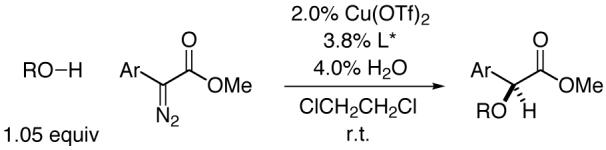
Remarkable advances have been reported in the discovery of methods for catalytic asymmetric insertion into C-H bonds.1 In contrast, there has been essentially no success in achieving corresponding reactions of O-H bonds (maximum ee: 8%)2-in fact, there has been only limited progress even with respect to diastereoselective processes.3,4 In this communication, we describe the first effective catalyst for enantioselective O-H insertions, thereby generating α-alkoxy and α-hydroxy carbonyl compounds in good ee (eq 1).5,6
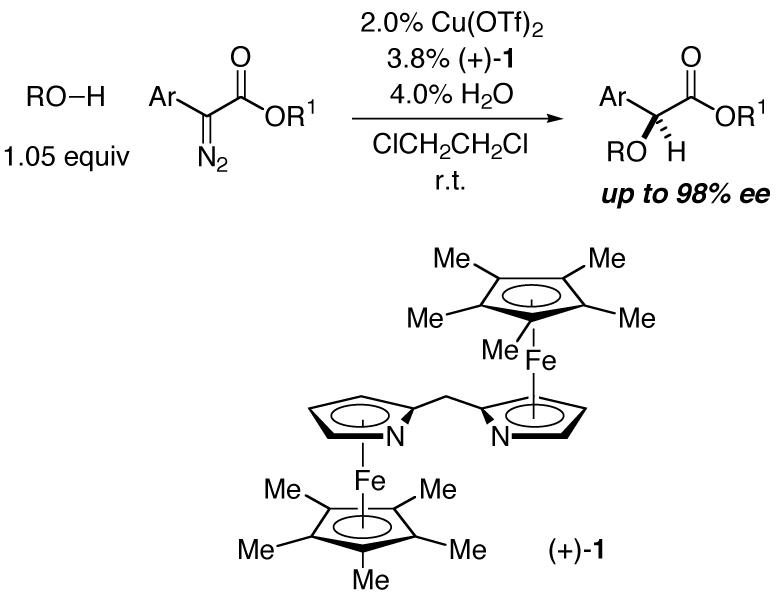 |
(1) |
Outlined in Table 1 are the effects of a number of reaction parameters on the yield and the ee for the copper-catalyzed coupling of ethanol with methyl α-diazo-α-phenylacetate.7 A key serendipitous discovery was that the addition of a small amount of water furnishes a much more enantioselective catalyst (entries 1 and 2 versus entry 3).8 A lower ligand:metal ratio leads to a lower yield and ee (entry 4), as does the use of chiral bisoxazoline, semicorrin, Pybox, DUPHOS, and BINAP ligands (entries 5-9). Under our standard conditions, in the absence of Cu(OTf)2, or in the presence of CuCl2 or CuCl, unsatisfactory yields and essentially no enantioselectivity are observed (entries 10-12). In contrast, CuPF6(CH3CN)4 provides an active, but somewhat less stereoselective, catalyst (entry 13 versus entry 1). Insertions conducted in CH2Cl2 proceed smoothly with fairly good enantioselectivity, whereas reactions in Et2O or toluene afford low ee (entries 14-16).9
Table 1.
Effect of a Range of Reaction Parameters on Catalytic Enantioselective O-H Insertions 
| entry | change from the standard conditions | yield (%)a,b | ee (%)a |
|---|---|---|---|
| 1 | none | 86 | 86 |
| 2 | 30% H2O instead of 4.0% H2O | 98 | 82 |
| 3 | no H2O instead of 4.0% H2O | 93 | 22 |
| 4 | 2.2% (+)-1 instead of 3.8% (+)-1 | 50 | 13 |
| 5 | 3.8% 2 instead of 3.8% (+)-1 | 70 | <2 |
| 6 | 3.8% 3 instead of 3.8% (+)-1 (4 h) | 72 | 40 |
| 7 | 3.8% (R)-i-Pr-Pybox instead of 3.8% (+)-1 (20 h) | 51 | 9 |
| 8 | 3.8% (R)-Et-DUPHOS instead of 3.8% (+)-1 | 45 | <2 |
| 9 | 3.8% (R)-BINAP instead of 3.8% (+)-1 (20 h) | 73 | <2 |
| 10 | no Cu(OTf)2 instead of 2.0% Cu(OTf)2 (24 h) | 0 | - |
| 11 | 2.0% CuCl2 instead of 2.0% Cu(OTf)2 (20 h) | 37 | <2 |
| 12 | 2.0% CuCl instead of 2.0% Cu(OTf)2 (20 h) | 39 | <2 |
| 13 | 2.0% CuPF6 (CH3CN)4 instead of 2.0% Cu(OTf)2 | 95 | 76 |
| 14 | CH2Cl2 instead of ClCH2CH2Cl | 92 | 72 |
| 15 | Et2O instead of ClCH2CH2Cl (4 h) | 71 | 7 |
| 16 | toluene instead of ClCH2CH2Cl (4 h) | 70 | 7 |
Average of two experiments.
