Abstract
Inhibitory neurotransmission is a critical determinant of neuronal network gain and dynamic range, suggesting that network properties are shaped by activity during development. A previous study demonstrated that sensorineural hearing loss (SNHL) in gerbils leads to smaller inhibitory potentials in L2/3 pyramidal neurons in the thalamorecipient auditory cortex, ACx. Here, we explored the mechanisms that account for proper maturation of γ-amino butyric acid (GABA)ergic transmission. SNHL was induced at postnatal day (P) 10, and whole-cell voltage-clamp recordings were obtained from layer 2/3 pyramidal neurons in thalamocortical slices at P16–19. SNHL led to an increase in the frequency of GABAzine-sensitive (antagonist) spontaneous (s) and miniature (m) inhibitory postsynaptic currents (IPSCs), accompanied by diminished amplitudes and longer durations. Consistent with this, the amplitudes of minimum-evoked IPSCs were also reduced while their durations were longer. The α1- and β2/3 subunit–specific agonists zolpidem and loreclezole increased control but not SNHL sIPSC durations. To test whether SNHL affected the maturation of GABAergic transmission, sIPSCs were recorded at P10. These sIPSCs resembled the long SNHL sIPSCs. Furthermore, zolpidem and loreclezole were ineffective in increasing their durations. Together, these data strongly suggest that the presynaptic release properties and expression of key postsynaptic GABAA receptor subunits are coregulated by hearing.
Keywords: α1 and β2/3 subunits, auditory cortex, development, GABAA receptor, hearing impairment
Introduction
Anatomically, inhibitory neocortical networks are outweighed by their excitatory counterparts by about 1:4, yet, if the integrity of inhibitory circuits is degraded, even a small amount, the properties of an entire network can be profoundly affected (Chagnac-Amitai and Connors 1989; Caspary et al. 1999; Cherubini and Conti 2001; Korpi et al. 2002; Mody and Pearce 2004; Farrant and Nusser 2005). Detailed studies on the intrinsic and synaptic properties of the inhibitory interneurons and their influence on the pre- as well as postsynaptic gain of the target cells are imperative for the evaluation of their contribution to normal development as well as pathological disorders. In the auditory cortex (ACx), for example, activation of γ-amino butyric acid (GABA) circuits leads to sharpening of onset responses and excitatory receptive field properties (Muller and Scheich 1988; Wang et al. 2000; Foeller et al. 2001). Such properties and the percepts that they support, such as speech discrimination in background noise, can be compromised by hearing loss, and this, in part, is due to a selective decline in inhibitory synapse function that shapes sound-driven response properties (Caspary et al. 2005).
The strength of inhibitory postsynaptic currents (IPSCs) represents, in part, the number of synaptic GABAA receptors and the specific array of subunits (Otis et al. 1994; Nusser et al. 1997, 1998). Modulation of the subunit expression and distribution, therefore, has profound consequences on the development and plasticity of inhibitory synapses (Fagiolini and Hensch 2000; Hensch 2005) and neural excitability under both physiological and pathophysiological conditions. In the mammalian brain, the most abundant pentameric receptors are comprised of α, β, and γ subunits; the most common stoichiometry within the neocortex is 2α:2β:1γ (Sieghart et al. 1999; Mehta and Ticku 1999; Knight et al. 2000).
The developmental expression of specific receptor subunits, and their distribution on a single neocortical or hippocampal neuron, can impart distinct functional properties. For example, experiments in “knock-in” mice show that while α1 subunits may participate in cortical plasticity, α2 subunits regulate neuronal firing (Fagiolini et al. 2004), presumably due to their abundance at synapses on the axon initial segment of pyramidal cells (Nusser et al. 1996; Fritschy et al. 1998). The profound changes in developmental expression of specific subunits (Laurie et al. 1992) may also underlie the ontogenic changes in inhibitory postsynaptic current (IPSC) kinetics (Vicini et al. 2001; Banks et al. 2002; Mody and Pearce 2004; Bosman et al. 2005; Huntsman and Huguenard 2006; Möhler 2006; Ing and Poulter 2007).
Experimental manipulations have shown that inhibitory neurotransmission is a critical determinant of neuronal network gain, suggesting that network properties are shaped by GABAergic transmission (Kilman et al. 2002; Burrone et al. 2002; Turrigiano 2007). Prior studies in the developing ventral auditory brain stem and midbrain have shown that the loss of cochlear activity for 1 to several days in vivo leads to a reduction of inhibitory synaptic gain due to diminished inhibitory postsynaptic potential (IPSP)/IPSC amplitudes and depolarized inhibitory reversal potential/current (Kotak and Sanes 1996; Vale and Sanes 2000). In the inferior colliculus (IC) neurons, such weakening of IPSCs is characterized by reduced inhibitory conductance and a compromised chloride cotransporter function (Vale and Sanes 2000; Vale et al. 2003). Furthermore, electrophysiological and quantitative electron microscopic (EM)-immunogold data show that developmental sensorineural hearing loss (SNHL) leads to a significant enhancement in excitatory synapse function in L2/3 pyramidal neurons of the ACx mediated by both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors (Kotak et al. 2005).
The current study addresses the question whether early auditory experience influences the development of GABAergic transmission. Our data show that the amplitudes of IPSCs decline following developmental SNHL. This was accompanied by the inability of the GABAA receptor subunits, α1 and β2/3, to respond to specific agonists. Moreover, increased frequency of spontaneous (s) IPSCs and miniature (m) IPSCs indicate parallel alterations at the presynaptic locus. Anatomically, quantitative EM-immunocytochemical assays reveal that presynaptic glutamic acid decarboxylase (GAD) increased and postsynaptic β2/3 subunit immunogold counts decreased following SNHL (Sarro E, Kotak VC, Sanes DH, Aoki C, unpublished data). Additionally, IPSCs from animals before hearing onset shared characteristics similar to SNHL IPSCs, including insensitivity to the subunit-specific agonists. Thus, SNHL may prevent the normal maturation of GABAergic transmission in the ACx.
