Abstract
BACKGROUND
No polymorphisms affecting serum FSH levels have been described in the human FSHB gene. We have identified a potential regulatory single nucleotide polymorphism (SNP, rs10835638; G/T) 211 bp upstream from the FSHB mRNA transcription start-site, located within a highly conserved region among placental mammals. We aimed to determine the correlation of carrier status of rs10835638 alternative alleles with serum FSH level in men, and testicular and hormonal parameters.
METHODS
A quantitative genetic association study using a cohort of healthy men (n = 554; age 19.2 ± 1.7 years) visiting the Centre of Andrology, Tartu University Hospital, Estonia.
RESULTS
Rs10835638 (allele frequencies: G 87.6%, T 12.4%) was significantly associated with serum FSH level (analysis of variance: F = 13.0, P = 0.0016, df = 1; regression testing for a linear trend: P = 0.0003). Subjects with the GG genotype exhibited higher FSH levels (3.37 ± 1.79 IU/l, n = 423) compared with heterozygotes (2.84 ± 1.54 IU/l, n = 125) (P = 0.0005), the group of T-allele carriers (GT+TT, 2.78 ± 1.51 IU/l, n = 131) (P = 0.0005) and TT-homozygotes (2.02 ± 0.81 IU/L, n = 6) (P = 0.031). Rs10835638 was also associated with significant (P < 0.05) reduction in free testosterone index and testes volume, but increased semen volume, sex hormone-binding globulin, serum testosterone and estradiol. LH and inhibin-B levels did not differ significantly between groups.
CONCLUSIONS
The identification of a regulatory SNP in FSHB promoter paves the way to study the effect of constitutively low FSH on male health and fertility. As FSH contributes to follicular development and sex steroid production in women, the role of this FSHB variant in female reproductive success is still to be addressed.
Keywords: human FSHB gene promoter, serum FSH level, men, regulatory single nucleotide polymorphism, testicular and hormonal parameters
Introduction
FSH is a pituitary-expressed glycoprotein hormone that regulates gonadal function in both sexes in mammals (Moyle and Campbell, 1996). In females, the role of FSH in regulating follicular development and sex steroid production is clear and well understood, and FSH is routinely used for the treatment of female infertility (Howles, 2000; McGee and Hsueh, 2000). In contrast, the role of FSH in males in regulating testicular function and spermatogenesis continues to be debated (Moudgal and Sairam, 1998; Plant and Marshall, 2001). Studies in transgenic animals have shown that FSH-deficient female mice are infertile and demonstrate small ovaries resulting from a block in folliculogenesis at the pre-antral stage; whereas male mice lacking FSH are fertile, although with reduction in testicular size, and sperm count and motility (Kumar et al., 1997).
FSH is composed of an α-subunit shared with other glycoprotein hormones and a specific β-subunit encoded by the FSHB gene. Human FSHB (4.2 kb) spans one non-coding and two translated exons encoding the 129-amino acid preprotein (Jameson et al., 1988) (Fig. 1a). Resequencing of FSHB (Lamminen et al., 2005; Grigorova et al., 2007) revealed extremely low variation of the gene and no non-synonymous mutations in all studied human populations. Consistently, only eight subjects with FSHB inactivating mutations have been described to date (Huhtaniemi, 2003; Berger et al., 2005). Female patients (n = 5) were suffering from primary amenorrhea, disturbed pubertal development and infertility. Male patients (n = 3) presented azoospermia and small testes, but the effect of inactivating mutations on pubertal development varied. The FSHB gene sequence has only two major variants worldwide (carried by up to 96.6% of each population sample) and exhibits a significant deviation from neutrality suggesting a possible effect of balancing selection (Grigorova et al., 2007). Although transcription of FSHB is rate-limiting for FSH production and controls most of FSH secretion (reviewed by Miller et al., 2002), there is a shortage of data on the transcriptional regulation of the human gene and so far no FSHB polymorphisms altering gene expression have been identified. In this study, we have screened evolutionary conserved regions upstream of the human FSHB gene and report the first promoter variant which is significantly associated with serum FSH levels in healthy young men.
