Abstract
Background
Early-life emotional stress may be associated with affective and cognitive disorders later in life, yet satisfactory animal models for studying the underlying mechanisms are limited. Because maternal presence and behavior critically influence molecular and behavioral stress responses in offspring, we sought to create a model of dysfunctional, fragmented maternal nurturing behavior that would, in turn, provoke chronic early-life stress in the offspring.
Methods
Dams’ nursing and nurturing behaviors were altered by limiting their ability to create satisfactory nests during postpartum days 2–9. Maternal behavior was observed throughout the diurnal cycle, and the frequency and duration of nurturing behaviors were scored. In addition, potential stress and anxiety of the dams were assessed using behavioral, molecular and hormonal measures.
Results
Both the quantity and the quality of dams’ care of their pups were profoundly influenced by restriction of nesting materials in their cages: licking/grooming activities decreased and the frequency of leaving the pups increased, resulting in fragmented interactions between the dams and pups. The abnormal activity patterns of the dams were accompanied by increased anxiety-like behavior in the open field, but not in the elevated plus maze tests. Additionally, dams’ plasma corticosterone levels and adrenal weights were augmented, suggesting chronic stress of these dams. By the end of the limited-nesting, stress-inducing period, hypothalamic corticotropin releasing hormone (CRH) mRNA expression was reduced in the limited-nesting dams, while arginine-vasopressin (AVP) mRNA levels were not significantly affected.
Conclusion
Limiting dams’ ability to construct a nest for their pups leads to an abnormal repertoire of nurturing behaviors, possibly as a result of chronic stress and mild anxiety of the dams. Because the fragmented and aberrant maternal behavior provoked chronic stress in the pups, the limited-nesting paradigm provides a useful tool for studying the mechanisms and consequences of such early-life stress experience in the offspring.
Keywords: Maternal behavior, stress, depression, CRH, vasopressin, programming
Introduction
The first week of life in the rat is crucial for appropriate maturation of the stress response and the underlying hypothalamo-pituitary adrenal (HPA) axis (Hess 1969; Meaney and Aitken 1985; Plotsky and Meaney 1993). The critical impact of maternal behavior and nurturing on functional and molecular components of the offspring’s CNS have been demonstrated in several species (e.g., Brunelli et al., 1994; Ziabreva et al., 2000), and maternal sensory input (i.e. licking and grooming) is necessary for proper development of HPA responses (Eghbal-Ahmadi et al., 1999; Caldji et al., 2000). Enhanced maternal care increases the ability of the pups to deal with future stressors throughout life (Liu et al., 1997; Avishai-Eliner et al., 2001a; Fenoglio et al., 2006a), reflected in relatively attenuated neuroendocrine stress responses. In contrast, deficient maternal care promotes exaggerated stress-responses later in life (Schmidt et al., 2004). Separation from the mother, particularly if recurrent, also promotes ‘hyperactive’ stress responses later in life (Plotsky and Meaney, 1993; Kehoe et al., 1998; Workel et al., 2001). These data suggest that both the quantity and quality of the dam’s behavior and care critically influence the developing HPA axis, as well as ‘program’ it for life (O’Reagan et al., 2001; Wellberg and Seckl 2001; Avishai-Eliner et al., 2002; Fenoglio et al., 2006a,b).
Whereas consistent and effective maternal care contribute to normal development of the stress response, deficient or abnormal care has been linked to human affective disorders (Sanchez et al., 2001; Heim et al., 2001, 2004; Charney and Manji, 2004), with associated dysfunction of the HPA axis. Indeed, an abnormal HPA axis is a hallmark of several depressive disorders, suggesting that perturbation of this axis, perhaps during a critical developmental period, may play a role in the pathogenesis of emotional and certain cognitive disorders (Nestler et al., 2002; Newport et al., 2004; Brunson et al., 2005; Fenoglio et al., 2006b). Interestingly, the abnormal HPA axis of depressed adults is ‘state-dependent’, i.e., is found during depressive epochs and is relieved, for example, by antidepressants. This suggests that the HPA axis in affected individuals has been rendered vulnerable, perhaps via its perturbation during a key developmental period.
Although the erratic and unpredictable nature of maternal behavior in neglect/abuse situations and the stress-like symptoms in victims of child abuse have been established, there has been a dearth of appropriate animal models for studying these phenomena (Nestler et al., 2002). Because a hallmark of abnormal maternal care in childhood abuse situations is considered to be fragmented or erratic behavior (Whipple et al., 1991), we sought to create such behavioral patterns in an animal model. Here we describe the alterations of dams’ nurturing activities using a novel model of chronic psychological stress, based on limiting the dam’s ability to construct a nest for her offspring. We also evaluated potential underlying neuroendocrine perturbations in the dams, including the expression of hypothalamic CRH (Walker et al., 2001) and AVP (Wigger et al., 2004).
Experimental Procedures
Animals
Sprague-Dawley derived rats (Zivic-Miller, Zelienople, PA) were housed in the federally approved UCI vivarium and maintained in quiet, temperature-controlled rooms on a 12 h light / dark cycle (6 pm lights off / 6 am lights on) with access to unlimited lab chow and water. Parturition was verified at 12 h intervals (date of birth = day 0). On the second day of the pups’ life (P2), litters were culled to 12 pups if necessary, and mixed among experimental groups with roughly equal gender distribution, to minimize potential inter-litter differences in pups’ behavior. This was accomplished during the assignment of each mixed litter to a cage environment, and was the only disturbance of the dams and pups until P9. A total of 16 dams with litters were utilized for experiments. Dams used for physiological experiments (assessment of corticosterone, adrenal and thymus weights, CRH and AVP mRNA) were sacrificed on postpartum day 9, between 8–10 AM (two to four hours after lights on) to minimize diurnal variability of stress hormones (Dallman et al., 1987). Dams used for behavior experiments (elevated plus maze, open field test) were sacrificed on the morning of postpartum day 10. All experiments were carried out according to NIH guidelines, and were approved by the Institutional Animal Care and Use Committee.
The limited nesting / early-life stress (ES) paradigm followed the procedure described in Gilles et al., 1996, Avishai-Eliner et al., 2001b, and Brunson et al., 2005. Briefly, early-life stress (ES) experimental dams raised their pups in cages with limited nesting / bedding material during the first postnatal week. Cage environments were altered on the morning of P2 by placing pups and dams in cages fitted with a plastic-coated aluminum mesh bottom, raised 2.0 cm from the cage floor to allow collection of urine and droppings. The provided nesting / bedding material consisted of one paper towel (approximately 0.09 cubic feet) that was used by the dam to construct a rudimentary nest area, and was not replaced. All cages were kept in a room with strong laminar air flow, that eliminated accumulation of ammonia and odors. Droppings fell through the mesh to the space below, and the mesh was generally kept clean by the dam. For the control group, normal-bedded cages contained approximately 0.33 cubic feet of Sanitized Chips. All litters were completely undisturbed, and bedding was not changed during P2-P9.
