Abstract
The pronounced convolution of the human cortex may be a morphological substrate that supports some of our species’ most distinctive cognitive abilities. Therefore, individual intelligence within humans might be modulated by the degree of folding in certain cortical regions. We applied advanced methods to analyze cortical convolution at high spatial resolution and correlated those measurements with intelligence quotients. Within a large sample of healthy adult subjects (n = 65), we detected the most prominent correlations in the left medial hemisphere. More specifically, intelligence scores were positively associated with the degree of folding in the temporo-occipital lobe, particularly in the outermost section of the posterior cingulate gyrus (retrosplenial areas). Thus, this region might be an important contributor toward individual intelligence, either via modulating pathways to (pre)frontal regions or by serving as a location for the convergence of information. Prominent gender differences within the right frontal cortex were observed; females showed uncorrected significant positive correlations and males showed a nonsignificant trend toward negative correlations. It is possible that formerly described gender differences in regional convolution are associated with differences in the underlying architecture. This might lead to the development of sexually dimorphic information processing strategies and affect the relationship between intelligence and cortical convolution.
Keywords: cortex, curvature, folding, IQ, MRI, sex
Introduction
The search for the biological substrate of cognitive functions and intellectual abilities dates back to the times of ancient Greece. The advent of modern imaging technologies and advanced statistics now provide unique opportunities to unravel the persisting mystery of the essence of human intelligence. To date, empirical evidence from imaging data has confirmed positive associations between general intellectual ability and a number of cerebral characteristics, such as the size of the brain, the volumes of (sub)cortical and cerebellar regions, the thickness of the corpus callosum, the amount of intracranial tissue (e.g., global and regional gray and white matter), as well as the thickness of the cortex (Andreasen et al. 1993; Wickett et al. 1994, 1996, 2000; Reiss et al. 1996; Flashman et al. 1997; Gur et al. 1999; Thompson et al. 2001; Posthuma et al. 2002; MacLullich et al. 2002; Wilke et al. 2003; Frangou et al. 2004; Haier et al. 2004, 2005; McDaniel 2005; Toga and Thompson 2005; Walhovd et al. 2005, 2006; Shaw et al. 2006; Luders et al. 2007; Narr, Woods, et al. 2007).
Another attractive candidate in the quest for brain-anatomical correlates of intelligence is the highly convoluted (gyrencephalic) surface of the cerebral cortex. Over the course of evolution, the cerebral cortex has grown considerably in surface area, which seems to be not only the result of a larger brain but, perhaps more important, of an increased folding of the brain's surface. For example, several studies revealed that larger primate brains are more convoluted than smaller ones (Zilles et al. 1989; Rilling and Insel 1999). In addition, cortical folding was reported to increase more rapidly with brain size among later evolved primates compared with earlier evolved primates (Zilles et al. 1988, 1989). It has been suggested that the high degree of cortical gyrification in humans may be a morphological substrate that supports some of our species’ most distinctive cognitive abilities. Consequently, individual intelligence within the human species might be modulated by the degree of convolution in certain cortical regions.
To our knowledge, this is the 1st study to examine the presence and direction of correlations between intelligence and local cortical convolution. We utilized a recently introduced automated whole-brain method that allows estimating the degree of gyrification across thousands of surface points (Gaser et al. 2006; Luders et al. 2006). Although it was reported that gyrification reaches a stable plateau shortly after birth (Haug 1987; Armstrong et al. 1995; Zilles et al. 1997), conflicting reports exist suggesting age-related increases of cortical complexity in frontal brain regions (Blanton et al. 2001). Reports are similarly controversial as to whether gyrification is different in men and women (Zilles et al. 1988; Nopoulos et al. 2000; Luders et al. 2004, 2006). Consequently, because age and gender may influence the relationships between intelligence and local cortical convolution, we analyzed our data while removing effects associated with age, and additionally explored gender-specific relationships between intelligence and local cortical convolution.