Material and Methods
Surgery for SNHL
All protocols were reviewed and approved by New York University Institutional Animal Care and Use Committee. As described previously (Vale and Sanes 2002), cochlear ablations were performed on gerbil (Meriones unguiculatus) pups at postnatal day 10 (P10), just before the onset of response to airborne sound. Gerbil pups were anesthetized (methoxyflurane), which was assessed by a lack of toe-pinch response. Each cochlea was then rapidly removed with a forceps. The wound was sealed, the pups were placed on a heating pad, and then transferred to the breeding pair. Pups were then reared for 6–9 days with their parents under conditions identical to those for control pups. The age of surgery was chosen based on evidence that anteroventral cochlear nucleus cell number is unaffected by cochlear ablation after P9 (Tierney and Moore 1997). Cochlea removal was confirmed post mortem by opening each cochlea under a dissecting microscope and finding the absence of cochlear tissue but gel foam in its place.
Thalamocortical Brain Slice Recordings and Pharmacology
Horizontal thalamocortical brain slices that retained the afferent projection from ventral medial geniculate nucleus (MGv) to the ACx (thalamorecipient cortex, ACx; Kotak et al. 2005) were generated from P8–20 gerbils. The artificial cerebrospinal fluid (ACSF) contained (in mM): 125 NaCl, 4 KCl, 1.2 KH2PO4, 1.3 MgSO4, 26 NaHCO3, 15 glucose, 2.4 CaCl2, and 0.4 L-ascorbic acid (pH 7.3 when bubbled with 95% O2–5% CO2). ACSF was superfused in the recording chamber at 3 mL/min at 32 ±1 °C. Whole-cell recordings were obtained from layer 2/3 pyramidal neurons that were located in the thalamorecipient cortex. Neurons were visually identified under infrared differential interface contrast (IR-DIC) optics prior to obtaining a GΩ seal. The location of individual neuron cell bodies varied from 125 to 400 μm from the pial surface. The data in this paper were collected from 104 recordings from control (n = 43), SNHL (n = 45), and prehearing (n = 16) neurons.
GABAergic Activity and Pharmacological Manipulations
To characterize GABAA receptor–mediated sIPSCs and minimum-evoked (me) IPSCs whole-cell voltage-clamp recordings were made at a bandwidth of 10 kHz. The internal patch solution contained (in mM): 100 KCl, 40 K-gluconate, 10 NaCl, 10 [4-(2-hydroxyethyl)-1-piperazineethanesulfonic free acid] (HEPES), 4 MgATP, 0.1 ethyleneglycol-bis(2-aminoethylether)-N,N,N‘,N‘-tetraacetic acid (EGTA), and 5 lidocaine derivative QX-314 (pH = 7.2 with KOH). Bipolar platinum wire stimulating electrodes were placed in the MGv and in upper cortical layer 4 (L4) approximately 100 μm from the recording site to stimulate the thalamocortical and intracortical pathways, respectively. These stimulating electrodes were fabricated from 0.004“ diameter teflon-coated platinum wires (A-M systems, Carlsborg, WA) inserted into an approximately 2”-long double-barrel glass electrode. The tips of the wires were bared of the teflon coat (the exposed tip was 0.002” diameter).
To isolate inhibitory GABAergic activity, 6,7-Dinitroquinoxaline-2,3-dione (DNQX) (20 μM) and 2-amino-5-phosphonopentanoate (AP-5) (50 μM) were added to the superfusing ACSF to block AMPA and NMDA receptors, respectively. The drugs were applied for a minimum of 8 min before recording inhibitory currents. For acquiring sIPSCs and mIPSCs, at least 10 sweeps of 30-s duration each were acquired for each neuron at holding potential close to the resting membrane potential; VHOLD = −60 mV (Kotak et al. 2005). The mIPSC recordings were obtained in the presence of 1 μM tetrodotoxin (TTX).
To examine whether the strength of putative monosynaptic inhibitory connections was altered following SNHL, me-IPSCs were recorded in normal ACSF in voltage-clamp conditions at VHOLD = −60 mV. Incremental stimulus intensities were delivered to L4 at 0.1 Hz until an evoked IPSC was discernible (Fig. 2A). Stimulation at this intensity produced ≥50% failures in eliciting IPSCs. The absolute stimulus intensity varied approximately 5% between preparations and ages. Increasing the minimal intensity by more than 5% decreased the failure rate and increased the IPSC amplitude and possible polysynaptic innervations. Therefore, the minimal stimulation intensity was kept constant throughout each recording. The failure rates were not analyzed.
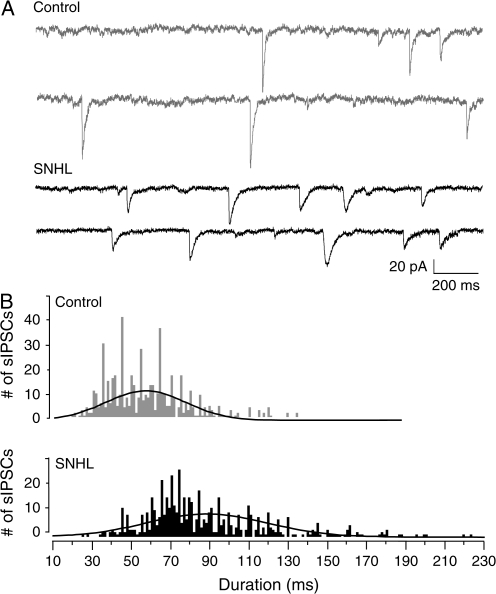
Figure 2.
SNHL sIPSC durations are long. (A) Two sweeps of sIPSCs recorded for 2 s each in a P17 control (top, 2 gray traces) and an age-matched SNHL neuron (bottom, black traces) at a holding potential of −60 mV. Each recording was acquired 10 s apart. Note that the durations of sIPSCs in the SNHL neuron are longer. (B) Distribution of all sIPSCs durations, 528 from 10 control neurons and 582 from 10 SNHL neurons, showing that the sIPSCs durations in SNHL neurons are significantly longer.
The function of the 2 GABAA receptor subunits was tested by the use of agonists. Zolpidem (100 nM, Tocris Biosciences, Bristol, UK) was applied to test for the presence of the α1 subunits, and loreclezole (10 μM, Tocris Biosciences) was applied to test for the presence of the β2/3 subunits. These agents were added to ACSF and bath applied for at least 15 min before recording the effect on sIPSC durations. These data were separately acquired in 2 second traces; at least 50 traces were recorded per neuron. Thus, they do not constitute a part of the sIPSC data on amplitudes and frequency recorded before. To validate that the recorded postsynaptic currents (sIPSCs, mIPSCs, and me-IPSCs) were due to GABAA receptor activity, bicuculline (20 μM, BIC methoiodide; Sigma, St Louis, MO) or GABAzine (500 nM-1μM, Tocris Biosciences) was applied at the end of some experiments (n = 6).
