Abstract
We have recently found that in the mouse cortex, activation of δ-opioid receptor (DOR) attenuates the disruption of K+ homeostasis induced by hypoxia or oxygen–glucose deprivation. This novel observation suggests that DOR may protect neurons from hypoxic/ischemic insults via the regulation of K+ homeostasis because the disruption of K+ homeostasis plays a critical role in neuronal injury under hypoxic/ischemic stress. The present study was performed to explore the ionic mechanism underlying the DOR-induced neuroprotection. Because anoxia causes Na+ influx and thus stimulates K+ leakage, we investigated whether DOR protects the cortex from anoxic K+ derangement by targeting the Na+-based K+ leakage. By using K+-sensitive microelectrodes in mouse cortical slices, we showed that 1) lowering Na+ concentration and substituting with impermeable N-methyl-D-glucamine caused a concentration-dependent attenuation of anoxic K+ derangement; 2) lowering Na+ concentration by substituting with permeable Li+ tended to potentiate the anoxic K+ derangement; and 3) the DOR-induced protection against the anoxic K+ responses was largely abolished by low-Na+ perfusion irrespective of the substituted cation. We conclude that external Na+ concentration greatly influences anoxic K+ derangement and that DOR activation likely attenuates anoxic K+ derangement induced by the Na+-activated mechanisms in the cortex.
Keywords: anoxia, cortex, δ-opioid receptor, K+ homeostasis, Na+ influx, neuroprotection
Introduction
Disruption of ionic homeostasis, including enhanced influx of Na+ and Ca2+ and efflux of K+ (Hansen 1985; Jiang and Haddad 1991; Friedman and Haddad 1994a; Müller and Somjen 2000a, 2000b; Martinez-Sanchez et al. 2004), has been generally regarded as an initial and key alteration in anoxia/ischemia-induced neuronal injury. Among cellular ions, K+ is the most abundant cation in the cytoplasm, and its sharp efflux has been shown to be closely associated with anoxia-induced depolarization, which is believed to be a crucial factor leading to neuronal death (Hansen 1985; Yu et al. 1997, 1999; Lauritzen et al. 2003; Liu et al. 2003; Wei et al. 2003; Yu 2003; Remillard and Yuan 2004). For example, sustained exposure to elevated extracellular K+ (simulating ischemic extracellular K+) causes significant neuronal death even under conditions of normoxia and abundant glucose supply (Takahashi et al. 1999), whereas blockade of K+ efflux has been shown to attenuate hypoxia- and ischemia-induced neuronal death (Huang et al. 2001; Liu et al. 2003; Wei et al. 2003). These findings suggest that maintaining cellular K+ homeostasis and inhibiting excessive K+ fluxes may be of therapeutic benefit in the treatment of stroke and related neurodegenerative conditions (Liu et al. 2003; Wei et al. 2003).
Our previous studies have demonstrated that activation of δ-opioid receptor (DOR) is neuroprotective against hypoxic and excitotoxic insults (Zhang et al. 2000, 2002, 2006; Ma et al. 2005). Others have also shown similar findings regarding neuroprotection against stress via DOR (Borlongan et al. 2004; Lim et al. 2004; Narita et al. 2006), suggesting that DOR plays an important role in neuronal survival during hypoxic/ischemic stress.
On exploring the underlying mechanisms, we demonstrated for the 1st time that DOR activation attenuates hypoxic and ischemic disruption of K+ homeostasis (Chao et al. 2006, 2007a, 2007b), that is, the massive K+ leakage that occurs in neurons in response to hypoxia/ischemia and triggers neuronal apoptosis and death (Yu et al. 1997, 1999; Liu et al. 2003; Wei et al. 2003). Our data also show that activation of DOR significantly inhibited disruption of K+ homeostasis induced by anoxic and ischemic conditions, whereas inhibition of DOR completely blocked the DOR effect on anoxic disruption of K+ homeostasis (Chao et al. 2007b). Moreover, we observed that in acute hypoxic/ischemic stress, absence of extracellular Ca2+ resulted in a significant attenuation of the anoxia-induced disruption of K+ homeostasis in the cortex, and the DOR protection from anoxic K+ derangement in the cortex was partially mediated via an inhibition of hypoxia-induced increase in Ca2+ entry–Ca2+-activated K+ (BK) channel activity (Chao et al. 2007a).
Many ionic factors may contribute to the onset and degree of the disruption of K+ homeostasis. The sum total of anoxic K+ efflux results from several components related to different mechanisms. At present, it is unknown whether the DOR protection from anoxic K+ leakage is dependent on multiple mechanisms or solely on the inhibition of the Ca2+-based component. Because Na+ influx is a major event accompanying the K+ derangement in response to hypoxic/ischemic stress (Műller and Somjen 2000a, 2000b), we asked whether the DOR protection is related to the regulation of the Na+-based component of anoxic K+ derangement.
We previously observed that DOR downregulation (Zhao et al. 2005) is associated with Na+ channel upregulation (Xia et al. 2003) and DOR activation attenuates hypoxic dysregulation of Na+ channels (Xia et al. 2001). More recently, we have found that activation of DOR inhibits Na+ currents in Xenopus oocytes cotransfected with Na+ channels and DOR. All these observations suggest a close interaction between DOR signals and Na+ homeostasis. We, therefore, hypothesized that the mechanism of the DOR protection against anoxic K+ derangement may be related to an inhibition of Na+ influx at the initial stage of neuronal responses. As a 1st step, we aimed in the present study to determine whether the DOR protection targets the Na+-based component of anoxic K+ derangement by testing the effect of DOR in low concentration of external Na+. Our data showed that lowering external Na+ concentration by substitution with a membrane impermeable organic cation reduced anoxic K+ derangement and that DOR activation could not then further attenuate the anoxic K+ derangement under such condition, suggesting that DOR activation attenuates anoxic K+ derangement by inhibiting a Na+ action potential–based component of anoxic K+ derangement.
Materials and Methods
Animals
Male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Yale University School of Medicine, which is accredited by the American Association for Accreditation for Laboratory Animal Care.
Chemicals and Reagents
N-(trimethylsilyl)dimethylamine (Fluka 41420), valinomycin (Fluka 94675), potassium tetrakis(p-chlorophenyl)borate (Fluka 60591), 2,3-dimethylnitrobenzene (Fluka 40870), N-methyl-D-glucamine (NMDG+), and LiCl were purchased from Sigma Chemicals Co. (St Louis, MO). H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP 512), a more specific and potent DOR agonist (Balboni et al. 2002), was provided by Dr Balboni. UFP 512 was prepared in high concentrations in low-Na+ artificial cerebrospinal fluid (ACSF) (Na+ was substituted with either NMDG+ or LiCl) as stock solution and diluted with low-Na+ ACSF to final concentration before experiments.
Slice Preparation
Slices of the frontoparietal cortex were prepared as described in our previous studies (Chao et al. 2007a, 2007b). Transverse cortical slices (400 μm) were cut from the brains of 24 to 32-day-old mice on a vibrotome containing carbogen (95% O2, 5% CO2)-saturated ice-cold standard ACSF. Slices were then transferred to an incubation holder placed in a beaker containing 150 ml ACSF vigorously aerated with carbogen at ∼35 °C. Standard ACSF consisted of (in millimoles / liter) NaCl 125, KCl 3.1, NaHCO3 26, CaCl2 2.4, MgSO4 1.3, NaH2PO4 1.25, and D-glucose 10 at pH 7.4. After an equilibration period of at least 90 min in carbogen-saturated ACSF at ∼35 °C, slices were used for recording. The recordings were made in the outer layer (corresponding to layer II and III) of the cortex.