Assessment of maternal behaviors
The activities of the dams were observed three times a day (0830, 1330, both referred to as the ‘light-phase’ because lights were on, and 1830, the ‘dark-phase’ when lights were off). Because activity levels in the rat vary throughout the day (lower activity during the light-phase and higher in the dark-phase), analyses were typically performed separately for the dark-phase and each of the two light-phase observation periods. An observer watched dams’ activities live at each time point for 75 minutes, on each day of the limited nesting period, using a modification of Myers procedure (Myers et al., 1989). Within each observation period, 25 consecutive epochs of activity were scored every third minute for the following parameters: dam in or out of the nest (i.e. on or off more than half of the litter), dam licking or grooming any pup, dam self-licking or grooming, dam eating or drinking, and dam nursing more than half of the litter. Types of nursing postures were also distinguished, using the following criteria: arched-back nursing was defined when the dam was domed over her pups and her hind legs obviously splayed; low nursing, in which the dam did not show obvious back-arching and/or leg extension; and side nursing, the dam was on her side with pups attached to her nipples.
The Observer program (Noldus Information Technology Version 5.0, 2003, Wageningen, the Netherlands) was used for formal analysis of sequential patterns (Noldus, 1991). Contingency tables were constructed, summarizing the transitions between all possible pairings of events for each mother / pup dyad. Conditional probabilities were calculated from these contingency tables for all possible pairs of events, by dividing the number of two-event sequences between a criterion and match event by the total number of sequences (i.e., opportunities for occurrence) for that criterion event.
Elevated Plus Maze and Open Field tests
To examine whether or not the dams exhibited ‘anxiety-like’ behaviors, reported to be associated with variation in maternal care in rodents (Olazabal and Young, 2005), five dams from each group (control and ES) were tested on the morning of postpartum day 9 using the elevated plus and open field paradigms. The elevated plus maze consisted of two open arms (50 × 10 cm) and two enclosed arms (50 × 10 × 40 cm), and was elevated 50 cm above the floor. The maze was arranged such that the two arms of each type were opposite each other (Pellow et al., 1985; Korte et al., 1999). Animals were placed in the center of the maze facing an enclosed arm at the start of the experiment. Each rat was allowed one 5-minute trial in the maze, and the maze was cleaned with 70% ethanol after each trial. Decreased time spent on open arms relative to closed arms, compared with control dams, was used as an index of anxiety. Locomotion, or the number of times the rat entered the open or closed arms was also recorded, as a measure of activity level. For the open field paradigm, the arena utilized was a 43.2 × 43.2 × 30.5 cm- plexiglass custom open field box (MED Associates Inc, St Albans, VT), consisting of three opaque white walls and one transparent wall, for live experimenter observation. Durations of time spent in the center of the field, of licking or grooming, freezing, and locomotion / rearing were measured during the 10 min trial, as indices of anxiety-like behavior (Galea et al., 2001). Both tests were conducted in a quiet, empty 20ft × 20ft room with no visual cues to distract the tested rat. The room was dimly lit with one 75 watt light bulb placed above the open field, for uniform illumination. Dams were allowed at least 1 hour between tests to recover in their home cages. All analyses were carried out ‘live’ and without knowledge of treatment group.
Physiological parameters of stress in the dams
Plasma corticosterone and adrenal gland weights, obtained at sacrifice, were used as measures of acute and chronic stress, respectively (Walker et al., 1992; Dallman et al., 1987). Dams were rapidly decapitated and trunk blood was collected within 30 seconds of cage disturbance to minimize handling effects on corticosterone levels. Plasma corticosterone levels were measured using a commercial radioimmunoassay kit (MP Biomedicals, Costa Mesa, CA).
In Situ Hybridization for CRH and AVP mRNA expression
In situ hybridization histochemistry (ISH) was conducted as described previously for deoxy-oligonucleotide probes (Yi and Baram, 1994; Avishai-Eliner et al., 2001a,b) with the exception that the CRH probe was a 39-bp oligomer, complementary to the coding region of the 13 most C-terminal amino acids of the CRH peptide. The AVP probe was a 48-bp deoxynucleotide oligomer, complementary to the last 16 amino acids of the glycopeptide region (Young et al., 1986). Both probes were labeled with 35S using routine terminyl deoxyneucleotide transferase methodology (Yi and Baram, 1994). Briefly, for the ISH, 20 µm brain sections were collected onto gelatin coated slides and stored at −80°C. Sections were brought to room temperature, air-dried and fixed in fresh 4% buffered paraformaldehyde for 20 min, followed by dehydration and rehydration through graded ethanols. Sections were exposed to 0.25% acetic anhydride and 0.1 M triethanolamine (pH 8) for 8 min and were dehydrated through graded ethanols. Pre-hybridization and hybridization steps were performed in a humidified chamber at 42°C in a solution of 50% formamide, 5 × SET, 0.2% SDS, 5 × Denhart’s, 0.5 mg/ml salmom sperm DNA, 0.25 mg/ml yeast tRNA, 100 mM dithiothreitol and 10% dextran sulfate. Following a 1 hour prehybridization, sections were hybridized overnight with 0.25 × 106 CPM of labeled probe. After hybridization, sections underwent serial washes at 42°C, most stringently at 0.3 × SSC for 30 min at room temperature. Sections were then dehydrated through increasing ethanol concentrations, air-dried and apposed to film (Kodak BioMax MR Film, Eastman Kodak Co., NY, USA), for 5–10 days for CRH and 1–5 hours for AVP.
ISH densitometric analysis
Semi-quantitative analyses of CRH and AVP mRNA were performed following ISH, without knowledge of experimental group, as described previously (Avishai-Eliner et al., 2001a,b). Digitized images of each brain section were analyzed using the ImageTool software program (University of Texas Health Sciences Center, San Antonia, TX). Densities were calibrated using 14C standards and are expressed in nCi/g, after correcting for background by subtracting the density of the hybridization signal over the corpus callosum. Two anatomically matched sections were chosen per animal, thus assuring that the size of the PVN area where messenger RNA was measured was similar in both groups The entire parvocellular region of PVN was outlined 5 times per section, and the signal in both sections was averaged to generate the final value for the animal (n = animal number).
