Atrial fibrillation (AF) is the most common arrhythmia in the United States, yet its mechanisms remain unclear (1). Seminal observations that premature beats from the pulmonary veins (PV) may initiate paroxysms of AF (2) led to the development of PV isolation for that condition. However, ablation is much less effective in persistent AF, largely because its pathophysiology is less understood (1). A major contemporary translational challenge is therefore to build upon insights from the animal laboratory to identify mechanisms that separate human persistent from paroxysmal AF.
Clinical Observations on AF Initiation
Focal Triggers or Tachycardias and the Autonomic Nervous System
Sympathetic or parasympathetic stimulation (1) may explain the importance of thoracic vein sites as AF-triggers (2), and may underlie vagally or adrenergicallymediated AF (3). In dogs, sympathovagal stimulation of PVs shortens action potential duration (APD) to cause early afterdepolarizations (EADs) and triggered beats (4). In support of this mechanism in humans, PV beats that trigger AF are likely focal (5), AF is less likely to recur if the PV antra are dennervated (3), and routine PV isolation often causes dennervation (6). Nevertheless, the mechanisms linking autonomic innervation with human AF are incompletely understood.
Atrial flutter is strongly associated with AF
Epidemiological studies show that patients with typical atrial flutter (AFL) often develop AF over time (7) while, in patients with paroxysmal AF, recent data suggest that co-existing AFL indicates ‘substrate’ that renders PV isolation less effective (8). These data are consistent with clinical experience that links AF with AFL, particularly with atypical macro-reentry (9).
Mechanistically, AFL could directly initiate AF (9), or facilitate AF by creating its ‘substrate’ (10). In dogs, typical AFL requires a short period of AF to cause functional block across the crista terminalis that protects isthmus-dependent reentry (11). However, answers to the inverse questions are less clear – does macro-reentry disorganize to human AF (12) or does microreentry perpetuate (“drive”) AF (13)?
Proposed Mechanisms for AF
It remains unclear whether human AF results from re-entrant or focal processes, whether both co-exist, or under what conditions one predominates. Elegant animal studies show that AF is self-facilitating by virtue of remodeling, in which refractory periods shorten and conduction may slow (14). Although this encourages reentry, by shortening tissue wavelength, it may also promote focal mechanisms, since shortened APD facilitates EAD-triggered activity in canine PVs (4).
Evidence for Multiwavelet reentry in Human AF (15)
Intraoperative mapping suggests that human AF represents multiple reentrant wavelets (16), as quantified by elegant electrophysiologic methods (17). Elimination of AF by the Maze surgery also supports this mechanism, although it has been suggested that Maze surgery also isolates the PVs and may thus eliminate potential triggers or drivers (18). Interestingly, human AF shows non-uniform spatial organization that may be consistent between patients (19,20). If multiwavelet reentry is a predominant mechanism, studies are needed to explain why reentry is confined to certain locations or why wavebreak occurs elsewhere. As described below, this organization may reflect dynamic regional spatial or temporal properties of the atria.
Evidence for Focal Drivers of Human AF
An alternative hypothesis is that rapid regular sources sustain human AF, by activating too fast for remaining tissue to keep pace (“fibrillatory conduction”). This mechanism has been shown in vitro in sheep (13) and in vivo in dogs (21), and could potentially co-exist with reentry.
“Drivers” of human AF have yet to be identified categorically, despite provocative observations. Sahadevan et al. (20) and Wu et al. (22) used intraoperative mapping to reveal sites of rapid regular activation in AF, often near the PVs. However, these sites were not ablated or otherwise perturbed, and so it is uncertain if they reflect “drivers” or local tissue properties.
Moreover, termination of paroxysmal AF by PV isolation does not prove that PVs are “drivers”, because PV isolation in any sequence slows AF progressively before termination (23). Indeed, the PVs may just as well represent preferred anchors for reentry. Furthermore, using short AF CL to identify a “driver” site is problematic, since this may also represent short refractoriness (14) or wave collision (24). An important study showed that sites of rapid AF, identified spectrally by sequential point mapping in patients with persistent AF, were unrelated to successful ablation (25).