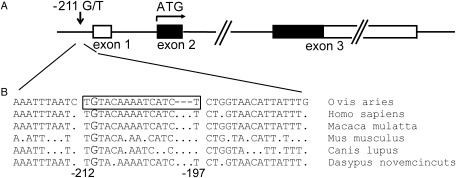
Figure 1:
Location of rs10835638 (G/T) (a) within the human FSHB genomic region and (b) on the comparative alignment of the conserved 5′ upstream sequence element shown to act as PRE in ovine Fshb promoter (Webster et al., 1995).
Black and white boxes indicate coding and non-coding exons E1–E3, respectively. The location of the rs10835638 (position −211) and the PRE element (from −212 to −197) is shown relative to transcription start-site of the human FSHB gene. The ovine PRE element (Webster et al., 1995) is indicated on the alignment as the boxed sequence and the nucleotide G corresponding to the position of the human polymorphism G/T is highlighted with larger font. Ovis aries, domestic sheep; Homo sapiens, human; Macaca mulatta, rhesus monkey; Mus musculus, house mouse; Canis lupus, dog; Dasypus novemcinctus, Nine-banded Armadillo.
Materials and Methods
Study group
The study has been approved by the Ethics Committee of Human Research of the University Clinic of Tartu, Estonia (permission no. 112/3, 27 January 2003). The study was carried out at the Centre of Andrology, Tartu University Hospital between May 2003 and June 2004, and included 578 young men who participated in a prospective study Environment and Reproductive Health (EU 6th FP project QLRT-2001-02911). Participation in the study was voluntary and informed consent was obtained from all study subjects. Principles of the study group formation have been described previously (Punab et al., 2002). In total, 24 subjects of the cohort with either severe genital pathologies (cryptochism n = 9) or with missing data (failed DNA extraction, n = 3; missing or incomplete sperm analysis, n = 9 or hormonal data, n = 3) were excluded from the analyses. The final number of eligible study subjects was 554 [mean age 19.2 ± 1.7 (SD) years]. All men were born and living in Estonia.
Hormone assays
Blood was drawn from the cubital vein between 8 a.m. and 10 a.m. after overnight fasting or light morning meal. Serum was extracted and stored at −80°C until it was sent frozen to the Department of Growth and Reproduction in Copenhagen, Rigshospitalet, Denmark, for analysis. Serum levels of FSH, LH and sex hormone-binding globulin (SHBG) were determined using a time-resolved immunofluorometric assay (Delfia, Wallac, Turku, Finland). Testosterone levels were determined using a time-resolved flouroimmunoassay (Delfia), estradiol (E2) by radioimmunoassay (Pantex, Santa Monica, CA, USA) and inhibin-B by a specific two-sided enzyme immunometric assay (Serotec, UK). Free testosterone index (FTI) was calculated as (testosterone/SHBG) × 100. All hormone assessments were done at the end of the study in order to reduce the influence of inter-assay variations. The intra- and inter-assay coefficients of variation (CV) for measurement of both FSH and LH were 3% and 4.5%, respectively. The intra- and inter-assay CVs for both testosterone and SHBG were <8% and <5%, for E2 and inhibin-B 7.5% and 13%, and 15% and 18%, respectively.
Semen analysis
Semen samples were obtained by masturbation and ejaculated into a sterile collection tube, in a private room near the laboratory. After ejaculation, the semen was incubated at 37°C for 30–40 min for liquefaction. The actual period of ejaculation abstinence was calculated as time in hours between current and previous ejaculation as reported by the men. Semen analysis was performed according to World Health Organization guidelines (World Health Organization, 1999). Semen volume was estimated by weighing the collection tube with the semen sample and subsequently subtracting the predetermined weight of the empty tube assuming 1 g = 1 ml. The motility assessment was performed in duplicate and the average value was calculated. For assessment of the sperm concentration, the samples were diluted in a solution of 0.6 mol/l NaHCO3 and 0.4% (v/v) formaldehyde in distilled water. The sperm concentration was assessed using the improved Neubauer haemocytometers. Total sperm count was calculated by multiplying semen volume by sperm concentration.