Statistical considerations
All analyses were performed without knowledge of treatment group (‘blindly’). Statistical significance was set at p < 0.05. The significance of differences in maternal care among groups was analyzed using two-way analysis of variance (ANOVA; examining effects of cage environment and postpartum day), and paired Student’s t-test, as appropriate. Unpaired Student’s t-tests were used for analysis of anxiety tests, adrenal weights, corticosterone levels, and mRNA levels of CRH and AVP. The Prism GraphPad (San Diego, CA) software package was utilized.
Results
Reduced nesting / bedding material provoked abnormal nurturing behaviors in the dams
Maternal licking and grooming of the pups have been extensively shown to be important parameters of nurturing behaviors associated with reduced stress responses of the offspring during adulthood (Liu et al., 1997; Caldji et al., 2000). Restricting the nesting material available to the early-stress (ES) dams prevented them from constructing adequate nests for their pups, and reduce the overall quality of their care, including licking and grooming activities. Comparing their behaviors to those of control dams, the total duration of licking / grooming during the dark-phase periods of observation was decreased for the ES dams, specifically during the first four days in the limited-nesting cages (postpartum days 2–5; Fig. 1A; two-way ANOVA, effect of cage environment, F = 4.66, p = 0.036). In contrast, the number of epochs during which dams licked / groomed the pups did not differ between the ES and control groups in either the dark or light phases (dark phase: p = 0.419; light phase: p = 0.065; paired t-tests). Thus, during the dark phase, dams spent less total time licking / grooming pups, but performed these activities during the same number of epochs as the controls. The duration of each nurturing bout was ultimately shorter, resulting in a more fragmented pattern of care. During postpartum days 6–8, licking and grooming behaviors diminished as a function of the pups’ age in both groups, consistent with previous reports (Champagne et al., 2003). No significant effect of the reduced nesting was noted during the light-phase period (Fig. 1B; two-way ANOVA, F = 1.69, p = 0.20).
Fig. 1.

Abnormal patterns of maternal nurturing behaviors of dams in cages with restricted nesting material (early-stress or ES). (A) Duration of ES dams’ licking and grooming (L&G) the pups was markedly decreased during dark-phase observation periods on postpartum days 2–5 (n = 7 dams per group, two-way ANOVA, effects of cage environment, F = 4.67, p < 0.05; + indicates the results of student’s t-test comparison of control and ES duration on P2). (B) During the light phase, there were no significant differences in L&G duration (two-way ANOVA, F = 1.686, p > 0.05). (C) Pups in restricted nesting cages were significantly more likely to be displaced from the nest (p < 0.05; student’s t-test). *denotes significant difference from the controls (p < 0.05).
Formal analysis of the dams’ behaviors demonstrated additional deviations of the limited-nesting group from the normal complex behavioral sequences constituting ‘nurturing’. Behavioral pattern analysis using the Observer program indicated that control dams tended to lick and groom their pups after carrying them, whereas ES dams did not: Control mothers exhibited either licking (75% conditional probability) or grooming (25% conditional probability) behavior after carrying their pups, whereas the limited nesting dams never exhibited licking or grooming (0% conditional probability for either) after carrying. This drastic alteration of sequence-pattern could not be a result of reduced licking and grooming rates, because only the duration of licking and grooming during the dark-phase was reduced in the limited-nesting paradigm.
The difficulty dams had constructing an adequate nest affected several parameters of the pups’ well-being (see Gilles et al., 1996; Avishai-Eliner et al., 2001b; Brunson et al., 2005). Immediately upon receipt of the single paper towel, ES dams shredded it and placed their pups on the resulting nest. They continued to manipulate this rudimentary nest throughout the chronic stress paradigm, and this persistent jostling often displaced the pups from the nest. Consequently, the average number of epochs in which pups were found outside the nest during the three daily 75 min observation periods was significantly higher in the limited-nesting cages (Fig. 1C; p = 0.007). Similarly, the average duration of pups being outside the nest was 3.80 ± 1.2 minutes in ES cages, compared with 1.33 ± 0.7 minutes in control cages, suggesting that the ES dams could be considered neglectful.
Maternal care was fragmented (inconsistent) in cages with reduced nesting material
ES dams left their pups and the nest area more frequently than controls (Fig. 2A,B). These mothers were observed to be away from pups for a significantly greater number of epochs during the light phase of postpartum days 2–5 (Fig. 2A; two-way ANOVA, effect of cage environment, F = 47.19, p = 0.0001; effect of postnatal day, F = 14.98, p = 0.001), as well as the dark phase (Fig. 2B; two-way ANOVA, effect of cage environment, F = 5.16, p = 0.028). Interestingly, during postpartum days 6–8, control and ES dams were similar in their frequency of leaving the nest. In addition, quantitative analysis in a sub-set of the dams (Noldus Observer program) demonstrated that ES dams spent significantly less time in contact with pups (49% ± 10%) compared to controls (74% ± 8%). The color-coded grids (Fig. 2C,D) represent the maternal behaviors from one dam in each group, and demonstrate the fragmented care provided by the ES dams compared to controls. For example, during both the light-phase (Fig. 2C) and dark-phase (Fig. 2D) of postpartum day 5, the duration of each bout of maternal activity was reduced in the ES environment (i.e., the activities were fragmented).
Fig. 2.