Data Collection and Analysis
Data were collected using a Macintosh G4 running a custom-designed IGOR (WaveMetrics, v6.0) macro called SLICE (Kotak et al. 2001). Evoked synaptic potentials and currents were analyzed off-line using a 2nd IGOR macro called SLICE ANALYSIS. Analysis was restricted to IPSC amplitudes that were ≥6 pA as these events could be reliably identified in baseline noise. sIPSC durations were manually analyzed off-line using the SLICE ANALYSIS program, by superimposing 1-ms vertical grids on traces. The time between the onset of the falling phase of the sIPSC and the return of the decay phase of the IPSC to the original baseline was considered as the total duration (ms). Any sIPSCs (2 or more) that summated before a single sIPSC decay returned to baseline were not considered in this analysis. To ascertain changes of sIPSC durations following experimental and pharmacological manipulations, analysis was restricted to IPSCs collected separately, majority (95%) of whose amplitudes varied between 20 and 80 pA (above). Thus, we biased this analysis such that there was no statistical difference between the control and SNHL sIPSC amplitudes (ca. 40 pA). This strategy discounted the amplitude-dependent fluctuations in sIPSC kinetic properties. Statistical tests (analysis of variance [ANOVA], Students' t-test for normally distributed data, or Wilcoxon nonparametric test for data that were not normally distributed) were performed using statistical software (JMP, SAS Institute, Cary, NC).
Results
Hearing Loss Alters sIPSCs and mIPSCs
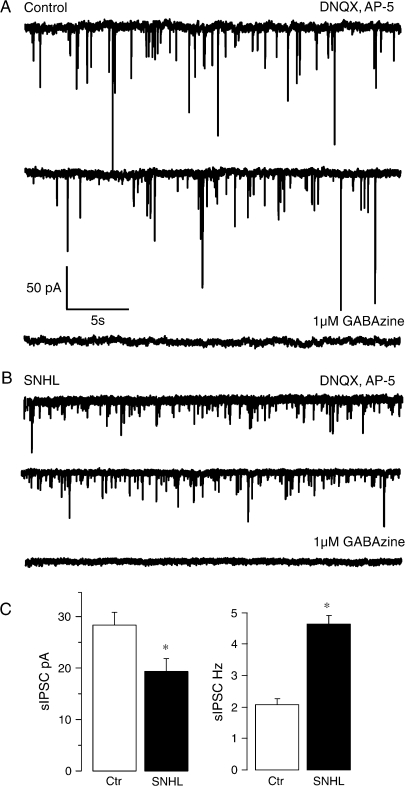
To determine whether hearing loss altered the inhibitory network properties, we 1st recorded sIPSCs under voltage-clamp conditions at a holding potential of −60 mV after blocking ionotropic glutamate receptor transmission. The results showed that the mean sIPSC frequency was increased while the mean amplitude was decreased significantly in SNHL neurons. (sIPSC frequency, mean ± standard error of the mean [SEM]: control, 2 ± 0.2 Hz vs. SNHL, 4.6 ± 0.2 Hz; t-test, t = 7.1; degrees of freedom [df] = 22, P = 0.0001; sIPSC amplitude, mean ± SEM: control, 28 ± 2 pA vs. SNHL, 19 ± 2 Hz; t-test, t = 2.6; df = 20, P = 0.01; Fig. 1). To further test whether these changes persisted in complete absence of presynaptic action potentials, TTX (500 nM) was added at the end of the recording session in some experiments. These results were comparable to sIPSCs, in that higher mIPSC frequency was accompanied by significantly reduced amplitudes for SNHL neurons (mIPSC frequency, mean ± SEM: control, 2.2 ± 0.4 Hz vs. SNHL, 5.2 ± 0.4 Hz; t-test, t = 4.5; df = 9, P = 0.002; mIPSC amplitude, mean ± SEM: control, 35 ± 3 pA vs. SNHL, 19 ± 3 Hz; t-test, t = 3.4; df = 9, P = 0.01; recordings not shown).
Figure 1.
SNHL sIPSC amplitudes are smaller and frequency is higher. (A, B) The top panel (A) shows 2 sweeps of sIPSCs recorded in the presence of DNQX and AP-5 for 30 s each in a P17 control neuron (A) and an age-matched SNHL neuron (B) at a holding potential of −60 mV. In both cases, each recording was acquired 1 min apart. Note that amplitudes of sIPSCs in the SNHL neuron are smaller, whereas the frequency is higher. In both control as well as SNHL recordings, addition of 1 μM GABAzine eliminates sIPSCs, showing that they were mediated by the activation of GABAA receptors. (C) Bar graphs summarizing the mean amplitude and frequency of sIPSCs recorded from 11 normal and 12 SNHL neurons. Note that the amplitude of mean sIPSCs is smaller in SNHL neurons (left panel, filled bar), whereas the mean frequency of sIPSCs is greater in SNHL neurons (right panel, filled bar). In this and all subsequent figures, asterisks indicate that the differences are significant (for statistics, see Results).
Hearing Loss Perturbs sIPSC Kinetics
Among other factors, sIPSC kinetic properties reflect underlying function of an array of GABAA receptor subunits. To measure durations, we used hundreds of control and SNHL sIPSCs that were separately recorded in 2-s sessions. To exclude the sIPSC amplitude-based variation in durations, we measured sIPSC durations of comparable amplitudes with an average of about 40 pA. This analysis showed that sIPSC durations were significantly longer in the SNHL group. (sIPSC duration, mean ms ± SEM: control, 58 ± 4 vs. SNHL, 89 ± 4; t = 5.6; df = 19, P = 0.0004; Fig. 2). The effect of hearing loss on sIPSC duration was thus independent of amplitudes because sIPSC amplitudes did not differ between the 2 groups; hence, it is discounted as a factor for this analysis (sIPSC amplitudes, mean pA ± SEM: control 39.6 ± 2, SNHL, 40 ± 3.3, P = 0.9).