As a result, it may be important to assess electrogram regularity as well as rate, and to use simultaneous multisite mapping, to circumvent some limitations of the spectral analysis of temporally-varying AF (figure 1) (24,26). A regular AF “driver” should have a high frequency yet narrow spectral DF, while surrounding tissue should not (13,21). We recently used this approach, with simultaneous multisite biatrial mapping (27), to identify sites from where a radial step-down in rate and regularity was seen (“centrifugal activation”) (28). These criteria have been used to define drivers in animal models (13,21) and, in our series, were seen in half of our patients. Proposed drivers lay near the PVs in patients with paroxysmal AF but elsewhere in those with persistent AF.
Figure 1. Fractionated Electrograms may indicate Wavelet Collision or Far Field Signals, as validated from monophasic action potentials.
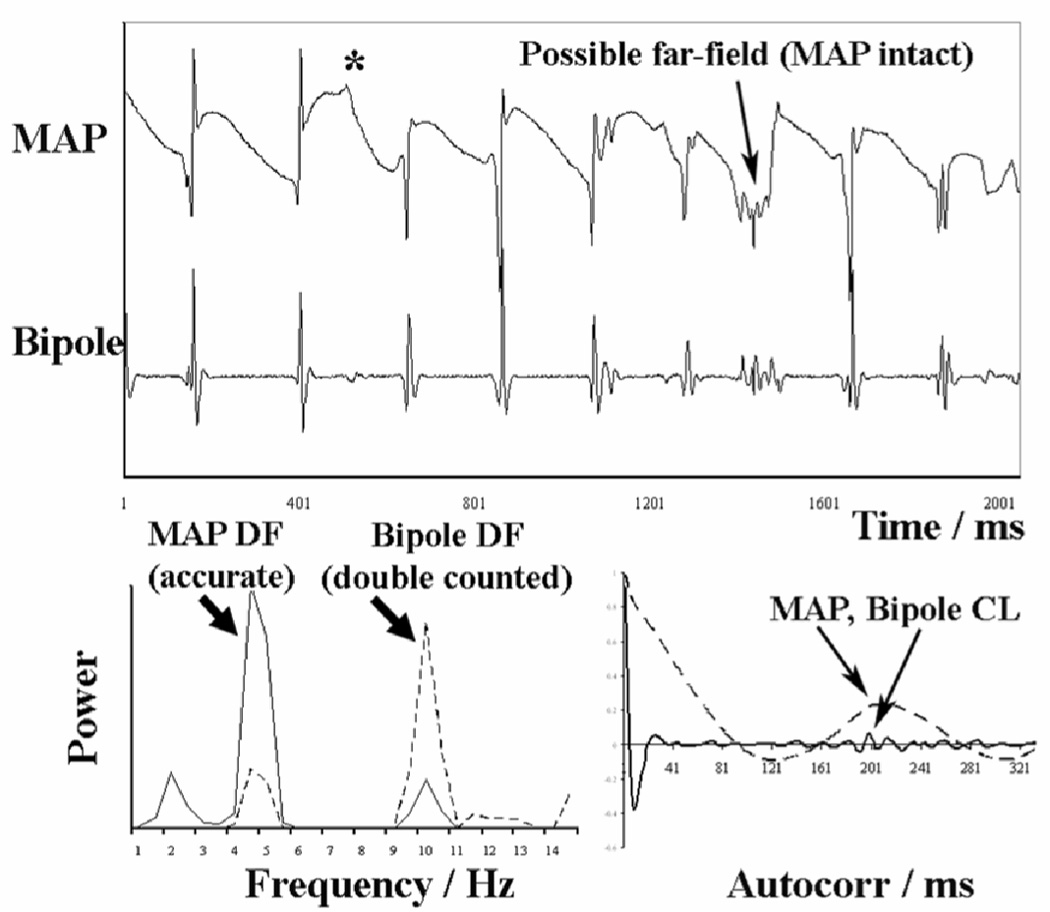
This far-field signal or noise may cause overestimation of the spectral DF (bottom left), that is avoided using time domain autocorrelation (reproduced from reference (24) with permission).
