relevant to the human condition. However, to date, models have mostly focused on behaviors mediated by subcortical systems. Here we evaluated the impact of long-term self-administration of cocaine in the rhesus monkey on cognitive performance. Tests included stimulus discrimination (SD)/reversal and delayed alternation tasks. The chronic cocaine animals showed marked deficits in ability to organize their behavior for maximal reward. This was demonstrated by an increased time needed to acquire SDs. Deficits were also indicated by an increased time to initially learn the delayed alternation task, and to adapt strategies for bypassing a reliance on working memory to respond accurately. Working memory per se (delay dependent performance) was not affected by chronic self-administration. This pattern of cognitive deficits suggests dysfunction that extends beyond localized prefrontal cortical areas. In particular, it appears that temporal cortical function is also compromised. This agrees with other recent clinical and preclinical findings, and suggests further study into addiction related dysfunction across more widespread cortical networks is warranted.
Keywords: addiction, cognition, prefrontal, primate, psychostimulant
Introduction
Psychostimulant use continues to be widely prevalent despite the significant costs to individuals and society (Substance Abuse and Mental Health Services Administration 2006). Cocaine and amphetamine users display impaired performance on a number of cognitive tests that specifically engage prefrontal cortex (O'Malley et al. 1992; van Gorp et al. 1999; Grant et al. 2000; Bechara et al. 2001; Rogers and Robbins 2001; Garavan and Stout 2005; Li et al. 2006). There is also a recent report of deficits consistent with temporal cortex dysfunction (Ersche et al. 2006). Cognitive deficits are dose related in terms of cumulative cocaine use (Bolla et al. 1999), and are consistent with models of addiction incorporating cortical dysfunction in addition to appetitive ideation (Bolla et al. 1998; Porrino and Lyons 2000; Dackis and O'Brien 2001; Goldstein and Volkow 2002; Bechara 2005; Garavan and Stout 2005; Schoenbaum et al. 2006). Importantly, cognitive function is a factor in treatment retention in cocaine dependence (Aharonovich et al. 2006). Thus, understanding the neurobiology of cognitive deficits resulting from chronic cocaine use may have significant clinical relevance.
In addressing the neurobiological basis of cognitive deficits associated with substance abuse, animal models with the highest face validity are needed. Nonhuman primate studies are particularly well suited to addressing the question of cognitive consequences of drug exposure, given the similarity of cognitive complexity between human and nonhuman primates, the similarity of neuroanatomical distribution of function, and the ability to use common investigative techniques such as brain imaging approaches. However, few studies have examined the long-term effects of prolonged exposure to cocaine on cognitive function in primates. One study (Jentsch et al. 2002) investigated the cognitive consequences of relatively short term investigator administered cocaine, and observed relatively selective deficits on a stimulus reversal task. However, there have not been any studies of cognition in monkeys following long-term drug self-administration. We undertook the present study to investigate cognitive performance of monkeys after long-term (2 years) self-administration of cocaine and its related psychoactive metabolite, cocaethylene, a compound equipotent to cocaine at inhibiting the dopamine transporter, but less potent at the noradrenergic and serotonergic transporters (Bradberry et al. 1993; Elsworth et al. 1993).
Because of a growing focus on prefrontal cortex dysfunction in addiction, we employed tasks that are dependent on intact orbitofrontal cortex and dorsolateral prefrontal cortex. To probe orbitofrontal functionality, a stimulus discrimination (SD)/reversal task was employed (Butter 1969; Jones and Mishkin 1972; Dias et al. 1996). The reversal component of the task is dependent on orbitofrontal cortex, whereas the SD component is typically believed to be more dependent on integrity of temporal cortex (Murray et al. 1993; Baxter and Murray 2001). For assessing dorsolateral prefrontal cortex, we employed a spatial delayed alternation task (Goldman and Rosvold 1970; Nixon and Passingham 1999). We hypothesized that extended cocaine use history would result in impaired performance of the stimulus reversal task and the delayed alternation task. We did not expect differences in SD performance.