Hearing Loss Decreases me-IPSCs
Our previous study showed that hearing loss significantly enhanced putative monosynaptic excitatory input originating from the MGv to L2/3 (Kotak et al. 2005). To determine whether the strength of putative monosynaptic inhibitory inputs from GABAergic interneurons to L2/3 pyramidal neurons was affected by SNHL, me-IPCSs (me-IPSCs) were recorded in the presence of blockers of ionotropic glutamate receptors, DNQX and AP-5. Over 100 intracortically evoked recordings were obtained from each neuron, of which about 50% responses contained an IPSC. The amplitudes of the 6 smallest me-IPSCs were averaged for each neuron. Comparison of these me-ISPCs revealed that activation of such putative unitary afferents produce significantly reduced IPSC amplitudes in SNHL neurons (me-IPSC amplitudes, mean pA ± SEM: normal, 14 ± 1.9 vs. SNHL, 7.7 ± 0.8; Wilcoxon's test, ν2 = 6.4; df = 20, P = 0.01; Fig. 3).
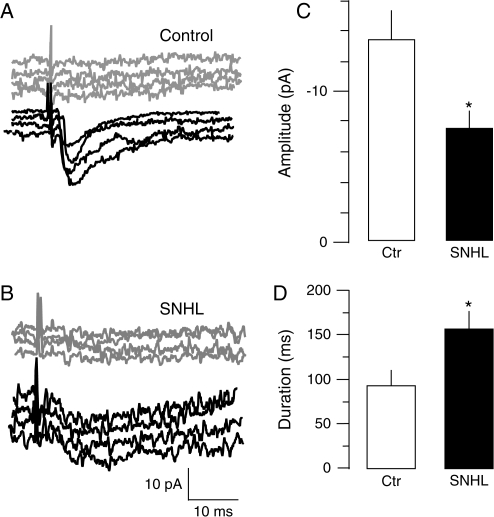
Figure 3.
SNHL me-IPSC amplitudes are smaller and durations are longer. (A) Intracortical me-IPSCs were recorded in voltage clamp at VHOLD of −60mV in the presence of ionotropic glutamate receptor blockers, DNQX and AP-5. me-IPSCs were elicited by stimulating L4 approximately 100 μm away from the L2/3 recording site at incremental intensities until the failures were replaced by responses. The intensity at which minimum IPSCs were discernible from failed responses (gray traces) was then chosen for successive recordings (black traces). Such minimal stimulation intensity produced a failure rate of 50% or more. (B) With the same method, note that me-IPSCs recorded from an age-matched SNHL neuron are smaller and longer (black traces). (C, D) Bar graphs summarizing the mean amplitude and duration of me-IPSCs recorded from 10 normal and 11 SNHL neurons. The amplitude of mean me-IPSCs is smaller in SNHL neurons (top panel, right, filled bar), whereas the mean duration of near me-IPSCs is longer in SNHL neurons (bottom, right panel, filled bar).
Because the duration of sIPSCs differed, we acquired additional data to compare me-IPSC durations. For this, IPSCs were examined slightly above minimum thresholds for control and SNHL neurons for consistent kinetic measurements. The stimulus intensity was adjusted about 5% above that which produced a 50% failure rate for these experiments. IPSC amplitudes between 15 and 40 pA were recorded, and thus, the mean amplitude did not differ between the 2 groups (near me-IPSC amplitudes, mean pA ± SEM: control, 20.5 ± 8.7 vs. SNHL, 19.1 ± 2.1, df = 13, P = 0.7). However, the IPSC durations were significantly longer in SNHL neurons (IPSC duration, mean ms ± SEM: control, 92.5 ± 14.5 vs. SNHL, 156.5 ± 20.4; t = 2.55; df = 13, P = 0.025; Fig. 3).
SNHL May Disrupt α1 and β2/3 Subunit Function
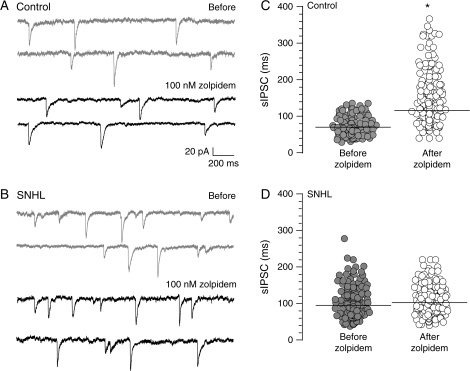
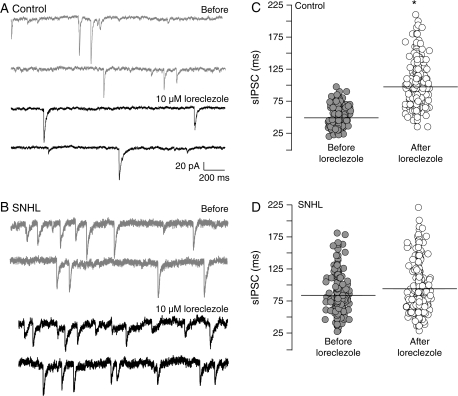
If the hearing loss–mediated increase in sIPSC duration involves a change in the expression of GABAA receptor subunits, then the efficacy of specific subunit agonists should be perturbed. To test this idea, agonists for GABAA receptor subunits known for their high affinity to the benzodiazapine-binding site (zolpidem, α1 agonist) or an anticonvulsant site (loreclezole, β2/3 agonist) were employed. Whereas zolpidem (100 nM) significantly prolonged sIPSC durations in control neurons, the agonist failed to enhance sIPSC durations in SNHL neurons (control sIPSC durations, mean ms ± SEM: before zolpidem, 69 ± 4 vs. after zolpidem, 114 ± 10; t = 5.6; df = 9, P = 0.003; SNHL sIPSC durations mean ms ± SEM, before zolpidem, 94 ± 6 vs. after zolpidem, 102 ± 7, t = 0.8, df = 9, P = 0.4; Fig. 4). Similarly, bath application of the β2/3 subunit agonist, loreclezole (10 μM), significantly lengthened sIPSC durations in control neurons but did not significantly affect sIPSC durations in SNHL neurons (control sIPSC durations, mean ms ± SEM: before loreclezole, 49 ± 2 vs. after loreclezole, 97 ± 5, Wilcoxon's test, χ2 = 6.8; P = 0.009; SNHL sIPSC durations, mean ms ± SEM: before loreclezole, 83 ± 3 vs. after loreclezole, 94 ± 12, Wilcoxon's test, χ2 = 0.01, P = 0.9; Fig. 5).
Figure 4.
