Abstract
The ventral portion of medial prefrontal cortex (vMPFC) is involved in contextual fear-conditioning expression in rats. In the present study, we investigated the role of local N-methyl-D-aspartic acid (NMDA) glutamate receptors and nitric oxide (NO) in vMPFC on the behavioral (freezing) and cardiovascular (increase of arterial pressure and heart rate) responses of rats exposed to a context fear conditioning. The results showed that both freezing and cardiovascular responses to contextual fear conditioning were reduced by bilateral administration of NMDA receptor antagonist LY235959 (4 nmol/200 nL) into the vMPFC before reexposition to conditioned chamber. Bilateral inhibition of neuronal NO synthase (nNOS) by local vMPFC administration of the Nω-propyl-L-arginine (N-propyl, 0.04 nmol/200 nL) or the NO scavenger carboxy-PTIO (1 nmol/200 nL) caused similar results, inhibiting the fear responses. We also investigated the effects of inhibiting glutamate- and NO-mediated neurotransmission in the vMPFC at the time of aversive context exposure on reexposure to the same context. It was observed that the 1st exposure results in a significant attenuation of the fear responses on reexposure in vehicle-treated animals, which was not modified by the drugs. The present results suggest that a vMPFC NMDA–NO pathway may play an important role on expression of contextual fear conditioning.
Keywords: cardiovascular system, fear conditioning, freezing, glutamatergic system, infralimbic cortex, nitric oxide
Introduction
Contextual fear conditioning is evoked by animal reexposure to an environment (context) that has been previously paired with an aversive or unpleasant stimulus. This is the case where a rat is reexposed to the chamber in which it has previously received electric footshocks (Blanchard RJ and Blanchard DC 1969; Fanselow 1980; LeDoux et al. 1988; Carrive 2000). In rats, contextual fear conditioning causes freezing immobility, and mean arterial pressure (MAP) and heart rate (HR) increases (Fanselow 1980; LeDoux et al. 1988; Carrive 2002; Resstel, Joca, Moreira, et al. 2006). Contextual fear conditioning is associated with a marked increase in neuronal activity in limbic structures involved with defense reactions, such as the ventral portion of medial prefrontal cortex (vMPFC) (Beck and Fibiger 1995).
The vMPFC is involved in the behavioral and cardiovascular responses associated with stress situations (Frysztak and Neafsey 1991, 1994; Blum et al. 2006; Resstel, Joca, Guimarães et al. 2006; Sierra-Mercado et al. 2006; Tavares and Corrêa 2006). We have recently showed that this structure is also important for the freezing and cardiovascular responses evoked by contextual fear-conditioning expression (Resstel, Joca, Guimarães, et al. 2006). However, which neurotransmitters are involved in these responses is still unclear.
Both glutamatergic terminals and N-methyl-D-aspartic acid (NMDA) receptors are present in the vMPFC of rats (Gigg et al. 1994; Nicolle and Baxter 2003). During stress conditions, glutamate levels are increased in the vMPFC (Moghaddam 1993). Moreover, the glutamatergic stimulation of the vMPFC was reported to modulate the parasympathetic component of baroreflex (Resstel and Corrêa 2006b), cardiac sympathetic activity (Resstel and Corrêa 2005), and blood pressure (Resstel and Corrêa 2006a). These cardiovascular responses were similar to those observed during defense responses (Nosaka 1996; Resstel, Joca, Guimarães, et al. 2006; Resstel, Joca, Moreira, et al. 2006; Tavares and Corrêa 2006). These results suggest that a glutamate-mediated neurotransmission in the vMPFC may play a role in fear-conditioning responses.
Glutamate acting on NMDA receptors in the central nervous system activates the enzyme neuronal nitric oxide synthase (nNOS), resulting in nitric oxide (NO) formation (Garthwaite et al. 1988; Garthwaite and Boulton 1995). The cardiovascular responses evoked by glutamate in the vMPFC depend on NMDA receptor–NO interaction (Resstel and Corrêa 2006a, 2006b). Therefore, it is possible that, similar to NMDA receptors, NO is also involved in fear-conditioning responses regulated by the vMPFC. To test this hypothesis, we examined the effects of bilateral injections of the selective NMDA antagonist LY235959, the selective neuronal NOS inhibitor Nω-propyl-L-arginine (N-propyl), or the NO scavenger carboxy-PTIO (c-PTIO) injected into the vMPFC of rats submitted to a contextual fear-conditioning protocol. The possible involvement of other subtypes of glutamate ionotropic receptors in this process was also investigated by injecting the selective non-NMDA ionotropic glutamate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) into the vMPFC. Finally, because memory expression depends on the context in which memory is retrieved (Spear 1973; Tulving and Thomson 1973) and reexposure to this context could engage either extinction or reconsolidation processes (Duvarci and Nader 2004), we also investigated the effects of inhibiting glutamate- and NO-mediated neurotransmission in the vMPFC at the time of aversive context exposure on reexposure to the same context.
Materials and Methods
Animal Preparation
One hundred and seven male Wistar rats weighing 230–270 g were used. Animals were kept in the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirão Preto, University of São Paulo. Rats were housed individually in plastic cages with free access to food and water and under a 12-h light:dark cycle (lights on at 06:30 h). The Institution's Animal Ethics Committee approved housing conditions and experimental procedures (process number: 215-2005).
