Abstract
Honey bees can distinguish nestmates from non-nestmates, directing aggressive responses toward non-nestmates and rarely attacking nestmates. Here we provide evidence that treatment with pilocarpine, a muscarinic agonist, significantly reduced the number of aggressive responses directed toward nestmates. By contrast, treatment with scopolamine, a muscarinic antagonist, significantly increased attacks on nestmates. Locomotor activity was not altered by these pharmacological treatments. When interpreted in light of known cholinergic pathways in the insect brain, our results provide the first evidence that cholinergic signaling via muscarinic receptors plays a role in olfaction-based social behavior in honey bees.
Keywords: acetylcholine, Apis mellifera, kin recognition, muscarinic receptor, pilocarpine, scopolamine
Honey bee workers use odor cues to distinguish nestmates from non-nestmates [6]. These cues are based on heritable (genetically determined composition of cuticular hydrocarbons and comb wax) and nonheritable environmental odors (floral oils, pollen) present in each hive in a unique combination [5, 10, 22]. All colony members share the same odor cue profile [9]. Guard bees positioned at the nest (hive) entrance accept or attack incoming bees based on this profile [4]. Bees attacked as foreign are pushed, bitten, and sometimes stung. Bees that are accepted pass unmolested into the hive [3]. Guarding at the hive entrance is a specialized task performed by a small fraction of workers in a colony [22], but numerous studies have shown that adult bees in general are significantly more likely to attack a non-nestmate than a nestmate [7].
In many mammals, such as voles [39] and sheep [25], kin recognition is based on learned odor cues. Pharmacological studies have shown that signaling via muscarinic receptors is required for odor-based kin recognition in some species [30]. Ewes, for example, form an exclusive olfactory bond with their lambs and do not allow unfamiliar lambs to suckle. Activity in cholinergic pathways is critical to the formation of this olfactory memory, as injection of ewes with the muscarinic antagonist scopolamine at the time of parturition significantly increased the proportion of ewes that accepted an alien lamb in a later selectivity test [26]. More recently, numerous studies in mammals have demonstrated that disruption of muscarinic signaling results in the disruption of many forms of odor memory [40].
Acetylcholine (ACh) also plays an important role in behavior, memory and neural plasticity in bees [16, 23, 27, 28, 35]. In the insect brain, ACh is a major neurotransmitter in sensory neurons and many interneuron populations [1, 24]. In particular, information from the antennal lobes (primary olfactory neuropil) is transmitted to the mushroom bodies, a higher order brain region involved in multimodal sensory integration and certain forms of learning and memory, by a cholinergic projection [11]. Intracranial injections of scopolamine 20 minutes after one trial classical conditioning of the proboscis extension reflex (CS, vanillin; US, drop of sugar water) significantly reduced the percentage of bees responding to the CS from 5 through 20 minutes after the injection, although responding returned to control levels one hour post-treatment [16]. Injections of scopolamine into the vertical (alpha) lobes of the mushroom bodies were particularly effective in disrupting retrieval of a learned association of an odor with a food reward [27].
In the present study, we used a well-established laboratory assay of nestmate recognition to assess possible cholinergic mediation of odor-based nestmate recognition in the bee [4, 5, 7, 8]. If signaling via muscarinic receptors is important for acquisition and/or retrieval of an odor template associated with nestmates, blockade of central muscarinic receptors with scopolamine is predicted to impair recognition of nestmates. Conversely, stimulation of brain muscarinic receptors with pilocarpine, a muscarinic agonist, is predicted to improve the ability to discriminate nestmates from non-nestmates. Honey bees for these studies were obtained from research apiaries maintained at the University of Illinois at Urbana Champaign (Urbana, IL, USA) and Wake Forest University (Winston-Salem, NC, USA). Behavioral tests were performed during the summers of 2004, 2005, and 2006. Experimental bees were derived from unrelated colonies containing a mixture of European races. Worker bees were obtained by removing honeycomb-filled brood frames containing pupae from source colonies in the field and placing them in an incubator (33 – 34º C, 85 – 95% RH). Bees that completed metamorphosis and emerged within a 24 hour period from a single colony were introduced in groups of 10 – 12 nestmates to plastic Petri dishes (90 mm diameter, 10 mm deep, with an 8 mm hole drilled in the lid for feeding tube access). We chose to work with young bees because, in preliminary studies using our test conditions, older bees (N = 188) with foraging experience almost never attacked nestmates, making it impossible to detect a treatment-related improvement in performance.