Induction of Anoxia in Cortical Slices
A slice was transferred to the recording chamber (Model RC-22C, Warner Instrument Co., Hamden, CT), which was perfused with carbogen-saturated ACSF (35.5 ± 0.5 °C) with a flow rate of ∼3 ml/min. Slices were completely submerged 0.5–1 mm below the ACSF surface in the tissue chamber and kept under normoxic conditions for at least 15 min at ∼35.5 °C before experimental measurements were taken.
Anoxia was induced by switching from the control superfusate (95% O2, 5% CO2) to 1 continuously bubbled with 95% N2 and 5% CO2. Each slice was subjected to a single period of anoxia that continued for about 1.5 min after the onset of anoxic depolarization (as assessed by a rapid increase in extracellular [K+] that usually occurs within 10 min after the onset of anoxia) or for a period of 20 min if anoxic depolarization did not occur.
Measurements of Extracellular Potassium
Extracellular K+ concentrations ([K+]e) were measured using K+-sensitive microelectrodes. K+-sensitive microelectrodes were prepared as described previously (Chao et al. 2007a, 2007b). Glass capillary-pulled electrodes were silanized by exposure to N-(trimethylsilyl)dimethylamine and baked at about 180 °C for at least 2 h. The microelectrode tips were then broken back to ∼2 μm. The internal filling solution (10 mM KCl) was injected from the back into the electrode. A column of optimized membrane phase (5% weight valinomycin, 2% potassium tetrakis(p-chlorophenyl)borate, and 93% 2,3-dimethylnitrobenzene, with height about 2 mm) was sucked into the microelectrode tips. The reference electrode was a Ag/AgCl bridge electrode embedded in 2% agar in 3 M KCl. Calibrations were carried out by detecting the responses generated in KCl solutions (1, 3.1, 5, 10, 20, 40, 80, 100, and 160 mM) in triplicate. To keep constant ionic strength in calibration solutions similar to that in interstitial fluid, various concentrations of NaCl were added to the calibration solutions in different potassium concentration. For each concentration, the average of voltage changes in 3 separate tests was used as the final voltage change. Over this range electrode response was near ideal, showing a logarithmic relationship to [K+]. The average slope of K+-sensitive electrodes was 55.08 ± 0.21 mV per log10 unit increase in [K+] at 25 °C (n = 81).
Electrical signals were monitored on an oscilloscope, recorded by a direct current (DC) amplifier (Model IE-210, LPF 200, Warner Instrument Co.), and digitized by an Axon mini-digitizer acquisition system (Model miniDigi 1A, Axon Instruments, Union City, CA) at a sampling rate of 100 Hz. The following parameters were derived to assess K+ homeostasis: 1) the latency of anoxia-induced [K+]e increase (latency), which was defined as a time period from the beginning of anoxia to the time point when anoxia induced a K+ electrode voltage change greater than 1 mV; 2) Maximal [K+]e ([K+]max), which was the peak change in extracellular potassium concentration induced by anoxia; 3) the rate of rise of [K+]e from latency level to peak (noted as latency-max rate of [K+]e), which was the ratio of the [K+]max and the time interval from the latency to the time point of anoxia-induced maximal [K+]e change; and 4) the undershooting of [K+]e (undershoot), which referred to the minimal value of [K+]e during reoxygenation.
After recording of a stable baseline for at least 5 min, the slices were subject to experimental treatments. The electrophysiological recordings were continuously performed at least 75 min. Because K+ homeostasis, including the resting and hypoxia-altered levels of extracellular K+ in the brain slices, has been well described previously (Jiang and Haddad 1991; Műller and Somjen 2000a, 2000b),we focused, in this work, on relative changes in extracellular K+ in cortical slices under stress with/without drug administration with all recordings being performed in the exact same conditions.
Low-Na+ Condition and Drug Administration
Either equimolar NMDG+ or LiCl was used as a substitute to lower Na+ (NaCl) in ACSF to a desired concentration (low-Na+ ACSF). For the former (NMDG+ as a substitute), solution was titrated to pH 7.4 with 10 M HCl.
The low-Na+ condition was provided to cortical slices by switching from standard ACSF to low-Na+ ACSF for 20 min before induction of anoxia and continued to the end of anoxic induction, which was controlled by a 6-channel valve-controlled solution perfusion system (Model VC-6, Warner Instrument Co.). Drugs were applied simultaneously under low-Na+ conditions.
Statistics
All data are expressed as mean ± standard error of the mean and the number of experiments (n) refers to the number of slices investigated. To ensure the independence of data, no more than 3 slices from the same mouse were used in the same experiments. To assess the significance, 2-tailed, unpaired Student's t-test was used for comparison of 2 experimental groups, and 1-way analysis of variance followed by Newman–Keuls test was used for multiple pairwise tests. Changes were identified as significant if the probability value was <0.05.
Results
Effect of Low-Na+ Superfusate Substituted with NMDG+ on Anoxic K+ Derangement
In these experiments, Na+ concentration in the external solution was decreased by substituting it with NMDG+, a membrane impermeable organic cation (Mroz and Lechene 1993). Prior to changing Na+ concentration, control experiments were performed with standard ACSF (Na+ 152.25 mM) (control, n = 11). Anoxia induced a dramatic K+ derangement characterized by an abrupt and large increase in extracellular potassium within 10 min of anoxia with an undershoot during reoxygenation (Fig. 1), which was the same as in our previous observations (Chao et al. 2007a, 2007b).
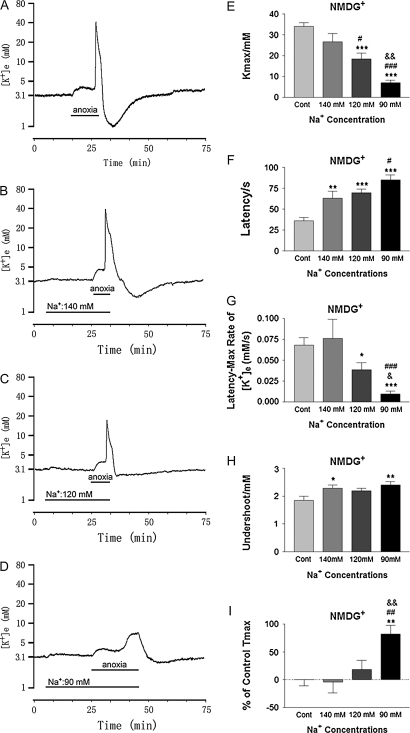
Figure 1.
Anoxic potassium derangement in different concentrations of external [Na+] substituted with NMDG+. Trace recordings of (A): Control (Cont), (B): [Na+] in 140 mM; (C): [Na+] in 120 mM; (D): [Na+] in 90 mM. (E–J) are statistical results of each recording parameter. *P < 0.05, **P < 0.01, ***P < 0.001 versus Cont; #P < 0.05, ##P < 0.01, ###P < 0.001 versus [Na+] in 140 mM; &P < 0.05, &&P < 0.01 versus [Na+] in 120 mM. Note that decrease in external [Na+] attenuated the anoxia-induced increase in [K+]e with the latency of response to anoxia elongated in a concentration-dependent manner.