Methods and Materials
Five adult Rhesus macaques (3 males, 2 females) with no previous psychoactive drug exposure (besides routine ketamine for veterinary purposes) were used in the present study. For all behavioral procedures, animals were placed in a behavioral chair (Primate Products, Redwood City, CA) using standard pole and collar methods. All animals were initially trained to lever press for food pellets with a visual cue indicating availability of a 1 g food pellet reinforcer. After establishment of food self-administration, the 2 animals that went on to self-administer drug had a vascular access port placed mid-scapula from which a catheter extended subcutaneously to either a femoral or internal jugular vein (Bradberry et al. 2000). One of the control animals (13-04) also had a vascular access port placed as a control for nonspecific effects of the surgery and maintenance of the port. All procedures were in accord with the NIH Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, revised 1987).
Food Self-Administration Training
Animals were placed in a behavioral chamber (Med-Associates) fitted with an operant panel, constructed in the laboratory, and to which the behavioral chair attached, as previously described (Bradberry et al. 2000). Med-Associates (Georgia, VT) software and hardware were used for data collection and control of all inputs and outputs. Animals were trained to self-administer food pellets under an FR1 lever response schedule of reinforcement using a visual discriminative stimulus (a light on the panel). Multiple food pellets could be obtained during the interval when the light on. The animals were judged to have acquired the food cue when, in 4 out of 5 sessions, the lever press rates with the light was on were 50% higher than with the light off. The 3 control animals continued to lever press for food for 113 ± 44 sessions following acquisition of the food cue.
Cocaine and Cocaethylene Self-Administration
Once lever pressing behavior for food was learned, 2 monkeys (1 male, 1 female) underwent 24 months of progressive ratio self-administration studies designed to compare the reinforcer efficacy of cocaine and cocaethylene. Intravenous self-administration procedures also took place in behavioral chairs in the same behavioral chambers as used for food self-administration. Details of the results from those studies will be presented elsewhere, but in brief, unit doses of 0, 0.025, 0.075, 0.25, 0.5, and 1.0 mg/kg cocaine hydrochloride (or the molar equivalent of cocaetheylene) were compared. During touch screen familiarization and SD/reversal task performance, animals continued to receive 1 weekly self-administration session on Friday afternoon, after all cognitive testing was complete for the week. During these weekly sessions, both animals received on average 3 mg/kg cocaine per session (average 6 infusions; unit dose 0.5 mg/kg). Cocaine self-administration was discontinued prior to delayed alternation testing. Mean total cumulative intake for study was 360 mg/kg cocaine, and 54 mg/kg cocaethylene (mass of the molar equivalent of cocaine hydrochloride). Individual values: Animal 15-04: 362 mg/kg cocaine; 90 mg/kg cocaethylene; Animal 19-04: 359 mg/kg cocaine; 13 mg/kg cocaethylene. For a detailed timeline of cocaine and cocaethylene intake, see Figure 1 in Supplementary Material, which also indicates that there was not a significant impact on body weights as a consequence of cocaine self-administration.
Cognitive Testing
All animals underwent training on touch screen based cognitive tasks in the following order: 1) touch screen familiarization; 2) SD and reversal task; 3) delayed alternation task. For details of total duration with each task, see Table 1 in Supplementary Material.
Touch Screen Familiarization
Animals were trained in a sound-attenuated chamber (Eckel Industries, Ontario, Canada, model AB4240) fitted with a 40 W houselight and white noise generator. All tasks were conducted on an 15″ touch screen (Elo systems CarrollTouch) that utilizes a grid of infrared sensors just off the surface to monitor touches. E-prime (Psychology Software Tools, Pittsburgh, PA) was used for all schedules of stimulus presentation and response recording. Animals were tested 5 days a week and were water regulated (from midday Sunday to Fri afternoon), with ad lib water over the weekend. They were supplemented in the afternoons to maintain adequate physiological needs (25 mL/kg/day). Initial training began with presentation of a 6.0 cm × 6.0 cm blue square stimulus in the middle of the touch screen (easy level). Upon successful execution of this task, the same size stimulus would appear at a random location in each trial (medium level). In the final stage of familiarization, the size of the stimulus varied (1.5–3.0 cm) as well as the location. After animals routinely scored above 90% correct on the easy and medium levels, they began training on the SD/reversal task.