Physical examination
Physical examination for assessment of genital pathology and testicular size was performed with the man in standing position. If necessary, pathologies were clarified further with the men in supine position. The orchidometer (made of birch wood, Pharmacia & Upjohn, Denmark) was used for assessment of testicular size. Participants of the study were examined by two investigators who, immediately before the study, had passed the special training for standardization of the clinical examination. The total testicular volume is the sum of the volume of the right and left testicles.
PCR and restriction fragment length polymorphism genotyping
The polymorphism located at the position −211 from FSHB mRNA start site (rs10835638) represents a restriction fragment length polymorphism. Thus, genotyping of the alternative alleles (major G/minor T) was conducted by a PCR (forward/reverse primers: 5′-GGAGCCAGATCATGAAATGTT-3′/ 5′-GACCAATGCTAGCCTGAAGC- 3′) and restriction enzyme digestion (TatI, Fermentas, Lithuania) approach. The uncut PCR product (364 bp) representing the T-allele was separated from the fragments (233 and 131 bp) resulting from digestion of the G-allele by electrophoreses in a 2% agarose gel with 1× Tris/Acetic acid/EDTA buffer. Allele frequencies were estimated and conformance with Hardy–Weinberg equilibrium was computed by an exact test (α = 0.05) using Genepop software (Version 3.4) (http://wbiomed.curtin.edu.au/genepop/).
Data analysis
Marker–Trait association tests for the association of rs10835638 with quantitative hormonal (FSH, LH, testosterone, SHBG and inhibin-B) and testicular (semen and combined testes volume, and sperm motility, concentration and count) parameters were performed using regression testing for a linear trend of marker alleles and one-way analysis of variance (ANOVA) based on marker genotypes. Analysis was adjusted by age and BMI (all parameters), and abstinence period (only semen parameters). The association tests were implemented in statistical analysis package JMP® 6.0.3 with Genomics module 2.0.6 (http://www.jmp.com/software/genomics/). Statistical differences between the carriers (TT homozygotes, n = 6; or TT+GT n = 131) and non-carriers (GG, n = 423) of the T-allele in age, abstinence period, BMI, hormonal and testicular parameters were assessed by non-parametric Mann–Whitney two-sided U-test, which compares the medians and the distribution of values. The advantage of Mann–Whitney U-test is that it allows differences in sample sizes among the compared groups. The analysis was performed with a web-based implementation of the Mann–Whitney U-test (http://eatworms.swmed.edu/~leon/stats/utest.html). A P-value of <0.05 was considered as significant and a P-value of <0.1 was considered suggestive.
Results
Identification of potential regulatory variant within the highly conserved FSHB promoter element
The human genome single nucleotide polymorphism (SNP) database (dbSNP—http://www.ncbi.nlm.gov) was screened for potential gene regulatory polymorphisms assigned to evolutionary conserved sequence elements upstream of the FSHB gene. The database search combined with comparative genomic analysis resulted in the identification of an uncharacterized SNP (rs10835638; G/T) 211 bp upstream from the FSHB mRNA (Genbank reference: NM_001018080.1, GI: 66528994) transcription start-site. This SNP is located within a region which is highly conserved among placental mammals (Fig. 1b) and is predicted to harbor a transcription regulatory element (UCSC Genome browser; http://genome.ucsc.edu/). Consistently, functional studies conducted with ovine Fshb 5′ flanking region have shown that the conserved element between −212 and −197 acts as progesterone responsive element (PRE; Fig. 1b) capable of enhancing gene transcription up to 9-fold (Webster et al., 1995). The conserved G nucleotide at position −211 was shown to be one of the critical positions for the proper functioning of this element (Webster et al., 1995).
