Abstract
BACKGROUND
Endometriosis is associated with an inflammatory response. Hence infliximab, an anti-TNF-α monoclonal antibody, might relieve pain.
METHODS
A randomized placebo-controlled trial was designed with 21 women with severe pain and a rectovaginal nodule of at least 1 cm. After 1 month of observation, three infusions of infliximab (5 mg/kg) or placebo were given. Surgery was performed 3 months later and follow-up continued for 6 months. The primary end-point was pain (dysmenorrhea, deep dyspareunia and non-menstrual pain) rated at each visit by the clinician and on a daily basis by the patient who in addition scored pain by visual analog pain scale and analgesia intake. Secondary end-points included the volume of the endometriotic nodule, pelvic tenderness and the visual appearance of endometriotic lesions at laparoscopy.
RESULTS
Pain severity decreased during the treatment by 30% in both the placebo (P < 0.001) and infliximab groups (P < 0.001). However, no effect of infliximab was observed for any of the outcome measures. After surgery, pain scores decreased in both groups to less than 20% of the initial value.
CONCLUSIONS
Infliximab appears not to affect pain associated with deep endometriosis. Treatment is associated with an important placebo effect. After surgery, pain decreases to less than 20%. Trials registration number ClinicalTrials.gov: NCT00604864.
Introduction
Endometriosis is associated with pelvic pain, especially if deep and/or ovarian cystic lesions are present (Koninckx et al., 1991b, 1994). Whether subtle lesions cause pain is not yet substantiated. The association of pain and typical endometriosis was derived from observational studies in which women with pelvic pain have a higher incidence of endometriosis (range: 40–80%) than women with infertility without pain (20–50%) or control groups (5–20%) (Koninckx et al., 1991b). Surgical ablation of typical endometriosis has been reported to decrease pain in several observational studies and in a randomized controlled trial (Sutton et al., 1994, 1997; Jacobson et al., 2001). The strong association between cystic ovarian and deep endometriosis with severe pelvic pain was invariably noted in observational studies, and logistic regression showed deep and cystic endometriosis to be the strongest predictors of pelvic pain (Koninckx and Martin, 1994; Vercellini et al., 1996). Following the surgical excision, pain invariably decreased by some 80% (Donnez et al., 1995; Koninckx and Martin, 1997; Brouwer and Woods, 2007; Darai et al., 2007; Kristensen and Kjer, 2007).
The pathophysiology of the association between endometriosis and pain is poorly understood. Several hypotheses have been put forward. First, as endometriosis is a hormonally responsive tissue similar to endometrium, it is reasonable to assume that mini-menstrual bleedings (Brosens, 1997) cause pain because of pelvic irritation by blood, by an inflammatory reaction or by the tension in the micro cysts of the lesions. This hypothesis is supported by a series of observations. During menstruation pain typically worsens and deep lesions feel tender (Koninckx et al., 1996a). At laparoscopy, endometriotic lesions were observed to bleed during menstruation (personal observation). Natural menopause and all medical treatments, which inactivate the endometrium (Vercellini et al., 1997, 2007a), prevent menstruation or decrease menstrual blood loss, including GnRH agonists (Shaw, 1991) and oral contraceptives decrease pain. Inflammatory reaction and neo angiogenesis (Oosterlynck et al., 1993) around endometriotic lesions have been observed during laparoscopy and by pathology. Secondly, in deep lesions, the observed endometriotic invasion of nerve fibers and the association with mast cells (Anaf et al., 2000, 2002, 2004, 2006) might explain the severe pain. Finally, especially deep endometriosis can cause sclerotic compression of ureters and of nerve fibers, as demonstrated by the excision of endometriosis surrounding the sciatic nerve (Batt et al., 2004; Possover and Chiantera, 2007) or the pudendal nerve in cases of menstrual sciatalgia or Alcock syndrome (Robert et al., 1998; Possover and Chiantera, 2007).
TNF-α, a pro-inflammatory cytokine, has been implicated in the pathophysiology of endometriosis (Agic et al., 2006). TNF-α levels are increased in peritoneal fluid of women with endometriosis (Bedaiwy et al., 2002), and the levels correlate with severity of disease (Richter et al., 2005). Peritoneal fluid TNF-α is produced locally by activated peritoneal macrophages (Koninckx et al., 1999). TNF-α induces IL-8 secretion by peritoneal mesothelial cells (Arici, 2002). The peritoneal fluid concentrations of TNF-α and IL-8 correlate with the size and the number of active peritoneal lesions (Bullimore, 2003). Serum TNF-α levels are increased, and monocytes from patients with endometriosis release more TNF-α in vitro compared with monocytes from controls (Braun et al., 1996). Peritoneal fluid levels of MCP-1 are increased in patients with endometriosis. TNF-α, IL-8 and MCP-1 drive an inflammatory Th-1 type response in the peritoneal fluid of patients with endometriosis (Akoum et al., 1995).