Methods
Subjects
We analyzed the brains of 30 men and 35 women from an overlapping sample of subjects who participated as healthy volunteers for a larger study aimed at examining changes in brain structure in schizophrenia (Narr, Bilder, et al. 2005; Narr, Toga, et al. 2005). Of note, the current sample is identical with the one used by Narr, Woods, et al. (2007) to examine relationships between intelligence and regional cortical gray matter thickness in healthy adults. The numbers of years of education ranged from 11 to 20 years (males: 11–18 years; females: 11–20 years; gender difference; P < 0.97). Age ranged between 16.2 and 44.2 years (males: 16.2–39.9 years; females: 18.6–44.2 years). There were no significant differences in age measurements between males and females. The significance values, as well as group-specific means and standard deviations of all age measures are shown in Table 1. Women were 97% dextral and men were 100% dextral, where handedness information was not available for 4 male subjects. Handedness was determined using a modified 20-item Edinburgh Handedness Inventory (Oldfield 1971), as described previously (Narr, Bilder, et al. 2007). Participants were recruited through local newspaper advertisements and community word of mouth and were determined to have no history of psychiatric illness as assessed by clinical interview using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM [4th edition]-R, nonpatient version (Spitzer et al. 1992). Study exclusion criteria included serious neurological or endocrine disorders, any medical condition or treatment known to affect the brain, or meeting DSM-IV criteria for mental retardation. The North Shore-Long Island Jewish Health System institutional review board (IRB) approved all procedures and informed written consent was obtained from all subjects. Additional approval for image processing and analysis was received from the UCLA IRB.
Table 1.
Group-specific means, standard deviations (SD), and significance values of gender differences in age and intelligence measures
| Age | Full-scale IQ | Performance IQ | Verbal IQ | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Combined sample (n = 65) | 28.2 ± 7.3 | 100.1 ± 12.7 | 99.9 ± 12.5 | 100.9 ± 13.4 |
| Males (n = 30) | 27.9 ± 7.1 | 100.3 ± 11.7 | 98.2 ± 13.0 | 101.9 ± 10.9 |
| Females (n = 35) | 28.5 ± 7.5 | 100.0 ± 13.7 | 101.3 ± 12.1 | 100.0 ± 15.4 |
| Gender difference | P ≤ 0.75 | P ≤ 0.94 | P ≤ 0.33 | P ≤ 0.56 |
Intelligence Assessments
To assess general intellectual ability, we employed the Wechsler Adult Intelligence Scale (WAIS-R) (Wechsler 1981). This highly reliable and valid test quantifies intelligence according to age-based norms that have been shown to correlate with academic and life success, as well as with measures of work performance and occupational level (Jensen 1998). Utilizing WAIS-R, we measured the full-scale intelligence quotient (IQ)—a composite score obtained from 11 subtests in verbal and performance categories—which is standardized in a United States population sample to have a mean of approximately 100 and standard deviation of approximately 15. In addition, we measured the performance IQ (from 5 subtests), as well as the verbal IQ (from 6 subtests) separately. In the present investigation, full-scale IQ scores ranged between 74 and 139 (males: 76–125; females: 74–139), whereas performance IQ scores ranged between 70 and 124 (males: 70–124; females: 79–123), and verbal IQ scores ranged between 71 and 148 (males: 82–124; females: 71–148). There were no significant differences in intelligence measurements between males and females. The significance values, as well as group-specific means and standard deviations of all intelligence measures, are shown in Table 1.
Image Acquisition and Preprocessing
High-resolution 3D-spoiled grass magnetic resonance images were obtained on a 1.5 Tesla scanner (General Electric, Milwaukee, WI) as a series of 124 contiguous 1.5-mm coronal brain slices (256 × 256 matrix, 0.86 mm × 0.86 mm in-plane resolution). Image volumes passed through a number of preprocessing steps that included 1) correction of head tilt and alignment by reorienting each volume into the standard position of the ICBM-305 average brain (Mazziotta et al. 1995) using a 6-parameter rigid-body transformation (Woods et al. 1998), 2) removal of nonbrain tissue and the cerebellum, 3) correction of intensity nonuniformity due to magnetic field inhomogeneities (Zijdenbos and Dawant 1994; Sled et al. 1998), and 4) extracting the cortical surface models for both hemispheres, each comprising of 65 536 surface points (MacDonald et al. 1994).