Seven days before the experiment, rats were anesthetized with tribromoethanol (250 mg/kg ip). After scalp anesthesia with 2% lidocaine, the skull was surgically exposed, and stainless steel guide cannulas (26 gauge) were implanted bilaterally in the vMPFC using a stereotaxic apparatus (Stoelting, IL). Coordinates for cannula implantation (AP = +2.2 mm; L = 2.8 mm from the medial suture, V = −3.3 mm from the skull with a lateral inclination of 23°) were selected from the rat brain atlas of Paxinos and Watson (1997). A control group of animals had stainless steel guide cannulas implanted bilaterally into surrounding structures of the vMPFC such as the cingulate cortex area 1 (AP = +1.2 mm; L = 1.5 mm from the medial suture, V = −2.3 mm from the skull), the corpus callosum (AP = +1.2 mm; L = 2.8 mm from the medial suture, V = −2.3 mm from the skull), and the tenia tecta (AP = +1.2 mm; L = 3 mm from the medial suture, V = −4.3 mm from the skull).
Cannulas were fixed to the skull with dental cement and 1 metal screw. One day before experiments, rats were anesthetized with tribromoethanol and a catheter (a 4-cm PE-10 segment heat bound to a 13-cm PE-50 segment, Clay-Adams, CA) was inserted into the abdominal aorta through the femoral artery for blood pressure recording. The catheter was tunneled under the skin and exteriorized on the animal's dorsum.
Drugs
LY235959 (Tocris), NBQX (Tocris), Nω-propyl-L-arginine (Tocris) and carboxy-PTIO (S)-3-Carboxy-4-hydroxyphenylglicine (c-PTIO, RBI), tribromoethanol (Aldrich), and urethane (Sigma) were dissolved in sterile artificial cerebrospinal fluid (composition: NaCl 100 mM, Na3PO4 2 mM, KCl 2.5 mM, MgCl2 1 mM, NaHCO3 27 mM, CaCl2 2.5 mM; pH = 7.4).
Fear Conditioning and Testing
Habituation, conditioning, and testing were carried out in 25 × 22 × 22 cm footshock chambers. The chambers had a grid floor composed of 18 stainless steel rods (2 mm in diameter), spaced 1.5 cm apart and wired to a shock generator (Automatic Reflex Conditioner, model 8572—Ugo Basile, Comerio, Italy). The chambers were cleaned with 70% ethanol, before and after use. Preconditioning started 1 week after guide cannula implantation and consisted of two 10-min-long preexposures (habituation) to the footshock chamber (1 in the morning and 1 in the afternoon). In the conditioning shock session, performed 24 h after the 1st habituation session, animals were divided into 2 experimental groups: nonconditioned and conditioned. The nonconditioned group was exposed to the footshock chamber for 10 min, but no shock was delivered. The conditioned group was submitted to a shock session consisting of 6 electric 2.5 mA/3-s footshocks (Resstel, Joca, Guimarães, et al. 2006; Resstel, Joca, Moreira, et al. 2006) delivered at 20-s to 1-min intervals. After the conditioning session, a catheter was implanted into the femoral artery for blood pressure and HR recording.
Cardiovascular and behavioral (freezing) responses evoked by conditioned emotional response to context were evaluated 1 day after the conditioning. The test session (day 1) consisted of a 10-min-long reexposure to the footshock chamber without shock delivery. Animals were transferred from the animal room to the procedure room (a different room was used for conditioning) in their home box. MAP and HR were recorded using an HP-7754A amplifier (Hewlett Packard, CA) connected to a signal acquisition board (Biopac M-100, CA) and computer processed. Rats were tested 1 at a time. After a few minutes of baseline recording, injections were performed into the vMPFC. Two 33-gauge needles (Small Parts, Miami Lakes, FL), 1 mm longer than the guide cannulas, connected to 10-μL syringe (7001KH, Hamilton Co, NV) through PE-10 tubing, were used. The needles were carefully inserted into the vMPFC guide cannulas, and the solutions were infused over a 15-s period in a final volume of 200 nL (each side). They remained in place for an additional 30-s period to prevent reflux. Ten minutes later, animals were replaced in the footshock chamber. Additional groups of animals were submitted to another 10-min-long reexposure to context, after 7 days of conditioning. With the exception of these animals (that were then tested twice in the aversively conditioned box), all animals were tested only once.
Freezing was evaluated during the test by an experimenter that sat 30 cm away from the footshock chamber and was blind to the treatment groups. Freezing was defined as the complete absence of movement while the animal assumed a characteristic tense posture (Fanselow 1980). We also evaluated animal activity during the test session by counting the number of crossings and rearings. A crossing was recorded when the animal crossed with 4 paws the imaginary line that divide the test chamber into 2 halves.
Experimental Design
Animals were divided into 2 groups: fear-conditioned rats and nonconditioned rats. Both groups received either 200 nL of vehicle or the specific NMDA receptor antagonist LY235959 (4 nmol, Resstel and Corrêa 2006a, 2006b), the specific non-NMDA receptor antagonist NBQX (4 nmol, equimolar dose to that of LY235959), the nNOS inhibitor N-propyl (0.04 nmol, Zhang et al. 1997; Resstel and Corrêa 2006a, 2006b), or the NO scavenger c-PTIO (1 nmol, Aguiar et al. 2006; Tavares et al. 2007) bilaterally injected into the vMPFC 10 min prior the test session. All doses were based on previous studies.
On day 7 (reexposition test), only fear-conditioned rats which had received drug administration (LY235959 and N-propyl) or vehicle prior the test (day 1) were reexposed to context. A nonexposed, control animal group, that also received LY235959, N-propyl, or vehicle injections on day 1, was exposed to the aversively conditioned context on day 7.