Each dish contained a small piece of comb (25 mm square) from their natal colony. Previous studies have shown that even one hour of contact with brood comb permits newly-emerged bees to learn the unique odor of their colony [8]. These dishes were stored in darkness (mimicking conditions within the hive) in the incubator for the next 5 days. Nestmates were defined as all bees that emerged from a brood frame derived from a single source on the same day; non-nestmates were bees that emerged on the same day from a brood frame obtained from a different source colony. To prevent cross-contamination of wax-based cues, all equipment and supplies that contacted comb were kept separate throughout the entire testing procedure.
Bees designated to serve as responders in nestmate recognition assays were treated orally with a 1:1 solution of sucrose and water (control), pilocarpine dissolved in sucrose (muscarinic agonist; Sigma P6503, 10−4 M), or scopolamine dissolved in sucrose (muscarinic antagonist; Sigma S1013, 10−3 M) for 5 days; bees designated to serve as stimulus bees were marked with a dot of paint (Testor’s PLA) on the dorsal thorax at the time the dishes were established and fed 50% (w/v) sucrose. Doses for the cholinergic drugs were 10-fold lower than the LD50 for each drug (data not shown). Pilocarpine and scopolamine have previously been shown to be active at the insect muscarinic receptor [15, 23, 38]; it should be noted that the honey bee genome encodes a single G-protein-coupled muscarinic-type cholinergic receptor, rather than the multiple subtypes characteristic of vertebrates [20, 21]. Feeding tubes (inverted 1.5 ml polypropylene microcentrifuge tubes with holes punched in the tip with an 18 gauge needle) were changed daily; fresh solutions were made weekly.
The assay used in this study assesses the response of a group of bees to a single introduced nestmate or non-nestmate. On the 5th day after the dishes were established, a single sucrose-fed nestmate or non-nestmate was added to each dish. The number of aggressive interactions was recorded over a 4 minute period in tests conducted in 2004, and over a 3 minute period in tests conducted in 2005/2006. Observations began immediately after the introduction. Behavioral observations were made under dim red illumination, to which bees are blind because they lack a red-sensitive photopigment [29]. A total of 722 (260 control, 243 pilocarpine, 219 scopolamine) dishes were tested. For statistical analysis, each dish was treated as a single data point; any observed grappling, biting, or stinging resulted in the dish being categorically scored as rejecting the introduced bee; dishes with no observed aggressive responses during the observation period were scored as accepting the introduced bee. Our expectation was that nestmates would be accepted and non-nestmates would be rejected. Most trials, even those scored as rejecting because of observed aggression, ended with acceptance of the introduced bee, but in some cases the introduced individual was killed. The G-squared statistic was calculated for the resulting 2 X 2 frequency tables using StatView 4.5.1 for Macintosh (Abacus Concepts, Inc.).
Locomotor responses were recorded in bees reared under similar conditions and treatment schedules. Locomotor activity was defined as the number of times any bee within the dish crossed a center line over a 4 minute period.
Control bees responded differently to nestmates than to non-nestmates (G-squared = 21.6, df = 1; p < 0.0001), as shown in previous studies [2]. Pilocarpine-treated bees also responded differently to nestmates and non-nestmates (G-squared = 63.9, df = 1; p < 0.0001), as did scopolamine-treated bees (G-squared 8.4, df = 1; p < 0.01).