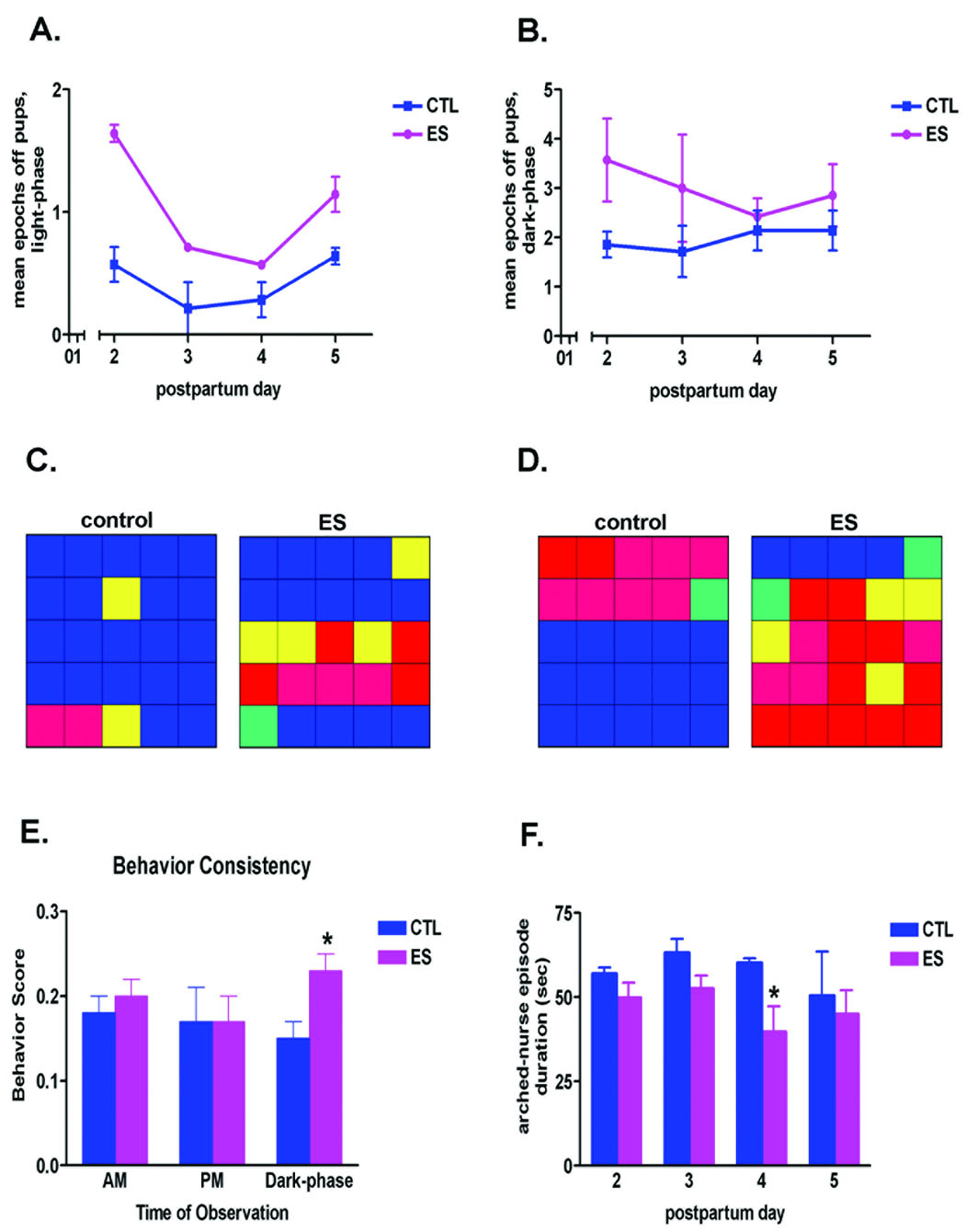
Maternal nurturing behaviors of dams maintained in cages with restricted nesting material (ES) were inconsistent and fragmented. (A,B) Mean number of epochs during which ES dams were found away from the pups was significantly higher during postpartum days 2–5, during both the light-phase (A; two-way ANOVA, effect of cage environment, F = 47.19, p < 0.001; effect of postnatal day, F = 14.98, p = 0.001) and the dark-phase (B; two-way ANOVA, effect of cage environment, F = 5.16, p < 0.05) observation periods (n = 7 per group). (C,D) Representative examples of maternal caring activities from one control and one ES dam, during the light-phase (C) and dark-phase (D) observations, performed on postpartum day 5. Each individual color depicts the predominant behavior during the epoch / square. Blue: nursing; red: away from pups (off pups or out of the nest); yellow: licking and grooming pups; pink: dam eating and drinking, away from pups; green: dam licking and grooming self, away from pups. This graphic representation illustrates the fact that in control dams, each behavior typically lasted for several consecutive 3 minute epochs, whereas ES dams tended to switch behaviors frequently and unpredictably. (E) The behavior of ES dams (n = 7 per group) was less consistent, with frequent switching from one type of activity to another, during the dark, high activity portion of the diurnal cycle. This erratic behavior is reflected by a higher behavior score, which denotes increased numbers of initiating a new behavior during consecutive observation epochs (see the Results for detailed description of this score). (F) Mean duration of arched-back nursing episodes was influenced by the ES cage environment throughout the light-phase of the first 4 days in this environment (two-way ANOVA, effect of cage environment, F = 5.67, p < 0.05). *p < 0.05; students t-test.
An additional approach to quantifying the fragmentation of the care provided by ES dams to their offspring was by defining a behavioral consistency score. Nurturing behaviors were evaluated during consecutive 3-minute epochs within each 75 minute observation period: If behaviors changed from one 3-minute epoch to the next, we assigned the epoch a ‘1'. If behaviors remained consistent, lasting for more than one epoch, we graded the second epoch a ‘0'. Thus, for 25 epochs, the maximal ‘fragmentation’ score, achievable if behavior changed at every epoch, was 24. We then divided this score by 24 (total possible number of behavior changes) obtaining a “behavior ratio” between 0–1. The higher the ratio, the more erratic the dam’s behavior; the lower this ratio, the more consistent the behavior pattern. Limited-nesting dams altered their behavior more frequently than control dams during the dark-phase period, when higher activity levels are observed in the rat (behavior score: control 0.15 ± 0.02 vs ES 0.23 ± 0.02; t-test, p < 0.05; Fig. 2E).
Selective types of nurturing behaviors were analyzed in more detail. For example, a dam’s frequency of arched-back nursing has been reported to correlate with the amounts of licking and grooming of pups, and to constitute a marker of effective nurturing (Caldji et al., 2000). To assess alterations in the consistency of arched-back nursing due to the ES cage environment, mean episode length of this behavior was compared between the two groups (Fig. 2F). When ES dams nursed in the arched-back position, the average duration of each nursing episode was significantly shorter than in controls during the light phase (two-way ANOVA, effect of cage environment, F = 5.67, p = 0.021; dark phase: two-way ANOVA, F = 1.48, p = 0.19). Combining all of the nursing types, nursing duration was longer for dams in normal cage environments compared with the ES dams during both light and dark phases (70% ± 8% time vs 48% ± 10% time; p < 0.05; note the expected overlap of nursing with duration of contact with the pups).