The anti-inflammatory effect of blocking TNF-α by monoclonal antibodies (e.g. infliximab) or by soluble TNF-α receptors (e.g. etanercept) has been demonstrated in vivo in animal models and also in the human. The clinical effectiveness of blocking TNF-α has been demonstrated in inflammation driven conditions including Crohn’s disease and rheumatoid arthritis but not in severe endometriosis (Shakiba and Falcone, 2006). In baboons with laparoscopically confirmed endometriosis, TNF-α blockade with p55 soluble TNF-α receptors results in inhibition of the development and growth of endometriotic implants (D’Hooghe et al., 2006). The size of peritoneal red lesions was decreased in comparison with a control group (Barrier et al., 2004), but there was no increase in pregnancy rates (Falconer et al., 2007). In rats with ectopically transplanted endometrial tissue, the administration of recombinant human TNF-α binding protein-1 (r-hTBP-1) resulted in defective development of implants compared with controls (D’Antonio et al., 2000).
TNF-α mediated inflammation may be a causal factor in the pain associated with endometriosis and blocking TNF-α appears to inhibit the development of the disease in animal models. We evaluated the effect of infliximab on pain in women with rectovaginal endometriosis at a dose proven to be effective in inflammatory bowel disease and rheumatoid arthritis.
Materials and Methods
Patients
All patients (aged 18–50 years) were recruited from a single, tertiary referral hospital at Leuven University, Belgium. All women suffered from pelvic pain and were scheduled for surgical excision of a rectovaginal endometriotic nodule of at least 1 cm in diameter with or without cystic ovarian endometriosis. None of the women had previously been operated for deep endometriosis. All women had a regular cycle (25–40 days) and moderate to severe pelvic pain as defined by their score on the Biberoglu–Behrman scale. If previously treated with hormonal medication, including progestagens, GnRH analogs, Danazol or oral contraceptives, at least 3 months had elapsed and they had at least two menstrual cycles since stopping treatment. The study was reviewed and approved by the institutional review board of KULeuven. Written informed consent was obtained from each subject.
If not sterilized the patient had to agree to use a double-barrier method of contraception for the duration of the study, and up to 6 months after receiving the last infusion with infliximab.
In view of infliximab’s known side-effect profile, the following were exclusion criteria: (i) old or currently active TB; (ii) evidence of serious infections in the previous 3 months; (iii) documented HIV infection, active hepatitis-B or C, or an opportunistic infection (e.g. herpes zoster, cytomegalovirus, pneumocystis carinii, aspergillosis, histoplasmosis or mycobacteria other than TB) in the previous 6 months; (iv) previous transplant surgery, a lymphoproliferative disorder or other malignancy; (v) positive cervical cytology in the previous 6 months; (vi) previous treatment with infliximab or, any drug known to affect TNF-α levels, e.g. pentoxifylline, thalidomide and etanercept, or any human/murine recombinant products; (vii) known allergy to murine products; (viii) recent use of other investigational drugs within 1 month of recruitment or within five half-lives of the investigational agent, whichever was longer and (ix) any hematological or biochemical abnormalities on routine screening. Subjects were also excluded if there was pelvic pathology on transvaginal ultrasound (TVU) scan other than small uterine fibroids (<4 cm in diameter) or an ovarian endometrioma or endometriotic nodule.
Study design
This study was a single center, randomized, double-blind, placebo-controlled pilot study in 21 women with deep endometriosis-associated pain in whom excisional surgery was planned as therapy. Randomization was performed in a 2–1 ratio for active and placebo treatments, respectively, 14 women thus receiving active treatment and seven receiving placebo. In one patient of the infliximab group, with a deep nodule diagnosed by TVU only, no deep endometriotic nodule was found during surgery. Both groups were similar for age, weight, height, blood pressure, heart rate, days of bleeding, age of menarche, number of pregnancies and spontaneous abortions, leucocyte and blood cell count and liver function tests. Patients receiving placebo (n = 7) and infliximab (n = 13) were 30.7 ± 5.5 and 28.4 ± 4.5 years old with a weight of 52.7 + 5.4 and 62.5 ± 7.4 kg (P = 0.002), a height of 161 + 5 and 162 + 5 cm, a cycle length of 29 + 2 and 32 + 5 days, a systolic blood pressure of 127 ± 15 and 120 ± 12 mm of mercury, a diastolic blood pressure of 78 ± 11 and 78 ± 7 mm of mercury and a heart rate of 79 ± 14 and 83 ± 12, respectively.
The study period consisted of 40 weeks, i.e. at least 4 weeks pre-treatment evaluation, a 12 week treatment period followed by surgery and 24 weeks follow-up period. Visits were scheduled at least 4 weeks before the start of treatment (Visit 1, Week 4), at the start of treatment (Visit 2, Week 0), then 2 (Visit 3), 4 (Visit 4), 8 (Visit 5) and 12 (Visit 6) weeks after the start of treatment and 6 (Visit 7) and 12 (Visit 8) weeks after surgery. Infliximab or placebo was administered as a slow infusion of 250 ml at the beginning of the cycle (Week 0 or Visit 2) and repeated after 2 (Visit 3) and 6 weeks (Visit 4) reflecting the typical induction treatment scheme of 0, 2 and 6 weeks given in other inflammatory pathologies, such as Crohn’s disease and rheumatoid arthritis. Women were monitored for adverse effects for 1 h post-infusion. A pregnancy test was performed prior to the infusion and on Week 8.