SD and Reversal Task
Each trial of the task started with a red square (6.0 cm × 6.0 cm) on the middle of the touch screen. Touching the red square led to 2 pictures (6.0 cm × 4.0 cm), selected randomly from a set of 40 bitmaps, that were randomly presented left and right of midline. Touching 1 stimulus (S+) led to a high reward (0.3 mL of water) while touching the other stimulus (S−) led to a low reward (0.1 mL of water). The 2 stimuli stayed on the touchscreen for another 0.5 s, and following a 1-s intertrial interval, the next trial was started. Responses outside of the 2 stimuli resulted in a blank screen and 3-s timeout. A SD was accomplished when the animal chose S+ in 18 out of 20 trials during the discrimination phase. The reward values associated with the stimuli were then reversed. Once the animal learned the new contingency (chose the new S+ for 18 out of 20 trials during the reversal phase), this was defined as the completion of a reversal, and a new pair of stimuli were presented. The 2 cocaine treated animals and 2 of the 3 controls were allowed to perform an unlimited number of trials in each session. After it became apparent that the cocaine treated animals would not perform as many trials as the control animals, a 200 trial limit per session was established for the third control animal (13-04).
During the initial touch screen training and testing on the SD/reversal task, the cocaine animals continued to receive weekly self-administration sessions on Friday afternoons after cognitive testing was done (avg 3 mg/kg cocaine from 6 infusions of 0.5 mg/kg). Based on the app. 30-min plasma half life of cocaine (Bradberry et al. 2000), no residual pharmacological effect would be expected after the weekend when testing resumed on Monday.
Delayed Alternation Task
The delayed alternation task was adapted from Nixon and Passingham (Nixon and Passingham 1999). Each session of the task was composed of 200 trials for all animals. Each trial started with a round white stimulus on a black background. Pressing that stimulus led to 2 white rectangles on the screen after a delay. At the first trial of a session, pressing of either rectangle led to a reward (0.75 mL of water). On the next trial, pressing the other rectangle led to the water reward and advancement to the next trial. This trial was counted as a correct trial. Pressing the same rectangle as the last trial led to no reward and advancement to the next trial. This trial was counted as an incorrect trial. Animals were first trained at 0 s delay to learn the alternation task. Once animals reached criterion performance of ≥80%, the delay increased to 3 s. A modification for initiating each trial was made after this initial stage of accomplishment, which was to begin each trial with 2 circles that had to be touched to bring up the alternating target locations. This was done in order to discourage set patterns of movement that could have served as positional cues, thereby reducing the need for performance to depend on visuospatial working memory. When criterion performance was reached at 3-s delay, the delays changed to random 0, 1, 3, 5, 10 s. At these random delays, performance was evaluated over 10 sessions for any differences in working memory. For 4 animals (2 from each group), performance on the delayed alternation task with the same delays was evaluated over at least 16 additional weeks to determine if delay dependent performance was stable over time, and to determine if there were any differences over time between groups. Prior to beginning to train on the delayed alternation task, animal 19-04 had received no cocaine for 6.5 months, whereas animal 15-04 had not received cocaine for 2 months.
Data Analysis
In order to evaluate the performance of learning to self-administer food pellets prior to any drug exposure, a t-test was used to compare the number of sessions to acquire the cue indicating food pellet availability.
For the SD/reversal task, performance was normalized for the number of trials for each animal. This was necessary because of differences between the cocaine and the first 2 control animals in the number of trials completed. The third control animal (13-04) was limited to 200 trials per session. Only trials from the discrimination phase were used in the calculation of SD/1000 trials. Trials that occurred after a SD (i.e., after the reward contingency had been reversed) were not used because they were not testing acquisition of an SD, but rather reversal performance. A t-test was used to compare the normalized number of SDs acquired for the first 10 sessions with >200 trials.
For the delayed alternation task, a t-test was used to compare the number of sessions to reach criterion performance (80% correct) at zero delay between control monkeys and monkeys with cocaine use history. A 2-way ANOVA with repeated measures (delay) was used to determine whether there was a main effect of treatment (cocaine or control), delay (0 s, 1 s, 3 s, 5 s, 10 s) or significant treatment and delay interaction upon the average accuracy over the first 10 sessions. The analysis was implemented in SAS PROC Mixed (Littell et al. 2006), where the degrees of freedom method was Kenward–Roger.