Quantitative association studies for rs10835638 with hormonal and testicular parameters
The putative regulatory polymorphism rs10835638 in FSHB promoter (Fig. 1) was genotyped for a cohort (n = 554) of young healthy Estonian men visiting the Andrology Center of the Tartu University Clinics, Estonia over a 2-year period. The genotyped cohort consisted of 423 major allele homozygotes (GG; 76.4%), 125 heterozygotes (GT; 22.6%) and 6 minor allele homozygotes (TT; 1.1%). The frequencies for G- and T-alleles were 87.6% and 12.4%, respectively.
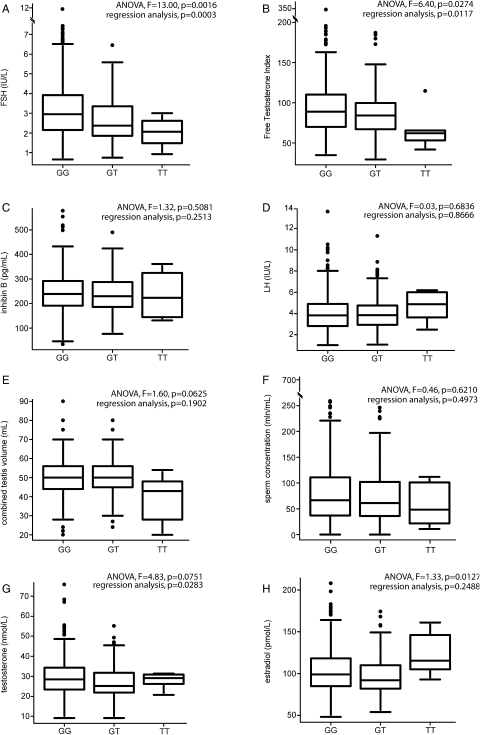
Association analysis using regression testing for a linear trend revealed a strong association of rs10835638 alleles with serum FSH levels (P = 0.0003; Table I). There is a gradient of declining FSH levels among the three subgroups of wild-type homozygotes (GG), heterozygotes (GT) and homozygotes for the minor allele (TT) of rs10835638 (ANOVA: F = 13.0, P = 0.0016, df = 1; Table I; Fig. 2). Significantly higher serum FSH was measured for the carriers of the GG genotype (3.37 ± 1.79 IU/l) compared with heterozygotes (2.84 ± 1.54 IU/l; Mann–Whitney U-test P = 0.0005), the joint group of T-allele carriers (GT+TT, n = 131; 2.78 ± 1.51 IU/l, P = 0.000502) and more remarkably compared with TT homozygotes (2.02 ± 0.81 IU/l; P = 0.031). Notably, the declining gradient of serum FSH among the GG, GT and TT genotype carriers was correlated with the calculated FTI (GG: 93.80 ± 35.00 > GT: 86.88 ± 30.16 > TT: 66.45 ± 25.15; ANOVA: F = 6.4, P = 0.0274, df = 1; Table I, Fig. 2). The GT and TT carriers exhibit different reasons for the lower FTI. In the case of GG versus GT, the difference is attributable to lower total testosterone while SHBG is the same; whereas for GG versus TT, the difference is attributable to the increase in SHBG (Table I).
Table I.
Characteristics of the study subjects grouped by rs10835638 (variant in human FSHB promoter) genotype.