Maternal behaviors normalized upon restoring nesting and bedding material to the cage
We examined whether the abnormal nurturing activities of the dams were a direct function of their presence within the limited-nesting cage environment, and whether this activity pattern normalized upon returning dam and offspring to a control cage environment. Thus, the reversibility of the effect of restricted nesting on dams’ nurturing activity was examined by continuing the observation period during postpartum day 9–15 (n = 4 dams / group). Basically, dams and offspring kept in ES cage during P2 to P9 were returned to a cage with normal bedding on day 9, and the parameters and patterns of maternal activities were evaluated as performed during the first experimental week. Remarkably, maternal care activities of ‘ex-ES’ dams normalized very rapidly, and were indistinguishable from those of control dams. For example, the frequency of epochs control dams left the nest was 1.29 ± 0.3, and of ‘ex-ES’ 1.5 ± 0.3; similarly, licking and grooming duration was comparable among control and ‘ex-ES’ dams: 49.05 ± 5.4 sec/epoch for control, and 42.74 ± 7.5 sec/epoch for ‘ex-ES’. Generally, nurturing behaviors diminished progressively as a function of pups’ age, as has been previously shown (Champagne et al, 2003), and this effect occurred to the same degree in control and ’ex-ES’ dams. These data suggest that whereas restricted nesting material alters maternal behaviors profoundly, this effect reverses rapidly upon restoration of a ‘normal’ environment. In other words, the ES paradigm does not exert enduring effects on the dams’ ability to care for their offspring once sufficient nesting is available.
The limited nesting cage environment had minor effects on dams’ anxiety levels
As demonstrated above, nurturing behaviors of dams housed with limited nesting materials were abnormal, with patterns of both reduced and fragmented interactions with their pups. This raised the possibility that anxiety, perhaps associated with activation of the neuroendocrine / behavioral stress responses (the limbic-HPA axis) was the underlying mechanism. To explore the biological mechanisms driving the these abnormal nurturing behaviors, we assessed anxiety-like behaviors and locomotor activity (often related to anxiety; Miyakawa et al., 2001; Olazabal and Young, 2005) in both groups. ES dams spent significantly less time in the center of an open field during the ten-minute trial (Fig. 3A, t-test, p = 0.043), consistent with increased anxiety. However, freezing, self-licking and grooming behaviors did not differ from those in controls (Fig. 3B; freezing: p = 0.42, licking and grooming: p = 0.57). In the elevated plus maze task, the two groups performed similarly in percent time spent on closed vs open arms (Fig. 3C; open: p = 0.97, closed: p = 1.0, t-test) as well as in the total number of arm entries (Fig. 3D; p = 0.64, t-test). In addition, no significant difference among groups was observed in the percentage of entries into open arms (control: 54.4% ± 3.6%, vs. ES: 47.3% ± 2.7%; p > 0.05, t-test). These findings suggest that the mechanisms leading to the abnormal patterns of nurturing behaviors in the ES dams are unlikely to be attributable to the slightly elevated anxiety behavior found exclusively in the open field. Therefore, we examined whether the aberrant cage environment engendered high levels of stress in the dams, that, in turn might have contributed to the dam’s abnormal patterns of nurturing behaviors.
Fig. 3.
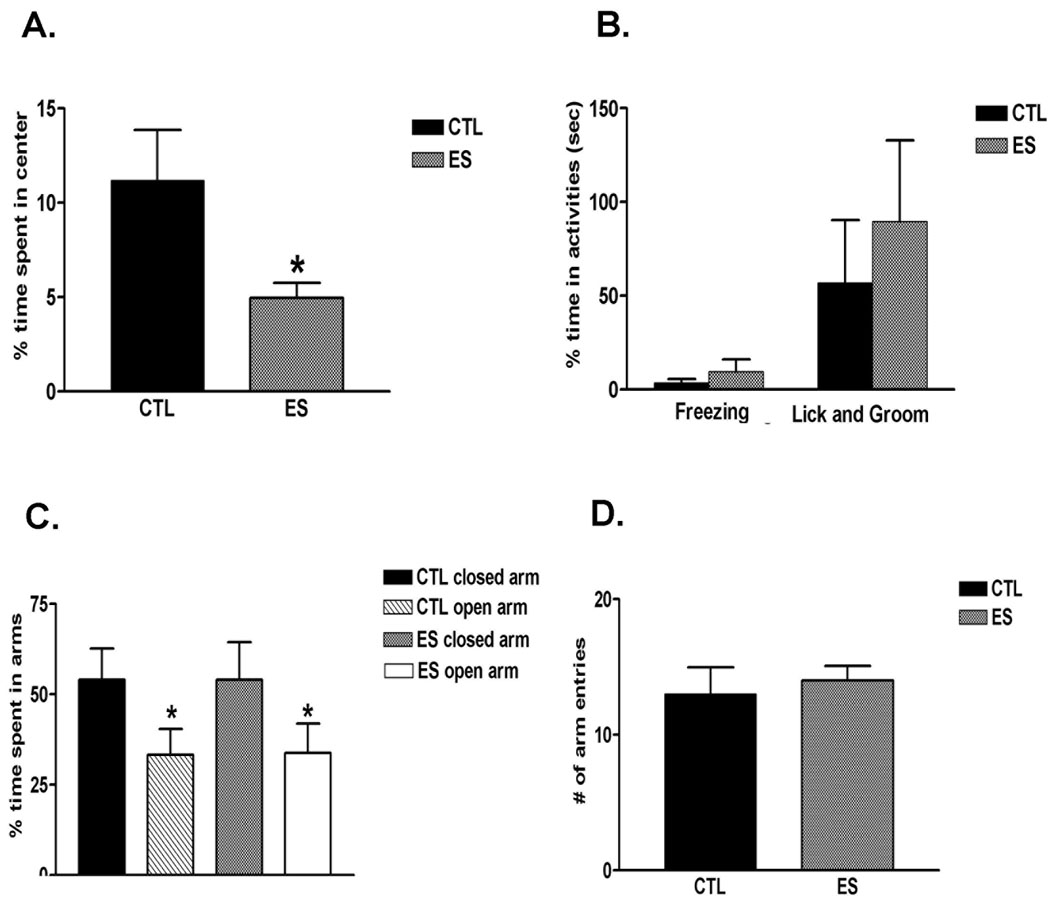
Dams rearing pups in restricted nesting material (ES) cages demonstrated increased anxiety in some but not other tests (n = 5 dams per group). In the open field task (A), percent time ES dams spent in the center of an open field chamber was significantly decreased (p < 0.05, t-test), without apparent alterations in freezing, licking and grooming behaviors (B). (C,D) Activities in the elevated plus maze were similar among control and ES dams; there were no differences in time spent on open vs. closed arms, nor in the number of arm entries., *p < 0.05; students t-test.