We also evaluated how performance on the delayed alternation task changed over time in the 2 drug exposed, and 2 of the control animals over the 16 additional weeks of testing. The principal outcome of interest was the rate of improvement in performance over the study duration. For each animal, the mean percent correct responses per week for each of the 5 delays was determined, and the geometric area under the curve (AUC) of these mean percent values versus delay interval was calculated for each animal for each week. AUC values were adjusted for baseline performance by subtracting out performance during the first week. These adjusted weekly values were the primary response variables. Initial graphical displays indicated that for each animal the adjusted weekly AUCs were linearly related to (week)1/2. The comparisons of the slopes between the 2 control and 2 drug treated animals used the 16 weekly postbaseline observations on each animal to compute a separate slope for each of the 4 animals. Those 4 slopes were then used to compare the control and drug treated groups.
To detect the effect of experimental group, 2 models were implemented. The main analysis was based on a multivariate analysis of covariance (MANCOVA) model. In this analysis, (week)1/2 was treated as a continuous covariate, and common slopes within experimental group were assumed with no intercept. The error terms were assumed to have a first-order autoregressive covariance structure and slope was a fixed effect (Neter et al. 1996). The analysis was implemented in SAS PROC Mixed (Littell et al. 2006), where the degrees of freedom method was Kenward–Roger. A secondary analysis was used to confirm the robustness of the main analysis. In the second analysis, for each individual animal, adjusted AUC was regressed on the (week)1/2, (without intercept) to obtain each animal's slope over time. Then an independent samples t-test (with 2 d.f.) was used to compare the slopes between the 2 experimental groups. The inference from this latter analysis confirmed those from the MANCOVA model. All statistical tests were 2 sided and conducted with an alpha level = 0.05.
Results
Acquisition of a SD Before and After Chronic Cocaine
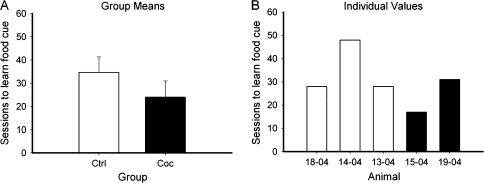
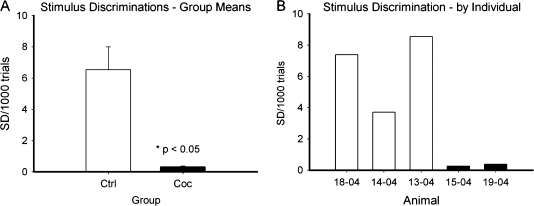
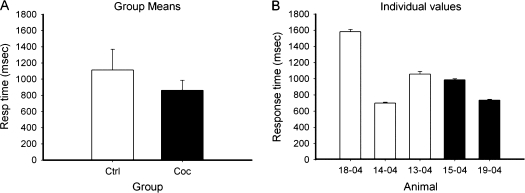
In order to compare the ability to acquire operant responding between the control and drug treated animals prior to the drug exposure, we retrospectively compared their ability to learn an operant task with a visual cue indicating food availability. There were no differences between groups in number of sessions to acquire the food cue (Fig. 1). After chronic cocaine (or additional food self-administration in the control group), animals were tested on the touch screen based SD/reversal task. Animals were tested for their ability to learn to discriminate between 2 visual stimuli (presented simultaneously) that represented different reward magnitudes (0.1 vs. 0.3 mL of water). The number of SDs acquired (normalized for number of trials) were significantly greater in the control group (Fig. 2) than the cocaine group. A comparison of response times (Fig. 3) indicated no difference between groups, suggesting there were no motivational differences.
Figure 1.
Control and cocaine groups were equivalent at learning an operant task prior to drug exposure. (A) Group means for number of sessions to acquire food cue discrimination (see Methods). (B) Individual values for sessions needed to acquire cue.
Figure 2.
Cocaine use history impaired acquisition of SD in the SD/reversal task. (A) Group means for SDs achieved, normalized for number of trials (mean ± SE); (B) individual values for SDs achieved.
Figure 3.