| GG (n = 423) |
GT (n = 127) |
TT (n = 6) |
P-values of statistical analysis using |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression testinga | ANOVAa | Mann–Whitney U-testb |
||||||||
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Allele effect | Genotype effect | TT versus GG | TT+GT versus GG | |
| Subject characteristics | ||||||||||
| Age (years) | 19.23 (1.73) | 18.68 (17.24–22.78) | 19.11 (1.68) | 18.65 (17.18–22.57) | 20.16 (2.56) | 19.56 (17.88–23.43) | 8.34 × 10−1 | 2.87 × 10−1 | 5.11 × 10−1 | 5.14 × 10−1 |
| Abstinence period (h) | 111.1 (52.07) | 94 (58–206) | 124.5 (60.95) | 112 (58.3–225.7) | 124.67 (77.99) | 97.5 (67–240.25) | 1.61 × 10−2 | 4.76 × 10−2 | 7.88 × 10−1 | 5.92 × 10−2 |
| Height (cm) | 180.74 (6.73) | 180 (170.1–192) | 180.06 (6.92) | 181 (168.3–191) | 178.17 (9.58) | 174.5 (170–191.5) | 4.40 × 10−1 | 5.25 × 10−1 | 7.72 × 10−1 | 4.84 × 10−1 |
| Weight (kg) | 73 (11.14) | 72 (60–90) | 72.54 (10.65) | 72 (60–90) | 75.5 (11.84) | 73.5 (63.75–90.25) | 8.69 × 10−1 | 8.15 × 10−1 | 7.33 × 10−1 | 7.46 × 10−1 |
| BMI | 22.33 (3.1) | 22.02 (18.81–27.44) | 22.37 (3.06) | 21.91 (18.57–27.58) | 23.66 (1.81) | 23.57 (21.82–25.99) | 6.87 × 10−1 | 5.75 × 10−1 | 8.26 × 10−2 | 9.63 × 10−1 |
| Hormone parameters | ||||||||||
| FSH (IU/l) | 3.37 (1.79) | 2.98 (1.29–6.74) | 2.84 (1.54) | 2.46 (1.14–5.4) | 2.02 (0.81) | 2.06 (1.06–2.91) | 3.00 × 10−4 | 1.60 × 10−3 | 3.10 × 10−2 | 5.02 × 10−4 |
| Inhibin-B (pg/ml) | 246.04 (79.44) | 239 (130.2–389.6) | 234.79 (79.32) | 227 (115.9–368.7) | 234.5 (94.35) | 223 (134.3–352.0) | 2.51 × 10−1 | 5.08 × 10−1 | 7.00 × 10−1 | 2.42 × 10−1 |
| LH (IU/l) | 4.08 (1.74) | 3.81 (1.84–7.32) | 4.08 (1.71) | 3.85 (1.73–7.48) | 4.68 (1.45) | 4.88 (2.76–6.15) | 8.67 × 10−1 | 6.84 × 10−1 | 2.52 × 10−1 | 8.31 × 10−1 |
| Testosterone (nmol/l) | 29.62 (9.08) | 28.4 (16.91–45.59) | 27.55 (9.29) | 25.2 (14.2–45.57) | 27.9 (4.00) | 29.1 (22.08–31.28) | 2.83 × 10−2 | 7.51 × 10−2 | 7.54 × 10−1 | 1.38 × 10−2 |
| Estradiol (pmol/l) | 102.84 (26) | 99 (68–145) | 97.55 (24.81) | 92 (64.3–141.4) | 122.67 (26.28) | 115.5 (96–157.25) | 2.49 × 10−1 | 1.27 × 10−2 | 5.98 × 10−2 | 5.02 × 10−2 |
| SHBG (nmol/l) | 34.2 (12.78) | 32 (18–55.9) | 34.25 (16.46) | 32 (18–51) | 45.5 (13.84) | 44 (30–63.75) | 3.94 × 10−1 | 1.22 × 10−1 | 3.40 × 10−2 | 8.74 × 10−1 |
| Free testosterone index | 93.8 (35) | 88.78 (50.36–150.48) | 86.88 (30.16) | 84 (45.93–139.1) | 66.45 (25.15) | 62.02 (44.48–102.21) | 1.17 × 10−2 | 2.74 × 10−2 | 1.64 × 10−2 | 3.30 × 10−2 |
| Testicular parameters | ||||||||||
| Total Testes volume (ml) | 50.59 (10.17) | 50 (36–70) | 50.07 (9.83) | 50 (35–69.1) | 39.33 (12.94) | 43 (22–52.5) | 2.07 × 10−1 | 2.50 × 10−2 | 3.92 × 10−2 | 6.24 × 10−1 |
| Seminal parameters | ||||||||||
| Semen volume (ml) | 3.4 (1.6) | 3.2 (1.2–6.3) | 3.43 (1.57) | 3.3 (1.2–6.04) | 4.93 (2.14) | 5.7 (1.88–6.73) | 1.90 × 10−1 | 6.25 × 10−2 | 4.66 × 10−2 | 3.68 × 10−1 |