To investigate the contribution of extracellular Na+-based neuronal factors to the anoxia-induced K+ derangement, we perfused the slices with ACSF containing different Na+ concentrations starting 20 min before induction of anoxia and continuing to the end of anoxic induction. As shown in Figure 1, lowering [Na+] from 152.25 to 140 mM significantly prolonged the latency of response to anoxia (P < 0.01) and decreased potassium undershoot (by 24.0 ± 6.3%) during reoxygenation (P < 0.05) in comparison to control (n = 10). There was no significant change in maximal [K+]e and the rate of rise of [K+]e in this condition (Fig. 1). When external Na+ was reduced to 120 and 90 mM (substituted with 32.25 mM and 62.25 mM NMDG+, respectively), most of parameters used for the assessment of K+ derangement were significantly changed (Fig. 1). In 120 mM of [Na+], for example, the anoxic increase in [K+]max was attenuated by 45.6 ± 7.9% (P < 0.001 vs. control), the latency of response to anoxia was prolonged by 92.8 ± 12.6% (P < 0.001), and the rate of rise of [K+]e from latency to peak decreased by 43.4 ± 12.1% (P < 0.05) (n = 15) (Fig. 1). Moreover, the undershoot tended to decrease (Fig. 1) though the difference was not statistically significant yet (P = 0.06) as compared with control. At 90 mM of external Na+, the anoxic responses were further attenuated with all 4 parameters being changed significantly (Fig. 1). Anoxic increase in [K+]max decreased from 33.97 ± 1.85 mM in the control to 7.10 ± 1.09 mM in 90 mM of external Na+ (P < 0.001), with a significantly prolonged latency of response to anoxia (85 ± 6 s vs. 36 ± 4 s in the control, P < 0.001) and a significant decrease in the rate of rise of [K+]e from latency to peak (0.009 ± 0.004 mM/s vs. 0.068 ± 0.009 mM/s in the control, P < 0.001) (n = 14). The potassium undershoot also decreased significantly during reoxygenation (P < 0.01) (n = 14). In addition, a significant delay in peak increase in [K+]e (% of control Tmax) was observed in the group of 90 mM external Na+ (Fig. 1I) (n = 14). In general, there was a concentration-dependent attenuation of anoxic K+ derangement in response to lowering Na+ concentration (Fig. 1), suggesting that external Na+ plays a critical role in anoxic K+ derangement.
Effect of Low-Na+ Superfusate Substituted with Li+ on Anoxic K+ Derangement
Because the impermeability of NMDG+ may influence Na+-mediated action potential, we applied another widely used substitute of Na+, Li+ that is permeable through Na+ channels nearly as well as Na+ itself (Hille 1972; Nikolakopoulos et al. 1998; Franceschetti et al. 2003; Nikolaeva et al. 2005), to explore whether Li+ has the same effect on anoxic K+ derangement as NMDG+ substitution of external Na+.
When the slices were perfused with ACSF containing 120 mM Na+ and supplemented with 32.25 mM of LiCl for 20 min before anoxia, [K+]e showed a slight increase from around 3.1 mM to 4.42 ± 0.16 mM (range from 3.81 to 5.58 mM) within the 1st 5 min (n = 11) in normoxia. The [K+]e then stayed at this level or tended to return to normal with the continuous perfusion of ACSF containing Li+ and low Na+ during the remaining perfusion period (n = 11) (Fig. 2B). This phenomenon was not seen in most of the slices studied with ACSF containing Na+ substituted by NMDG+.
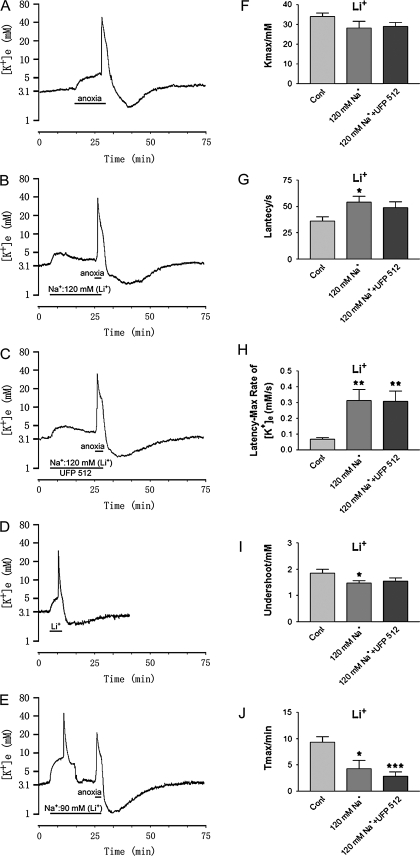
Figure 2.
Effect of lowering external [Na+] by substituting with Li+ on anoxia-induced K+ derangement. Trace recordings of (A) Control (Cont), (B) [Na+] in 120 mM; (C) [Na+] in 120 mM + UFP 512 (1 μM); (D) Li+ (62.5 mM)-evoked change in K+ activities under low external Na+ condition (90 mM) in ACSF; (E) anoxic change in K+ activities under low-Na+ condition (90 mM) in ACSF. (F–I) are statistical results of each recording parameter. *P < 0.05, **P < 0.01, ***P < 0.001 versus Cont. Note that perfusion with low-Na+ solution by substitution with Li+ induced an increase in [K+]e, particularly in 90 mM Na+ with 62.25 mM Li+. Perfusion of 120 mM Na+ ACSF with UFP 512 (1 μM) had no appreciable effect on anoxic K+ derangement in cortical slices.
Under perfusion with the Li+-substituted low-Na+ solution, a short period of anoxia (4.3 ± 1.6 min vs. 9.3 ± 1.1 min of anoxia in normal Na+ concentration, P < 0.05) was sufficient to induce a major increase in extracellular K+ with a prolongation of the latency of response to anoxia (P < 0.05, n = 11) (Fig. 2). However, the anoxia-induced increase in maximal [K+]e was not significantly different from that of the control (P > 0.05, n = 11). Also, both the rate of rise of [K+]e to peak and the undershoot during reoxygenation were not attenuated at all by the low-Na+ perfusion. Instead, they significantly increased (P < 0.01 vs. control; P < 0.05 vs. control, respectively, n = 11) (Fig. 2).
Further reduction of external Na+ to 90 mM by substitution with 62.25 mM Li+, led to a significant increase in [K+]e in normoxia in all 7 slices studied (Fig. 2D). Within 4.2 ± 0.6 min [K+]max was 29.90 ± 3.39 mM and the undershoot was 1.98 ± 0.10 mM in recovery from Li+-induced change in extracellular activity (n = 7), similar to the changes induced by anoxia. After at least 30-min recovery in normoxia and perfusing the slices with carbongen-saturated standard ACSF, the Li+-substituted low-Na+ ACSF repeatedly induced the same response (Fig. 2E). With this low-Na+ ACSF, anoxia induced an increase in [K+]e within 2 min (Fig. 2E). Because of incomplete recovery from the Li+-induced K+ disruption during 20-min perfusion (90 mM Na+ plus 62.25 mM Li+), it was extremely difficult, if not impossible, to determine the accurate changes in the anoxia-induced increase in [K+]e, which varied greatly and it was impracticable to be analyzed quantitatively (n = 7).