Response times did not differ between groups. (A) Group means for response times during SD trials (mean ± SE); (B) individual values for response times (mean ± SE).
The number of trials engaged in during the SD task (excluding reversal trials) did not differ between the 2 groups (2599 ± 922 for the controls, and 3078 ± 593 for the cocaine animals). The number of SD trials was not fewer for cocaine animals (despite fewer overall trials), because control animals acquired SDs, and then did trials during the reversal phase of the task. Cocaine treated animals did not acquire SDs to enable them to move on to the reversal phase, thus, almost all of their trials were in the discrimination phase. Total trials for both phases were 5046 ± 1670 for controls, and 3240 ± 580 for cocaine animals.
Due to the greatly reduced number of discriminations achieved by the cocaine animals, there was not a sufficient number of stimulus reversals achieved by the cocaine group to permit a valid statistical comparison of the number of reversals accomplished.
Delayed Alternation Task performance
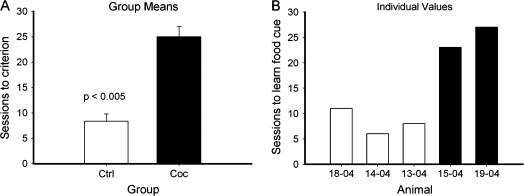
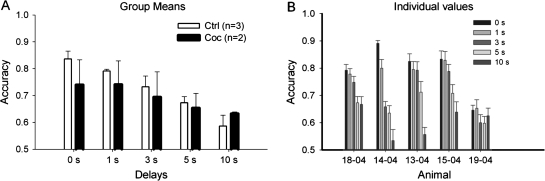
In the delayed alternation task, cocaine animals required significantly more sessions than the control animals to reach the criterion performance (80%) at zero delay (P < 0.05; Fig. 4). Following task acquisition, the varying delays were introduced, and randomly presented across the 200 trials per session. For the average accuracy of the first 10 sessions with random delays from 0 to 10 s, 2-way repeated ANOVA showed a main effect of delay (F(4,12) = 8.84, P < 0.01) but not treatment (F(1,3) = 0.45, P = 0.55) or delay * treatment (F(4,12) = 1.28, P = 0.33), indicating that there was not a significant difference in working memory, as reflected in the dependence of performance on delay. Figure 5 shows the mean performance of the 2 groups across delay interval. Though there was no significant difference in delay dependent performance, there was considerable difference between the 2 chronic cocaine animals in that 1 showed a clear delay dependency, whereas the other did not, as can be seen in panel B, which presents the values for the individual animals.
Figure 4.
Cocaine use history impaired acquisition of alternation at zero delay. (A) Sessions to acquire alternation at 0-s delay (mean ± SE). (B) Sessions to acquire alternation at 0-s delay for individual animals.
Figure 5.
Effects of cocaine use history on delayed alternation task with random delays. (A) Accuracy of the first 10 sessions (mean ± SE). (B) Accuracy of the first 10 sessions for individual animals.
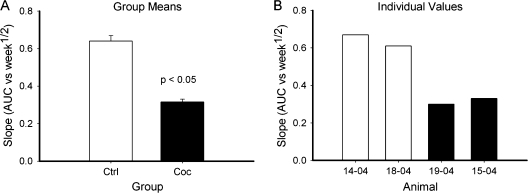
We also examined performance on the delayed alternation task over a period of 17 weeks in 4 of the animals (2 control and 2 cocaine). Using the values of the adjusted weekly AUC (see Data Analysis), we compared how the animal's performance changed over time by a MANCOVA model. There was an increase in performance over time in both groups as indicated by a positive slope of adjusted AUC versus (week)1/2. However, the control animals improved at a significantly greater rate as indicated by the difference in slopes (t(10,2) = 5.89, P = 0.001, Fig. 6). Figure 2 in the Supplementary Material presents the completed AUC versus time plots from which the slopes of Figure 6 were obtained.
Figure 6.
Control animals showed more improvement over time in delayed task performance than cocaine animals. Ordinate represents the slope of the geometric AUC of delay dependent performance versus (week)1/2 over 17 weeks. (A) Group means. (B) Individual values.