At each visit and during the follow-up period, safety was monitored through standard blood tests, vital signs and breast examination. Adverse events were reviewed by a Safety Monitoring Committee every 3 months.
The primary end-point was the effect of infliximab treatment upon pelvic pain including the intake of pain killers. Secondary end-points included the volume of endometriotic nodules assessed clinically and on TVU, the macroscopical appearance of endometriotic lesions during surgery and the extend of endometriosis. The revised American Fertility (rAFS) classification system was not used to score endometriosis since the severity of deep endometriosis is poorly reflected in the rAFS score.
Clinical evaluations of endometriosis and pain assessment
Pain was assessed by one gynecologist (PK) at each visit using a modified Biberoglu–Behrman scale, scoring from 0 (no pain) to 3 (severe pain) dysmenorrhea, deep dyspareunia, chronic pelvic pain, pelvic tenderness and pelvic induration. In addition the patients independently recorded daily dyspareunia, dysmenorrhea and pelvic pain and the intake of Ibuprofen 100 mg tablets. In addition, they recorded weekly by visual analog pain scale (VAS), the average maximum tolerated pain (i.e. before intake of a pain killer) over the last 7 days. Ibuprofen was taken as required up to a maximum of 2.4 g/day; additional pain medication was permitted with documentation.
A TVU was performed during the screening period and at Visits 2, 3, 5 and 6 to measure deep endometriosis volume and endometrial thickness (Timmerman et al., 2002). Patients recorded daily the amount of vaginal blood loss.
Treatments, randomization and blinding
Infliximab an IgG monoclonal anti-TNF antibody was supplied by Centocor as a lyophilized solid containing 100 mg of infliximab IgG, 0.5 g of sucrose, 6.1 mg of dibasic sodium phosphate dihydrate, 2.2 mg of monobasic sodium phosphate monohydrate and 0.5 mg of polysorbate 80 in a 20 ml vial for reconstitution in 10 ml of sterile water for injection. The placebo was supplied as a lyophilized solid containing 0.5 g of sucrose, 6.1 mg of dibasic sodium phosphate dihydrate, 2.2 mg of monobasic sodium phosphate monohydrate and 0.5 mg of polysorbate 80 in a 20 ml vial for reconstitution in 10 ml of sterile water. The vials of infliximab/placebo were supplied by Centocor, stored at 2–8°C and reconstituted immediately before each administration.
All investigators, research nurses and patients were blinded throughout the study. Randomization (prepared by Centocor Paris) was performed by consecutive sealed envelopes opened by the pharmacist prior to the preparation of medication. Randomization code was broken only after the database had been locked.
Surgery and other procedures
Surgery for endometriosis was performed as reported (Koninckx and Barlow, 1999) 4–6 weeks after the last infliximab dose. A follow-up visit was planned 4–9 weeks after surgery (Visit 7) and a final visit to assess safety was performed between 26 and 29 weeks (Visit 8). During surgery, an endometrial biopsy was taken and subsequently processed for routine pathology.
Statistics
This study was an exploratory trial, and thus not powered to detect small differences. An important decrease of pain would have been detected, since with a SD of 2, a difference in BB score of 3 would have been detected with a power of 90%. To detect small differences, e.g. of one point with a power of 80% a RCT of 120 women would have been necessary.
Statistical analysis was performed with the SAS system (SAS/STAT users guide, 1988). Wilcoxon Rank sum test was used to evaluate differences of means at each visit for each variable. Since in this study obviously two possible effects were present simultaneously, i.e. the effect of Infliximab treatment and the effect of placebo or surgery, a two-way analysis of variance (proc GLM, general linear methods) was performed, analysing simultaneously for each end-point the effect of treatment (control or anti-TNF-α) and the placebo or surgery effect (the difference between two treatment periods). Baseline was defined as the mean of Visits 1 and 2, the early treatment period as the mean of Visits 3 and 4 and the late treatment period as mean of Visits 5 and 6. For the effect of surgery, baseline, early and late treatment periods were compared with the post-operative period (mean of Visits 7 and 8).
Since pain was exacerbated during menstruation, the menstrual period, defined as the first 4 days of the cycle was analysed separately. A menstrual cycle was defined as starting with the first day of bleeding and lasting for 28 days.
The effect upon pain was evaluated from the 5 BB entries made by the physician, and the 3 BB entries, the VAS scale and the intake of ibuprofen and other painkillers recorded by the patients. Analysis of pain killer intake was done twice: a first analysis was done considering only the ibuprofen intake (the number of tablets of ibuprofen taken); in a second analysis other painkiller intake was taken into account: if other painkillers had been taken the number of tablets taken was added to the number of ibuprofen tablets taken; if other strong pain killers had been injected, e.g. a morphine product, these injections were arbitrarily scored as six tablets in order to obtain the total score. In addition, the effect of treatment upon two calculated total pain scores combining the individual BB entries and pain killer intake was calculated. A first total pain score consisted of the sum of dysmenorrhea, deep dyspareunia and pelvic pain (both as assessed by the clinician and by the patients). In order to take also the ibuprofen intake into account, a second total pain score was calculated by multiplying the total daily pain score with the number +1 (in order to avoid to multiply by zero) of ibuprofen tablets taken during that day.