Measurement of Local Cortical Convolution
As described previously (Gaser et al. 2006; Luders et al. 2006), local cortical convolution was evaluated by measuring mean curvature (Do Carmo 1976) across thousands of vertices on the lateral and medial surfaces of each individual cortical model. Mean curvature (Tcurvature) at a given point is defined as
where 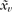 is the centroid of the neighbors of vertex v, Bv is the average distance from the centroid of each of the neighbors,
is the centroid of the neighbors of vertex v, Bv is the average distance from the centroid of each of the neighbors, 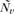 is the normal vector of vertex v, and “·” is the vector product operator (MacDonald 1998). In other words, mean curvature can be thought of as the mean angular deviation from a planar surface for points on a small closed loop surrounding a vertex. As demonstrated previously, increased values of (smoothed absolute) mean curvature reliably reflect increases in the amplitude and frequency of the folding of a surface (Luders et al. 2006).
is the normal vector of vertex v, and “·” is the vector product operator (MacDonald 1998). In other words, mean curvature can be thought of as the mean angular deviation from a planar surface for points on a small closed loop surrounding a vertex. As demonstrated previously, increased values of (smoothed absolute) mean curvature reliably reflect increases in the amplitude and frequency of the folding of a surface (Luders et al. 2006).
In the present investigation, we estimated the local cortical convolution in a 3-step approach: First, we calculated the mean curvature (in degrees) for 65 536 vertex points across the lateral and medial cortical surface resulting in large positive values for local maxima (corresponding to gyri) and large negative values for local minima (corresponding to sulci), followed by averaging curvature values within a geodesic distance of 3 mm (Step I). We then calculated the absolute value of the average mean curvature resulting in curvature values greater than or equal to zero (Step II). Finally, absolute mean curvature values were smoothed using a surface-based heat kernel smoothing filter (Chung et al. 2005) with a full width at half maximum of 20 mm (Step III). The subsequent statistical analyses were conducted on smoothed absolute mean curvature values, hereafter referred to as mean curvature, which indicates the local degree of cortical convolution.
Relationship between Cortical Convolution and Intelligence
Statistical relationships were examined at all 65 536 surface points for the whole group, and also at 65 536 surface points within male and female subjects separately. That is, 1st we calculated the Pearson correlation coefficients (r) between full-scale, verbal, and performance IQ measures and mean curvature values at each vertex point for the combined sample (n = 65), whereas removing the variance associated with age. More specifically, we generated maps indicating the direction of the correlation (r-map) and its significance (P-map), both uncorrected at P < 0.01 and corrected for multiple comparisons, using false discovery rate (FDR) at P < 0.05 (Benjamini and Hochberg 1995). Subsequently, we mapped the correlations between full-scale, verbal, and performance IQ measures and mean curvature values within females (n = 35) and males (n = 30) separately, after removing the partial effects of age. Finally, we analyzed the effect of gender on the slopes of the regression in order to determine the significance of the gender difference with respect to the relationship between mean curvature and full-scale, verbal, and performance IQ. All statistical mapping results were projected onto the 3D group-averaged surface models. For descriptive purposes, we also investigated the correlation between age and mean curvature at 65 536 surface points across the lateral and medial cortex within the combined sample, and in males and females separately (Supplementary Fig. 1).
Results
Relationship between Cortical Convolution and Full-Scale IQ within the Combined Sample
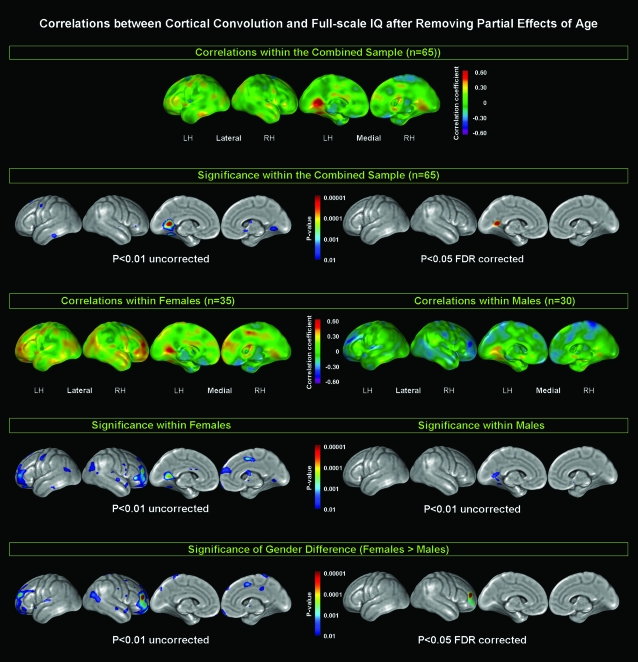
As demonstrated in Figure 1, significant negative correlations between full-scale IQ and convolution were completely absent (where the 1st row shows the correlation coefficients and the 2nd row shows the respective significance values). However, significant uncorrected positive correlations were detected in a few isolated left-hemispheric (LH) and right-hemispheric (RH) cortical regions across the lateral and medial surface (2nd row; left panel). The respective Broadman areas (BA) for the observed significant clusters are summarized in Supplementary Table 1. Only 1 cluster in the left medial hemisphere comprising the outermost posterior portion of the cingulate gyrus (retrosplenial areas: BA 26, 29, and 30) survived corrections for multiple comparisons using FDR (2nd row; right panel). To further illustrate relationships between cortical thickness and full-scale IQ and also to ensure that Pearson correlation coefficients are not unduly influenced by outliers, we sampled the cortical point that showed the highest P value (located in the left posterior cingulate gyrus). The correlations between full-scale IQ and mean curvature measures obtained from this same surface point in each subject are shown in Supplementary Figure 2 (r = 0.51, P < 0.001).