SNHL disrupts α1 subunit function. (A) Two sweeps of sIPSCs recorded for 2 s each in a P17 control (top, 2 gray traces) at a holding potential of −60 mV. Each recording was acquired 10 s apart. Note that GABAA receptor α1 subunit–specific agonist, zolpidem, prolongs sIPSC durations (bottom, 2 black traces). (B) Note that in an age-matched SNHL neuron, zolpidem application does not produce an increase in sIPSC durations. (C, D) Distribution of all sIPSCs durations, 470 from 5 control neurons and 572 from 5 SNHL neurons, showing that the sIPSCs durations are significantly prolonged by zolpidem treatment in control neurons but not in SNHL neurons. Horizontal bars indicate mean sIPSC durations.
Figure 5.
SNHL disrupts β2/3 subunit function. (A) Two sweeps of sIPSCs recorded for 2 s each in a P17 control (top, 2 gray traces) at a holding potential of −60 mV. Each recording was acquired 10 s apart. Note that GABAA receptor β2/3 subunit–specific agonist, loreclezole, prolongs sIPSC durations (bottom, 2 black traces). (B) Note that in an age-matched SNHL neuron, loreclezole application does not produce an increase in sIPSC durations. (C, D) Distribution of all sIPSCs durations, 564 from 5 control neurons and 608 from 5 SNHL neurons, showing that the sIPSCs durations are significantly prolonged by loreclezole application in control neurons but not in SNHL neurons. Horizontal bars indicate mean sIPSC durations.
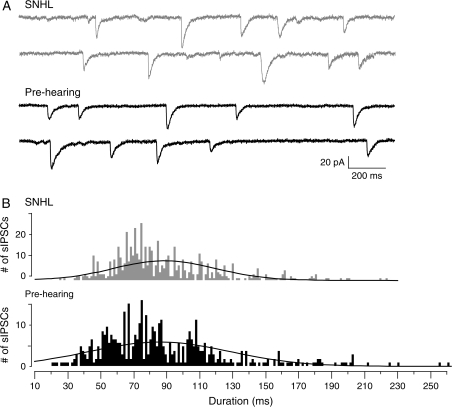
We therefore tested the hypothesis that SNHL may perturb the maturation of processes that underlie shortening of sIPSC durations following hearing onset. Additional recordings from animals before hearing onset (prehearing; P8–10) were therefore performed. The results showed that the sIPSC durations resembled the prolonged duration sIPSCs in SNHL neurons, when compared with controls. A 3-way comparison of means revealed that there was no significant difference between the sIPSC durations between SNHL and prehearing neurons, while the control sIPSC duration differed significantly from both groups (ANOVA, F = 15.2, df = 27; sIPSC durations, mean ms ± SEM: prehearing, 90.4 ± 6.5 vs. SNHL, 89 ± 4, t = 0.2, df = 17, P = 0.8; prehearing, 90.4 ± 6.5 vs. control, 59 ± 3.8; t = 4.1, df = 17, P = 0.0005, Fig. 6). This effect was independent of the amplitudes of sIPSCs (ANOVA, F = 0.2, df = 20, P = 0.8). Recordings of near me-IPSCs from prehearing neurons also showed that the durations were similar to SNHL me-IPSCs (IPSC durations, mean ms ± SEM: prehearing, 154.8 ± 19.5 vs. SNHL, 156.5 ± 20.4, t = 0.061, df = 13, P = 0.95).
Figure 6.
Prehearing IPSC durations are long. (A) Two sweeps of sIPSCs recorded for 2 s each in a P17 SNHL (top, 2 gray traces, taken from Figure 2) and a P10 prehearing neuron (bottom, 2 black traces) at a holding potential of −60 mV. Each recording was acquired 10 s apart. Note that the durations of sIPSCs in the prehearing neuron are long as in the SNHL neuron. (B) Distribution of all sIPSCs durations, 582 from 10 SNHL neurons and 469 from 8 prehearing neurons, showing the close resemblance between the sIPSC durations of prehearing and SNHL neurons. For comparison, see control sIPSC durations in Figure 2.
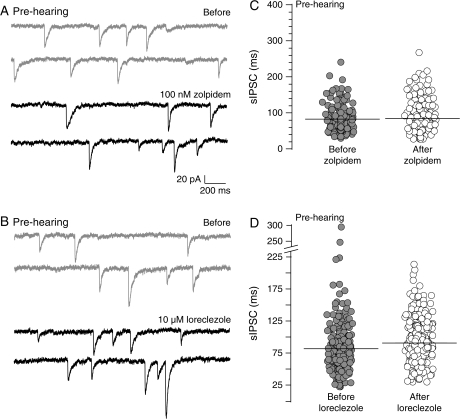
To further test whether the reduced sensitivity of the SNHL sIPSCs to the α1 and β2/3 subunit agonists reflects an immature state, the agents were tested in prehearing neurons. Similar to SNHL, sIPSCs from prehearing animals did not show a significant change in duration after zolpidem or loreclezole applications (prehearing sIPSC durations, mean ms ± SEM; before zolpidem, 82.5 ± 4.3 vs. after zolpidem, 84.2 ± 6.0, t-test, t = 0.2, df = 7, P = 0.8; before loreclezole, 79.0 ± 3.8 vs. after loreclezole, 90.0 ± 7.4, t-test, t = 1.3, df = 7, P = 0.2; Fig. 7).
Figure 7.
Prehearing IPSC durations are insensitive to α1 and β2/3 subunit agonists. (A) Two sweeps of sIPSCs recorded for 2 s each in a P10 prehearing neuron (top, 2 gray traces) at a holding potential of −60 mV. Each recording was acquired 10 s apart. Note that the GABAA receptor α1 subunit–specific agonist, zolpidem, does not prolong sIPSC durations (2 black traces). (B) Note that in another age-matched prehearing neuron, the application of the β2/3 agonist, loreclezole, does not prolong sIPSC durations. (C, D) Distribution of all sIPSC durations, from all prehearing neurons showing that the sIPSCs durations are not prolonged by zolpidem (581 sIPSCs from 4 neurons) or loreclezole (544 sIPSCs from 4 neurons). This result is similar to that obtained for SNHL neurons (see Figs 4 and 5).