Both an intention to treat analysis, considering all 21 patients included, and an analysis of the 20 women with endometriosis was performed. Since results were identical only the latter will be given in the manuscript. Means and SE are given unless indicated otherwise. For the safety analysis, obviously all 14 women treated with Infliximab were included.
Results are presented as mean + SE. Spearman correlation was used for correlation analysis.
Results
These women with endometriosis had very severe pain before treatment with a total BB score of 12.2 on a scale of 15 (Figs 1 and 2). The baseline BB scorings by the clinician were calculated as 2.7 + 0.09 for dysmenorrhea, 2.3 + 0.19 for deep dyspareunia, 2.2 + 0.12 for chronic pain, 2.2 + 0.14 for pelvic tenderness, 2.8 + 0.07 for pelvic induration, with a sum of the pain scores of 7.1 + 0.24 and a total BB score of 12.2 + 0.28.
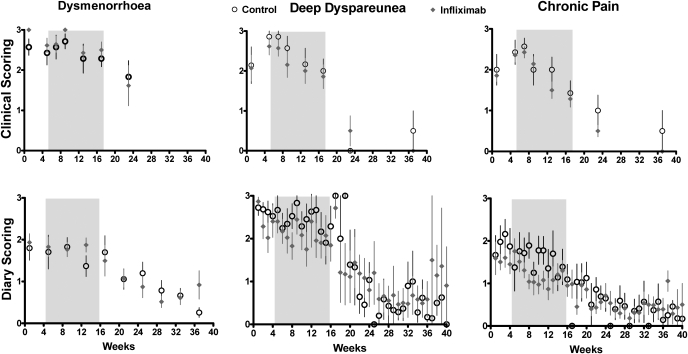
Figure 1:
Dysmenorrhea, deep dyspareunia and chronic pelvic pain, as assessed by the clinician (upper graphs) and by the patient in her diary, before treatment, during the 12 week treatment period (shaded area) and after surgery.
Baseline versus early treatment: NS, baseline versus late treatment: >0.003 for all. Baseline and late treatment versus post-surgery: P < 0.001 for all. Infliximab: NS for all.
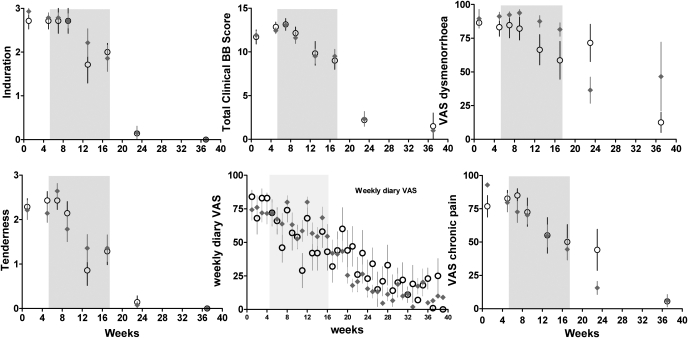
Figure 2:
Induration, pelvic tenderness and total Biberoglu–Behrman score together with VAS scales as recorded at each visit and weekly by the patient in her diary, before treatment, during the 12 week treatment period (shaded area) and after surgery.
Baseline versus early treatment, NS; baseline versus late treatment, >0.003 for all except NS for VAS dysmenorrhea. Baseline and late treatment versus post-surgery: P < 0.001 for all. Infliximab: NS for all.
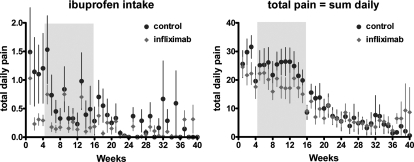
As shown in Figs 1, 2 and 3, during the 12 week treatment period a similar exponential decrease in pain of some 25–30% was observed in both the control and the infliximab group, compatible with a placebo effect. No statistically significant differences between placebo and infliximab treatment could be identified. The strong and significant placebo effect and the absence of an infliximab effect were consistently found for all pain estimations in this study, i.e. the five scorings by the clinician and the calculated total scores (i.e. dysmenorrhea, deep dyspareunia, chronic pain, pelvic tenderness, the sum of the 3 BB pain scores and the total BB score) and the pain recorded in the dairy by the patients (i.e. the 3 BB scores, the visual analog scales, the pain killer intake and both calculated total scores, i.e. with or without taking pain killer intake into account). The placebo effect was not significant for the early treatment period (Visits 3 and 4) but highly significant for the late treatment period (Visits 5 and 6) both by two way analysis of variance (P < 0.003 to 0.001 for all) and when the placebo (Wilcoxon, P < 0.001) and the infliximab group (Wilcoxon, P < 0.001) were analysed separately.
Figure 3:
Ibuprofen intake and total pain calculated from the patient dairy.