Figure 1.
Relationships between cortical convolution and full-scale IQ, after removing the variance associated with age. Larger positive values of correlation coefficients are color-coded in yellow, orange, and red; negative values appear in light blue, dark blue, and purple (1st and 3rd row). Significance values, both uncorrected at P < 0.01 and FDR corrected at P < 0.05 (if significant) associated with the observed correlations are indexed in color, with gray indicating areas of no significance (2nd, 4th, and 5th row). Rows 1 and 2 illustrate findings for the combined sample (n = 65). Rows 3 and 4 illustrate findings within females (n = 35; left panel) and males (n = 30; right panel) separately. Row 5 illustrates the significance of the gender differences (females > males).
Relationship between Cortical Convolution and Full-Scale IQ within Females and Males
As demonstrated in Figure 1 (3rd row), visual inspection appears to indicate that females (left panel) and males (right panel) differ considerably with respect to the spatial distribution and the direction of the relationships between convolution and full-scale IQ. That is, although cortical convolution and intelligence are predominantly positively related within the female group (depicted in warmer colors [yellow, orange, red]), mainly negative relationships appear present within the male group (reflected in cooler colors [light blue, dark blue, purple]). Mapping the regional significance of these effects (4th row) shows that a number of clusters are significantly positively correlated in females. Males, on the other hand, did not reveal any significant correlations on the lateral surface and only a small set of temporo-occipital clusters showing significant positive correlations on the medial surface (see also Supplementary Table 1). No significance cluster in females nor males survived corrections for multiple comparisons using FDR (maps not shown).
The last row in Figure 1 (see also Supplementary Table 2) demonstrates the gender difference with respect to the relationship between cortical convolution and full-scale intelligence, where associations between cortical convolution and intelligence appeared more pronounced in females than in males (females > males). Although this effect was detected in several cortical regions across the lateral and medial surface (left panel), only a cluster in the right prefrontal (BA 10) survived corrections for multiple comparisons using FDR (right panel). There were no regions where associations between cortical convolution and intelligence were more pronounced in males than in females.
Relationship between Cortical Convolution and Verbal IQ and Performance IQ
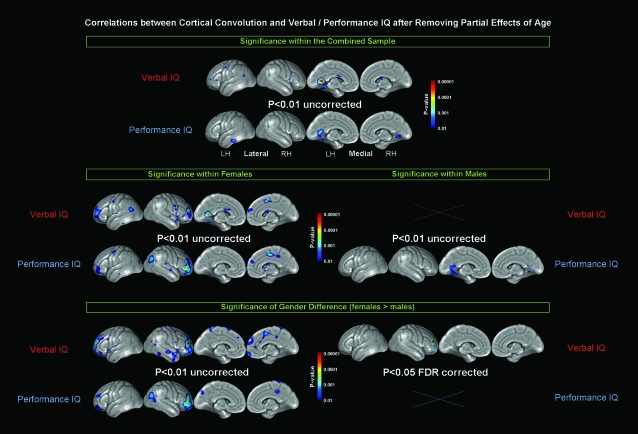
As demonstrated in Figure 2 (1st and 2nd row), the significance profiles indicating the positive correlations between cortical convolution and verbal/performance IQ within the whole group show a striking similarity to outcomes based on full-scale IQ correlations. That is, although significant negative correlations were completely absent, significant uncorrected positive correlations were most pronounced in the left medial temporo-occipital lobe (with peak values in the outermost posterior section of the cingulate gyrus). Similar correlation profiles were detected in females for verbal IQ (left panel; 3rd row) and in males for performance IQ (right panel; 4th row). With respect to verbal IQ in males (right panel; 3rd row) significant correlations were completely absent (maps not shown). Interestingly, with respect to performance IQ in females (left panel; 4th row), we detected the most significant positive correlations in the right prefrontal lobe. However, none of these significance clusters survived corrections for multiple comparisons using FDR (maps not shown). As further illustrated in Figure 2, gender effects (females > males) became obvious in several cortical regions both for verbal IQ (5th row) and performance IQ (6th row) without correcting for multiple comparisons (left panel). However, only the cluster in the right prefrontal cortex survived FDR correction for verbal IQ measures, but not for performance measures (right panel).