Histological Procedure
At the end of the experiments, the rats were anesthetized with urethane (1.25 g/kg, ip), and 200 nL of 1% Evan's blue dye was bilaterally injected into the vMPFC to mark the injection site. The chest was surgically opened; the descending aorta occluded; the right atrium severed; and the brain perfused with 10% formalin through the left ventricle. Brains were postfixed for 24 h at 4 °C, and 40-μm sections were cut using a cryostat (CM-1900, Leica, Wetzlar, Germany). Serial brain sections were stained with 1% neutral red and injection sites determined using the rat brain atlas of Paxinos and Watson (1997) as reference.
Data Analysis
MAP and HR values were continuously recorded during the 5-min period before and the 10-min period after exposure to the footshock chamber. Data were expressed as means ± standard error of the mean of MAP or HR changes (respectively ΔMAP and ΔHR) sampled at 60-s intervals. Points sampled during the 300 s before exposure were used as control baseline value. MAP and HR changes were analyzed using 3-way analysis of variance (ANOVA) with group (conditioned or nonconditioned) and treatment (drug or vehicle) as main independent factors and time as repeated measurement. When interaction between the factors was observed, groups were compared at specific times using the Bonferroni's post hoc test.
Freezing was expressed as percentage of the whole test period (600 s). Freezing, crossings, and rearings were analyzed using 2-way ANOVA with condition (conditioned or nonconditioned) and treatment (drug or vehicle) as the 2 factors. When interaction between the factors was observed, specific 1-way ANOVA followed by the Bonferroni post hoc test was performed.
The cardiovascular and behavior responses observed at reexposition test (day 7) were analyzed as described above, with day of exposition (day 1 or day 7) and treatment (drug or vehicle) as main independent factors. P < 0.05 was assumed as statistically significant.
Results
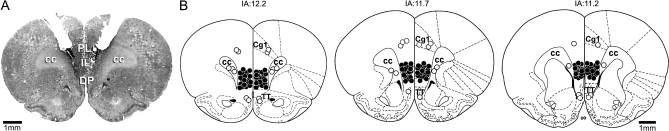
Diagrammatic representation indicating the injection sites of vehicle and all drugs and a photomicrograph showing a representative bilateral injection site in the vMPFC are presented in Figure 1.
Figure 1.
(A) Photomicrograph of a coronal brain section showing bilateral microinjections sites in the vMPFC. (B) Diagrammatic representation based on the rat brain atlas of Paxinos and Watson (1997) indicating the drugs sites into the vMPFC (closed circles). Animals with drug injection sites outside the vMPFC were represented by opened circles. Cg1, cingulate cortex area 1; PL, prelimbic cortex; IL, infralimbic cortex; DP, dorsal peduncular cortex; cc, corpus callosum; and TT, tenia tecta. IA, interaural.
Compared with the group (n = 5), which received vehicle into the vMPFC, no changes were observed in behavior (F3,19 = 0.1, P > 0.05) and cardiovascular responses (MAP: F3,285 = 0.64, P > 0.05 and HR: F3,285 = 0.62, P > 0.05) when LY, N-propyl, or c-PTIO were microinjected into structures vMPFC surrounding such as the cingulate cortex area 1 (n = 6), the corpus callosum (n = 6), or the tenia tecta (n = 6) during the chamber reexposition test.
Behavioral Responses to Contextual Fear Conditioning
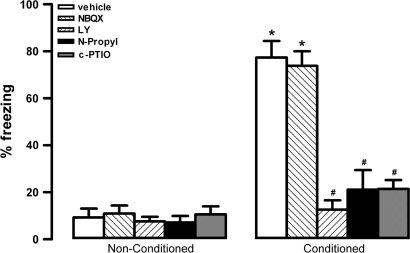
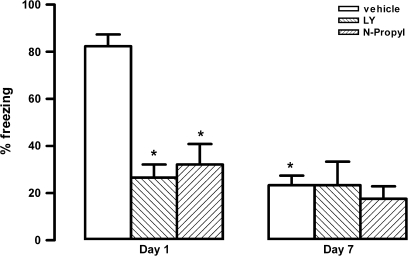
There were significant effects of condition (F4,40 = 29.3, P < 0.001), treatment (F1,40 = 34.2, P < 0.001), and interaction between the 2 factors (F4,40 = 25.1, P < 0.001) on time spent in freezing behavior. Vehicle-treated rats pre-exposed to shocks (conditioned group) spent more time in freezing than non–pre-exposed controls (nonconditioned group) during the reexposure to context (Fig. 2). One-way ANOVA indicated a significant effect for treatment on conditioned animals (F4,20 = 16, P < 0.001). The bilateral injection into the vMPFC of LY (n = 5), N-propyl (n = 5), or c-PTIO (n = 5) reduced the time spent in freezing on conditioned animals when compared with respective vehicle-treated group (n = 5, Fig. 2). NBQX (n = 5) did not change time spent in freezing of conditioned animals (P > 0.05, Fig. 2). In nonconditioned animals, no significant treatment effect was found (F4,20 = 0.3, P > 0.05, Fig. 2).
Figure 2.
Effects of bilateral microinjection of 200 nL of vehicle, 4 nmol of LY235959 (LY), 4 nmol of NBQX, 0.04 nmol of N-propyl, or 1 nmol of c-PTIO in the percentage of time spent in freezing behavior of nonconditioned and fear-conditioned rats (n = 5/group). Columns represent the mean and bars the SEM, *P < 0.05 (compared with vehicle nonconditioned group) and #P < 0.05 (compared with vehicle-conditioned group).