Discussion
This study investigated cognitive/learning tasks in monkeys following long-term cocaine self-administration. Our findings indicate that striking cognitive deficits result. In addition to the widely observed clinical indicators of prefrontal cortical dysfunction, our results suggest more widespread deficits that implicate temporal cortical dysfunction as well. Before cocaine self-administration, the cocaine and control groups did not show any significant differences in learning to respond to a cue associated with food reward availability. After chronic cocaine self-administration, there was a decreased ability to discriminate 2 stimuli associated with different reward amounts. In the delayed spatial alternation task, the monkeys with cocaine use history showed impairments at learning the task at zero delay, and in adoption of adaptive strategies to maximize performance. The 2 groups did not differ in delay dependent (working memory) performance. Thus, our hypothesis that relatively selective prefrontal cortex dependent deficits would be seen was not supported, with more extensive deficits observed instead.
SD/Reversal Performance
There was no difference in operant performance prior to cocaine self-administration. However, following chronic drug self-administration, we observed a striking deficit in the ability of the cocaine treated animals to preferentially respond to the stimulus associated with the higher reward. In order to determine if there were motivational differences between groups, response times in the SD trials were compared. There were no significant differences, suggesting that the 2 groups were equivalently engaged in the task and motivated by the water reward. However, cocaine exposed animals would engage in fewer trials than control animals. It is unclear why, but in addition to possibilities such as impaired sustained attention, impaired motivation as a consequence of altered subcortical mechanisms cannot be ruled out.
The only previous published work on the effects of cocaine exposure on SD/reversal learning in monkeys is that of Jentsch et al. (2002), who found that repeated investigator administration of cocaine over a 2-week period produced impairments in reversal learning, without affecting novel object discrimination. Our observation of an expansion of deficits to the acquisition of a SD is likely related to the increased duration, route of administration, and cumulative exposure in the present study. Whereas Jentsch et al. administered cocaine (2 or 4 mg/kg) to monkeys daily for 14 days via an intramuscular route, the monkeys in this study self-administered cocaine i.v. over a period of 2 years with mean cumulative intakes of 360 mg/kg cocaine. Thus, our monkeys received higher cumulative doses over a much longer period, and via a route that is associated with greater neurobiological impact (Bradberry 2002; Samaha et al. 2002). The extensive exposure of the monkeys in this report increases the relevance for comparison with clinical cases of long-term cocaine abuse.
The majority of clinical investigations into cognitive deficits in drug users have focused on prefrontal cortex (including anterior cingulate, dorsolateral prefrontal, and medial and lateral orbitofrontal cortex (O'Malley et al. 1992; Bolla et al. 1999; van Gorp et al. 1999; Grant et al. 2000; Bechara et al. 2001; Rogers and Robbins 2001; Garavan and Stout 2005; Li et al. 2006). However, nonprefrontal cortical regions are also implicated. For example, a clinical imaging study has shown that a group of cocaine users with an average of 13 years cocaine use had a decreased gray matter concentration in their temporal cortices (Franklin et al. 2002), and cognitive deficits consistent with temporal cortical damage have been observed following long-term psychostimulant abuse (Ersche et al. 2006). Our observation of decreased performance at acquiring a novel object discrimination is consistent with temporal cortex deficits following long-term cocaine self-administration. Brain lesion studies have shown that lesions of medial temporal cortical structures impaired the acquisition of novel object discrimination (Murray et al. 1993; Baxter and Murray 2001), and recent work on functional activity in rhesus monkey brain following extended cocaine self-administration has demonstrated cumulative dose dependent effects in the temporal lobe, including amygdala, hippocampus, and surrounding cortical areas (Beveridge et al. 2006).
We had hypothesized that deficits in orbitofrontal cortex dependent cognition (reversal of a learned SD) would be observed, consistent with the preclinical and clinical literature cited above. At present, we cannot speak to reversal performance given the limited capacity of the chronic cocaine animals to establish initial discriminations. Further work with a larger group of animals and/or modified procedures will help to clarify if the effects of self-administered cocaine also produce deficits in reversal performance.