Figure 2.
Relationships between cortical convolution and verbal/performance IQ, after removing the variance associated with age. Illustrated are uncorrected findings at P < 0.01 (if significant) and FDR corrected findings at P < 0.05 (if significant). Rows 1 and 2 illustrate findings for males and females combined (n = 65). Rows 3 and 4 illustrate findings within females (n = 35; left panel) and males (n = 30; right panel) separately. Rows 5 and 6 illustrate the significance of the gender differences (females > males).
Discussion
We observed significant positive correlations between intelligence and cortical convolution in the outermost posterior section of the left cingulate gyrus, near the junction of the medial temporal and occipital lobe. Significant negative correlations were completely absent. This observation supports previous in vivo and post mortem findings of predominantly positive associations between intelligence and brain morphology, as summarized elsewhere (Haier et al. 2005; Witelson et al. 2006; Narr, Woods, et al. 2007)
Potential Mechanisms for the Correlation between Intelligence and Convolution
It could be argued that the more pronounced the regional convolution of the cortex, the larger the surface area for this region, which corresponds to an increased number of neurons within a certain radius. More neurons within a particular area might facilitate an efficient processing of information, which in turn, might be beneficial for cognitive performance. The idea that an increased number of neurons might be advantageous for cognitive performance is in line with previously reported positive associations between intelligence and gray matter (Andreasen et al. 1993; Reiss et al. 1996; Gur et al. 1999; Thompson et al. 2001; Wilke et al. 2003; Frangou et al. 2004; Haier et al. 2004), and also between intelligence and cortical thickness (Narr, Woods, et al. 2007). On the other hand, Haier et al. (1988, 1992) demonstrated that intelligence measures and glucose consumption are negatively correlated, supporting the assumption that more intelligent individuals use their neurons more efficiently. Consequently, even if enhanced convolution is associated with an increase in neuronal numbers, fewer neurons may not necessarily be associated with reduced cognitive performance. Thus, the detected positive correlation between cortical convolution and intelligence might be only partly driven by increased neuronal numbers in the regions implicated. This assumption is also supported by the observation that significance profiles indicating positives correlations between cortical convolution and intelligence (as observed in the current study) only partly overlap with significance profiles indicating positive correlations between cortical thickness and intelligence in the same sample (Narr, Woods, et al. 2007). It is likely that the IQ relationships observed for cortical convolution and cortical thickness within similar regions might simply underscore the relevance of the structural integrity of these regions for intellectual ability, and are not necessarily present due to a link between cerebral curvature and thickness (or neuronal numbers). Thus, we believe that other micro-anatomical characteristics (possibly in addition to changes in neuronal numbers) need to be considered as the link between intelligence measures and cerebral macro-anatomy. For example, the regional degree and pattern of the convolution of the cortex has been suggested to reflect regional interconnectivity or neuronal circuitry (Caviness 1975; Goldman-Rakic 1981; Rakic 1988; Rademacher et al. 1993; Watson et al. 1993; Roland and Zilles 1994; Van Essen 1997). That is, certain constellations of axons and dendrites in brain regions relevant for processing cognitive information might benefit intellectual abilities and thus contribute to our observation of positive correlations between intelligence and cortical convolution. The precise nature of these advantageous underlying architectonic constellations, however, remains to be established and requires further investigation. As suggested by Toro and Burnod (2005), the position of primary convolutions may be determined both by the location of architectonic inhomogeneities or by the initial geometry of the cortex, where secondary and tertiary convolutions are an effect of the preceding primary convolutions, and thus, not necessarily related to architecture at all. Similarly, they proposed that gyri and sulci differ significantly with respect to laminar thickness. Our curvature measurement approach does not allow us to distinguish between primary, secondary, and tertiary convolutions; nor between sulci and gyri; nor between a cortical region with a large number of shallow convolutions and 1 with only a small number of deep convolutions. Thus, positive correlations between cortical convolution and intelligence, as observed in the current study, are based on considering a conglomerate of several aspects of convolution (and thus, different architectonic features). This clearly makes it difficult, at this point, to propose advantageous underlying architectonic constellations that promote intellectual abilities. Notwithstanding, correlations between convolution measures and intelligence data should become evident in cortical regions that are involved in mediating cognitive function.