| Sperm conc (106/ml) | 87.41 (77.14) | 70 (7–219.9) | 83.39 (83.54) | 62 (10.3–225.7) | 57.17 (41.98) | 48.5 (13.75–109.25) | 4.97 × 10−1 | 6.21 × 10−1 | 3.55 × 10−1 | 3.68 × 10−1 |
| A+B motility (%) | 55.86 (13.19) | 57 (32.1–75) | 57.34 (11.72) | 58.5 (41–76.75) | 59.33 (9.33) | 61 (48–69) | 1.92 × 10−1 | 4.26 × 10−1 | 5.80 × 10−1 | 3.54 × 10−1 |
aRegression testing for a linear trend of marker alleles and one-way analysis of variance (ANOVA; tests for all traits df = 1) for genotype–trait associations were performed with the following covariates: age and BMI (all analysis), abstinence time (seminal parameters).
bMann–Whitney U-test (two-tailed) testing the difference between the medians and distributions of the study parameters for two subgroups.
Significant difference has been highlighted: P < 0.1, P < 0.05, P< 0.001.
SHBG: sex hormone-binding globulin.
Figure 2:
Boxblots for the distribution of (a) serum FSH, (b) free androgen index, (c) Inhibin-B, (d) LH, (e) combined testicular volume (left + right), (f) sperm concentration, (g) testosterone and (h) estradiol in study subjects subgrouped according to their FSHB promoter SNP rs10835638 genotype, either as GG (n = 423), GT (n = 125) or TT (n = 6) individuals.
The boxes represent the 25th and 75th percentiles; whiskers are lines extending from each end of the box covering the extent of the data on 1.5× inter-quartile range. The median value is denoted as the line that bisects the boxes. Circles represent the outlier values. For each boxplot are shown P-values of Marker–Parameter association analysis: one-way ANOVA and regression testing for a linear trend.
In addition to reduced FSH and FTI, the data suggested that rs10835638 minor allele homozygosity (TT) might affect also other markers of male reproductive function (Fig. 2, Table I). This subgroup was characterized by significant reduction in combined testes volume (GG: 50.59 ± 10.17≈GT: 50.07 ± 9.83 > TT: 39.33 ± 12.94 ml; ANOVA: F = 1.6, P = 0.0250, df = 1), but significantly increased E2 (GG: 102.84 ± 26.00≈GT: 97.55 ± 24.81 < TT: 122.67 ± 26.28; ANOVA: F = 1.33, P = 0.0127, df = 1) and semen volume (GG: 3.4 ± 1.6≈GT: 3.43 ± 1.57 < TT: 4.03 ± 2.14 ml; ANOVA: F = 1.72, P = 0.0625, df = 1). For the TT homozygotes, we also observed elevated serum LH, SHBG and BMI as well as reductions in average sperm concentration and testosterone, but in ANOVA analysis these differences among subgroups did not reach statistical significance (Table I, Fig. 2). No effect was detected on serum inhibin-B level, or sperm count and motility. We are aware that due to the low number of subjects with TT genotype (n = 6), the latter observations relative to this group should be handled with caution until replicated in a larger cohort.