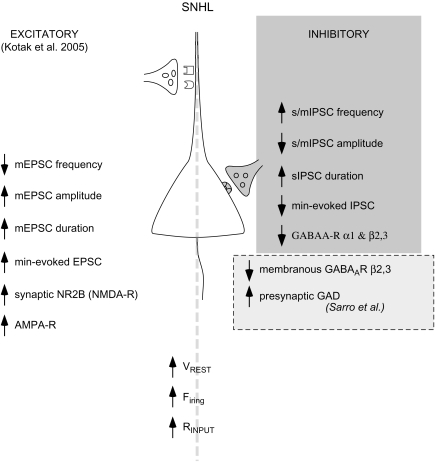
Figure 8 is a schematic diagram summarizing the present findings on GABAergic transmission following SNHL, along with our previous findings on changes depicting heightened intrinsic and excitatory synaptic properties (Kotak et al. 2005) and 2 changes assayed at an EM-immunocytochemical level (Sarro E, Kotak VC, Sanes DH, Aoki C, unpublished data).
Figure 8.
Summary sketch of a L2/3 SNHL pyramidal neuron. This sketch highlights the present findings that hearing loss leads to alterations in inhibitory synaptic properties (top right panel, gray). Further, they accompany coadjustments in the passive and active intrinsic (bottom panel) and excitatory synaptic properties (bottom left panel). An upward arrow indicates increased whereas a downward arrow indicates decreased function. For example, SNHL caused a rise in resting membrane potential (VREST), firing properties, and input resistance (RINPUT; bottom panel; Kotak et al. 2005). For thalamocortical synapses, SNHL decreased the release probability (mEPSC frequency), whereas increasing mEPSC and thalamically evoked minimum-EPSC amplitudes and enhancing current carried by the NR2B subunits of the NMDA receptor (left panel). In contrast, the present study shows increased GABA release probability (higher sIPSCs and mIPSCs frequency) is accompanied by a decrease in sIPSC and me-IPSC amplitudes. Further, an inability of α1 and β2/3 subunit–specific agonists to alter sIPSCs recorded from SNHL and prehearing neurons sugests an arrest in the maturation of GABAergic transmission. The bottom right panel (dashed box) displays an observation derived from EM-immunocytochemistry (Sarro E, Kotak VC, Sanes DH, Aoki C, unpublished data) that postsynaptic β2/3 subunit distribution is disrupted. In addition, the presynaptic terminals may synthesize/release more GABA. The cumulative outcome of such robust homeostatic alterations following hearing loss may adjust the cortical network at a new set point in anticipation that peripheral activity will be restored.
Discussion
Deafness and GABAergic Properties
Despite the strides made by behavioral and by in vivo physiology experiments, we are just beginning to understand the biophysical and synaptic alterations in the ACx that attend moderate or severe hearing loss. A previous report demonstrated that SNHL elevates excitability in L2/3 pyramidal neurons as reflected by changes in the passive membrane properties, increased thalamocortical excitatory drive, and diminished maximum-evoked IPSPs (Kotak et al. 2005). A fundamental question is whether inhibitory synapse function is altered at both pre- and postsynaptic loci. The present study shows that SNHL prevents the normal maturation of presynaptic release and postsynaptic GABA receptor function. Specifically, while the presynaptic terminals may release GABA more frequently (Fig. 1), the diminished amplitudes of unitary afferent-evoked inhibitory currents indicate inadequate postsynaptic GABAA receptor activation (Figs 1–3, Cherubini and Conti 2001; Nusser et al. 1997, 1998). Futhermore, prolonged sIPSCs and evoked IPSCs, resembling those recorded in prehearing animals (P10), suggest that SNHL prevents the maturation of inhibitory synaptic properties. These altered IPSC kinetics are well correlated with the diminished efficacy of the α1 and β2/3 subunit–specific agonists in SNHL neurons (Figs 4–5) and prehearing neurons (Fig. 7). We argue that hearing experience is critical for the emergence of mature pre- and postsynaptic properties of GABAergic transmission in the ACx, and this involves key subunits integral to the postsynaptic GABAA receptors.
Complete hearing loss is expected to affect endogenous patterns of activity across the ascending auditory pathway and key integrating elements in the brain stem, midbrain, and thalamus. For example, prior to the onset of hearing, gerbil IC neurons display spontaneous activity (Kotak and Sanes 1995). Maintained discharge at the level of the eighth nerve and ventral cochlear nucleus (VCN) originates within the cochlea of adult and juvenile mammals (Bock and Webster 1974; Koerber et al. 1966; Tucci et al. 1999; Sheperd et al. 1999; Tucci et al. 2001; Lee et al. 2001; Cook et al. 2002., Yu et al. 2005) and pre- and posthatched birds (Born and Rubel 1988; Born et al. 1991; Lippe 1994). An alteration in VCN spontaneous activity is expected to have an impact on IC electrical activity. In the gerbil, 2-deoxyglucose studies demonstrate that the metabolic activity is decreased in the contralateral lobe of the IC following either conductive hearing loss or SNHL, even in relative silence (Tucci et al. 1999, 2001). Together, these findings suggest that cochlear ablation, either in infancy or adulthood, results in a significant loss of peripheral action potentials and neurotransmission during the period immediately after ablation, and these factors likely influenced the inhibitory properties we assayed in the cortex.
Partial or total loss of activity in the somatosensory, visual, and auditory systems indicates downregulation of GABAergic transmission, and our data concur with these findings. For example, blocking action potentials by chronically exposing cultured neurons with tetrodotoxin leads to a significant decline in mIPSC amplitudes due to a decrease in the average number of open GABA-gated channels and their inappropriate clustering at the postsynaptic membrane that includes β subunits (Kilman et al. 2002). We observed a similar reduction in the amplitudes of sIPSC and mIPSC in SNHL neurons suggesting decreased GABAA receptor function is likely associated with a lower number of GABAA receptors (Nusser et al. 1997, 1998). The diminished sIPSC and mIPSC and putative unitary IPSC amplitudes in SNHL neurons (Figs 1 and 2) correspond with a decline in inhibitory conductance in the IC following bilateral SNHL (Vale and Sanes 2000). It is also possible that decreased inhibitory response amplitudes were due to depolarization of the IPSP reversal potential as shown in the lateral superior olivary and IC neurons (Kotak and Sanes 1996; Vale and Sanes 2000; Vale et al. 2003), although we did not test this. Similarly, weakened connections between fast-spiking GABAergic interneurons and star pyramidal neurons are observed in L4 of visually deprived animals (Maffei et al. 2004). Our observations may additionally explain the reduced amplitude IPSPs when maximum number of afferents are activated (Kotak et al. 2005).