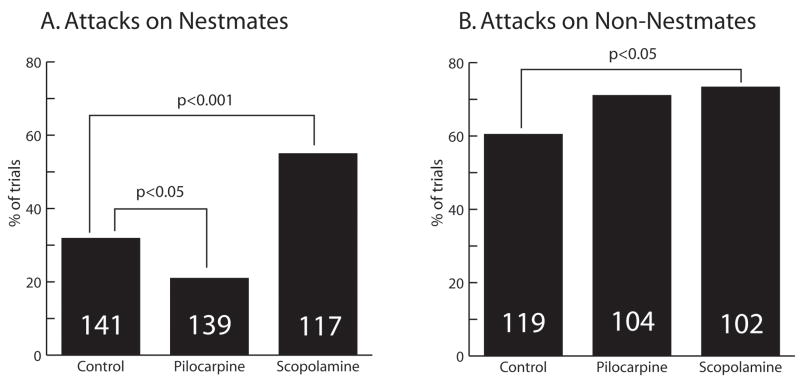
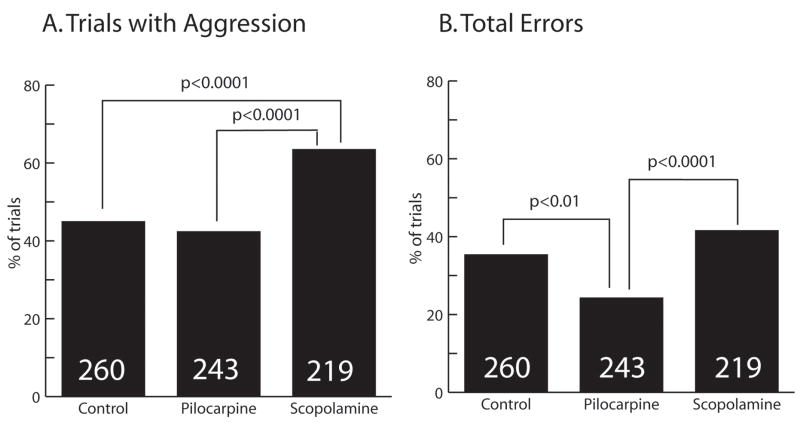
Further analyses, however, revealed important differences among the three test groups (Fig. 1). Pilocarpine-treated bees were significantly less likely to react aggressively toward a nestmate than control bees (G-squared = 4.4, df = 1, p < 0.05). Pilocarpine-treated bees also made fewer total errors (rejection of nestmate + acceptance of non-nestmate) than did controls (G-squared = 7.4, df = 1; p < 0.01) (Fig. 2). They did not differ from controls on the overall proportion of trials featuring displays of aggression.
Fig. 1.
Percentage of trials resulting in attacks on an unfamiliar stimulus bee. The G-squared statistic was used to analyze contingency tables. Comparisons yielding a statistically significant difference and associated p-values are indicated by brackets. Numbers in bars indicate the number of dishes tested. A. Attacks on nestmates. B. Attacks on non-nestmates.
Fig. 2.
Summary of responses to all introductions. The G-squared statistic was used to analyze contingency tables. Comparisons yielding a statistically significant difference and associated p-values are indicated by brackets. Numbers in bars indicate the number of dishes tested. A. Percentage of all introductions (nestmates and non-nestmates combined) eliciting an aggressive response. B. Percentage of trials in which errors (rejection of nestmate, acceptance of non-nestmate) occurred.
Scopolamine-treated bees displayed a different response profile (Fig. 1). Compared with nestmates, scopolamine-treated bees were significantly more likely to display aggressive responses towards nestmates (G-squared = 13.7, df = 1, p < 0.001) and non-nestmates (G-squared = 4.2, df = 1, p < 0.05). Scopolamine-treated bees displayed aggressive behavior on a greater proportion of trials than did controls (G-squared = 16.4; df = 1; p < 0.0001) and pilocarpine-treated bees (G-squared = 20.7; df = 1, p < 0.0001) (Fig. 2). Scopolamine-treated bees made significantly more errors than pilocarpine-treated bees, but did not differ from controls in total errors (Fig. 2). There was no difference in locomotor activity displayed by control, pilocarpine- and scopolamine-treated bees [total line crosses per dish of 10 bees per 4 minute test (mean ± SEM): control, N = 220, 8.36 ± 3.27; pilocarpine, N = 236, 8.47 ± 3.38; scopolamine, N = 197, 7.94 ± 3.00; F (2, 650) = 1.573, p > 0.05].
The main results can be summarized as follows. The effect of pilocarpine treatment was an improvement in the ability, relative to controls, to respond differentially to nestmates and non-nestmates. The improvement consisted of a reduction in attacks on nestmates rather than an increase in attacks on non-nestmates. By contrast, scopolamine-treated bees directed significantly more aggression to both nestmates and non-nestmates than either control or pilocarpine-treated bees. Scopolamine-treated bees were the only group more likely to attack than accept a nestmate. The lack of difference in locomotor scores across treatment groups suggests that the reduction in attacks on nestmates observed in pilocarpine-treated bees did not simply reflect an increased number of physical interactions with other bees in the test dish that would have provided more opportunities to encounter the odor profile of the stimulus bee.