Physiological and molecular parameters of chronic stress are apparent in ES dams
In previous studies, we have described the negative consequences of aberrant maternal care provoked by the limited nesting cages on the offspring that were reared in these cages for one week, ending on postnatal day 9. In essence, when examined on day of life 9, these pups had increased adrenal gland weights, a modestly lower body weight, higher basal AM corticosterone levels and enhanced HPA axis responsiveness to acute stressors compared to controls (Gilles et al., 1996; Avishai-Eliner et al., 2001b; Brunson et al., 2005). Here we examined the basis of the deficient nurturing behavior of the dams, considering the hypothesis that it was attributable to chronic stress of the dams. Therefore, we tested the dams for the same parameters of acute and chronic stress. On postpartum day 9, body weights of dams did not distinguish the ES from control dams (control dams: 324.8 ± 21.25 g; ES dams: 314 ± 6.54 g; p = 0.64). In contrast, ES dams’ adrenal weight per body weight, a hallmark of chronic stress (Walker et al., 1992), was increased (Fig. 4A, p = 0.026; t-test). Note that thymus weights were similar among control and ES dams (controls: 62.0 ± 8.52 g, n = 5; ES: 75.6 ± 15.03 g, n = 5; p = 0.45). Morning plasma corticosterone levels (obtained ~2 hours after lights-on) of ES dams were significantly higher than those of dams in normal-bedded cages (Fig. 4B), and for each individual dam, seemed to correlate with adrenal weight (r2 = 0.86; n = 4). These data suggest that the abnormal nurturing behavior of the ES dams was associated with--and perhaps a result of--the stressful effects of the cage environment. Alternatively, stress, mild anxiety, and abnormal nurturing behaviors could be independent, unrelated consequences of this environmental manipulation.
Fig. 4.
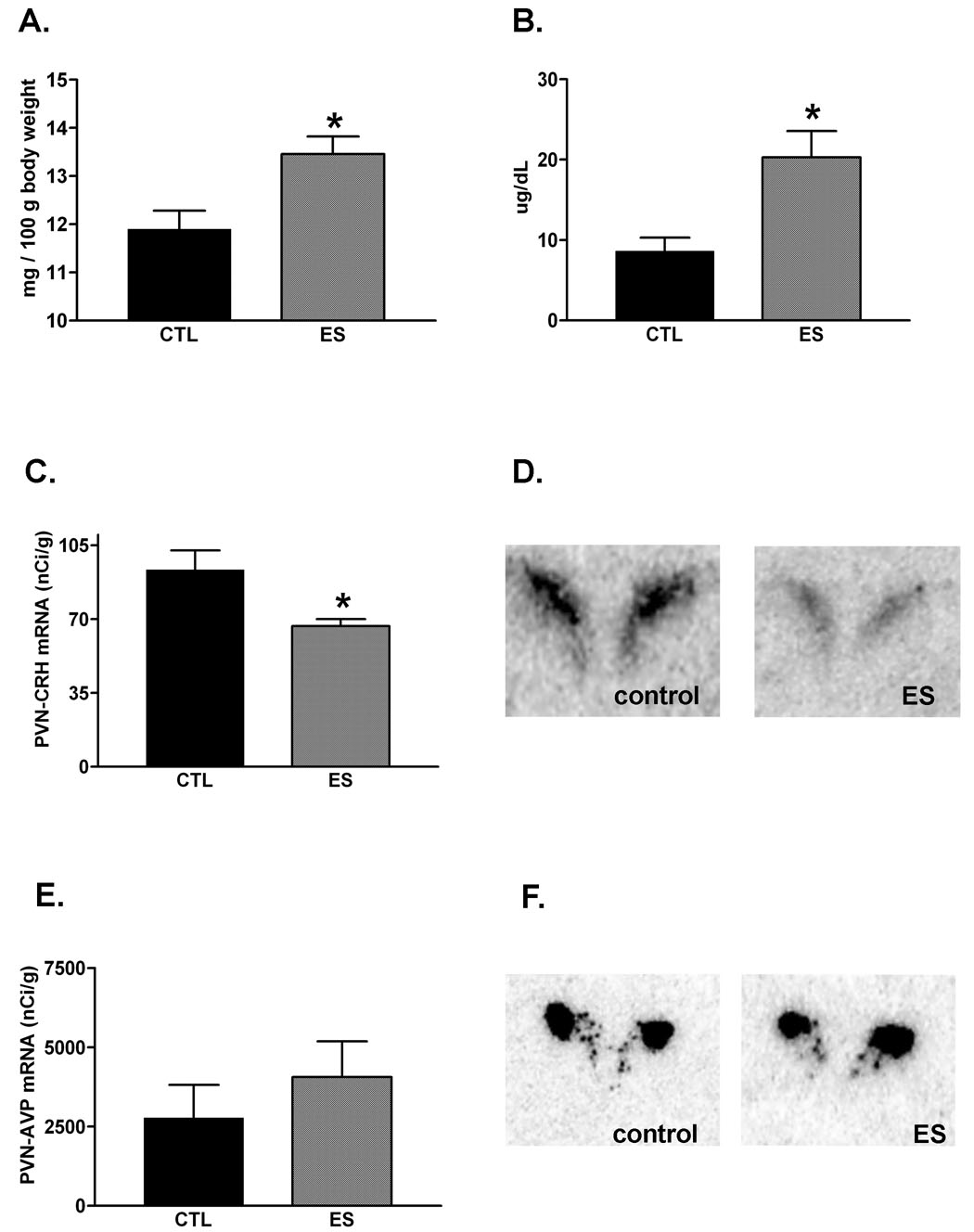
Physiological and molecular parameters of chronic stress were apparent in dams that reared their offspring in restricted nesting material (ES) cages. (A) Mean adrenal gland weight per body weight was higher than in controls (p < 0.05), and body weight was not significantly altered (see text). (B) Basal AM plasma corticosterone levels were significantly elevated in ES dams compared with controls (n = 4 dams / group; p < 0.05). (C,D) Corticotropin-releasing hormone (CRH) expression in the hypothalamic paraventricular nucleus PVN was reduced on postpartum day 9, following a week of the ES cage environment (p < 0.05). (E,F) However, there were no apparent changes in the expression of arginine-vasopressin (AVP). *p < 0.05; students t-test.
To further characterize the effects of the limited nesting environment on the dams’ HPA axis, the expression of CRH and AVP mRNA were determined in the hypothalamic PVN. As shown in Figure 4C, CRH mRNA levels in PVN were significantly reduced in ES dams, compared to controls (p = 0.047, t-test). In contrast, semi-quantitative analysis of AVP mRNA in the region corresponding to the medial parvocellular PVN did not suggest a significant effect of the limited-nesting cage environment (Fig. 4D; p = 0.09, t-test).