Spatial Delayed Alternation/Working Memory
Delay dependent performance on a spatial delayed alternation task is believed to be dependent on intact dorsolateral prefrontal cortex in rhesus monkeys (Goldman and Rosvold 1970). We employed this task (modeled on a touch screen task developed by Nixon and Passingham 1999) in order to determine if the cocaine animals displayed working memory deficits. There is some ambiguity in the clinical literature in this regard. Some clinical studies have shown working memory deficits in drug abusing populations (Bechara and Martin 2004; Kubler et al. 2005), suggesting impaired dorsolateral prefrontal cortex which the delayed alternation task is dependent upon. However, many reports indicate no deficits in cocaine users (Bolla et al. 1999; Grant et al. 2000; Aharonovich et al. 2006) on the Wisconsin Card Sort task which is dependent on dorsolateral prefrontal cortex in humans, as delayed alternation is in monkeys.
We observed deficits in the ability of animals to learn the delayed alternation task many months after cessation of cocaine exposure, consistent with clinical studies that cognitive deficits in former drug users are persistent (Clark et al. 2006; Ersche et al. 2006). In the delayed alternation task, monkeys with a cocaine self-administration history showed an impaired ability to learn alternation at zero delay. In rats, it has been shown that lesions of the temporal cortex resulted in significant impairments on acquisition of a spatial alternation task (Ramirez et al. 2007), suggesting involvement of the temporal cortex in the acquisition of an alternation task. Thus, the deficits in task acquisition at zero delay, like the SD deficits discussed above, may be associated with temporal cortical dysfunction. There is also the possibility that prefrontal cortical mechanisms could underlie the observed deficits, given the extensive clinical evidence of anterior cingulate dysfunction in addiction, and the role of the anterior cingulate in the temporal organization of behavior (Garavan and Stout 2005; Rushworth et al. 2007).
When variable intratrial delays were added after acquisition of the alternation task, monkeys with a cocaine use history did not show significant impairments compared with control monkeys, suggesting working memory was not affected consistently. It is interesting that in 1 of the cocaine animals (19-04), there was no dependency of performance on delay, similar to an observation in human addicts whose deficits on a delayed nonmatch to sample task were delay independent (Bechara and Martin 2004). The limited size of the population sampled does not permit the definitive conclusion that working memory is unaffected by chronic cocaine self-administration at present.
Similar to the observed deficits at learning the delayed alternation task at zero delay, when animals were allowed to engage in the task daily (5 days per week) for 17 weeks, the cocaine exposed animals showed reduced improvement over time relative to controls. The improvement is presumably not due to development of greater working memory, but rather to development of adaptive (cheating) strategies such as the use of positional cueing in which the animals signal to themselves where the response should be by either placement of (e.g.) their hand, or by the use of orchestrated patterns of movement. Like our other observations, this deficit also is reminiscent of the generally poorer functionality at maximizing effectiveness of interacting with the environment shown by drug abusers.
Potential Subcortical Involvement
The deficits displayed by chronic self-administering animals in this study implicate cortical dysfunction. However, subcortical mechanisms could also be involved. For instance, in monkeys, lesion of the caudate nucleus, a major efferent target of prefrontal cortex, will result in many similar deficits as lesion of the prefrontal itself (Goldman and Rosvold 1972), and striatal lesions have been shown to disrupt acquisition of a complex visual stimulus response in rats (Reading et al. 1991). Likewise, acute pharmacological intervention at striatal targets of mesocortical areas such as the amygdala and hippocampus can similarly disrupt reward related learning as when the cortical areas are disrupted (Kelley 2004), suggesting that reward related learning is dependent on a distributed corticostriatal network for “for assessing the temporal relationship of sensory and motor events” (Kelley 2004). Because of ventral striatal dopamine facilitation of reinforced behavior (Salamone et al. 2005), and the ability of cocaine to produce enduring alterations in dopaminergic systems in primates (Nader et al. 2006), this is also a potential mechanism for subcortical changes to contribute to the effects we have observed, especially the unwillingness of chronic cocaine animals to engage in as many trials as control animals.