Different Effects of NMDG+ and Li+ Substitutes on Anoxic K+ Derangement
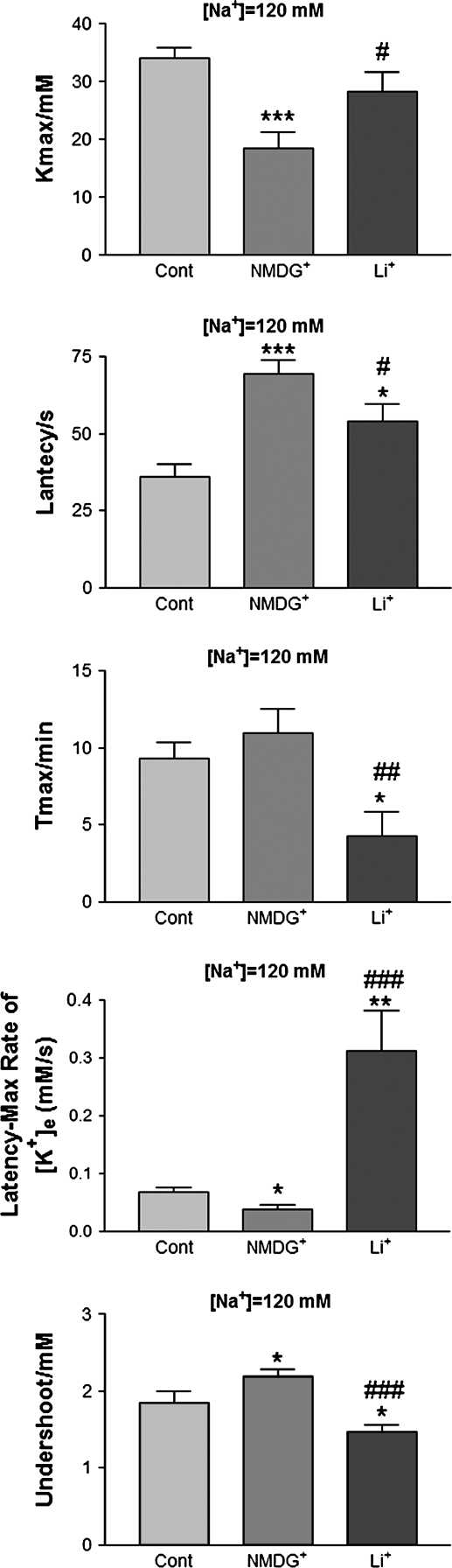
Because NMDG+ and Li+ as substitutes of external Na+ had different effects on the K+ derangement in cortical slices, it is important to characterize their differences in response to anoxic stress. Therefore, we quantitatively compared the effects of NMDG+ and Li+ in 120 mM Na+ ACSF in which the slices had a measurable K+ response to anoxia. As seen in Figure 3, substituting Na+ with NMDG+ greatly attenuated the anoxia-induced increase in peak [K+]e (P < 0.001), the rate of rise of [K+]e to peak (P < 0.05), and undershoot of potassium during reoxygenation (P < 0.05) (n = 15). In sharp contrast, substituting Na+ with Li+ did not significantly affect the anoxia-induced increase in peak [K+]e (P > 0.05 vs. control, P < 0.01 vs. NMDG+). Both the anoxia-induced increase in the rate of rise of [K+]e to peak and potassium undershoot during reoxygenation were not attenuated at all (n = 11). Instead, they were potentiated by the Li+-substituted low-Na+ perfusion (P < 0.01 vs. control, P < 0.001 vs. NMDG+; P < 0.05 vs. control, P < 0.001 vs. NMDG+, respectively) (n = 11) (Fig. 3). Furthermore, the anoxic peak [K+]e was observed about 2.2 times earlier in the Li+-substituted low-Na+ perfusion than in the NMDG+-substituted low-Na+ perfusion (P <0.05 vs. control, P < 0.01 vs. NMDG+) (Fig. 3). The only similarity between the NMDG+ and Li+ substitution was that they both prolonged the latency of response to anoxia (P < 0.001, NMDG+ vs. control and P < 0.05, Li+ vs. control). However, the prolongation of the latency of response to anoxia was more pronounced in the case of NMDG+ (n = 15) than Li+ (n = 11) (P < 0.05). These findings indicate that substituting Na+ with NMDG+ seemed to attenuate anoxic K+ derangement, whereas substituting Na+ with Li+ tended to potentiate the anoxic K+ derangement in cortical slices.
Figure 3.
Substituting external [Na+] with NMDG+ and Li+ has different effects on anoxia-induced K+ derangement. *P < 0.05, **P < 0.01, ***P < 0.001 versus Cont; #P < 0.05, ##P < 0.01, ###P < 0.001 versus NMDG+. Note that substituting Na+ with NMDG+ greatly attenuated the anoxia-induced increase in peak [K+]e, the rate of rise of [K+]e to peak, and undershoot of potassium during reoxygenation, whereas low-Na+ perfusion by substituting with Li+ potentiated anoxia-induced increase in the rate of rise of [K+]e to peak and potassium undershoot during reoxygenation.
Effect of DOR Activation on Anoxic K+ Derangement under Low-Na+ Perfusion
We have recently shown that DOR activation stabilizes the anoxia-induced disruption of K+ homeostasis in cortical slices at “normal” Na+ concentration (Chao et al. 2007a, 2007b). To determine whether DOR activation has the same effect in a low-Na+ condition, we applied UFP 512 (1 μM), a specific and potent DOR agonist (Balboni et al. 2002; Chao et al. 2007a), to cortical slices concurrently with perfusion of ACSF with 120 mM Na+ substituted with either NMDG+ or Li+.
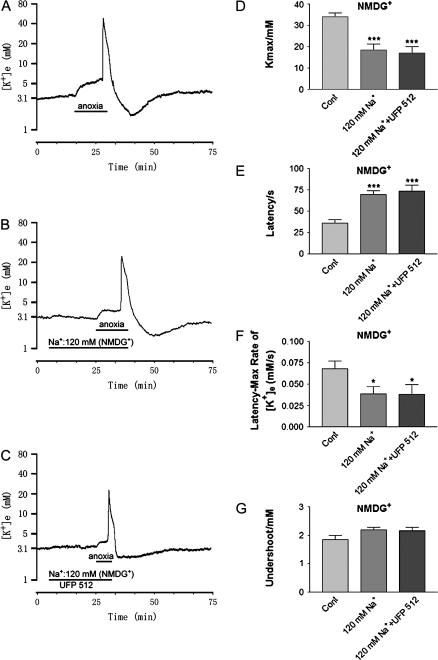
One μM of UFP 512, which significantly attenuates the anoxia-induced disruption of K+ homeostasis in normal Na+ ACSF (Chao et al. 2007a), reduced neither the anoxic increase in [K+]max nor the rate of rise of [K+]e to peak in both NMDG+ and Li+ -substituted perfusions (n = 13, P > 0.05 either NMDG+ or Li+-substituted 120 mM Na+ solution plus UFP 512 vs. 120 mM Na+ alone) (Figs. 2F and 4D). The latency of response to anoxia was not significantly prolonged (Figs. 2G and 4E) and occurrence of peak K+ increase (Tmax) was not delayed in perfusions of 120 mM Na+ ACSF with UFP 512 (1 μM) (P > 0.05 either NMDG+- or Li+-substituted 120 mM Na+ solution plus UFP 512 vs. 120 mM Na+ alone) (n = 13, for both NMDG+- and Li+-substituted low-Na+ solution). Also, DOR activation had no appreciable effect on the potassium undershoot with perfusion of ACSF with 120 mM Na+ substituted with either NMDG+ or Li+ during reoxygenation. (Figs. 2I and 4G).
Figure 4.
Effect of DOR agonist UFP 512 on anoxic K+ derangement in low external Na+ (120 mM) condition substituted with NMDG+. Trace recordings of (A) control (Cont), (B) [Na+] in 120 mM; (C) [Na+] in 120 mM + UFP 512 (1 μM). (D–F) are statistical results of each recording parameter. *P < 0.05, ***P < 0.001 versus Cont. Note that perfusions of 120 mM Na+ ACSF with UFP 512 (1 μM) had no appreciable effect on anoxic K+ derangement in cortical slices.