Our results provide the first evidence that cholinergic signaling via muscarinic receptors is an important neurochemical substrate for odor-based nestmate recognition in worker honey bees, as has been previously demonstrated in mammals. The inhibition of aggressive responses towards an unfamiliar stimulus bee requires matching of odor cues from the stimulus bee to a learned odor profile, sometimes referred to as a template. This process necessarily involves detection of odor cues through receptors on the antennae and mouthparts. In other insects, and presumably in the honey bee, cholinergic projections carry these signals to the antennal lobes and the gustatory regions of the subesophageal ganglion [14, 18, 19, 34, 37]. Cholinergic projection neurons in turn convey information about chemical signals to the lateral horn and mushroom bodies of the protocerebrum [11, 41]. Both muscarinic and nicotinic receptors for acetylcholine are present in these regions of the bee brain [1, 24]. The results of our scopolamine treatments indicate the importance of signaling via the muscarinic receptors for odor-based recognition of nestmates, although it is not possible, given that the antagonist was orally administered, to assign the effects of receptor blockade to a particular neuroanatomical locus, such as the antennal lobes or the mushroom bodies. We did not test the possible role of signaling via nicotinic receptors in this assay. Important follow-up studies include treatments designed to separate the effects of chronic treatment with the agonist and antagonist from the acute effects of these treatments, and studies that ask if scopolamine can block not only the actions of endogenous acetylcholine but also the effects of treatment with pilocarpine.
Given that past studies suggest that scopolamine injections into the bee protocerebrum impact primarily retrieval of stored information and not acquisition [16, 27], one explanation for the present results, especially the increase in overall aggression we observed, is that bees could not retrieve a previously-learned template in the presence of scopolamine and hence treated all introduced bees as non-nestmates. Alternatively, scopolamine may have simply reduced the flow of information from the stimulus bee into the protocerebrum; an information deficit could lead to the perception of a mismatch between the cues presented by the stimulus bee and the stored template, resulting in enhanced aggression.
Bees treated with pilocarpine made significantly fewer errors when confronted with a nestmate. We envision that ingested pilocarpine either acts independently or sums with endogenous acetylcholine released at central synapses in olfactory-processing regions to increase attention to olfactory stimuli. It is notable that the effect of pilocarpine was not an overall enhancement of sensitivity, as pilocarpine-treated bees did not have an increased probability of attacking non-nestmates. This finding may reflect a ceiling effect, as control bees attacked on approximately 60% of non-nestmate trials in our study. It is also possible, despite lack of close genetic relatedness, that some of the non-nestmate bees used in these studies presented odor profiles with a high degree of overlap with the learned templates of our test bees. Alternatively, it has been argued that natural selection favors the ability to vary the conspecific acceptance threshold according to context [31, 36]. The laboratory assay used in this study, performed with bees younger than typical guard bees, may present a context that prefers acceptance errors over rejection errors, imposing a biological ceiling that neurochemical manipulations cannot raise. A previous study using a slightly different behavioral assay for nestmate recognition suggested that acute treatment of honey bees with injected octopamine agonists, 2,3-xylylaminomethyl-2'-imidazoline and N'-(4-chloro-o-tolyl)-N- methylformamidine, reduced the probability of attacks on nestmates while simultaneously increasing the probability of attacks on non-nestmates [32]. In contrast to the present study, bees in this study were housed in groups of 3 in dishes with no wax comb present; they were also tested at a slightly younger age. These factors resulted in a much lower level of aggressive responses by untreated controls towards non-nestmates (approximately 30% attacked vs. 60% attacked in the present study).
Octopamine has numerous roles in insect physiology, including coordination of “fight-or-flight” responses and facilitation of the acquisition and retrieval of olfactory associations [12, 13, 17, 33]. Receptors for acetylcholine and octopamine are present both in the antennal lobes and the calyces of the protocerebral mushroom bodies. Our results imply that, as would be expected, neural circuits mediating odor-based nestmate recognition overlap with previously-described neural circuits mediating olfactory learning in insects.
In summary, we provide here evidence that treatment with pilocarpine significantly reduced the number of aggressive responses directed toward nestmates, while treatment with scopolamine significantly increased attacks on nestmates. These results are consistent with the hypothesis that cholinergic signaling via muscarinic receptors plays a role in nestmate recognition in honey bees.