Discussion
The main findings of these studies are: (1) Limiting a dam’s ability to nest provokes abnormal nurturing behaviors towards her offspring. (2) A key element of this abnormal maternal behavior involves its inconsistent and fragmented nature. (3) The dams’ abnormal nurturing behaviors are associated with, and perhaps a result of, changes in physiological and psychological indicators of chronic stress; these include increased plasma corticosterone, hypertrophic adrenal glands, reduced hypothalamic CRH gene expression, and increased anxiety in an open field. Because these maternal behaviors lead to persistent derangements in the pups, the limited-nesting paradigm may provide a useful model for early-life, maternal-dependent emotional stress that recapitulates key aspects of the human condition.
Both human and animal studies have identified sensory and nurturing input from the mother as a major regulator of the neonate’s levels of stress (Hofer, 1994; Champagne et al., 2003; Heim et al., 2004; Fenoglio et al., 2006a). In the human, studies of emotional stress, particularly related to neglect / abuse early in life, suggest that in these situations, the mother is typically present, but her behavior is abnormal (Whipple et al., 1991; Koenen et al., 2003; Kendall-Tacket, 2007). In contrast, animal models of early-life stress have generally involved isolation–or separation–from the mother, identifying long-lasting behavioral abnormalities associated with HPA axis alterations (Harlow and Suomi 1971; Kehoe et al., 1998; Sanchez et al., 2001). Indeed, removal of the mother provokes stress in the pups, and, additionally, results in an impaired HPA axis long-term (Plotsky and Meaney, 1993; Kehoe et al., 1998). Either a single prolonged maternal separation (e.g., Avishai-Eliner et al 1999; Schmidt et al., 2004 and many others), or repeated, shorter (180 minute) epochs of isolation have been used (e.g., Plotsky and Meaney, 1993; Huot et al., 2002), and the results have depended on the duration of the separation as well as on the pups’ age during it (van Oers et al., 1998). Here, we chose to recapitulate an important element of the human condition -- the generation of early-life ‘emotional stress’ by a present mother.
A hallmark of maternal behavior in neglect / abuse situations is its unpredictable and fragmented quality (Whipple and Webster-Stratton, 1991; Gaudin et al., 1996). The limited nesting paradigm described here seems to re-create this pattern, which is characterized by shortened bouts of each nurturing behavior and frequent shifts between behaviors as well as an apparent reduction in the drive or ability of the dam to keep the pups in the nest area. Interestingly, some of these aberrant behaviors were more prominent during the active circadian (dark) phase, consistent with the notion that the perception of the abnormal environment influenced maternal behaviors in an ongoing manner.
The precise mechanisms driving the abnormal behavior of the ES dams are not fully resolved: for example, considering the possibility that ES dams were ’anxious’, we found abnormal behavior in a single measure of this trait (duration of time spent in the center of the open field), but not in others (all measures of the elevated plus maze). This seems to exclude major anxiety as a key basis of the profound and persistent deranged nurturing pattern throughout the week of limited-nesting period. Alternatively, ES dams seemed to demonstrate chronic stress. This was manifest by increased adrenal weight (with no significant change in body weight) and elevated AM plasma corticosterone. It is therefore likely that maternal stress was translated into abnormal care, which, in turn, led to chronic stress in the pups.
The specific aberration of maternal care that led to the stress in the pups merits discussion. It has been recently shown that a quantitative reduction of maternal nurturing activities does not, in itself, result in altered HPA axis in the offspring (Macri et al., 2004). In the current experiments, a quantitative reduction of the duration of maternal nurturing behavior was observed (specifically in the dark phase), but this was associated with a profound qualitative derangement of these activity patterns: increased frequency of leaving the nest area led to fragmentation of each nursing / caring episode, so that the duration of each interaction with the pups was both short and unpredictable (variable). In addition, the normal sequence of nurturing activities (e.g., licking after carrying) was disrupted. Therefore, it is likely that the combined derangement of both quality and quantity of the dams’ care, rather than the quantitative reduction in the overall care, led to the profound and long-lasting change in the offspring’s HPA axis (Gilles et al., 1996; Avishai-Eliner et al., 2001b; Brunson et al., 2005).
The basis for the persistence of the stress in the offspring might also relate to the inconsistent, unpredictable behaviors of the dams. These, in essence, constituted a source of ongoing, non-habituating stress. The HPA axis possesses a remarkable ability to process (Bhatnagar and Dallman 1998) and adapt to repeated stresses (Vazquez, 1998). Teleologically, this might contribute to ‘coping’ with daily or chronic stress (McEwen, 2001). However, exposure to random, unpredictable stressors prevents this habituation (Isgor et al., 2004). Although the HPA axis of 2–9 day old pups is immature (e.g., Walker et al., 1997; Avishai-Eliner et al., 2002) and mechanisms of habituation at this age are not fully understood (Walker and Dallman 1993; Hatalski et al., 1998), it is tempting to speculate that the unpredictable and fragmented nature of the maternal behavior may lead to non-habituating stress in these pups, with enduring consequences (Brunson et al., 2005; Fenoglio et al., 2006b).
It is intriguing to speculate about the origin of the dams’ stress, and specifically whether it was a function of the pups’ altered behavior in the abnormal cage environment (e.g., Brunelli et al., 1994; Walker et al., 2003). The lack of an appropriate nest might have influenced independently the dams and pups, which could result also in an altered interaction among them (Walker et al., 2004), creating a ‘vicious cycle’ of abnormal behavior and stress in both.
The current studies examined several parameters of the dams’ stress axis, and found reduced CRH mRNA expression in PVN, without significant change in AVP mRNA. Reduced CRH in PVN has been reported after recurrent stress in adult rats (Harbuz et al., 1994; Ma and Lightman, 1998). These authors and others (Whitnall, 1989) also found that the ACTH-stimulating role of CRH is partially usurped by AVP (Volpi et al., 2004). Thus, AVP expression ‘invades’ neurons that classically express CRH alone in the medial parvocellular PVN (Whitnall, 1989). Here we did not see alteration of the pattern of AVP expression in either classically magnocellular nor parvocellular sub-regions of the PVN. However, we did not perform single-cell immunocytochemistry to exclude potential minor changes of AVP distribution.