Implications from the Regional Specificity of the Observed Correlations
Within the combined sample, we detected a robust relationship between intelligence and the degree of folding in the medial temporo-occipital lobe, particularly within the outermost portions of the posterior cingulate, bilaterally. Significance was confirmed by FDR correction for full-scale IQ measurements (but not for verbal or performance IQ measurements) in the left hemisphere only. Positive correlations in the left medial temporo-occipital lobe were also detected when males and females were analyzed separately, albeit below the threshold of significance when corrected for multiple comparisons. With respect to the location of this cluster, our observation agrees with other recently published findings based on assessing relationships between intelligence and cortical thickness in the same data set (Narr, Woods, et al. 2007). In accordance with our observation, this previous study detected pronounced positive correlations bilaterally in the medial temporo-occipital lobe within the whole sample (with a more extended cluster in the left hemisphere). Notwithstanding, Narr, Woods, et al. (2007) also reported relationships with IQ in a number of additional regions, pronounced within the whole sample and in females (e.g., the lateral prefrontal, inferior temporal and occipital lobes). Several factors may explain why significant correlations with intelligence, in the current study, were only observed for the medial temporo-occipital lobe after correction for multiple comparisons. Differences in results are deemed at least partially attributable to the use of different morphological measurements for assessing brain structure-intelligence relationships. That is, findings from different studies capturing unique aspects of brain morphology are only partially comparable because some imaging measures may be more closely related to the functional correlate of interest. Consequently, corresponding or similar results for cortical convolution and cortical thickness relationships with intelligence, might be attributable to the shared sources or sensitivity of the different approaches (e.g., regional number of neurons), whereas deviant findings might result from the unique features inherent to each approach. It is also possible that interindividual variations of cortical convolution could be greater within specific regions with respect to others, whereas variability profiles are narrower for gray matter density or thickness measures. Thus, effect sizes could differ within regions depending on the imaging measure examined. Visual inspections of the color-coded correlation maps actually appear to indicate strong positive correlations in females (and moderate positive correlation within the combined sample) within the left and/or right prefrontal lobe. This is in agreement with previous findings of significant positive correlations between cerebral gray matter and intelligence within (pre)frontal regions (Reiss et al. 1996; Flashman et al. 1997; Thompson et al. 2001; Wilke et al. 2003; Haier et al. 2004, 2005; Frangou et al. 2004; Narr, Woods, et al. 2007). However, although these prefrontal clusters in the current study were significant within females (albeit uncorrected), they were below the threshold of significance within the combined sample due to the presence of negative associations within males.
In contrast, both males and females demonstrated large positive correlations within the left medial temporo-occipital lobe, and thus this region was highly significant when examining the combined sample, with the findings in the left hemisphere surviving corrections for multiple comparisons. The strong agreement between both studies and also between males and females with respect to the significance of the medial temporo-occipital lobe emphasizes that this particular region may play an essential role in the modulation of intelligence. In support of this hypothesis, Plomin and Kosslyn (2001), pointed out that, although it is possible that a single fundamental brain characteristic such as frontal gray matter volume is responsible for the general cognitive ability, it seems more likely that many brain processes (and areas) are involved. On the one hand, certain regions within the medial temporo-occipital cortex might substantially contribute to cognitive outcomes via modulating pathways to (pre)frontal regions. On the other hand, as suggested previously (Haier et al. 2003), the temporo-occipital region of the medial cortex might be fundamental in determining individual differences in intelligence “by serving as a brain site in which higher-order language-based concepts converge with the incoming visual information stream.” Further evidence for the involvement of posterior brain regions in the modulation of higher cognitive information is provided by recently published findings of positive correlations between intelligence and the thickness of the corpus callosum in posterior callosal sections in a subject sample partly overlapping with the current data set (Luders et al. 2007).