These data suggest that the DOR-induced protection against the anoxic K+ responses in cortical slices was largely abolished by low-Na+ perfusion.
Discussion
The major findings of the present work are that in mouse cortical slices, 1) there was a concentration-dependent attenuation of anoxic K+ derangement in response to lowering Na+ concentration by substitution with NMDG+; 2) lowering Na+ concentration by substitution with NMDG+ attenuated anoxic K+ derangement, whereas lowering Na+ concentration by substitution with Li+ tended to potentiate the anoxic K+ derangement; and 3) DOR-induced protection against the anoxic K+ responses was largely abolished by low-Na+ perfusion irrespective of substituting ion.
Anoxic Disruption of K+ Homeostasis and Neuronal Injury
Neuronal function is critically dependent on maintenance of the electrochemical distribution of ions (e.g., Na+, K+, Ca2+, and Cl−) across the membrane. A disruption of cellular ionic environment is considered to be a crucial event in the pathophysiology of brain ischemia/hypoxia (Hansen 1985). There are considerably longer or sustained alterations in ionic concentrations in neurons, including enhanced Na+ influx and K+ efflux (Jiang and Haddad 1991; Friedman and Haddad 1994a; Calabresi et al. 1999; Müller and Somjen 2000a, 2000b; Martinez-Sanchez et al. 2004; Sheldon et al. 2004). Despite the well-established role of Na+ and Ca2+ (Friedman and Haddad 1994b; Probert et al. 1997; Hasbani et al. 1998; Breder et al. 2000), K+ efflux also plays an important role in the neuronal death that occurs in various conditions including hypoxia/ischemia (Yu et al. 1997, 1999; Yu 2003; Huang et al. 2001; Liu et al. 2003; Wei et al. 2003). For instance, activation of ionotropic glutamate receptors, even when inward cation influx is decreased by lowering extracellular Na+ and Ca2+, induced a large outward K+ current; this caused loss of about 50–80% intraneuronal K+ and led to great shrinkage of cell body and consequently neuronal apoptosis (Yu et al. 1999; Xiao et al. 2001). Blockade of excessive K+ efflux through K+ channels and from the intracellular compartment attenuates hypoxia- and ischemia-induced neuronal death (Huang et al. 2001; Liu et al. 2003; Wei et al. 2003), suggesting that inhibition of K+ fluxes and maintaining cellular K+ homeostasis may be of therapeutic benefit in the treatment of stroke and related neurodegenerative conditions (Liu et al. 2003; Wei et al. 2003). Therefore, the DOR attenuation of K+ leakage is a protective strategy against neuronal injury in the cortex.
The Role of External Na+ in Anoxic K+ Derangement
Na+ is the predominant ion in the extracellular space. The changes in extracellular Na+, [Na+]o, widely affect cellular function, for example, neuronal excitability, intracellular Ca2+ homeostasis, pH stability, and glutamate uptake by altering the operating mode of Na+/Ca2+ exchange, Na+/H+ exchange, and Na+-glutamate cotransport during hypoxic/ischemic stress (Calabresi et al. 1999; Zhang and Lipton 1999; Sheldon and Church 2004; Nikolaeva et al. 2005; Camacho and Massieu 2006; Kiedrowski 2007; Rojas et al. 2007). It is, however, unclear whether it affects K+ homeostasis during hypoxia/ischemia. In hippocampal slices, the Na+ channel blocker tetrodotoxin (TTX) postponed hypoxic depolarization but only slightly decreased sharp changes in extracellular K+ and Na+ (Müller and Somjen 2000a, 2000b). In the present study, we showed that lowering Na+ concentration by substituting with NMDG+ attenuated anoxic K+ derangement, whereas lowering Na+ concentration by substituting with Li+ tended to potentiate the anoxic K+ derangement in cortical slices. These results suggest that external Na+ indeed affects anoxic K+ derangement. In dopaminergic neurons of rat substantia nigra, brief hypoxia induces intracellular Na+ rise and an outward K+ current, which can be depressed by lowering extracellular Na+ concentration by substituting with choline chloride (Guatteo et al. 1998b), another widely used membrane impermeable cation (Franceschetti et al. 2003). With 90 mM Na+ ACSF perfusion in our study, the reduced magnitude in [K+]e (46%) and delayed onset of the abrupt sharp [K+]e rise (2.2-fold delay) during anoxia are also parallel to the results of Müller and Somjen (2000a), in which they showed a 2.4-fold delay of onset and 42% reduction in magnitude of anoxia-induced extracellular DC potential shift (which is closely related to anoxic [K+]e increase, see Hansen 1985, Chao et al. 2007b) in hippocampal slices perfused with NMDG+-substituted 90 mM Na+ solution. Taken together, our observations on the NMDG+-substituted low-Na+ ACSF suggest that lowering [Na+]o and subsequently decreasing Na+ entry attenuate anoxic/ischemic K+ derangement and subsequent neuronal death. In support of this idea is the findings that blocking sodium channels with TTX or lidocaine greatly attenuates hypoxic/ischemic intracellular Na+ increase and K+ loss in hippocampal and hypoglossal neurons (Jiang and Haddad 1991; Lopachin et al. 2001; Raley-Susman et al. 2001) as well as prevents hypoxia/ischemia-induced neuronal injury and cell death (Friedman and Haddad 1994b; Probert et al. 1997; Banasiak et al. 2004). Although there may be differences among neuronal subtypes in terms of the effect of altered extracellular/intracellular Na+ on activities of Na+/Ca2+, Na+/H+ exchange and Na+-glutamate cotransport during hypoxia (Calabresi et al. 1999; Zhang and Lipton 1999; Sheldon and Church 2004; Nikolaeva et al. 2005; Camacho and Massieu 2006; Kiedrowski 2007; Rojas et al. 2007), our results demonstrate that lowering Na+ concentration by substitution with NMDG+ attenuated anoxic K+ derangement in the cortex.
The increase in extracellular K+ could be the result of increased K+ efflux, decreased K+ influx, or both. The anoxic increase in [K+]e is, to a great extent, secondary to neuronal loss of intracellular K+ (Jiang and Haddad 1991). The proposed mechanisms of contribution to the anoxia-induced K+ efflux from neurons include an increase in K+ leakage due to increased excitability and action potential formation, inhibition of Na+–K+ adenosine triphosphatase (ATPase), and activation of KATP channels, BK channels, and Na+-activated K+ channels (KNa channels) (Jiang and Haddad 1991; Reid and Paterson 1996; Murai et al. 1997; Erdemli et al. 1998; Lopachin et al. 2001, Bhattacharjee and Kaczmarek 2005; Chao et al. 2007a). NMDG+ is a membrane impermeable organic cation. Lowering external Na+ with NMDG+ replacement may reduce Na+ influx and lower neuronal excitability because of a reduction of driving force for inward sodium current during anoxia (Calabresi et al. 1999; Sheldon et al. 2004). As a result, it may 1) inhibit K+ leakage via Na+-activated K+ channels (Franceschetti et al. 2003); 2) attenuate anoxic increase in excitability and action potential; 3) reduce energy consumption for the maintenance of Na+ and K+ equilibriums; 4) delay or minimize the opening of KATP channels; and 5) decrease glutamate release from reversed glutamate transports and presynaptic membrane (Camacho and Massieu 2006; Rojas et al. 2007), all of which contribute to K+ efflux in pathophysiological conditions such as hypoxia/ischemia (Yu et al. 1997, 1999; Műller and Somjen 2000a, 2000b; Lopachin et al. 2001; Raley-Susman et al. 2001; Xiao et al. 2001; Bhattacharjee and Kaczmarek 2005). Indeed, it has been demonstrated that when Na+ is replaced with membrane impermeable reagents (e.g., NMDG+), neuronal membrane becomes less depolarized during ischemia and hyperpolarized in N-methyl-D-aspartate (NMDA) receptor activation and the glutamate-elicited K+ efflux occurs at a much lower rate and only in the presence of Ca2+ (Calabresi et al. 1999; Kiedrowski 1999).