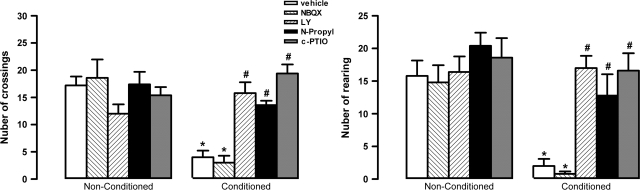
Two-way ANOVA indicated significant effects of conditioning (F1,40 = 14.3, P < 0.01), treatment (F4,40 = 35.4, P < 0.001), and interaction (F4,40 = 19.2, P < 0.001) on the number of crossings. Further analysis showed that vehicle-treated rats pre-exposed to shocks (n = 5) present a small number of crossings compared with non–pre-exposed controls (n = 5, t = 6.5, df = 8, P < 0.01, Fig. 3).
Figure 3.
Effects of bilateral microinjection of 200 nL of vehicle, 4 nmol of LY235959 (LY), 4 nmol of NBQX, 0.04 nmol of N-propyl, or 1 nmol of c-PTIO in the number of crossings and rearings of nonconditioned or fear-conditioned rats (n = 5/group). *P < 0.05 (compared with vehicle nonconditioned group) and #P < 0.05 (compared with vehicle-conditioned group). Further specifications as in figure 2.
Similar effects of conditioning (F1,40 = 22.3, P < 0.001), treatment (F4,40 = 18.5, P < 0.001), and interaction (F4,40 = 22.4, P < 0.001) were observed on the number of rearings. Vehicle-treated rats pre-exposed to shocks (n = 5) showed a smaller number of rearings than non–pre-exposed controls (n = 5, t = 5.3, P < 0.001, df = 8, Fig. 3).
Bilateral injection of LY, N-propyl, and c-PTIO into the vMPFC significantly increased the number of crossings (F4,20 = 25.4, P < 0.001) and rearings (F4,20 = 13.4, P < 0.001) of conditioned animals when compared with vehicle-treated animals (Fig. 4). No drug effect was found in nonconditioned animals (crossings, F4,20 = 1.3; rearings, F4,20 = 0.8, P > 0.05, ig. 3).
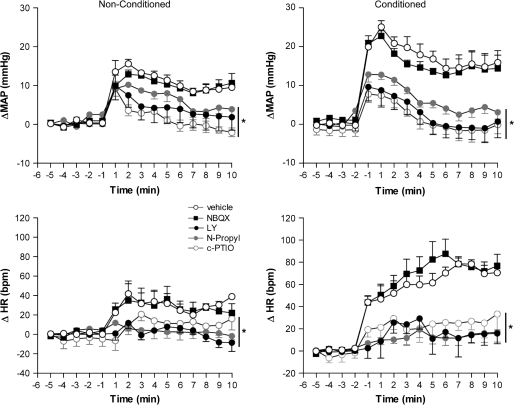
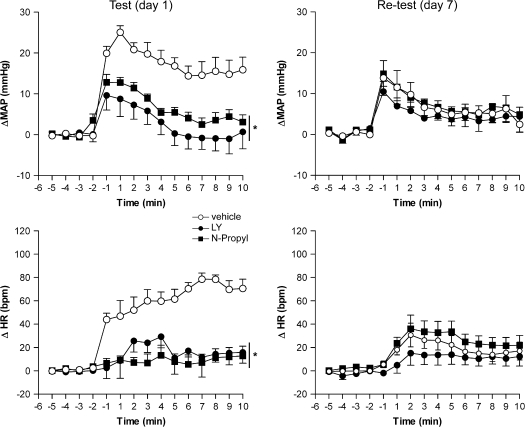
Figure 4.
Time course of the effects of bilateral microinjection of 200 nL of vehicle, 4 nmol of LY235959 (LY), 4 nmol of NBQX, 0.04 nmol of N-propyl, or 1 nmol of c-PTIO in the mean arterial pressure (ΔMAP) and heart rate (ΔHR) recorded in nonconditioned and fear-conditioned rats (n = 5/group). Asterisk indicates significant treatment effect (P < 0.05) compared with vehicle- and NBQX-treated animals. Further specifications as in figure 2.
Cardiovascular Responses to Contextual Fear Conditioning
In both group, bilateral injection of LY, N-propyl, NBQX, and c-PTIO into the vMPFC had no effect on basal levels of MAP and HR.
There were significant effects of condition, treatment, and condition versus time interaction on both HR (condition: F1,39 = 28.4, P < 0.01; condition vs. time: F14,546 = 9.8) and MAP (condition: F1,39 = 4.5, P < 0.05; condition vs. time: F14,546 = 13.7, P < 0.01). For these variables, there were also significant effects of treatment (HR: F4,39 = 16.1, P < 0.01; MAP: F4,39 = 15.6, P < 0.01), condition versus treatment (HR: F4,39 = 2.4, P > 0.05; MAP: F4,39 = 2.1, P > 0.05), and condition versus treatment versus time interactions on HR (F56,546 = 1.5, P < 0.05) but not in MAP (F56,546 = 1.2, P > 0.05).
In the nonconditioned group, reexposure to the context induced an increase in HR and MAP, although smaller than that observed in the conditioned group (MAP: F1,120 = 64.3, P < 0.01 and HR: F1,120 = 104.8, P < 0.01) (Fig. 4). Treatment effects were significant on both conditioned (MAP: F1,136 = 159.5, P < 0.001 and HR: F1,136 = 164.7, P < 0.001) and nonconditioned (MAP: F1,136 = 34.7, P < 0.001 and HR: F1,136 = 104.8, P < 0.001) groups. In these groups, bilateral injection of LY, N-propyl, and c-PTIO into the vMPFC significantly reduced the increase in MAP and HR. NBQX did not significantly affect HR and MAP in any experimental group (Fig. 4).