No effect of treatment was found upon the mean diameters of the nodules as measured by TVU. For the placebo group (n = 7) and the infliximab group (n = 13), the diameters were 15.2 ± 4.6 and 13.6 ± 3.2 mm, during Visit 3, 15.1 ± 5.12 and 14.25 ± 3.5 mm, during Visit 5, 13.5 ± 4.9 and 15.6 ± 3.5 mm and during Visit 6, 13.2 + 3.4 and 15.1 + 2.38 mm. Endometrial thickness was not affected by treatment, being at baseline and at the end of the treatment period for the infliximab group 5.2 (range 1.3–18) and 7.8 (1.9–11) mm and for the placebo group 7.7 (1.8–23) and 8.2 (3–15) mm, respectively.
During surgery, no obvious differences were observed in the extend of the disease, the macroscopic aspect of endometriosis (vascularization and sclerosis), the duration of surgery and bleeding estimates. Routine pathology (FC) also showed no obvious differences between the two groups.
After surgery, all pain estimates decreased to less than 10% of the baseline in both groups (Figs 1 and 2), and no differences were found between the placebo and the infliximab group (two-way analysis of variance). This decrease of pain after surgery was highly significant (P < 0.001) for all pain estimates at all visits.
During treatment all side-effects were reviewed by the safety committee. One patient had 2 days after the second infliximab infusion an acute tonsillitis, which resolved quickly by antibiotic treatment. One patient had a mild (infliximab) infusion reaction during the 3rd infusion. One patient developed an acute leukemia 4 months after the last infliximab infusion. Two infliximab patients, who failed to use contraception, became pregnant 6 and 10 weeks after surgery, respectively; one miscarried spontaneously at 10 weeks and the other delivered a healthy baby at term. One infliximab patient had myalgia for a few days after the second infusion and another infliximab patient had a bad taste in the mouth for a few days; both events were considered unrelated to the drug by the safety review committee. There were no AEs reported in the placebo patients.
A Spearman correlation between all entries of the whole dataset was performed. We realize that this crude analysis is strictly incorrect since all entries were used as independent variables, and that this blind analysis carries the risk of spurious correlations. Since correlations were so strong, some seem worth mentioning. There was a strong intercorrelation (P < 0.001 for all) between all pain estimates by the physician (dysmenorrhea, deep dyspareunea and chronic pain) and by the patients (dysmenorrhea, deep dyspareunea, chronic pain, VAS scales and ibuprofen intake), suggesting that pain estimates are not entirely independent. Over the whole observation period, the infliximab group had a slightly higher intake of pain killers (P < 0.01), and higher dysmenorrhea scores (P < 0.001 for physician assessment, for patient VAS scales and for patient diary). The volume of the nodule assessed by TVU correlated with the pelvic induration assessed by the physician (P = 0.04) but surprisingly not with any of the pain estimates nor with ibuprofen intake. Unexpected correlations were found between weight and temperature (P < 0.001), between temperature and dysmenorrhea (P < 0.001) and deep dyspareunea (P < 0.001), and between pulse rate and pain estimates (P < 0.001).
Discussion
To the best of our knowledge, this is the first randomized, placebo-controlled trial to assess the effect of an anti-TNF-α drug in the treatment of deep endometriosis-associated pain. Women with deep lesions were chosen as the study group for two reasons. First, these lesions were diagnosed on clinical examination rather than at laparoscopy and the women participated in the study while awaiting definitive surgical treatment. Second, for deep endometriosis the association between pain and endometriosis is much stronger than for other disease types (Koninckx et al., 1991a; Vercellini et al., 2007b). Women with deep endometriosis have severe pain close to the maximum on a BB painscale, the painful nodules can be confirmed by clinical exam and less than 5% is pain free (Koninckx et al., 1996b). A study of women with typical lesions only would carry a much higher risk of including women in whom pain is not caused by the endometriosis. In typical endometriosis pain is highly variable, is rarely very severe and 30–50% of women are pain free. Women with deep endometriosis may thus be a preferred group to evaluate drug effects on endometriosis-associated pain.
The observed placebo effect was unexpectedly high in these women, and is consistent with previous reports albeit in women with less severe pain (Sutton et al., 1994, 1997). Placebo effect was observed for all pain measures, including tenderness on pelvic examination. The absence of effect upon pelvic induration, indirectly confirms that the effect on pain was a placebo effect and that the clinician remained objective.
The strong placebo effect can be explained by the patient perception of the importance of an intravenous infusion followed by the close observation in hospital for 1 h. In addition, the enthusiasm of the researchers who became convinced of the efficacy of infliximab could have been conveyed to the patients. Indeed, two-thirds of the patients reported a greater than 50% decrease in pain, with little effect in the remaining third. Understanding the mechanisms involved in this important decrease of pain by more than 50% (Fuente-Fernandez et al., 2006; Pacheco-Lopez et al., 2006; Beauregard, 2007; Benedetti, 2007; Koshi and Short, 2007; Lidstone and Stoessl, 2007; Olshansky, 2007) could lead to clinical effective treatments of endometriosis related pain (Rostkowska-Nadolska, 2007).