Replacing external Na+ with Li+ induced a substantially different effect on anoxia-induced K+ derangement. Chemically, Li+, unlike NMDG+, is permeable across the membrane via multiple transport pathways, in particular the sodium channels through which Li+ passes nearly as well as Na+ itself (Hille 1972; Hemsworth et al. 1997; Nikolakopoulos et al. 1998; Franceschetti et al. 2003; Montezinho et al. 2004; Nikolaeva et al. 2005). Because of a reduction in driving force for inward sodium current, lowering external Na+ and substitution with Li+ may reduce anoxia-induced Na+ influx, similar to that with NMDG+. This may greatly attenuate the increase in KNa channel activity. Indeed, it has been demonstrated that Li+ has no direct effect on K+ channels activated by increased intracellular Na+ concentration (Bischoff et al. 1998; Franceschetti et al. 2003) although it can permeate Na+ channels (Hille 1972; Nikolakopoulos et al. 1998; Franceschetti et al. 2003; Nikolaeva et al. 2005). However, Li+ gradually depolarizes membrane potential (Grafe et al. 1983; Kiedrowski 1999; Franceschetti et al. 2003), broadens action potential (Mayer et al. 1984; Colino et al. 1998), positively shifts afterpotential (Franceschetti et al. 2003; Liu and Leung 2004), and progressively potentiates the high-frequency firing (Franceschetti et al. 2003). Moreover, Li+ also presynaptically enhances excitatory synaptic transmission (Grafe et al. 1983; Evans et al. 1990; Colino et al. 1998). All these effects could lead to an increase in K+ efflux, which counteracts the potential decrease in Na+-dependent K+ efflux (through KNa channels and by ATP depletion-induced inhibition of Na+–K+ ATPase) with low external Na+ during hypoxia. All these mechanisms can therefore explain the net K+ efflux increase in response to anoxia under low-Na+ condition with Li+ substitution.
The Li+-evoked increase in [K+]e during normoxia is very likely caused solely by Li+ influx through Na+ channels and/or the “side effect” of Li+ ions on the electrogenic Na+–K+ pump under resting condition (Grafe et al. 1983). The physiochemical properties of Li+ and Na+ are similar but the atomic radius of Li+ is less than that of Na+ (Page and Di Cera 2006), which favors Li+ entry. Therefore, under resting conditions, Li+ can easily enter neurons within 1 min after reaching the extracellular membrane surface, depolarize membrane potential, and decrease intracellular Na+ concentration (Thomas et al. 1975; Grafe et al. 1982; Nikolakopoulos et al. 1998). As a result, the activity of the Na+–K+ pump is decreased by less intracellular Na+ stimulation, and consequently, [K+]e increases.
Ionic Mechanism Underlying the DOR Protection against Anoxic K+ Derangement
We have shown that in the presence of standard ACSF, DOR activation greatly attenuates anoxia/oxygen–glucose deprivation-induced disruption of K+ homeostasis in the cortical slices (Chao et al. 2006, 2007a, 2007b). In sharp contrast, DOR had little effect on anoxic K+ derangement when external Na+ was lowered by substitution with either Li+ or NMDG+ as shown in the present work, suggesting that the DOR activation may target Na+-based mechanisms to protect the cortex against anoxic K+ derangement.
A potential mechanism that could explain our finding is that DOR may inhibit excessive Na+ entry and thus reduce cellular excitability and action potential during anoxia. Indeed, electrophysiological evidence has suggested that activation of DOR depresses both spontaneous and stimulus-evoked action potential discharge as well as the amplitudes of stimulus-evoked excitatory postsynaptic potentials/currents of neocortical neurons (Stanzione et al. 1989; Tanaka and North 1994; Ostermeier et al. 2000). Such depression may decrease K+ leakage because of a decrease in the Na+-based action potentials (Műller and Somjen 2000a, 2000b; Raley-Susman et al. 2001; Lopachin et al. 2001). The reason that DOR could not attenuate anoxic K+ derangement in low Na+ is possibly because low-Na+ perfusion actually mimics the effect of DOR activation, rendering the anoxia-induced Na+ influx much less than in the normal Na+ condition. Therefore, DOR signals do not further inhibit Na+ entry in the condition without excessive Na+ influx. This mechanism is supported by our findings with NMDG+, which show that reduction of Na+ entry with impermeable cation NMDG+ decreased anoxic K+ derangement, whereas DOR activation no longer attenuated the anoxic K+ derangement in this condition. Moreover, DOR activation could not induce any inhibitory effect on anoxic K+ derangement in the condition of low Na+ with Li+ substitution although Li+, unlike NMDG+, promotes the generation of action potential (Grafe et al. 1983; Kiedrowski 1999; Franceschetti et al. 2003). This is very likely because DOR signals target excessive Na+ but not Li+ influx. This conclusion was further supported by our recent observations showing that blocking Na+ entry through Na+ channels and glutaminergic NMDA receptors abolished DOR attenuation on anoxic K+ derangement.
The DOR-mediated reduction of Na+ entry might lead to an attenuation of KNa channel activity and other Na+-activated mechanisms. When these Na+-based mechanisms are inhibited, such as in low Na+ with NMDG+ substitution, DOR signals could not further reduce anoxic K+ derangement. The observations on the Li+ substitution lend more support for this possibility. Although DOR regulates the release and uptake of glutamate, thereby reducing glutamate excitability (Tanaka and North 1994; Ostermeier et al. 2000; Xia et al. 2006), it did not reduce anoxic K+ derangement in low Na+ by substitution with Li+ that potentiates excitatory synaptic transmission (Grafe et al. 1983; Evans et al. 1990; Colino et al. 1998). This is likely because the anoxic K+ derangement in low Na+ by substitution with Li+ relies more on other mechanisms and not on excessive Na+ entry. Furthermore, we observed that the DOR protection from anoxic K+ derangement is not affected by blocking the KATP channels (Chao et al. 2007a) in spite of the fact that KATP channels also play an important role in anoxic K+ leakage (Jiang and Haddad 1991; Reid and Paterson 1996; Guatteo et al. 1998a). All these observations prompt us to conclude that the DOR-mediated inhibition on Na+ entry and subsequent Na+-triggered activities constitute a major mechanism underlying the DOR protection against anoxic K+ derangement in the cortex though we cannot rule out other possibilities at this stage.
In terms of the subsequent Na+-triggered activities, it is unclear which cellular activities were affected by DOR activation during the attenuation of anoxic K+ derangement. However, we have a clue showing that DOR activation may also reduce Ca2+ influx for the attenuation of anoxic K+ derangement (Chao et al. 2007a). Indeed, anoxia/ischemia triggers a great amount of Na+ and Ca2+ influxes, whereas DOR activation inhibits both Ca2+ current (Toselli et al. 1997, 1999; Adams and Trequattrini 1998; Acosta and López 1999) and Na+ current (Kang XZ, Gu QB, Ding GH, Wang YW, Xia Y., unpublished data). Because Na+ influx plays a prominent role in the ischemia-induced depolarization (Calabresi et al. 1999) and triggers cytosolic [Ca2+] elevations in neurons (Friedman and Haddad 1993; Zhang and Lipton 1999; Nikolaeva et al. 2005; Kiedrowski 2007), the anoxia-induced increase in Na+ influx may serve as an upstream signal for the elevation of cytosolic Ca2+ in the cortex and DOR activation may inhibit the ionic reactions in series.