Behavioral and Cardiovascular Responses to Contextual Fear Conditioning During a Second Reexposure to the Aversive Context
Seven days after the conditioning session, conditioned animals that received vehicle, LY, or N-propyl on day 1 (test) were reexposed to the context again. There were significant effects of treatment (F2,24 = 11.4, P < 0.01), day of exposition (F1,24 = 21.9, P < 0.01), and interaction between the 2 factors (F2,24 = 9.8, P < 0.01) on time spent in freezing behavior. Vehicle-treated rats showed a significant decrease in freezing on day 7 compared with day 1 (n = 5, P < 0.01, Fig. 5). Bilateral injection into the vMPFC of LY (n = 5) and N-propyl (n = 5) reduced the time spent in freezing when compared with respective vehicle-treated group on day 1 (F2,12 = 21.4, P < 0.001). This reduction was maintained when the same animals were tested on day 7 (F2,12 = 0.7, P > 0.05, Fig. 5). The autonomic responses of these animals were shown in Figure 6. There were significant effects of treatment, day of exposition, and interaction between the 2 factors on both HR (treatment: F2,120 = 131.3, P < 0.01; day of exposition: F1,120 = 10.36, P < 0.01; interaction: F14,120 = 4.9, P < 0.01) and MAP (treatment: F2,120 = 123.4, P < 0.01; day of exposition: F1,120 = 9.3, P < 0.01; interaction: F14,120 = 3.4, P < 0.01). Similar to the behavioral responses, control animals showed decreased cardiovascular responses on day 7 (MAP: F1,120 = 119.3, P < 0.01 and HR: F1,120 = 243.2, P < 0.001) (Fig. 6). Bilateral injection into the vMPFC of LY (n = 5) and N-propyl (n = 5) reduced the cardiovascular responses when compared with control group on day 1 (MAP: F2,180 = 87.1, P < 0.001 and HR: F2,180 = 112.7, P < 0.001). This reduction was also maintained when the same animals were tested on day 7 (MAP: F2,180 = 1.3, P > 0.05 and HR: F1,180 = 1.8, P > 0.05, Fig. 6).
Figure 5.
Effects of bilateral microinjection of 200 nL of vehicle, 4 nmol of LY235959 (LY), or 0.04 nmol of N-propyl in the percentage of time spent in freezing behavior of fear-conditioned rats (n = 5/group) 10 min before the 1st reexposition of context (day 1) and 6 days before the 2nd reexposition to context (day 7). Columns represent the mean and bars the SEM, *P < 0.05, compared with vehicle-conditioned group in day 1.
Figure 6.
Time course of the effects of bilateral microinjection of 200 nL of vehicle, 4 nmol of LY235959 (LY), or N-propyl in the mean arterial pressure (ΔMAP) and heart rate (ΔHR) recorded in fear-conditioned rats (n = 5/group) 10 min before the 1st reexposition of context (day 1) and 6 days before the 2nd reexposition to context (day 7). Asterisk indicates significant treatment effect (P < 0.05) compared with vehicle-treated animals.
There were no differences among animals that received vehicle, LY or N-Propyl (n=8, each group) on day 1 and were exposed to the context on day 7. However, they all showed freezing (table 1) and cardiovascular responses (data not shown) similar to vehicle-treated animals tested 24 h after conditioning (P > 0.05).
Table 1.
Effects of bilateral microinjection of 200 nL of vehicle (tested at day 1 and day 7 or only in day 7), 4 nmol of LY235959 (LY), or 0.04 nmol of N-propyl in the percentage of time spent in freezing behavior of fear-conditioned rats 10 min before the 1st reexposition of context (day 1) and 6 days before the 2nd reexposition to context (day 7)
| Group | Freezing (%) |
|
| Day 1 | Day 7 | |
| Vehicle (n = 5) | 74 ± 5 | 13 ± 4* |
| Vehicle (n = 8) | Not exposed | 80 ± 3 |
| LY (n = 8) | Not exposed | 74 ± 5 |
| N-propyl (n = 8) | Not exposed | 82 ± 4 |
Data represent the mean ± standard error of the mean.
P < 0.05 compared with vehicle in day 1.
Discussion
The present results showed that reexposure 1 or 7 days to a context previously paired with aversive (footshock) stimulus induces an almost immediate increase in MAP and HR. These effects were associated with significant behavioral changes, with the conditioned animals spending almost 80% of the time in freezing behavior. Therefore, the present study was able to reproduce the behavioral and autonomic responses typically observed in studies employing contextual fear-conditioning paradigms (Carrive 2000; Zhang et al. 2004, Resstel, Joca, Guimarães, et al. 2006; Resstel, Joca, Moreira, et al. 2006).