Complex fractionated atrial electrograms (CFAE) may also be mechanistically important to human AF (29). In intraoperative unipolar mapping studies, fractionation identified sites of slow conduction (16). It has recently been proposed that CFAE during human sinus rhythm results from vagal stimulation (30), and CFAE during AF in dogs reflects autonomic stimulation (31). Nevertheless, because CFAE may also reflect wavelet collision, far-field signals or noise (figure 1), the role of CFAE sites to human AF remains unclear. We found no relationship between CFAE and sites of centrifugal step-down in rate and organization in our series of patients (28).
Validating Proposed “Drivers” of Human AF
A major clinical challenge is to prove that any proposed “driver” sustains AF. Naturally, terminating AF by ablation at this site would provide compelling evidence. However, elimination of one source may allow the emergence of alternate perpetuating mechanisms. Indeed, this may explain why termination of persistent AF typically requires ablation of sequential AF ‘sources’(32). Quantifying fibrillatory conduction (i.e. stepdown in rate and organization) may also validate that a candidate site perpetuates AF. In sheep, the radial increase in cycle length from a driver is 33 ms per cm tissue (13); studies should determine if human AF exhibits analogous decay constants.
Dynamic Tissue-Level Mechanisms for AF Initiation
An important unresolved question is why regular atrial tachycardia or macroreentry degenerate to fibrillation. Much work has addressed this issue in the ventricle (33,34), and similar considerations may apply to AF.
Structural Non-Uniformities (1) may result in dispersion of repolarization and slow conduction that enable unidirectional block and reentry. This is relevant to ablation because ridges and scar tissue can be identified by real-time ultrasound, CT imaging and electroanatomic mapping (35). On the other hand, individuals without AF show similar non-uniformities (1). Moreover, a static anatomic substrate does not explain the sporadic nature of AF. For these reasons, it is increasingly felt that dynamic spatial or temporal factors help initiate fibrillation (33,34).
Spatial Dynamics
Variability in activation sequence provides a potential mechanism for rapid regular tachycardias (e.g. near the PVs) to result in collision, wavebreak and AF. Indeed, recent studies have demonstrated variations in activation sequence in otherwise regular atrial macro-reentry.
In typical AFL, activation is extremely regular at the cavotricuspid isthmus, yet bi-atrial mapping shows wavefront variability and collision some distance away in the left atrium (36). We recently extended these observations by showing greater cycle-to- cycle variability in atypical than typical macro-reentry (37). Notably, variations were most marked contralateral to sites of entrainment and successful ablation (38), supporting reports by Jais et al. of right atrial variations from left atrial macro-reentry (39). To support a mechanism for AF, studies should examine whether wavelet variability at sites including the coronary sinus, Bachmann’s bundle and/or the inter-atrial septum (40) becomes exaggerated at faster rates and lead to wavebreak.
Temporal Dynamics
Rate-related variations in repolarization and conduction are increasingly recognized factors in the initiation of fibrillation (33,34). Restitution is the relationship of APD to the diastolic interval from the preceding beat. Notably, APD restitution slope > 1 (“steep”) may cause self-amplifying oscillations: a premature beat shortens diastolic interval yet shortens APD to a greater extent, thus lengthening diastolic interval and APD and so on, leading to alternans that may eventually cause wavebreak and fibrillation (33,34).
Atrial APD restitution is indeed steep in patients with AF compared to control subjects (41). Furthermore, APD alternans may directly precede human AF (12). In patients with typical AFL, we found that APD alternans at the cavotricuspid isthmus precedes paced and spontaneous transitions to AF (figure 2). Prior to AF, APD alternans became exaggerated, leading to conduction block and period multupling (12). These data were strengthened by the lack of alternans at slower rates, the consistent emergence of alternans prior to AF, and the lack of AP alternans in patients without AF even at fast rates. Indeed, computational modeling has suggested that alternans is a general initiating mechanism for human AF (42). Because human action potentials may alternate without steep APD restitution (43), both remain potential therapeutic targets.