In summary, external Na+ concentration greatly influenced anoxia-induced disruption of K+ homeostasis with NMDG+ and Li+, as impermeable and permeable Na+ substitutes, respectively, having different effects. The DOR-induced protection against anoxic disruption of K+ homeostasis was largely abolished by low-Na+ perfusion, either with NMDG+ or Li+ substitution. We conclude that DOR activation can attenuate anoxic K+ derangement induced by Na+-activated mechanisms.
Funding
National Institutes of Health (HD-34852) and American Heart Association (0755993T) to Y.X.; University of Cagliari grant to G.B.
Acknowledgments
Conflict of Interest: None declared.
References
- Acosta CG, López HS. δ opioid receptor modulation of several voltage-dependent Ca2+ currents in rat sensory neurons. J Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DJ, Trequattrini C. Opioid receptor-mediated inhibition of ω-conotoxin GVIA-sensitive calcium channel currents in rat intracardiac neurons. J Neurophysiol. 1998;79:753–762. doi: 10.1152/jn.1998.79.2.753. [DOI] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bischoff U, Vogel W, Safronov BV. Na+-activated K+ channels in small dorsal root ganglion neurons of rat. J Physiol. 1998;510:743–754. doi: 10.1111/j.1469-7793.1998.743bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: linking hibernation and neuroprotection. Front Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- Breder J, Sabelhaus CF, Opitz T, Reymann KG, Schrőder UH. Inhibition of different pathways influencing Na+ homeostasis protects organotypic hippocampal slice cultures from hypoxic/hypoglycemic injury. Neuropharmacology. 2000;39:1779–1787. doi: 10.1016/s0028-3908(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Marfia GA, Centonze D, Pisani A, Bernardi G. Sodium influx plays a major role in the membrane depolarization induced by oxygen and glucose deprivation in rat striatal spiny neurons. Stroke. 1999;30:171–179. doi: 10.1161/01.str.30.1.171. [DOI] [PubMed] [Google Scholar]
- Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37:11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Chao D, Bazzy-Asaad A, Balboni G, Xia Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007a;212:60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- Chao D, Donnelly D, Feng Y, Bazzy-Asaad A, Xia Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2007b;27:356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- Chao D, Qian H, Ghassemi F, Chen J, Xia Y. Transgenic overexpression of delta-opioid receptor protects the cortex from anoxic disruption of ionic homeostasis. 2006 [Internet]. Program No. 87.19/MM68, 2006 Neuroscience Meeting Planner, Society for Neuroscience, 2006 Online [cited 2007 Dec 27]. Available from http://www.abstractsonline.com. [Google Scholar]
- Colino A, García-Seoane JJ, Valentín A. Action potential broadening induced by lithium may cause a presynaptic enhancement of excitatory synaptic transmission in neonatal rat hippocampus. Eur J Neurosci. 1998;10:2433–2443. doi: 10.1046/j.1460-9568.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Erdemli G, Xu YZ, Krnjevic K. Potassium conductance causing hyperpolarization of CA1 hippocampal neurons during hypoxia. J Neurophysiol. 1998;80:2378–2390. doi: 10.1152/jn.1998.80.5.2378. [DOI] [PubMed] [Google Scholar]
- Evans MS, Zorumski CF, Clifford DB. Lithium enhances neuronal muscarinic excitation by presynaptic facilitation. Neuroscience. 1990;38:457–468. doi: 10.1016/0306-4522(90)90042-3. [DOI] [PubMed] [Google Scholar]
- Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, Avanzini G, Magistretti J. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol. 2003;89:2101–2111. doi: 10.1152/jn.00695.2002. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Haddad GG. Major differences in Ca2+i response to anoxia between neonatal and adult rat CA1 neurons: role of Ca2+o and Na+o. J Neurosci. 1993;13:63–72. doi: 10.1523/JNEUROSCI.13-01-00063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res. 1994a;663:329–334. doi: 10.1016/0006-8993(94)91281-5. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Haddad GG. Removal of extracellular sodium prevents anoxia-induced injury in freshly dissociated rat CA1 hippocampal neurons. Brain Res. 1994b;641:57–64. doi: 10.1016/0006-8993(94)91815-5. [DOI] [PubMed] [Google Scholar]
- Grafe P, Reddy MM, Emmert H, Ten Bruggencate G. Effects of lithium on electrical activity and potassium ion distribution in the vertebrate central nervous system. Brain Res. 1983;279:65–76. doi: 10.1016/0006-8993(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Grafe P, Rimpel J, Reddy MM, Ten Bruggencate G. Lithium distribution across the membrane of motoneurons in the isolated frog spinal cord. Pflügers Arch. 1982;393:297–301. doi: 10.1007/BF00581413. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Federici M, Siniscalchi A, Knőpfel T, Mercuri NB, Bernardi G. Whole cell patch-clamp recordings of rat midbrain dopaminergic neurons isolate a sulphonylurea- and ATP-sensitive component of potassium currents activated by hypoxia. J Neurophysiol. 1998a;79:1239–1245. doi: 10.1152/jn.1998.79.3.1239. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knöpfel T. Intracellular sodium and calcium homeostasis during hypoxia in dopamine neurons of rat substantia nigra pars compacta. J Neurophysiol. 1998b;80:2237–2243. doi: 10.1152/jn.1998.80.5.2237. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- Hemsworth PD, Whalley DW, Rasmussen HH. Electrogenic Li+/Li+ exchange mediated by the Na+-K+ pump in rabbit cardiac myocytes. Am J Physiol. 1997;272:C1186–C1192. doi: 10.1152/ajpcell.1997.272.4.C1186. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972;59:637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gao TM, Gong LW, Zhuang ZY, Li X. Potassium channel blocker TEA prevents CA1 hippocampal injury following transient forebrain ischemia in adult rats. Neurosci Lett. 2001;305:83–86. doi: 10.1016/s0304-3940(01)01821-3. [DOI] [PubMed] [Google Scholar]
- Jiang C, Haddad GG. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991;66:103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. N-methyl-D-aspartate excitotoxicity: relationships among plasma membrane potential, Na+/Ca2+ exchange, mitochondrial Ca2+ overload, and cytoplasmic concentrations of Ca2+, H+, and K+ Mol Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. Critical role of sodium in cytosolic [Ca2+] elevations in cultured hippocampal CA1 neurons during anoxic depolarization. J Neurochem. 2007;100:915–923. doi: 10.1111/j.1471-4159.2006.04308.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honoré E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis: role of TASK leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Lim YJ, Zheng S, Zuo Z. Morphine preconditions Purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004;100:562–568. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmer E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Leung LS. Sodium-activated potassium conductance participates in the depolarizing afterpotential following a single action potential in rat hippocampal CA1 pyramidal cells. Brain Res. 2004;1023:185–192. doi: 10.1016/j.brainres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lopachin RM, Gaughan CL, Lehning EJ, Weber ML, Taylor CP. Effects of ion channel blockade on the distribution of Na, K, Ca, and other elements in oxygen-glucose deprived CA1 hippocampal neurons. Neuroscience. 2001;103:971–983. doi: 10.1016/s0306-4522(01)00035-5. [DOI] [PubMed] [Google Scholar]
- Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen sensitive δ-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchez M, Striggow F, Schroder UH, Kahlert S, Reymann KG, Reiser G. Na+ and Ca2+ homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience. 2004;128:729–740. doi: 10.1016/j.neuroscience.2004.06.074. [DOI] [PubMed] [Google Scholar]
- Mayer M, Crunelli V, Kemp JA. Lithium ions increase action potential duration of mammalian neurons. Brain Res. 1984;293:173–177. doi: 10.1016/0006-8993(84)91466-5. [DOI] [PubMed] [Google Scholar]
- Montezinho LP, Duarte CB, Fonseca CP, Glinka Y, Layden B, Mota de Freitas D, Geraldes CFGC, Castro MMCA. Intracellular lithium and cyclic AMP levels are mutually regulated in neuronal cells. J Neurochem. 2004;90:920–930. doi: 10.1111/j.1471-4159.2004.02551.x. [DOI] [PubMed] [Google Scholar]
- Mroz E, Lechene C. Extracellular N-methyl-D-glucamine leads to loss of hair-cell sodium, potassium, and chloride. Hear Res. 1993;70:146–150. doi: 10.1016/0378-5955(93)90152-q. [DOI] [PubMed] [Google Scholar]
- Műller M, Somjen GG. Na+ dependence and the role of glutamate receptors and Na+ channels in ion fluxes during hypoxia of rat hippocampal slices. J Neurophysiol. 2000a;84:1869–1880. doi: 10.1152/jn.2000.84.4.1869. [DOI] [PubMed] [Google Scholar]
- Műller M, Somjen GG. Na+ and K+ concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. J Neurophysiol. 2000b;83:735–745. doi: 10.1152/jn.2000.83.2.735. [DOI] [PubMed] [Google Scholar]
- Murai Y, Ishibashi H, Koyama S, Akaike N. Ca2+-activated K+ currents in rat locus coeruleus neurons induced by experimental ischemia, anoxia, and hypoglycemia. J Neurophysiol. 1997;78:2674–2681. doi: 10.1152/jn.1997.78.5.2674. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of δ-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci. 2005;25:9960–9967. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakopoulos J, Zachariah C, Mota de Freitas D, Stubbs EB, Jr, Ramasamy R, Castro MMCA, Geraldes CFGC. 7Li nuclear magnetic resonance study for the determination of Li+ properties in neuroblastoma SH-SY5Y cells. J Neurochem. 1998;71:1676–1684. doi: 10.1046/j.1471-4159.1998.71041676.x. [DOI] [PubMed] [Google Scholar]
- Ostermeier AM, Schlosser B, Schwender D, Sutor B. Activation of μ- and δ-opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology. 2000;93:1053–1063. doi: 10.1097/00000542-200010000-00029. [DOI] [PubMed] [Google Scholar]
- Page MJ, Di Cera E. Role of Na+ and K+ in enzyme function. Physiol Rev. 2006;86:1049–1092. doi: 10.1152/physrev.00008.2006. [DOI] [PubMed] [Google Scholar]
- Probert AW, Borosky S, Marcoux FW, Taylor P. Sodium channel modulators prevent oxygen and glucose deprivation injury and glutamate release in rat neocortical cultures. Neuropharmacology. 1997;36:1031–1038. doi: 10.1016/s0028-3908(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J Neurophysiol. 2001;86:2715–2726. doi: 10.1152/jn.2001.86.6.2715. [DOI] [PubMed] [Google Scholar]
- Reid JM, Paterson DJ. Role of K+ in regulating hypoxic cerebral blood flow in the rat: effect of glibenclamide and ouabain. Am J Physiol. 1996;270:H45–H52. doi: 10.1152/ajpheart.1996.270.1.H45. [DOI] [PubMed] [Google Scholar]
- Remillard CV, Yuan JXJ. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca2+i-induced Ca2+ release in rat cerebellar type-1 astrocytes. J Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Church J. Reduced contribution from Na+/H+ exchange to acid extrusion during anoxia in adult rat hippocampal CA1 neurons. J Neurochem. 2004;88:594–603. doi: 10.1046/j.1471-4159.2003.02169.x. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Diarra A, Cheng YM, Church J. Sodium influx pathways during and after anoxia in rat hippocampal neurons. J Neurosci. 2004;24:11057–11069. doi: 10.1523/JNEUROSCI.2829-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzione P, Stefani A, Calabresi P, Mercuri NB, Bernardi G. Met- and leu-enkephalins inhibit rat cortical neurons intracellularly recorded in vivo while morphine excites them: evidence for naloxone-sensitive and naloxone-insensitive effects. Exp Brain Res. 1989;77:302–308. doi: 10.1007/BF00274987. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shibata M, Fukunchi Y. Role of sodium ion influx in depolarization-induced neuronal cell death by high KCl or veratridine. Eur J Pharmacol. 1999;372:297–304. doi: 10.1016/s0014-2999(99)00208-3. [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA. Opioid actions on rat anterior cingulated cortex neurons in vitro. J Neurosci. 1994;14:1106–1113. doi: 10.1523/JNEUROSCI.14-03-01106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC, Simon W, Oehme M. Lithium accumulation by snail neurons measured by a new Li+-sensitive microelectrode. Nature. 1975;258:754–756. doi: 10.1038/258754a0. [DOI] [PubMed] [Google Scholar]
- Toselli M, Tosetti P, Taglietti V. μ and δ opioid receptor activation inhibits ω-conotoxin-sensitive calcium channels in a voltage- and time-dependent mode in the human neuroblastoma cell line SH-SY5Y. Pflügers Arch. 1997;433:587–596. doi: 10.1007/s004240050318. [DOI] [PubMed] [Google Scholar]
- Toselli M, Tosetti P, Taglietti V. Kinetic study of N-type calcium current modulation by δ-opioid receptor activation in the mammalian cell line NG108-15. Biophys J. 1999;76:2560–2574. doi: 10.1016/S0006-3495(99)77409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Yu SP, Gottron F, Snider BJ, Zipfei GJ, Choi DW. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- Xia P, Pei G, Schwarz W. Regulation of the glutamate transporter EAAC1 by expression and activation of delta-opioid receptor. Eur J Neurosci. 2006;24:87–93. doi: 10.1111/j.1460-9568.2006.04897.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Cao H, Zhang JH, Chen NY, Siegel K, Agulnik M, Haddad GG. Effect of δ-opioid receptor activation on Na+ channel expression in cortical neurons subjected to prolonged hypoxia in culture. 2001 [Internet]. Program No.740.6 SfN Abstract. Society for Neuroscience Online [cited 2007 Dec 27]. Available from http://sfn.scholarone.com. [Google Scholar]
- Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J Neurophysiol. 2003;89:229–236. doi: 10.1152/jn.00488.2002. [DOI] [PubMed] [Google Scholar]
- Xiao AY, Homma M, Wang XQ, Wang X, Yu SP. Role of K+ efflux in apoptosis induced by AMPA and kainate in mouse cortical neurons. Neuroscience. 2001;108:61–67. doi: 10.1016/s0306-4522(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enchancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Strasser U, Tian M, Choi DW. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284:336–339. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through δ-opioid receptor. Stroke. 2006;37:1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Gibney GT, Zhao P, Xia Y. Neuroprotective role of δ-opioid receptors in the cortical neurons. Am J Physiol. 2002;282:C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Haddad GG, Xia Y. δ-, but not μ- and κ-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885:143–153. doi: 10.1016/s0006-8993(00)02906-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lipton P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Ma MC, Qian H, Xia Y. Down-regulation of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53:442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]