The MPFC is anatomically defined as the cortical area receiving afferent projections from the medial dorsal thalamic nucleus. Iontophoretic studies identified L-glutamine as the neurotransmitter in the MPFC projection from the medial dorsal thalamic nucleus (Gigg et al. 1992). In addition, binding experiments showed that ionotropic glutamate receptors are present in the vMPFC (Nicolle and Baxter 2003). Our results showed that intra-vMPFC administration of LY235959, a selective NMDA receptor antagonist, blocked the cardiovascular and freezing responses of conditioned rats exposed to the aversive context, corroborating previous observations (Benveniste and Mayer 1991; Dhruva et al. 1998). To exclude a possible interference of LY235959 on non-NMDA glutamate receptors, we pretreated rats with the selective non-NMDA antagonist NBQX (Ohmori et al. 1996). NBQX is more potent than 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor–mediated effects (Yu and Miller 1995). It was previously reported by Dhruva et al. (1998) that AMPA receptors were blocked by a 5-fold lower dose of NBQX than that of AP-7 (a selective NMDA receptors antagonist less potent than LY235959) necessary to block NMDA receptors. The pretreatment with NBQX in dose equimolar to that used of LY235959 failed to affect the fear-conditioning responses, suggesting that the effects of LY235959 on fear-conditioning expression do not involve activation of non-NMDA receptors. This observation further reinforces the idea of a major involvement of NMDA receptors in the expression of fear-conditioning responses. However, considering that only a single dose of NBQX was used, it is not possible to rule out the involvement of non-NMDA receptors in these responses. Because a previous study has shown that administration of a NMDA receptor antagonist into the vMPFC before the conditioning test has no effect on freezing induced by a tone Conditioned Stimulus presentation (Burgos-Robles et al. 2007), NMDA receptors located in the vMPFC seems to be preferentially associated with emotional responses evoked by fear conditioning to context rather than specific cues. Anyway, at least for contextual fear conditioning, our results argue the current view that NMDA receptors of the vMPFC are only involved in the extinction of fear responses.
Previous studies using Fos-like imunoreactivity showed that the vMPFC is strongly activated by contextual fear (Beck and Fibiger 1995). Moreover, vMPFC excitotoxic lesions or chemical inhibition (Frysztak and Neafsey 1991, 1994; Blum et al. 2006; Sierra-Mercado et al. 2006) or neurotransmission inhibition (Resstel, Joca, Guimarães, et al. 2006) attenuate fear-related responses in aversive conditioning, elevated-plus maze, and burying tests (Shah and Treit 2003), suggesting that this cortical region has a significant role in wide varieties of fear and anxiety responses.
Probably reflecting the stress induced by our experimental conditions, nonconditioned rats also presented a significant, although smaller, increase in MAP and HR. However, practically no freezing response was observed in these animals. Reinforcing the possible involvement of NMDA receptors and NO synthesis in the vMPFC with cardiovascular responses to stress (Tavares and Corrêa 2006), NMDA receptor antagonist or drugs that interfere with NO-mediated neurotransmission attenuated these changes in MAP and HR.
The involvement of NMDA receptors in the vMPFC with cardiovascular responses has been investigated by several studies. L-glutamate administration into the vMPFC evoked MAP, HR, and sympathetic activity increases (Verberne 1996; Resstel and Corrêa 2005) through NMDA receptors (Resstel and Corrêa 2006b). Tachycardia, MAP and sympathetic tonus increases are associated with emotional arousal and stress responses (Berntson et al. 1998; Cacioppo et al. 1998; Carrive 2000). Also, the parasympathetic component of the baroreflex is inhibited during stress situations (Nosaka and Murata 1989; Neafsey 1990; Nosaka 1996), an effect that involves the vMPFC (Resstel et al. 2004, 2005). This effect is also mediated by local NMDA receptors (Resstel and Corrêa 2006a) and is characterized by a reduction of the parasympathetic activity (Resstel and Corrêa 2005). Thus, during defensive reactions, activation of vMPFC NMDA receptors could be modulating the bradycardic reflex and facilitating tachycardic responses.
Garthwaite et al. (1988) 1st reported that L-glutamate, acting on NMDA receptors in cerebellar cells, releases NO. In recent years, many electrophysiological and biochemical findings have suggested that NO modulates neuronal synaptic function in the central nervous system (Prast and Philippu 2001). NO production by nNOS correlates with activation of NMDA, but not of other, receptors (Garthwaite and Boulton 1995). This puzzling correlation was clarified when Brenman and Bredt (1997) reported that nNOS is connected to NMDA receptors via a postsynaptic density protein named PSD-95, being directly exposed to the influx of Ca2+ caused by activation of NMDA receptors. The Ca2+ transients that arise from activation of other receptors are presumable too diluted when they reach the vicinity of the enzyme nNOS.
To investigate a possible involvement of NO formation in contextual fear-conditioning responses, we microinjected the selective nNOS inhibitor N-propyl into the vMPFC. The drug abolished the behavior and cardiovascular responses evoked by aversive chamber reexposure. N-propyl was reported to be 3000 and 150 fold more potent to inhibit nNOS than inducible or endothelial NOS, respectively (Hevel et al. 1991; Martasek et al. 1996; Zhang et al. 1997). Confirming the involvement of NO in vMPFC effects, the contextual fear-conditioning responses were also blocked by c-PTIO, a NO scavenger, at a dose that was able to produce anxiolytic-like effects after injection into the dorsolateral periaqueductal gray (Aguiar et al. 2006). NO acts primarily as an intercellular messenger (Guix et al. 2005). It diffuses rapidly and influences target cells within a spatial sphere of approximately 0.3–0.4 mm of diameter (Wood and Garthwaite 1994; Lancaster 1997). Thus, NO can influence neuronal or glial cells far away from its neuron source (Wood and Garthwaite 1994; Lancaster 1997). Because c-PTIO is a cell membrane–impermeable compound (Ko and Kelly 1999), the present findings indicate that both NOS activation and extracellular release of NO in the vMPFC are involved in conditioned emotional responses.