The lack of efficacy for infliximab as a treatment for deep endometriosis-associated pain was unexpected because of the widely held belief that inflammation is a major cause of pain in endometriosis (Vercellini et al., 2007a). However, the pathophysiology of the severe pain associated with deep endometriosis may be different from that caused by typical lesions, i.e. inflammation might be more important in superficial peritoneal endometriosis whereas nerve invasion or compression is more important in deep lesions (Anaf et al., 2000, 2002, 2004, 2006). This absence of effect upon pain is consistent with the absence of effect during surgery or after examination by routine pathology. Whether another treatment protocol or a higher dose would be effective cannot be excluded.
We have scrutinized the literature on the medical treatment of endometriosis-associated pain. The evidence of efficacy may be weak as the blinding in most studies appears inadequate. Researchers and patients were able to guess whether individuals were randomized to placebo or active treatment if menstruation was prevented or if there were major side-effects such as hot flushes, or recognizable physical signs such as vaginal atrophy. In addition, conclusions are usually based upon a reduction in the total pain score and all drugs that abolish menstruation will thus by definition be effective in reducing dysmenorrhea. Given the strong correlation between pain symptoms it remains uncertain whether these treatments are effective for all pain symptoms associated with endometriosis or simply dysmenorrhea alone.
The correlations found should be regarded cautiously since the study is small, and since some might be spurious. It was surprising not to find a correlation between the size of the nodules measured by TVU and the pain estimates. Second, that dysmenorrhea correlated negatively with age and age of menarche is an intriguing observation as are the systematic correlations of pain estimates with temperature, blood pressure and pulse rate. That anticipation of a clinical exam could have increased blood pressure and pulse rate more in women with more severe pain remains speculative.
When designing this study, we were concerned about the theoretical possibility of increasing post-operative complication rates as a result of infliximab’s effect on the immune system. The existing evidence was reassuring, i.e. there were no differences in length of hospital stay or surgical complications between patients with Crohn’s disease who received infliximab 2 months before surgery (n = 22) or 1 month after surgery (n = 13) compared with matched controls (Parsi et al., 2002). Since then, two retrospective studies in patients with Crohn’s disease have similarly concluded that the use of infliximab, as well as steroids or immunomodulators, before abdominal surgery does not increase the risk for post-operative complications (Colombel et al., 2004; Marchal et al., 2004). In our study, there were no differences in post-operative complication rates between the groups.
Given that we only recruited women with deep endometriosis, we accept the possibility that anti-TNF-α treatment might have an effect on other types of endometriosis, e.g. minimal disease with a marked inflammatory component. However, we urge caution about conducting a study to evaluate the effect of anti-TNF-α drugs in such patients. We are not convinced that the potential benefits outweigh the risk of serious side-effects, the high cost of anti-TNF-α drugs and the ethical problems associated with not treating minimal disease surgically at the time of diagnosis.
This study confirmed the effectiveness of surgical excision of deep endometriosis upon pain (Koninckx and Martin, 1994; Redwine and Wright, 2001; Wright and Redwine, 2002; Donnez et al., 2004; Garry, 2004). We do not know to what extend a placebo effect contributes to the surgical outcome.
In conclusion, infliximab appears to have no important beneficial effect upon pain associated with deep endometriosis. We observed an overall placebo effect of 25%, which reached over 50% in two-thirds of the women, emphasizing the need for well conducted, double-blind, randomized, placebo-controlled studies when evaluating novel treatments for pain. The efficacy of surgical excision upon pain was confirmed, as was the safety of infliximab treatment in the pre-operative period.
Funding
This trial was performed as an investigator initiated trial. Support was received from Centocor.
Acknowledgements
We thank Centocor for supporting this trial conducted as a phase II exploratory proof of concept trial with full external monitoring. We do thank the members of the safety committee, Paul Rutgeers, Rene Westhovens and Christel Meuleman for their advice and help with the few side-effects observed. We thank Bart Scheerder, DF Jansen and Desiree Jansen for independent statistical evaluation of these data. We thank the members of the clinical trial unit and the CRO for conducting and monitoring this trial. The trial was previously registered in Europe with EUDRACT number: EU-0053/endometriosis.