Figure 2. Alternans of Action Potentials Preceding AF.
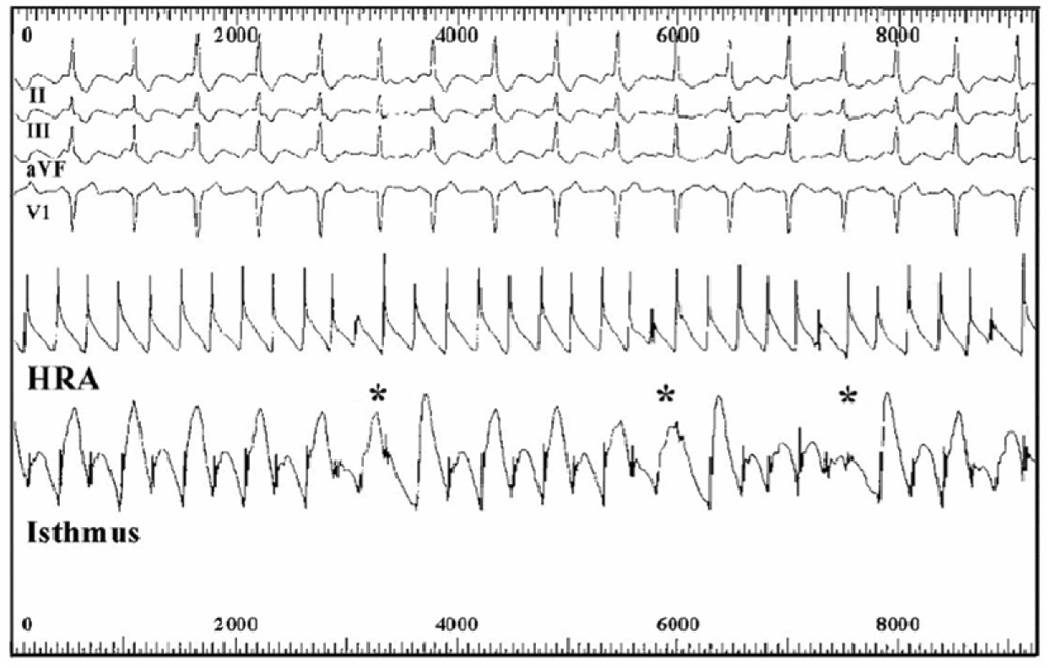
The patient showed typical AFL with APD alternans at the cavotricupid isthmus (but not high right atrium, HRA), that became exaggerated in amplitude preceding conduction block (asterisked), period multupling and AF (reproduced from reference (12) with permission from the American Heart Association).
Further studies are needed to explain the sporadic onset of AF, and whether altered spatial or temporal dynamics by autonomic variations (3,4,31), or stretch (44),or a critical site or origin or timing of a trigger beat, conspire to initiate AF.
Summary
Several mechanisms may explain AF in animal models, but the extent to which they operate in human paroxysmal or persistent AF is undefined. AF may result from reentrant or focal mechanisms yet, in either case, the mechanisms enabling single beats or regular tachycardias to cause fibrillation with regional organization are unclear. These questions are increasingly relevant as advances in imaging, mapping and ablation allow us to translate these concepts to the bedside for improved therapies for AF.
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health (K23 HL70529, R01 HL83359) and Doris Duke Clinical Foundation to SMN, and by a grant from the American College of Cardiology Foundation/Merck to DEK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. Dr. Narayan is co-inventor of a patent (U.S. # 7,123,954) in the analysis of atrial tachyarrhythmias, and is on the speaker’s bureau for Boston Scientific and St Jude Medical Corporations.
Disclosures
The authors report no disclosures.