Determined by GC versus a calibrated internal standard.
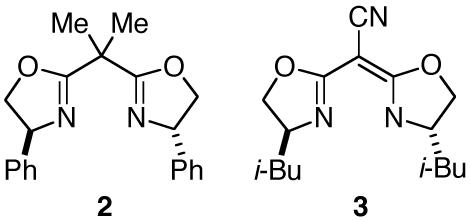
We have examined the impact of the structure of the alcohol on the yield and the ee of copper/bisazaferrocene-catalyzed asymmetric O-H insertion reactions (Table 2). The steric demand of the alkyl group plays an important role, with ethanol furnishing the best results among the four simple alcohols described in entries 1-4. Among ethanol derivatives, dramatically different outcomes are obtained depending on the substituents on the remote carbon-e.g., 2-trimethylsilylethanol reacts in excellent yield and ee (entry 5), whereas 2,2,2-trifluoroethanol does not undergo insertion (entry 6). 2-Trimethylsilylethanol is a particularly attractive substrate, since the insertion product can be deprotected to provide the α-hydroxy ester in high yield without racemization (eq 2). Reactions of benzyl alcohols proceed with fairly good enantioselectivity (entries 7 and 8), although insertions into allyl alcohol and phenol afford unsatisfactory results (entries 9 and 10).10,11
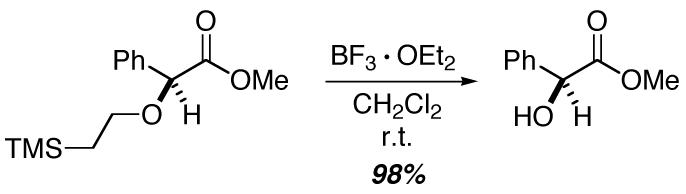 |
(2) |
Table 2.
Catalytic Enantioselective O-H Insertions: Dependence of ee on the Choice of Alcohol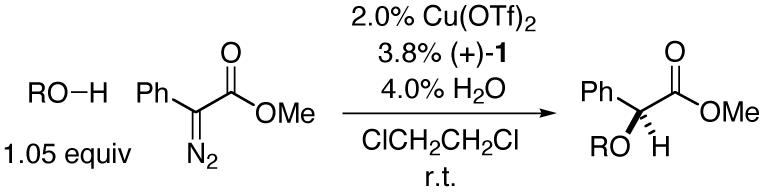
| entry | R | yield (%)a,b | ee (%)a |
|---|---|---|---|
| 1 | Me | 86 | 69 |
| 2 | Et | 85 | 87 |
| 3 | i-Pr | 76 | 68 |
| 4 | t-Bu | <2 | - |
| 5 | CH2CH2TMS | 94 | 90 |
| 6 | CH2CF3 | <2 | - |
| 7 | Bn | 86 | 77 |
| 8 | p-methoxybenzyl | 87 | 82 |
| 9 | allyl | 77 | 27 |
| 10 | Ph | 56 | 11c |
Average of two experiments.
Isolated yield.
The opposite stereoisomer is produced.
Copper/bisazaferrocene-catalyzed insertions into the O-H bond of 2-trimethylsilylethanol proceed in high yield and generally good enantioselectivity for a range of α-diazo esters (Table 3). Thus, the aromatic ring can be substituted in the ortho (entries 2-5), meta (entries 6 and 7), or para (entries 8-13) positions, and it can be electronically diverse (for an exception, see entry 13). Furthermore, bicyclic substituents are tolerated (entries 14 and 15), as is a heterocycle (entry 16).12
Table 3.