Acknowledgments
We thank K. Pruiett, D.A. Rauch, and L. M. Qi for assistance in the field; J. Portnoy and S. Babcock for assistance with behavioral testing; and members of the Robinson and Fahrbach laboratories for comments on the manuscript. This work was supported by a Developmental Psychobiology and Neurobiology Training Grant Fellowship from NIH to the University of Illinois at Urbana-Champaign (NI) and NIH Award GM073644 (GER and SEF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bicker G. Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microsc Res Tech. 1999;45:174–183. doi: 10.1002/(SICI)1097-0029(19990501)45:3<174::AID-JEMT5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Breed MD. Nestmate recognition in honey bees. Anim Behav. 1983;31:86–91. [Google Scholar]
- 3.Breed MD, Bennett B. Kin recognition in highly eusocial insects. In: Fletcher DJC, Michener CD, editors. Kin Recognition. Wiley; New York: 1987. pp. 243–285. [Google Scholar]
- 4.Breed MD, Diaz PH, Lucero KD. Olfactory processing in honeybee, Apis mellifera, nestmate recognition. Anim Behav. 2004;68:921–928. [Google Scholar]
- 5.Breed MD, Garry MF, Pearce AN, Hibbard BE, Bjostad LB, REPage J. The role of wax comb in honey bee nestmate recognition. Anim Behav. 1995;50:489–496. [Google Scholar]
- 6.Breed MD, Guzmán-Novoa E, Hunt GJ. Defensive behavior of honey bees: Organization, genetics, and comparisons with other bees. Annu Rev Entomol. 2004;49:271–298. doi: 10.1146/annurev.ento.49.061802.123155. [DOI] [PubMed] [Google Scholar]
- 7.Breed MD, Smith TA, Torres A. Role of guard honey bees (Hymenoptera: Apidae) in nestmate discrimination and replacement of removed guards. Ann Entomol Soc Am. 1992;85:633–637. [Google Scholar]
- 8.Breed MD, Stiller TM. Honey bee, Apis mellifera, nestmate discrimination: hydrocarbon effects and the evolutionary implications of comb choice. Anim Behav. 1992;43:875–883. [Google Scholar]
- 9.Breed MD, Williams KR, Fewell JH. Comb wax mediates the acquisition of nest-mate recognition cues in honey bees. Proc Nat Acad Sci USA. 1988;85:8766–8769. doi: 10.1073/pnas.85.22.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs SG, Ratnieks FL. Recognition of conspecifics by honeybee guards uses nonheritable cues acquired in the adult stage. Anim Behav. 1999;58:643–648. doi: 10.1006/anbe.1999.1177. [DOI] [PubMed] [Google Scholar]
- 11.Fahrbach SE. Structure of the mushroom bodies of the insect brain. Ann Rev Entomol. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- 12.Fahrbach SE, Mesce KA. ″ Neuroethoendocrinology ″: Integration of field and laboratory studies in insect neuroendocrinology. Horm Behav. 2005;48:352–359. doi: 10.1016/j.yhbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Farooqui T, Robinson K, Vaessin H, Smith BH. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci. 2003;23:5370–5380. doi: 10.1523/JNEUROSCI.23-12-05370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galizia CG, Menzel R. Odour perception in honeybees: coding information in glomerular patterns. Curr Opin Neurobiol. 2000;10:504–510. doi: 10.1016/s0959-4388(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 15.Gauglitz S, Pflüger HJ. Cholinergic transmission via central synapses in the locust nervous system. J Comp Physiol [A] 2001;187:825–836. doi: 10.1007/s00359-001-0253-y. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier M, Lozano VC, Zaoujal A, Richard D. Effects of intracranial injections of scopolamine on olfactory conditioning in the honeybee. Behav Brain Res. 1994;63:145–149. doi: 10.1016/0166-4328(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 17.Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson BS, Anton S. Function and morphology of the antennal lobe: New developments. Annu Rev Entomol. 2000;45:203–231. doi: 10.1146/annurev.ento.45.1.203. [DOI] [PubMed] [Google Scholar]
- 19.Homberg U, Hoskins SG, Hildebrand JG. Distribution of acetylcholinesterase activity in the deutocerebrum of the sphinx moth Manduca sexta. Cell Tissue Res. 1995;279:249–259. doi: 10.1007/BF00318481. [DOI] [PubMed] [Google Scholar]