Our data also suggest that at the presynaptic level, SNHL neurons appear to release more GABA (Fig. 1; and increased mIPSCs frequency, results), and this correlates with a significantly enhanced GAD immunoreactivity quantified ultrastructurally at the inhibitory terminals that impinge on L2/3 pyramidal neurons (Sarro E, Kotak VC, Sanes DH, Aoki C, unpublished data). In lieu of an enhanced presynaptic GABA release probaility, it is possible that the altered kinetics in sIPSCs we observe may additionally be caused by postsynaptic GABAA receptor desensitization (Jones and Westbrook 1995), although this hypothesis remains to be tested.
Development and GABAergic Properties
The strong resemblance of sIPSC characteristics in SNHL neurons with those recorded before hearing onset (Fig. 6) implies an arrest in the normal expression of GABAergic transmission at pre- and postsynaptic loci. We found that SNHL leads to longer duration sIPSCs (Fig. 2) and near me-IPSCs (Fig. 3), which resemble long IPSCs before hearing onset (Fig. 6). Developmental shortening of IPSCs is consistent with long mIPSCs recorded during early development from rat cerebellar granule cell and mouse cerebellar stellate neurons (Tia et al. 1996; Nusser et al. 1997, Vicini et al. 2001). In stellate neurons, for example, mIPSCs at P11 are long, whereas at P35, they decay 5 times faster (Vicini et al. 2001).
The subunit-specific agonists zolpidem for α1 subunits (Pritchett and Seeburg 1990; Wafford et al. 1994; Luddens et al. 1995; Rudolph and Möhler 2004) and loreclezole for β2/3 subunits (Wingrove et al. 1994; Bacci et al. 2003) target GABAA receptor subunits that affect IPSC kinetics. Both α1 and β2/3 subunits are activated by synaptically released GABA (Amin and Weiss 1993; Connolly et al. 1996; Baumann et al. 2003). Functions of these subunits in L2/3 pyramidal neurons can be assayed by the magnitude of effect of these 2 agents, which act to enhance IPSC durations (Figs 4 and 5, Bacci et al. 2003). Importantly, these agents can be used to assess whether the subunit expression undergoes developmental changes in parallel to the alterations in the amplitudes and kinetics of IPSCs. For example, in cerebellar inhibitory synapses, mIPSCs recorded in α1 subunit–deficient mice at P35 are as slow as those at P11 suggesting that lack of α1 subunit insertion in postsynaptic receptors is responsible for the prolonged inhibitory synaptic currents. Also, in recombinant systems, rapid application of GABA to outside-out excised patches generates faster currents with α1β2γ2 than with other subunit combinations (Verdoorn 1994; Gingrich et al. 1995; Lavoie et al. 1997; McClellan and Twyman 1999), which agrees with the predominant stoichiometry (α1β2γ2) of the GABAA receptor in mammalian cortex (Korpi et al. 2002; Möhler 2006; Huntsman and Huguenard 2006). Similarly, a progressive shortening of mIPSCs in thalamic relay neurons during the 1st postnatal month is accompanied by an increased expression of α1 subunit transcripts and decrease in the α2 transcripts. Further, thalamic organotypic cultured neurons with an overexpressed α1 subunit showed faster mIPSCs (Okada et al. 2000). Similar to the α1 subunit, the β2/3 subunits may emerge during postnatal development. For example, IPSC sensitivity to loreclezole increases across postnatal life in rat dentate granule cells (Kapur and MacDonald 1999), rat medial septum/diagonal band neurons (Hsiao et al. 1998), and mouse cerebellar granule cultured neurons (Ortinski et al. 2004). In fact, the developmental upregulation of β2/3 subunit function may depend on α1 subunit expression; the effect of loreclezole is compromised in IPSCs from α1 subunit −/− mice (Ortinski et al. 2004).
Consistent with these studies, our data also suggest that the α1 and β2/3 subunits are upregulated during development. This is shown by the significant augmentation of sIPSCs in control P17–19 neurons by zolpidem and loreclezole and in contrast the inability of these agonists to potentiate sIPSC durations in prehearing animals (Fig. 6). Similarly, the inability of zolpidem and loreclezole to augment SNHL sIPSCs strongly implies that SNHL may halt the normal expression of the α1 and β2/3 subunits on L2/3 cells that otherwise would impart shorter IPSC kinetics (Fig. 2, Okada et al. 2000; Banks et al. 2002; Amin and Weiss 1993; Wafford et al. 1994; Gingrich et al. 1995; Jones and Westbrook, 1995; Baumann et al. 2003). Significantly lower β2/3 immunogold counts observed in SNHL neurons at the normal postsynaptic membraneous site supports this deduction (Sarro E, Kotak VC, Sanes DH, Aoki C, unpublished data). Importantly, similar to an area-specific expression of the α1 subunits in developing rat neocortex that may be mediated via activity (Paysan et al. 1997), the expression and proper distribution of the α1 and β2/3 subunits appear to be regulated by auditory activity.
The prolonged sIPSCs observed in prehearing animals and the inability of the subunit-specific agonists to affect their durations implies that α1 and β2/3 are not predominantly expressed in the ACx before hearing onset. This raises an important question: what other subunits may underlie such long IPSCs that may persist in an absence of acoustically driven activity? It is known that the developmental expression of the α1 subunit mRNA in the cerebellum is very low during early life (Bovolin et al. 1992; Laurie et al. 1992). Other subunits such as α5 (Dunning et al. 1999), α2, and α3 are likely to mediate long sIPSCs similar to those found early in development. As a matter of fact, mRNA for α2 and α3 subunits are observed in early development (Laurie et al. 1992).