The vMPFC is implicated in both learning and memory processes (Kesner et al. 1996; Ragozzino et al. 1998) and in the regulation of emotional behaviors (Morgan et al. 1993; Jinks and McGregor 1997; Quirk et al. 2000). It plays a crucial role in interfacing limbic and neocortical nuclei involved in fear responses (Sesack et al. 1989; Takagishi and Chiba 1991; Conde et al. 1995). Efferent projections connect the vMPFC to structures involved in fear-conditioning expression such as the periaqueductal gray matter, amygdala, hippocampus, and hypothalamus (Sesack et al. 1989; Fendt and Fanselow 1999; LeDoux 2000; Murphy et al. 2000). The vMPFC receives information about context and electrical shock associations through afferents from the amygdala and the hippocampus (Jay and Witter 1991). Both the hippocampus and the amygdala have been implicated in the associative processes underlying formation of conditioned fear (Fanselow and LeDoux 1999; LeDoux 2000; Bast et al. 2003). Moreover, lesions of the hippocampus or amygdala decreased conditioned fear cardiovascular responses (Galeno et al. 1984; Gentile et al. 1986; Iwata et al. 1986; LeDoux et al. 1988; Kim and Fanselow 1992; Maren and Fanselow 1997; Maren et al. 1997; Anagnostaras et al. 1999).
The basolateral amygdala sends glutamatergic projections to the central amygdala (Pitkanen et al. 1997). This nucleus is the main source of amygdala outputs to the brain stem and hypothalamic sites (Holstege et al. 1996) that mediate the behavioral and autonomic responses associated with fear (LeDoux et al. 1988; De Oca et al. 1998). Projections from the central amygdala are thought to mediate behavioral components of conditioned fear reactions (LeDoux et al. 1988; Helmstetter 1992). It has been proposed that vMPFC modulates conditioned fear responses via its projections to the amygdala (al Maskati and Zbrozyna 1989; Morgan et al. 1993; Rosenkranz and Grace 2001; Royer and Paré 2002). In addition, Quirk et al. (2003) observed that stimulation of the vMPFC alters the central amygdala neurons activity to basolateral amygdala inputs provide direct physiological support for the hypothesis that the vMPFC modulates fear responses by amygdala output (Garcia et al. 1999; LeDoux 2000).
Memory expression depends on the context in which memory is retrieved (Spear 1973; Tulving and Thomson 1973). In addition, reexposure to this context could engage either extinction or reconsolidation processes, with the latter initiating a 2nd time-dependent memory formation process (Duvarci and Nader 2004). To investigate a possible role of NMDA/NO-mediated neurotransmission in the vMPFC in these processes, we reexposed rats that had received LY235959 or N-propyl prior the test (day 1) to the aversive context 6 days later. The results showed that the 1st exposure causes a significant attenuation of the freezing and cardiovascular responses on reexposure, which was not modified by the drugs. However, if the animals have just received the drug treatments on day 1 and exposed to the aversive context on day 7, they will show similar freezing and cardiovascular responses to vehicle-treated animals exposed on day 1 after conditioning. This shows that the conditioning effects last for at least 1 week and are not modified by blocking of NMDA- or NO-mediated neurotransmission in vMPFC 1 day after conditioning. They also suggest that under our experimental conditions the 1st reexposure to the aversively conditioned context engages extinction processes that does not depend on NMDA/NO neurotransmission in the vMPFC at the time of reexposure. Corroborating these results, studies employing reversible blockade (Sierra-Mercado et al. 2006), NMDA receptor antagonism (Burgos-Robles et al. 2007), or protein synthesis inhibition (Santini et al. 2004) of vMPFC at the time of reexposure of the conditioned stimulus, failed to show any impairment in fear extinction.
In conclusion, the present results suggest that NMDA glutamate receptors and NO formation in the vMPFC play an important role on the expression of the behavioral and cardiovascular responses observed during fear evoked by contextual conditioning.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (postdoctoral fellowship 151931/2005-4 to L.B.M.R.); Fundação de Amparo à Pesquisa do Estado de São Paulo; CNPq.
Acknowledgments
The authors wish to thank Ivanilda A.C. Fortunato for technical help. Conflict of Interest: None declared.