Effects of Gender on the Correlations between Intelligence and Convolution
We observed pronounced differences between males and females in frontal regions bilaterally, although only the right prefrontal cluster (BA 10) survived corrections for multiple comparisons using FDR for full-scale and verbal IQ measurements (but not for performance IQ measurements). The spatial location of the present gender effect appears to support prior interpretations that the (pre)frontal cortex is an integral component for cognitive outcomes (Duncan and Owen 2000; Duncan et al. 2000). Our findings indicate that cortical convolution in female brains changes more rapidly and/or in a different direction with increasing IQ than it does in male brains. Although an increase in statistical power might account for the stronger relationship in females compared with males (given that there were 35 females/30 males), limited statistical power is not likely to cause a shift in the direction of the correlation (positive in females/negative in males). It is possible that formerly described gender differences in regional convolution (Luders et al. 2004, 2006) mediate the relationship between intelligence and its underlying anatomical correlates. (Of note, although these previously detected gender effects were based on scaled data after applying 12-parameter transformations [whereas using unscaled data based on 6-parameter transformations in the current study], we could demonstrate that mean curvature is relatively invariant to applying different kinds of linear transformations; Luders et al. 2006.) Interestingly, when utilizing the same curvature measurement in a different sample examining sexual dimorphism in cortical convolution specifically, we observed an increased gyrification in female brains compared with male brains in a number of cortical regions, but with the most pronounced effects in the frontal lobes (Luders et al. 2006). This observation is in agreement with previous findings of significantly increased cortical complexity in females in the right frontal lobe (Luders et al. 2004). If sexually dimorphic information processing strategies develop as a result of differences in the underlying architecture and neural connectivity of specific brain regions, it might affect the measured relationship between intelligence and cortical convolution in men and women, as observed in the prefrontal cortex. In line with these assumptions, recent functional magnetic resonance imaging analyses detected gender-specific patterns of activation in the prefrontal cortex during a variety of cognitive tasks. Males, for example, had a significantly greater blood oxygen level–dependent signal magnitude bilaterally in the dorsolateral prefrontal cortex during a word generation task than females (Bell et al. 2006). Moreover, although males showed bilateral activation or right-sided dominance, females showed activation predominantly in the left hemisphere in the lateral prefrontal cortex during verbal working memory tasks (Speck et al. 2000).
Another intriguing finding is that significant correlations (positive or negative) were completely absent in males when correlating verbal IQ with cortical convolution. Less strong verbal skills in men, as often reported in the literature (Halpern 1992; Kimura 1999), may mediate these outcomes. Nevertheless, gender-specific cognitive abilities are not likely to provide a sufficient explanation for our findings because men and women in the present analysis did not differ in verbal IQ (nor in full-scale and performance IQ). Interestingly, although empirical evidence from imaging data has confirmed predominantly positive associations between general intellectual ability and brain-anatomical characteristics, trends for absent (or even negative) correlations in males have been reported. For example, Gur et al. (1999) reported that the correlation between intracranial volume and verbal performance was significant in women, but not significant in men. Moreover, analyzing the relationship between cognitive performance and white matter volume, they revealed significantly steeper slopes in women (Gur et al. 1999). When analyzing the relationship between cortical thickness and full-scale intelligence, Narr, Woods, et al. (2007) observed less pronounced positive correlations in men than in women, and a few clusters of negative correlations in male brains that were completely absent in female brains. Witelson et al. (2006) revealed that visuospatial ability was positively correlated with cerebral volume in women, although in men, there was a nonsignificant trend toward a negative correlation. Finally, Jung et al. (2005) discovered that a combination of frontal and occipito-parietal white matter neurometabolites (N-acetylaspartate [NAA]) strongly predicted intellectual outcomes in females but not in males. Comparable findings exist from previous analyses, where frontal lobe NAA was related to verbal ability in women but not in men (Pfleiderer et al. 2004).