Catalytic Enantioselective O-H Insertions: Scope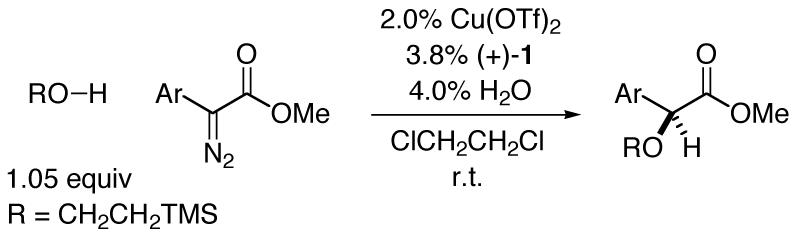
| entry | Ar | yield (%)a,b | ee (%)a |
|---|---|---|---|
| 1 | Ph | 94 | 90 |
| 2 | (2-OMe)C6H4 | 90 | 96 |
| 3c | (2-Me)C6H4 | 94 | 79 |
| 4 | (2-Cl)C6H4 | 96 | 96 |
| 5 | (2-F)C6H4 | 98 | 98 |
| 6c | (3-OMe)C6H4 | 96 | 89 |
| 7 | (3-Cl)C6H4 | 92 | 65 |
| 8 | (4-OMe)C6H4 | 85 | 86 |
| 9 | (4-NHAc)C6H4 | 89 | 87 (99)d |
| 10 | (4-Ph)C6H4 | 91 | 86 |
| 11c | (4-Br)C6H4 | 95 | 79 |
| 12 | (4-F)C6H4 | 92 | 89 |
| 13 | (4-CF3)C6H4 | 90 | 21 |
| 14 | 2-naphthyl | 93 | 84 |
| 15 |
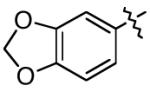
|
88 | 89 |
| 16 | 3-thienyl | 88 | 88 |
Average of two experiments.
Isolated yield.
Due to ease of synthesis, the ethyl ester was used.
Value in parentheses: ee after one recrystallization.
Although we have not yet conducted detailed mechanistic studies, we have made two observations worthy of mention. First, product ee correlates linearly with catalyst ee.13 Second, there is a substantial preference for O-H, rather than O-D, insertion (eq 3).14
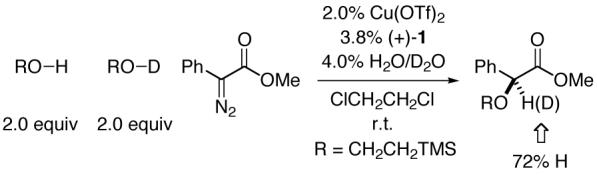 |
(3) |
In summary, we have developed the first effective method for catalytic enantioselective insertions into O-H bonds. Thus, a copper/bisazaferrocene catalyst couples alcohols such as 2-trimethylsilylethanol with α-aryl-α-diazo esters in high yield and generally good ee. Additional investigations of this and related processes are underway.
Supplementary Material
The first effective method for catalytic enantioselective insertions into O-H bonds has been developed. Specifically, a copper/bisazaferrocene catalyst couples alcohols such as 2-trimethylsilylethanol with α-aryl-α-diazo esters in high yield and good ee.
Acknowledgment
This paper is dedicated to Prof. JoAnne Stubbe on the occasion of her 60th birthday. Support has been provided by the NIH (National Institute of General Medical Sciences: R01-GM66960), Deutsche Forschungsgemeinschaft (postdoctoral fellowship to T.C.M.), Merck Research Laboratories, and Novartis. Funding for the MIT Department of Chemistry Instrumentation Facility has been furnished in part by NIH IS10RR13886 and NSF DBI-9729592.
References
- (1).(a) For reviews and leading references, see: Davies HML, Long MS. Angew. Chem. Int. Ed. 2005;44:3518–3520. doi: 10.1002/anie.200500554. Davies HML. In: Comprehensive Asymmetric Catalysis. Supplement 1. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Springer; New York: 2004. pp. 83–94. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis. Springer; New York: 1999. pp. 513–603. Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861–2903. doi: 10.1021/cr0200217. Müller P, Fuirt C. Chem. Rev. 2003;103:2905–2919. doi: 10.1021/cr020043t.