References
- Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- Akoum A, Lemay A, Brunet C, Hebert J, Bergeron J, Maheux R, Quesnel G, Villeneuve M. Secretion of monocyte chemotactic protein-1 by cytokine-stimulated endometrial cells of women with endometriosis. Fertil Steril. 1995;63:322–328. doi: 10.1016/s0015-0282(16)57363-4. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15:1744–1750. doi: 10.1093/humrep/15.8.1744. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–1900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- Anaf V, El Nakadi I, Simon P, Van de Stadt J, Fayt I, Simonart T, Noel JC. Preferential infiltration of large bowel endometriosis along the nerves of the colon. Hum Reprod. 2004;19:996–1002. doi: 10.1093/humrep/deh150. [DOI] [PubMed] [Google Scholar]
- Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noel JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006;86:1336–1343. doi: 10.1016/j.fertnstert.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–109. doi: 10.1111/j.1749-6632.2002.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst AM. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril. 2004;81(Suppl 1):775–779. doi: 10.1016/j.fertnstert.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Batt RE, Yeh J, Koninckx PR. Asymmetric distribution of sciatic nerve endometriosis. Obstet Gynecol. 2004;103:400–401. doi: 10.1097/01.aog.0000112921.14894.04. [DOI] [PubMed] [Google Scholar]
- Beauregard M. Mind does really matter: evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog Neurobiol. 2007;81:218–236. doi: 10.1016/j.pneurobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Placebo and endogenous mechanisms of analgesia. Handb Exp Pharmacol. 2007;177:393–413. doi: 10.1007/978-3-540-33823-9_14. [DOI] [PubMed] [Google Scholar]
- Braun DP, Gebel H, House R, Rana N, Dmowski NP. Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis. Fertil Steril. 1996;65:1125–1129. [PubMed] [Google Scholar]
- Brosens IA. Endometriosis—a disease because it is characterized by bleeding. Am J Obstet Gynecol. 1997;176:263–267. doi: 10.1016/s0002-9378(97)70482-4. [DOI] [PubMed] [Google Scholar]
- Brouwer R, Woods RJ. Rectal endometriosis: results of radical excision and review of published work. ANZ J Surg. 2007;77:562–571. doi: 10.1111/j.1445-2197.2007.04153.x. [DOI] [PubMed] [Google Scholar]
- Bullimore DW. Endometriosis is sustained by tumour necrosis factor-alpha. Med Hypotheses. 2003;60:84–88. doi: 10.1016/s0306-9877(02)00336-5. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Loftus EV, Jr, Tremaine WJ, Pemberton JH, Wolff BG, Young-Fadok T, Harmsen WS, Schleck CD, Sandborn WJ. Early postoperative complications are not increased in patients with Crohn’s disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol. 2004;99:878–883. doi: 10.1111/j.1572-0241.2004.04148.x. [DOI] [PubMed] [Google Scholar]
- D’Antonio M, Martelli F, Peano S, Papoian R, Borrelli F. Ability of recombinant human TNF binding protein-1 (r-hTBP-1) to inhibit the development of experimentally-induced endometriosis in rats. J Reprod Immunol. 2000;48:81–98. doi: 10.1016/s0165-0378(00)00073-5. [DOI] [PubMed] [Google Scholar]
- D’Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74:131–136. doi: 10.1095/biolreprod.105.043349. [DOI] [PubMed] [Google Scholar]
- Darai E, Ackerman G, Bazot M, Rouzier R, Dubernard G. Laparoscopic segmental colorectal resection for endometriosis: limits and complications. Surg Endosc. 2007;21:1572–1577. doi: 10.1007/s00464-006-9160-1. [DOI] [PubMed] [Google Scholar]
- Donnez J, Nisolle M, Casanasroux F, Bassil S, Anaf V. Rectovaginal septum, endometriosis or adenomyosis: laparoscopic management in a series of 231 patients. Hum Reprod. 1995;10:630–635. doi: 10.1093/oxfordjournals.humrep.a136001. [DOI] [PubMed] [Google Scholar]
- Donnez J, Pirard C, Smets M, Jadoul P, Squifflet J. Surgical management of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:329–348. doi: 10.1016/j.bpobgyn.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Song XY, Cornillie FJ, Bergqvist A, Fried G, D’Hooghe TM. Effects of anti-TNF-mAb treatment on pregnancy in baboons with induced endometriosis. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.05.062. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Fuente-Fernandez R, Lidstone S, Stoessl AJ. Placebo effect and dopamine release. J Neural Transm Suppl. 2006:415–418. doi: 10.1007/978-3-211-45295-0_62. [DOI] [PubMed] [Google Scholar]
- Garry R. The effectiveness of laparoscopic excision of endometriosis. Curr Opin Obstet Gynecol. 2004;16:299–303. doi: 10.1097/01.gco.0000136496.95075.79. [DOI] [PubMed] [Google Scholar]
- Jacobson TZ, Barlow DH, Garry R, Koninckx PR. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2001;4:CD001300. doi: 10.1002/14651858.CD001300. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Barlow D. Principles and management of endometriosis: surgical treatment. In: Shoham Z, Howles CM, Jacobs HS, editors. Female infertility therapy: Current practice. London: Martin Dunitz; 1999. pp. 373–389. [Google Scholar]
- Koninckx PR, Martin D. Treatment of deeply infiltrating endometriosis. Curr Opin Obstet Gynecol. 1994;6:231–241. [PubMed] [Google Scholar]
- Koninckx PR, Martin DC. Surgical treatment of deeply infiltrating endometriosis. In: Sutton C, editor. Gynecological Endoscopic Surgery. London: Chapman and Hall; 1997. pp. 19–35. [Google Scholar]
- Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;a 55:759–765. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;b 55:759–765. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Oosterlynck D, D’Hooghe T, Meuleman C. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann N Y Acad Sci. 1994;734:333–341. doi: 10.1111/j.1749-6632.1994.tb21763.x. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;a 65:280–287. [PubMed] [Google Scholar]
- Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;b 65:280–287. [PubMed] [Google Scholar]
- Koninckx PR, Kennedy SH, Barlow DH. Pathogenesis of endometriosis: the role of peritoneal fluid. Gynecol Obstet Invest. 1999;47(Suppl 1):23–33. doi: 10.1159/000052856. [DOI] [PubMed] [Google Scholar]
- Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Pract. 2007;7:4–20. doi: 10.1111/j.1533-2500.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- Kristensen J, Kjer JJ. Laparoscopic laser resection of rectovaginal pouch and rectovaginal septum endometriosis: the impact on pelvic pain and quality of life. Acta Obstet Gynecol Scand. 2007;86:1467–1471. doi: 10.1080/00016340701645006. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Stoessl AJ. Understanding the placebo effect: contributions from neuroimaging. Mol Imaging Biol. 2007;9:176–185. doi: 10.1007/s11307-007-0086-3. [DOI] [PubMed] [Google Scholar]
- Marchal L, D’Haens G, Van Assche G, Vermeire S, Noman M, Ferrante M, Hiele M, Bueno De Mesquita M, D’Hoore A, Penninckx F, et al. The risk of post-operative complications associated with infliximab therapy for Crohn’s disease: a controlled cohort study. Aliment Pharmacol Ther. 2004;19:749–754. doi: 10.1111/j.1365-2036.2004.01904.x. [DOI] [PubMed] [Google Scholar]
- Olshansky B. Placebo and nocebo in cardiovascular health: implications for healthcare, research, and the doctor-patient relationship. J Am Coll Cardiol. 2007;49:415–421. doi: 10.1016/j.jacc.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril. 1993;59:778–782. doi: 10.1016/s0015-0282(16)55859-2. [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Engler H, Niemi MB, Schedlowski M. Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav Immun. 2006;20:430–446. doi: 10.1016/j.bbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Parsi MA, Achkar JP, Richardson S, Katz J, Hammel JP, Lashner BA, Brzezinski A. Predictors of response to infliximab in patients with Crohn’s disease. Gastroenterology. 2002;123:707–713. doi: 10.1053/gast.2002.35390. [DOI] [PubMed] [Google Scholar]
- Possover M, Chiantera V. Isolated infiltrative endometriosis of the sciatic nerve: a report of three patients. Fertil Steril. 2007;87:417–419. doi: 10.1016/j.fertnstert.2006.05.084. [DOI] [PubMed] [Google Scholar]
- Redwine DB, Wright JT. Laparoscopic treatment of complete obliteration of the cul-de-sac associated with endometriosis: long-term follow-up of en bloc resection. Fertil Steril. 2001;76:358–365. doi: 10.1016/s0015-0282(01)01913-6. [DOI] [PubMed] [Google Scholar]
- Richter ON, Dorn C, Rosing B, Flaskamp C, Ulrich U. Tumor necrosis factor alpha secretion by peritoneal macrophages in patients with endometriosis. Arch Gynecol Obstet. 2005;271:143–147. doi: 10.1007/s00404-003-0591-9. [DOI] [PubMed] [Google Scholar]
- Robert R, Prat-Pradal D, Labat JJ, Bensignor M, Raoul S, Rebai R, Leborgne J. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Surg Radiol Anat. 1998;20:93–98. doi: 10.1007/BF01628908. [DOI] [PubMed] [Google Scholar]
- Rostkowska-Nadolska B. Can the placebo be the cure? Wiad Lek. 2007;60:201–204. [PubMed] [Google Scholar]
- SAS/STAT users guide. 1988 Release 6.03 level 0045. [Google Scholar]
- Shakiba K, Falcone T. Tumour necrosis factor-alpha blockers: potential limitations in the management of advanced endometriosis? A case report. Hum Reprod. 2006;21:2417–2420. doi: 10.1093/humrep/del179. [DOI] [PubMed] [Google Scholar]
- Shaw RW. Endometriosis: the next ten years. Br J Clin Pract Symp Suppl. 1991;72:59–55. [PubMed] [Google Scholar]
- Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- Sutton CJ, Pooley AS, Ewen SP, Haines P. Follow-up report on a randomized controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal to moderate endometriosis. Fertil Steril. 1997;68:1070–1074. doi: 10.1016/s0015-0282(97)00403-2. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Deprest J, Bourne T. Ultrasound characteristics of endometriosis. In: Timmerman D, Deprest J, Bourne T, editors. Ultrasound and Endoscopic Surgery in Obstetrics and Gynecology. Berlin: Springer Verlag; 2002. pp. 189–195. [Google Scholar]
- Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996;65:299–304. [PubMed] [Google Scholar]
- Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68:393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Abbiati A, Daguati R, Crosignani PG, Somigliana E, Vigano P. Endometriosis: current and future medical therapies. Best Pract Res Clin Obstet Gynaecol. 2008;22:275–306. doi: 10.1016/j.bpobgyn.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;b 22:266–271. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- Wright JT, Redwine DB. Radical conservative surgery for recto-vaginal endometriosis. Fertil Steril. 2002;77(Suppl 1):S40. [Google Scholar]