Cortical Excitability and GABAergic Properties
The coadjustments of GABAergic function at pre- and postsynaptic loci are in contrast with the amplified strength of the thalamocortical synapses following SNHL. Decreased probability of glutamate release (miniature excitatory postsynaptic currents [mEPSCs]) was accompanied by increased AMPAergic and NMDAergic participation (me-EPSCs) and higher NR2B immunogold counts at the asymmetric synapses (Kotak et al. 2005). Coupled with altered membrane properties favoring heightened excitability, the significant decline in postsynaptic GABAergic function may support higher discharge rates and thus alter the network properties of the ACx. Figure 8 shows a diagrammatic sketch of a L2/3 pyramidal SNHL neuron summarizing the present and previous findings. Although this model represents synaptic and biophysical properties following SNHL, it may apply to a range of conditions that decrease sound-evoked activity (e.g., the hair cell loss due to ototoxic drugs, aging, or noise-induced trauma, Syka 2002; Ling et al. 2005). Such weakened inhibition may eventually lead to flawed communication among neurons and a disorganized tonotopy (Kaur et al. 2004). For example, even mild to moderate forms of conductive hearing loss produces synaptic modifications within the ACx (Xu et al. 2007), and reduced GABAergic transmission may contribute. The current findings may also explain, in part, the changes that result from partial hair cell damage including the unmasking of excitatory sidebands and discharge characteristics (Irvine and Rajan 1997; Rajan 1998; Salvi et al. 2000; Wang, Ding, et al. 2002; Wang, McFadden, et al. 2002). Diminished inhibitory transmission may also contribute to temporal jitter to acoustic cues; for example, both the excitatory synaptic and spike precision are compromised at auditory nerve–bushy cell synapses in the cochlear nucleus of early-onset hearing-impaired mice (Wang and Manis 2005). The net effects of such pre- and postsynaptic modifications upon ACx networks and their input–output functions remain to be identified. The overall downscaling of inhibitory gain shown for SNHL neurons may be associated with various clinical conditions such as epilepsy, tinnitus, and presbycusis that are also characterized by reduced GABAergic gain. In addition to the use of implant-based prostheses, attempts to design targeted drugs with specific affinity toward distinct GABAA receptor subunits holds potential to alleviate deficits for the hearing impaired.
Funding
National Institute of Health DC006864 (to D.H.S. and V.C.K.).
Acknowledgments
Conflict of Interest: None declared.
References
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince D. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophysiol. 2005;94:338–346. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- Bock GR, Webster WR. Spontaneous activity of single units in the inferior colliculus of anesthetized and unanesthetized cats. Brain Res. 1974;76:150–154. doi: 10.1016/0006-8993(74)90521-6. [DOI] [PubMed] [Google Scholar]
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Born DE, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: presynaptic action potentials regulate protein synthesis in nucleus magnocellularis neurons. J Neurosci. 1988;8:901–919. doi: 10.1523/JNEUROSCI.08-03-00901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolin P, Santi MR, Puia G, Costa E, Grayson D. Expression patterns of gamma-aminobutyric acid type A receptor subunit mRNAs in primary cultures of granule neurons and astrocytes from neonatal rat cerebella. Proc Natl Acad Sci USA. 1992;89:9344–9348. doi: 10.1073/pnas.89.19.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy MN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory input. J Neurosci. 2005;25:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989;61:747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Cook RD, Hung TY, Miller RL, Smith DW, Tucci DL. Effects of conductive hearing loss on auditory nerve activity in gerbil. Hear Res. 2002;164:127–137. doi: 10.1016/s0378-5955(01)00424-5. [DOI] [PubMed] [Google Scholar]
- Dunning DD, Hoover CL, Soltesz I, Smith MA, O'Dowd DK. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABA(A)-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Mahoney JC, West JR, Frye GD. Development of GABAA receptors on medial septum/diagonal band (MS/DB) neurons after postnatal ethanol exposure. Brain Res. 1998;810:100–113. doi: 10.1016/s0006-8993(98)00891-9. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor beta1 subunit. J Physiol. 2006;572:459–475. doi: 10.1113/jphysiol.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing T, Poulter MO. Diversity of GABA(A) receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci. 2007;25:723–734. doi: 10.1111/j.1460-9568.2007.05331.x. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R. Injury-induced reorganization of frequency maps in adult auditory cortex: the role of unmasking of normally inhibited inputs. Acta Otolaryngol Suppl. 1997;532:39–45. doi: 10.3109/00016489709126143. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kapur J, MacDonald RL. Postnatal development of hippocampal dentate granule cell γ-aminobutyric acid A receptor pharmacological properties. Mol Pharmacol. 1999;55:444–452. [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniatures IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber KC, Pfeiffer RR, Warr WB, Kiang NY. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp Neurol. 1966;16:119–130. doi: 10.1016/0014-4886(66)90091-4. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABAA receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kotak VC, DiMattina C, Sanes DH. GABAB and Trk receptor signaling mediates long-lasting inhibitory synaptic depression. J Neurophysiol. 2001;86:536–540. doi: 10.1152/jn.2001.86.1.536. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol. 1995;74:1611–1620. doi: 10.1152/jn.1995.74.4.1611. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSPs. J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Stephanson FA, Tallman JF, Ramabahdran TV. Monospecific antibodies as probes for the stoichiometry of recombinant GABA(A)receptors. Receptors Channels. 2000;7:213–226. [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73:2518–25256. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddens H, Korpi ER, Seeburg PH. GABAA/benzodiazepine receptor heterogeneity: neurophysiological implications. Neuropharmacology. 1995;34:245–254. doi: 10.1016/0028-3908(94)00158-o. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Muller CM, Scheich H. Contribution of GABAergic inhibition to the response characteristics of auditory units in the avian forebrain. J Neurophysiol. 1988;59:1673–1689. doi: 10.1152/jn.1988.59.6.1673. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–111144. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABA(A) receptor alpha subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Kentaroh T, Zhanyan F, Vicini S. Expression of distinct α subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paysan J, Kossel A, Bolz J, Fritschy JM. Area-specific regulation of gamma-aminobutyric acid type A receptor subtypes by thalamic afferents in developing rat neocortex. Proc Natl Acad Sci USA. 1997;94:6995–7000. doi: 10.1073/pnas.94.13.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographical map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Baxi JH, Hardie NA. Response of inferior colliculus neurons to electrical stimulation of the auditory nerve in neonatally deafened cats. J Neurophysiol. 1999;82:1363–1380. doi: 10.1152/jn.1999.82.3.1363. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Höger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABA(A) receptor alpha 6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney TS, Moore DR. Naturally occurring neuron death during postnatal development of the gerbil ventral cochlear nucleus begins at the onset of hearing. J Comp Neurol. 1997;387:421–429. [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope. 1999;109:1359–1371. doi: 10.1097/00005537-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res. 2001;155:124–132. doi: 10.1016/s0378-5955(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Quirk K, McKernan RM, Wingrove PB, Whiting PJ, Kemp JA. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;285:95–98. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;19:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus end bulb synapse during age-related hearing loss in mice. J Neurophysiol. 2005;94:1814–1824. doi: 10.1152/jn.00374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the gamma-aminobutyric acid type A receptor is determined by a single amino acid in the beta 2 and beta 3 subunit. Proc Natl Acad Sci USA. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in the developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–968. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]