- (2).Bulugahapitiya P, Landais Y, Parra-Rapado L, Planchenault D, Weber V. J. Org. Chem. 1997;62:1630–1641. 8% ee, 59% yield. [Google Scholar]
- (3).(a) For examples of diastereoselective O-H insertion reactions, see: Aller E, Cox GG, Miller DJ, Moody CJ. Tetrahedron Lett. 1994;35:5949–5952. Aller E, Brown DS, Cox GG, Miller DJ, Moody CJ. J. Org. Chem. 1995;60:4449–4460. Shi G.-q., Cao Z.-y., Cai W.-l. Tetrahedron. 1995;51:5011–5018. Bulugahapitiya P, Landais Y, Parra-Rapado L, Planchenault D, Weber V. J. Org. Chem. 1997;62:1630–1641. Miller DJ, Moody CJ, Morfitt CN. Aust. J. Chem. 1999;52:97–107. Pansare SV, Jain RP, Bhattacharyya A. Tetrahedron Lett. 1999;40:5255–5258. Moody CJ, Morfitt CN, Slawin AMZ. Tetrahedron: Asymmetry. 2001;12:1657–1661. Jiang N, Wang J, Chan ASC. Tetrahedron Lett. 2001;42:8511–8513. Doyle MP, Yan M. Tetrahedron Lett. 2002;43:5929–5931. Im CY, Okuyama T, Sugimura T. Chem. Lett. 2005:1328–1329. Bolm C, Saladin S, Classen A, Kasyan A, Veri E, Raabe G. Synlett. 2005:461–464.
- (4).(a) For reviews of catalyzed insertions of diazo compounds into O-H bonds, see: Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. Wiley; New York: 1998. Chapters 8.3 and 8.4. Miller DJ, Moody CJ. Tetrahedron. 1995;50:10811–10843.
- (5).(a) For leading references to the synthesis and the utility of α-hydroxycarbonyl compounds, see: Janey JM. Angew. Chem. Int. Ed. 2005;44:4292–4300. doi: 10.1002/anie.200462314. Chen B-C, Zhou P, Davis FA, Ciganek E. Org. React. 2003;62:1–356.
- (6).(a) For previous applications of bisazaferrocene ligands in asymmetric catalysis, see: Lo MM-C, Fu GC. J. Am. Chem. Soc. 1998;120:10270–10271. Lo MM-C, Fu GC. Tetrahedron. 2001;57:2621–2634. Lo MM-C, Fu GC. J. Am. Chem. Soc. 2002;124:4572–4573. doi: 10.1021/ja025833z.
- (7).For transition metal-catalyzed insertions of diazo compounds into O-H bonds, rhodium, not copper, complexes have generally been the catalysts of choice (see Reference 4). For a recent report of an effective copperbased catalyst, see: Morilla ME, Molina MJ, Diaz-Requejo MM, Belderrain TR, Nicasio MC, Trofimenko S, Perez PJ. Organometallics. 2003;22:2914–2918.
- (8).We do not yet understand the role that water is playing in these reactions.
- (9).Reactions of α-diazo esters derived from primary alcohols (rather than secondary alcohols, tertiary alcohols, or phenols) proceed with the highest yield and enantioselectivity. Insertions by α-diazo ketones and amides furnish low ee.
- (10).For the reaction of allyl alcohol, we observe no cyclopropanation of the olefin (see also Reference 7).
- (11).Under our standard conditions, when water is employed as a substrate, O-H insertion occurs in moderate yield (∼55%) and low ee (∼15% ee). Triphenylsilanol and triethylsilanol are unreactive.
- (12).Notes: (a) Highly electron-rich α-aryl-α-diazo carbonyl compounds are relatively unstable, and they are not suitable substrates under our standard conditions. Insertions of α-pyridyl-α-diazo esters proceed in low ee. (b) Reaction of an alkenyl-substituted (α-styryl) diazoacetate leads to the formation of the desired product in 27% yield and 13% ee. We have not yet attempted to optimize this process. (c) Under our standard conditions, α-alkyl-α-diazoacetates undergo a 1,2-H shift to furnish α,β-unsaturated esters. (d) For the insertion depicted in entry 1 of Table 3, decreasing the catalyst loading to 0.5% Cu(OTf)2/0.95% 1 leads to a drop in ee (76% ee, 96% yield).
- (13).For a review of non-linear effects in asymmetric catalysis, see: Kagan HB, Luukas TO. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Springer; New York: 1999. Chapter 4.1.
- (14).Insertions into the O-D bond of deuterated alcohols furnish a route to enantioenriched α-deuterio-α-hydroxy esters (e.g., methyl α-diazo-α-phenylacetate + TMSCH2CH2OD: 94% yield, 78% ee).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