Although research findings from different laboratories and across different samples provide powerful evidence for gender-specific relationships between cognitive performance and underlying morphological substrates, we observed that the magnitude and direction of the correlation were similar in men and women within the medial temporo-occipital lobe. That is, both groups showed positive associations between intelligence and convolution in both hemispheres, with larger effects in the LH compared with the RH. Against the background of prominent gender differences across the remainder of the cortex, the striking resemblance of men and women in the medial temporo-occipital lobe remains puzzling and will stimulate future research, especially with respect to its role in determining and/or modulating cognitive outcomes.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Center for Research Resources grant (P41 RR13642); the National Institute of Mental Health grant (RO1 MH60374); the National Institutes of Health (NIH) Roadmap Initiative grant (P20 RR020750); the National Library of Medicine grant (R01 LM05639); the NIH Roadmap for Medical Research, grant (U54 RR021813) entitled Center for Computational Biology (CCB); and a Career Development Award (K01 MH073990-01A1), to K.L.N.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Andreasen NC, Flaum M, Swayze V, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, Singerman JD, McCracken JT, Toga AW. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr Mechanical model of brain convolutional development. Science. 1975;189:18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage. 2005;25:1256–1265. doi: 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Do Carmo MP. Differential geometry of curves and surfaces. Englewood Cliffs (NJ): Prentice Hall; 1976. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seltz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Am J Ophthalmol. 2000;130:687. doi: 10.1016/s0002-9394(00)00752-2. [DOI] [PubMed] [Google Scholar]
- Flashman LA, Andreasen NC, Flaum M, Swayze VW. Intelligence and regional brain volumes in normal controls. Intelligence. 1997;25:149–160. [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Bellugi U, Galaburda AM, Korenberg JR, et al. Increased local gyrification mapped in Williams syndrome. Neuroimage. 2006;33:46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci. 1981;1:721–735. doi: 10.1523/JNEUROSCI.01-07-00721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Peak J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;11:199–218. [Google Scholar]
- Haier RJ, White NS, Alkire MT. Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence. 2003;31:429–441. [Google Scholar]
- Halpern DF. Sex differences in cognitive abilities. New York: Erlbaum; 1992. [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: the science of mental ability. Westport (CT): Praeger Publishers; 1998. [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, Sibbitt WL, Brooks WM. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26:965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and cognition. Cambridge (MA): MIT Press; 1999. [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, Toga AW. Gender differences in cortical complexity. Nat Neurosci. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29:1224–1230. doi: 10.1016/j.neuroimage.2005.08.049. [DOI] [PubMed] [Google Scholar]
- MacDonald D A method for identifying geometrically simple surfaces from three dimensional images. PhD thesis, McGill University; [Google Scholar]
- MacDonald D, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance imaging. Proc SPIE. 1994;2359:160–169. [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Narr KL, Bilder RM, Luders E, Thompson PM, Woods RP, Robinson D, Szeszko PR, Dimtcheva T, Gurbani M, Toga AW. Asymmetries of cortical shape: Effects of handedness, sex and schizophrenia. Neuroimage. 2007;34:939–948. doi: 10.1016/j.neuroimage.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O'Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, Michael N. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience. 2004;123:1053–1058. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Plomin R, Kosslyn SM. Genes, brain and cognition. Nat Neurosci. 2001;4:1153–1154. doi: 10.1038/nn1201-1153. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Caviness VS, Jr, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Roland PE, Zilles K. Brain atlases—a new research tool. Trends Neurosci. 1994;17:458–467. doi: 10.1016/0166-2236(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11:2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). History, rationale, and description 1. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annu Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb Cortex. 2005;15:1900–1913. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I. Regional cortical thickness matters in recall after months more than minutes. Neuroimage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, Salat D, Quinn BT, Makris N, Dale AM. Cortical volume and speed-of-processing are complementary in prediction of performance intelligence. Neuropsychologia. 2005;43:704–713. doi: 10.1016/j.neuropsychologia.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, revised: manual. New York: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- Wickett JC, Vernon PA, Lee DH. In-vivo brain size, head perimeter, and intelligence in a sample of healthy adult females. Person Individ Differ. 1994;16:831–838. [Google Scholar]
- Wickett JC, Vernon PA, Lee DH. General intelligence and brain volume in a sample of healthy adult male siblings. Int J Psychol. 1996;31:32425–32425. [Google Scholar]
- Wickett JC, Vernon PA, Lee DH. Relationships between factors of intelligence and brain volume. Person Individ Differ. 2000;29:1095–1122. [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20:202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129:386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Langemann C, Amunts K, Morosan P, Palomero-Gallagher N, Schormann T, Mohlberg H, Bürgel U, Steinmetz H, et al. A quantitative analysis of sulci in the human cerebral cortex: development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum Brain Mapp. 1997;5:218–221. doi: 10.1002/(SICI)1097-0193(1997)5:4<218::AID-HBM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.