- 20.Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulme EC, Birdsall NJM, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 22.Hunt GJ. Flight and fight: A comparative view of the neurophysiology and genetics of honey bee defensive behavior. J Insect Physiol. 2007;53:399–410. doi: 10.1016/j.jinsphys.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc Nat Acad Sci USA. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreissl S, Bicker G. Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J Comp Neurol. 1989;286:71–84. doi: 10.1002/cne.902860105. [DOI] [PubMed] [Google Scholar]
- 25.Levy F, Kendrick KM, Goode JA, Guevara-Guzman R, Keverne EB. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: changes with maternal experience and effects on acetylcholine, gamma-aminobutyric acid, glutamate and noradrenaline release. Brain Res. 1995;669:197–206. doi: 10.1016/0006-8993(94)01236-b. [DOI] [PubMed] [Google Scholar]
- 26.Levy F, Richard P, Meurisse M, Ravel N. Scopolamine impairs the ability of parturient ewes to learn to recognise their lambs. Psychopharmacol. 1997;129:85–90. doi: 10.1007/s002130050166. [DOI] [PubMed] [Google Scholar]
- 27.Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. J Comp Physiol A. 2001;187:249–254. doi: 10.1007/s003590100196. [DOI] [PubMed] [Google Scholar]
- 28.Lozano VC, Gauthier M. Effects of the muscarinic antagonists atropine and pirenzepine on olfactory conditioning in the honeybee. Pharmacol Biochem Behav. 1998;59:903–907. doi: 10.1016/s0091-3057(97)00524-8. [DOI] [PubMed] [Google Scholar]
- 29.Menzel R, Blakers M. Colour receptors in the bee eye – morphology and spectral sensitivity. J Comp Physiol A. 1976;108:11–33. [Google Scholar]
- 30.Prediger RD, De-Mello N, Takahashi RN. Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. Eur J Pharmacol. 2006;531:176–182. doi: 10.1016/j.ejphar.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Reeve HK. The evolution of conspecific acceptance thresholds. Am Nat. 1989;133:407–435. [Google Scholar]
- 32.Robinson GE, Heuser LM, Le Conte Y, Lenquette F, Hollingworth RM. Neurochemicals aid bee nestmate recognition. Nature. 1999;399:534–535. [Google Scholar]
- 33.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 34.Schröter U, Menzel R. A new ascending sensory tract to the calyces of the honeybee mushroom body, the subesophageal-calycal tract. J Comp Neurol. 2003;465:168–178. doi: 10.1002/cne.10843. [DOI] [PubMed] [Google Scholar]
- 35.Shapira M, Thompson CK, Soreq H, Robinson GE. Changes in neuronal acetylcholinesterase gene expression and division of labor in honey bee colonies. J Mol Neurosci. 2001;17:1–12. doi: 10.1385/JMN:17:1:1. [DOI] [PubMed] [Google Scholar]
- 36.Starks PT, Fischer DJ, Watson RE, Melikian GL, Nath SD. Context-dependent nestmate discrimination in the paper wasp, Polistes dominulus: A critical test of the optimal acceptance threshold model. Anim Behav. 1998;56:449–458. doi: 10.1006/anbe.1998.0778. [DOI] [PubMed] [Google Scholar]
- 37.Torkkeli PH, Widmer A, Meisner S. Expression of muscarinic acetylcholine receptors and choline acetyltransferase enzyme in cultured antennal sensory neurons and non-neural cells of the developing moth Manduca sexta. J Neurobiol. 2005;62:316–329. doi: 10.1002/neu.20097. [DOI] [PubMed] [Google Scholar]
- 38.Trimmer BA, Weeks JC. Effects of nicotinic and muscarinic agents on an identified motoneurone and its direct afferent inputs in larval Manduca sexta. J Exp Biol. 1989;144:303–337. [Google Scholar]
- 39.Williams JR, Slotnick BM, Kirkpatrick BW, Carter CS. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiol Behav. 1992;52:635–639. doi: 10.1016/0031-9384(92)90390-n. [DOI] [PubMed] [Google Scholar]
- 40.Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learn Mem. 2004;11:23–34. doi: 10.1101/lm.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasuyama K, Salvaterra PM. Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microsc Res Tech. 1999;45:65–79. doi: 10.1002/(SICI)1097-0029(19990415)45:2<65::AID-JEMT2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]