ReferencesTSC1
- 1.Calkins H, Brugada J, Packer D, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation.European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Scoiety (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical Stimulation to Identify Neural Elements on the Heart: Their Role in Atrial Fibrillation. Journal of Interventional Cardiac Electrophysiology. 2005;13:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 4.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Arentz T, Haegeli L, Sanders P, et al. High-density mapping of spontaneous pulmonary vein activity initiating atrial fibrillation in humans. J Cardiovasc Electrophys. 2007;18:31–38. doi: 10.1111/j.1540-8167.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 6.Verma A, Saliba W, Lakkireddy D, et al. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm. 2007;4:1177–1182. doi: 10.1016/j.hrthm.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Halligan SC, Gersh BJ, Brown RD, Jr, et al. The Natural History of Lone Atrial Flutter. Ann Intern Med. 2004;140:265–268. doi: 10.7326/0003-4819-140-4-200402170-00008. [DOI] [PubMed] [Google Scholar]
- 8.Moreira W, Timmermans C, Wellens HJ, et al. Can common-type atrial flutter be a sign of an arrhythmogenic substrate in paroxysmal atrial fibrillation? Clinical and ablative consequences in patients with coexistent paroxysmal atrial fibrillation/atrial flutter. Circulation. 2007;116:2786–2792. doi: 10.1161/CIRCULATIONAHA.107.711622. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Mangat I, Glatter KA, et al. Mechanism of conversion of atypical right atrial flutter to atrial fibrillation. Am J Cardiol. 2003;91:46–52. doi: 10.1016/s0002-9149(02)02996-x. [DOI] [PubMed] [Google Scholar]
- 10.Franz MR, Karasik PL, Li C, et al. Electrical Remodeling of the Human Atrium: Similar Effects in Patients with Chronic Atrial Fibrillation and Atrial Flutter. J Am Coll Cardiol. 1997;30:1785–1792. doi: 10.1016/s0735-1097(97)00385-9. [DOI] [PubMed] [Google Scholar]
- 11.Waldo AL. The Interrelationship Between Atrial Fibrillation and Atrial Flutter. Progress in Cardiovascular Diseases. 2005;48:41–56. doi: 10.1016/j.pcad.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Narayan SM, Bode F, Karasik PL, et al. Alternans Of Atrial Action Potentials As A Precursor Of Atrial Fibrillation. Circulation. 2002b;106:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 13.Mandapati R, Skanes A, Chen J, et al. Stable Microreentrant Sources as a Mechanism of Atrial Fibrillation in the Isolated Sheep Heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Allessie MA, Ausma J, Schotten U. Electrical, Contractile and Structural Remodeling during Atrial Fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 15.Moe GK, Rheinboldt W, Abildskov J. A computer model of atrial fibrillation. American Heart Journal. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 16.Konings K, Smeets J, Penn O, et al. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 17.Botteron GW, Smith JM. Quantitative assessment of the spatial organization of atrial fibrillation in the intact human heart. Circulation. 1996;93:513–518. doi: 10.1161/01.cir.93.3.513. [DOI] [PubMed] [Google Scholar]
- 18.Cox JL. The central controversy surrounding the interventional-surgical treatment of atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2005;129:1–4. doi: 10.1016/j.jtcvs.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Lazar S, Dixit S, Callans D, et al. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006;3:889–895. doi: 10.1016/j.hrthm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Sahadevan J, Ryu K, Peltz L, et al. Epicardial Mapping of Chronic Atrial Fibrillation in Patients: Preliminary Observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 21.Ryu K, Shroff SC, Sahadevan J, et al. Mapping of Atrial Activation During Sustained Atrial Fibrillation in Dogs with Rapid Ventricular Pacing Induced Heart Failure: Evidence for a Role of Driver Regions. Journal of Cardiovascular Electrophysiology. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu T-J, Doshi RN, Huang H-LA, et al. Simultaneous Biatrial Computerized Mapping During Permanent Atrial Fibrillation in Patients with Organic Heart Disease. J. Cardiovasc. Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 23.Haissaguerre M, Sanders P, Hocini M, et al. Changes in Atrial Fibrillation Cycle Length and Inducibility During Catheter Ablation and Their Relation to Outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Krummen DE, Kahn AM, et al. Evaluating Fluctuations in Human Atrial Fibrillatory Cycle Length Using Monophasic Action Potentials. Pacing Clin Electrophysiol. 2006d;29:1209–1218. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanders P, Berenfeld O, Hocini M, et al. Spectral Analysis Identifies Sites of High-Frequency Activity Maintaining Atrial Fibrillation in Humans. Circulation. 2005a;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 26.Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm. 2006;3:1295–1305. doi: 10.1016/j.hrthm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Brown JP, Krummen DE, Feld GK, et al. Using Electrocardiographic Activation Time and Diastolic Intervals to Separate Focal from Macroreentrant Atrial Tachycardias. J Am Coll Cardiol. 2007;49:1965–1973. doi: 10.1016/j.jacc.2006.10.080. [DOI] [PubMed] [Google Scholar]
- 28.Krummen D, Bullinga J, Narayan S. Centrifugal Organization and Rate Step-Down: A Novel Method for Identification of Potential Drivers in Human Atrial Fibrillation (abstract) Circulation. 2007;116:II_394. [Google Scholar]
- 29.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004a;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 30.Lellouche N, Buch E, Celigoj A, et al. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 2007;50:1324–1331. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Scherlag B, Edwards J, et al. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophys. 2007;18:83–90. doi: 10.1111/j.1540-8167.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 32.Haissaguerre M, Sanders P, Hocini M, et al. Catheter Ablation of Long-Lasting Persistent Atrial Fibrillation: Critical Structures for Termination. Journal of Cardiovascular Electrophysiology. 2005a;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JN, Karma A, Shiferaw Y, et al. From Pulsus to Pulseless: The Saga of Cardiac Alternans (Review) Circ Res. 2006;98:1244. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 34.Narayan SM. T-Wave Alternans and The Susceptibility to Ventricular Arrhythmias: State of the Art Paper. J Am Coll Cardiol. 2006a;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 35.Chang S, Tai CL, Y J, Wongcharoen W, et al. The role of left atrial muscular bundles in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2007;50:964–973. doi: 10.1016/j.jacc.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez L-M, Timmermans C, Nabar A, et al. Biatrial Activation in Isthmus-Dependent Atrial Flutter. Circulation. 2001;104:2545–2550. doi: 10.1161/hc4601.097996. [DOI] [PubMed] [Google Scholar]
- 37.Krummen DE, Feld GK, Narayan SM. Diagnostic Accuracy of Irregularly Irregular RR Intervals in Separating Atrial Fibrillation from Atrial Flutter. Am J Cardiol. 2006a;98:209–214. doi: 10.1016/j.amjcard.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 38.Narayan SM, Hassankhani A, Feld GK, et al. Separating Non-Isthmus From Isthmus Dependent Atrial Flutter Using Wavefront Variability. J Am Coll Cardiol. 2005b;45:1269–1279. doi: 10.1016/j.jacc.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 39.Jais P, Shah DC, Haissaguerre M, et al. Mapping and ablation of left atrial flutters. Circulation. 2000;101:2928–2934. doi: 10.1161/01.cir.101.25.2928. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Hertervig EJ, Kongstad O, et al. Deterioration of interatrial conduction in patients with paroxysmal atrial fibrillation: Electroanatomic mapping of the right atrium and coronary sinus. Heart Rhythm. 2004;1:548–553. doi: 10.1016/j.hrthm.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Kim B-S, Kim Y-H, Hwang G-S, et al. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002;39:1329–1336. doi: 10.1016/s0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y, Xie F, Stein K, et al. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation. 2007;115:2094–2102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 43.Narayan SM, Franz MR, Kim J, et al. T-wave Alternans, Restitution of Ventricular Action Potential Duration and Outcome. J Am Coll Cardiol. 2007f;50:2385–2392. doi: 10.1016/j.jacc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Kalifa J, Jalife J, Zaitsev AV, et al. Intra-Atrial Pressure Increases Rate and Organization of Waves Emanating From the Superior Pulmonary Veins During Atrial Fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]