References
- Aguiar DC, Moreira FA, Guimarães FS. Flight reactions induced by injection of glutamate N-methyl-d-aspartate receptor agonist into the rat dorsolateral periaqueductal gray are not dependent on endogenous nitric oxide. Pharmacol Biochem Behav. 2006;83:296–301. doi: 10.1016/j.pbb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Auton Nerv Syst. 1989;28:117–125. doi: 10.1016/0165-1838(89)90084-2. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Structure-activity analysis of binding kinetics for NMDA receptor competitive antagonists: the influence of conformational restriction. Br J Pharmacol. 1991;104:207–221. doi: 10.1111/j.1476-5381.1991.tb12409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Blum S, Runyan JD, Dash PK. Inhibition of prefrontal protein synthesis following recall does not disrupt memory for trace fear conditioning. BMC Neurosci. 2006;7:67. doi: 10.1186/1471-2202-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr Opin Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Res. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- Carrive P. Cardiovascular and behavioural components of conditioned fear to context after ganglionic and alpha-adrenergic blockade. Auton Neurosci. 2002;98:90–93. doi: 10.1016/s1566-0702(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A, Bhatnagar T, Sapru HN. Cardiovascular responses to microinjections of glutamate into the nucleus tractus solitarii of unanesthetized supracollicular decerebrate rats. Brain Res. 1998;801:88–100. doi: 10.1016/s0006-8993(98)00550-2. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1:418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Galeno TM, Van Hoesen GW, Brody MJ. Central amygdaloid nucleus lesion attenuates exaggerated hemodynamic responses to noise stress in the spontaneously hypertensive rat. Brain Res. 1984;291:249–259. doi: 10.1016/0006-8993(84)91257-5. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gentile CG, Jarrell TW, Teich A, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in the retention of differential pavlovian conditioning of bradycardia in rabbits. Behav Brain Res. 1986;20:263–273. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Gigg J, Tan AM, Finch DM. Glutamatergic excitatory responses of anterior cingulate neurons to stimulation of the mediodorsal thalamus and their regulation by GABA: an in vivo iontophoretic study. Cereb Cortex. 1992;2:477–484. doi: 10.1093/cercor/2.6.477. [DOI] [PubMed] [Google Scholar]
- Gigg J, Tan AM, Finch DM. Glutamatergic hippocampal formation projections to prefrontal cortex in the rat are regulated by GABAergic inhibition and show convergence with glutamatergic projections from the limbic thalamus. Hippocampus. 1994;4:189–198. doi: 10.1002/hipo.450040209. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci. 1992;106:518–528. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Hevel JM, White KA, Marletta MA. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991;266:22789–22791. [PubMed] [Google Scholar]
- Holstege G, Bandler R, Saper CB. The emotional motor system. Prog Brain Res. 1996;107:3–6. doi: 10.1016/s0079-6123(08)61855-5. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Ko GY, Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:6784–6794. doi: 10.1523/JNEUROSCI.19-16-06784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Martasek P, Liu Q, Liu J, Roman LJ, Gross SS, Sessa WC, Masters BS. Characterization of bovine endothelial nitric oxide synthase expressed in E. coli. Biochem Biophys Res Commun. 1996;219:359–365. doi: 10.1006/bbrc.1996.0238. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Pezze M, Feldon J, Heidbreder C. Differential involvement of dopamine in the shell and core of the nucleus accumbens in the expression of latent inhibition to an aversively conditioned stimulus. Neuroscience. 2000;97:469–477. doi: 10.1016/s0306-4522(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Baxter MG. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. Eur J Neurosci. 2003;18:3335–3342. doi: 10.1111/j.1460-9568.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- Nosaka S. Modifications of arterial baroreflexes: obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn J Physiol. 1996;46:271–288. doi: 10.2170/jjphysiol.46.271. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Murata K. Somatosensory inhibition of vagal baroreflex bradycardia: afferent nervous mechanisms. Am J Physiol. 1989;257:R829–R838. doi: 10.1152/ajpregu.1989.257.4.R829. [DOI] [PubMed] [Google Scholar]
- Ohmori J, Shimizu-Sasamata M, Okada M, Sakamoto S. Novel AMPA receptor antagonists: synthesis and structure-activity relationships of 1-hydroxy-7-(1H-imidazol-1-yl)-6-nitro-2,3(1H,4H)- quinoxalinedione and related compounds. J Med Chem. 1996;39:3971–3979. doi: 10.1021/jm960387+. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Sydney (Australia): Academic Press; 1997. [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Corrêa FM. Pressor and tachycardic responses evoked by microinjections of L-glutamate into the medial prefrontal cortex of unanaesthetized rats. Eur J Neurosci. 2005;21:2513–2520. doi: 10.1111/j.1460-9568.2005.04078.x. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM. Injection of l-glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor—nitric oxide in rat. Neuropharmacology. 2006a;51:160–167. doi: 10.1016/j.neuropharm.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Corrêa FM. Medial prefrontal cortex NMDA receptors and nitric oxide modulate the parasympathetic component of the baroreflex. Eur J Neurosci. 2006b;23:481–488. doi: 10.1111/j.1460-9568.2005.04566.x. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Fernandes KB, Corrêa FM. Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res. 2004;1015:136–144. doi: 10.1016/j.brainres.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Fernandes KB, Corrêa FM. Alpha-adrenergic and muscarinic cholinergic receptors are not involved in the modulation of the parasympathetic baroreflex by the medial prefrontal cortex in rats. Life Sci. 2005;77:1441–1451. doi: 10.1016/j.lfs.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Guimarães FG, Corrêa FM. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143:377–385. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Moreira FA, Corrêa FM, Guimarães FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006;172:294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Spear NE. Retrieval of memory in animals. Psychol Rev. 1973;80:163–194. [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Corrêa FM. Role of the medial prefrontal cortex in cardiovascular responses to acute restraint in rats. Neuroscience. 2006;143:231–240. doi: 10.1016/j.neuroscience.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Resstel LB, Corrêa FM. Interaction between glutamatergic and nitrergic mechanisms mediating cardiovascular responses to l-glutamate injection in the diagonal band of Broca in anesthetized rats. Life Sci. 2007;81:855–862. doi: 10.1016/j.lfs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352–373. [Google Scholar]
- Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol. 1996;270:R713–R719. doi: 10.1152/ajpregu.1996.270.4.R713. [DOI] [PubMed] [Google Scholar]
- Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res. 1995;692:190–194. doi: 10.1016/0006-8993(95)00665-d. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by N omega-propyl-L-arginine. J Med Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Murphy CA, Feldon J. Behavioural and cardiovascular responses during latent inhibition of conditioned fear: measurement by telemetry and conditioned freezing. Behav Brain Res. 2004;154:199–209. doi: 10.1016/j.bbr.2004.02.016. [DOI] [PubMed] [Google Scholar]