Potential Neurochemical Mechanisms
Cocaine alters extracellular levels of dopamine, norepinephrine, and serotonin acutely. However, the altered cognition observed herein was not dependent on presence of the drug, and in the case of deficits of delayed alternation performance, several months had passed since last exposure. This suggests that drug exposure has produced long lasting alterations in cortical synaptic organization, and/or in circuit interactions that underlie successful association of environmental stimuli with rewards, and temporal organization of behavior needed, e.g., to perform the delayed alternation task. Long-term alterations in dopaminergic signaling can alter corticostriatal glutamatergic signaling in the striatum (Surmeier et al. 2007), and repeated psychostimulant exposure has been shown to alter excitatory amino acid receptor expression in prefrontal cortex itself (Sun et al. 2005). Given the postulated role of dopamine systems in alerting wider neuronal networks to rewards (Schultz 2007), and the striking effects of cocaine on dopaminergic systems in primates (Bradberry et al. 2000; Nader et al. 2006; Bradberry 2007), it must also be considered that cocaine exposed animals could have dysfunctional dopaminergic responses to rewards, thereby reducing the contrast between stimuli associated with the different reward values. There is also increasing evidence of corelease of glutamate and dopamine, with suggestions that glutamate is more likely to mediate fast signaling needed for precise correlation of stimuli and rewards (Lapish et al. 2006). Thus, alterations in dopamine neuronal function could also be mediated by alterations in the corelease of glutamate. The possibility of altered responsiveness to an unchanged dopaminergic signal is also possible (Peterson et al. 2006).
Other potential mechanisms for our observations include changes in noradrenergic systems, shown to be altered in primates after long-term cocaine self-administration in forebrain areas that include temporal cortex (Beveridge et al. 2005). There is a growing appreciation of the role of locus coeruleus norepinephrine in organization of reinforced behavior (Aston-Jones and Cohen 2005), therefore, norepinephrine systems must also be considered as a potential contributor to the observed cognitive dysfunction.
A clear limitation of the current study is the small sample number, an inherent difficulty in conducting nonhuman primate studies. However, the small variability within groups and relatively large differences between groups suggests that the differences we see are meaningful. Given the increased phylogenetic proximity of monkeys to humans and the resultant similarity of cortical anatomy and function, future investigations into the underlying neurobiological basis of the cognitive dysfunction observed in this model should help to inform the clinical understanding of addiction.
Funding
National Institutes of Health (DA 10331, MH 78789); and the Veteran's Administration Biomedical Laboratory Research and Development Service.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Supplementary Material
Acknowledgments
Excellent technical assistance was provided by Dustin Kerr and Kate Gurnsey. Conflict of Interest: None declared.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Impairments in visual discrimination learning and recognition memory produced by neurotoxic lesions of rhinal cortex in rhesus monkeys. Eur J Neurosci. 2001;13:1228–1238. doi: 10.1046/j.0953-816x.2001.01491.x. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl). 2005;180:781–788. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Dynamics of extracellular dopamine in the acute and chronic actions of cocaine. Neuroscientist. 2002;8:315–322. doi: 10.1177/107385840200800407. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl). 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treatment. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Jatlow P, Roth RH. Serotonin involvement in cocaine sensitization: clues from studies with cocaine analogs. Drug Dev Res. 1993;30:189–200. [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. The effects of selective caudate lesions in infant and juvenile Rhesus monkeys. Brain Res. 1972;43:53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex [Review] Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De la Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Judson Chandler L. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. Cary (NC): SAS Institute; 2006. [Google Scholar]
- Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Wasserman W, Nachtsheim CJ. Applied linear statistical models. Chicago: Irwin; 1996. [Google Scholar]
- Nixon PD, Passingham RE. The cerebellum and cognition: cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. Eur J Neurosci. 1999;11:4070–4080. doi: 10.1046/j.1460-9568.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex. 2000;10:326–333. doi: 10.1093/cercor/10.3.326. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, Campbell D, Poulton W, Barton C, Swails J, Geghman K, Courchesne SL, Wentworth S. Bilateral entorhinal cortex lesions impair acquisition of delayed spatial alternation in rats. Neurobiol Learn Mem. 2007;87:264–268. doi: 10.1016/j.nlm.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB, Robbins TW. Dissociable roles of the ventral, medial and lateral striatum on the acquisition and performance of a complex visual stimulus-response habit. Behav Brain Res. 1991;45:147–161. doi: 10.1016/s0166-4328(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2006. [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.