Whereas the data presented above is consistent with a significant role of chronic stress in the dams as a ‘driver’ of their abnormal behaviors, other molecular and neuroendocrine changes in the dams might also influence their nurturing behaviors (Neumann, 2003; Bosch et al., 2004). For example, we did not measure oxytocin (Bosch et al., 2004), or the receptors for oxytocin and vasopressin (Francis et al., 2002; Takayanagi et al., 2005; Olazabal and Young, 2006), all of which play major roles in governing maternal behavior. Clearly, analyses of the oxytocin and vasopressin systems, and perhaps others, should be a focus of future studies.
In summary, a model of dysfunctional maternal nurturing behaviors is described here, that it is based on limiting nesting material in the cage. The paradigm influences the quality as well as the quantity of the dams’ nurturing activities, recapitulating an important element of human early-life neglect and abuse. Because a week of exposure to this disrupted maternal care leads to long-lasting effects on the offspring that resemble those in human survivors of early-life neglect / abuse (Bremner and Vermetten 2001; Brunson et al., 2005; Nelson et al., 2007) the model should provide a useful tool for studying long-term consequences of early-life chronic stress that is related to abnormal maternal care.
Acknowledgments
The authors acknowledge Natalie Korthamar for excellent technical assistance with the maternal behavioral analysis and Joy Calara for editorial contributions. Supported by NIH NS28912 and MH73136 (TZB).
List of Abbreviations
- ANOVA
Analysis of Variance
- HPA
hypothalamo-pituitary-adrenal
- ES
early-stress; limited nesting animal model
- P
postpartum day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Dev Brain Res. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinol. 2001a;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001b;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;6:319–329. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neurosci. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neurosci. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol. 1994;108:298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psych. 2000;48(12):1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol & Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE 2004. 2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19(10):3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front Neuroendocrinol. 2006a;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006b;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol relieves depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122(1):1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gaudin JM, Jr, Polansky NA, Kilpatrick AC, Shilton P. Family functioning in neglectful families. Child Abuse Negl. 1996;20:363–367. doi: 10.1016/0145-2134(96)00005-1. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz MS, Jessop DS, Lightman SL, Chowdrey HS. The effects of restraint or hypertonic saline stress on corticotrophin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, Sprague-Dawley and Wistar strains of rat. Brain Res. 1994;667(1):6–12. doi: 10.1016/0006-8993(94)91707-8. [DOI] [PubMed] [Google Scholar]
- Harlow JF, Suomi SJ. Social recovery by isolation-reared monkeys. Proc Natl Acad Sci. 1971;68:1534–1538. doi: 10.1073/pnas.68.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Gurguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psych. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacol. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hess JL, Denenberg VH, Zarrow M, Peiffer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiol Behav. 1969;4:102–109. [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Triano L, Callahan M, Rappolt G. Adult rats stressed as neonates show exaggerated behavioral responses to both pharmacological and environmental challenges. Behav Neurosci. 1998;112:116–125. doi: 10.1037//0735-7044.112.1.116. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA. Violence against women and the perinatal period: the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse. 2007;8:344–353. doi: 10.1177/1524838007304406. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol. 2003;15:297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- Korte SM, De Boer SF, Bohus B. Fear-potentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning. Stress. 1999;3:27–40. doi: 10.3109/10253899909001110. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J Physiol. 1998;510(Pt 2):605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrí S, Mason GJ, Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci. 2004 Aug;20(4):1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: Temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yama M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressure. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psych. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-Adrenal responses to standard and low-dose dexamethasone suppression test in adult survivors of child abuse. Biol Psych. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Noldus LPJJ. The Observer: a software system for collection and analysis of observational data. Behavioral Research Methods, Instruments and Computers. 1991;23:415–429. [Google Scholar]
- Olazabal DE, Young LJ. Variability in "spontaneous" maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47:166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate "spontaneous" maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- O’Reagan D, Welburg LL, Holmes MC, Seckl JR. Glucocorticoid programming of pituitary-adrenal function: mechanisms and physiological consequences. Semin Neonatal. 2001;6:319–329. doi: 10.1053/siny.2001.0067. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;12:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van Woezik JH, Levine S, de Kloet ER, Oitzl MS. The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. J Neuroendocrinol. 2004;16:53–57. doi: 10.1111/j.1365-2826.2004.01123.x. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;23:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinol. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G. Regulation of vasopressin V1b receptors and stress adaptation. Ann NY Acad Sci. 2004;1018:293–301. doi: 10.1196/annals.1296.035. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Stern JS, Dallman MF. Obese Zucker (fa/fa) rats exhibit normal target sensitivity to corticosterone and increased drive to adrenocorticotropin during the diurnal trough. Endocrinol. 1992;131:2629–2637. doi: 10.1210/endo.131.6.1332842. [DOI] [PubMed] [Google Scholar]
- Walker CD, Dallman MF. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinol. 1993;132:1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]
- Walker CD, Tankosic P, Tilders FJ, Burlet A. Immunotargeted lesions of paraventricular CRF and AVP neurons in developing rats reveal the pattern of maturation of these systems and their functional importance. J Neuroendocrinol. 1997;9:25–41. doi: 10.1046/j.1365-2826.1997.00544.x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Tilders FJ, Burlet A. Increased colocalization of corticotropin-releasing factor and arginine vasopressin in paraventricular neurones of the hypothalamus in lactating rats: evidence from immunotargeted lesions and immunohistochemistry. J Neuroendocrinol. 2001;13(1):74–85. doi: 10.1046/j.1365-2826.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res Dev Brain Res. 2003;140:253–261. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, et al. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J Psychiatry Neurosci. 2004;29:364–382. [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Whipple EE, Webster-Stratton C. The role of parental stress in physically abusive families. Child Abuse Negl. 1991;15:279–291. doi: 10.1016/0145-2134(91)90072-l. [DOI] [PubMed] [Google Scholar]
- Whitnall MH. Stress selectively activates the vasopressin-containing subset of corticotropin-releasing hormone neurons. Neuroendocrinol. 1989;50(6):702–707. doi: 10.1159/000125302. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sánchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacol. 2004;29(1):1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Workel JO, Oitzl MS, Fluttert M, Lesscher H, Karssen A, de Kloet ER. Differential and age-dependent effects of maternal deprivation on the hypothalamic-pituitary-adrenal axis of brown norway rats from youth to senescence. J Neuroendocrinol. 2001;13(7):569–580. doi: 10.1046/j.1365-2826.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide's gene expression. Endocrinol. 1994;135(6):2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986;387(3):231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Ziabreva I, Schnabel R, Braun K. Parental deprivation induces N-methyl-D-aspartate-receptor upregulation in limbic brain areas of Octodon degus: protective role of the maternal call. Neural Plast. 2000;7:233–244. doi: 10.1155/NP.2000.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