Discussion
There is a growing consensus that the genetic component of complex traits may reflect a different spectrum of sequence variants than the missense and nonsense mutations that dominate monogenetic diseases and traits. Within this spectrum, gene variants that alter gene expression are thought to play an important role. Herewith we report the first human FSHB polymorphism (rs10835638; G/T; −211 from mRNA transcription start) showing significant association with serum FSH hormone levels in men. This variant is located in the evolutionary conserved PRE-element 5′ upstream of the FSHB gene transcription site, a regulatory element demonstrated to be functionally active in controlling mammalian FSHB expression (Webster et al., 1995). Compared with the wild-type homozygotes (GG), the heterozygotes (GT) and the homozygotes (TT) for the alternative allele had on average 0.53 IU/l (15.7%) and 1.35 IU/l (40%) lower levels of FSH in their bloodstream, respectively. The differential effect of the two alleles (G, T) on FSHB gene expression is supported by an independent data set from a large-scale study focusing on the functional analysis of common human promoter polymorphisms across 170 genes (Hoogendoorn et al., 2003). Tested by the luciferase assay (Hoogendoorn et al., 2003), the relative activity of the FSHB proximal promoter carrying the rs10835638 T-allele was only half (46–58%; P < 0.0005) compared with the activity of the wild-type promoter variant with the G-allele in cell lines JEG-3 and TE671, known to have progesterone-responsive regulation of transcription (An et al., 2005; Yie et al., 2006). The complex mechanism controlling transcription of the FSHB gene is critical for proper regulation of the production of FSH hormone (Miller et al., 2002). Most of the knowledge about the transcriptional regulation of FSHB gene comes from the studies of ovine (Webster et al., 1995; Strahl et al., 1997; Huang et al., 2001) and murine Fshb promoters (Coss et al., 2004; Lamba et al., 2006). An important role of progesterone-responsive regulation in FSHB transcription is further supported by the data from progesterone receptor knockout mice showing moderately but significantly lower serum FSH levels compared with wild-type counterparts (Schneider et al., 2005). There is limited experimental data addressing the transcriptional regulation of the human FSHB gene. Identification of a regulatory polymorphism in human FSHB promoter co-localizing with a previously mapped ovine Fshb PRE element suggests that in addition to the high conservation of the FSHB gene and its function among mammals, physiologically relevant 5′ upstream promoter elements might also be conserved.
Among the healthy young men, the T-allele carriers of the FSHB promoter SNP had decreased serum FSH levels and reduction in combined testes volume, but no difference in inhibin-B and sperm parameters compared with the wild-type variant carriers. The smaller testes volume of the TT homozygotes is concordant with the role of FSH as the stimulator of seminiferous tubule growth during development in primates, and subsequent determination of adult testicular size (Arslan et al., 1993; Marshall and Plant, 1996; Phillip et al., 1998). Consistently, the murine Fshb knockout model has small testes and decreased Sertoli cell number (Kumar et al., 1997). Lack of correlation between the drop of FSH levels and testes volume in T-allele carriers and levels of serum inhibin-B is in concordance with the suggestion that the Sertoli cell retains a significant capacity for activity which is independent of direct hormonal regulation (Abel et al., 2008). The lower FTI and the increase in semen volume and BMI as well as some serum hormone levels for the T-allele carriers suggest additional regulatory effects of FSH, beyond gonadal development and function, on male hormonal balance and physiology.
In this first report, we only studied the male phenotype associated with the identified FSHB promoter polymorphism as the cyclic variation of FSH in women makes it complicated to reliably conduct a genetic association study with serum hormone levels. Strong evidence for a functional consequence of the FSHB promoter polymorphism, and the magnitude of its effects, encourages us to proceed in asking whether differences are seen in cycle-matched serum samples from women with different genotypes. Previous studies have shown that in women, the FSH receptor (FSHR) variants determined by two common non-synonymous changes in exon 10 are associated with basal serum FSH level and are contributing to the FSH sensitivities during the menstrual cycle and different cycle lengths (reviewed by Gromoll and Simoni, 2005). In contrast, the FSHR SNPs had no effect on serum levels of FSH and other clinical parameters in men with either normal or impaired spermatogenesis. The outcome of a combined FSHB/FHSR SNP association study in men and women may have therapeutic implications for fertility treatment.
In summary, although there is convincing evidence for the role of FSH in Sertoli cell proliferation, its functional significance in spermatogenesis and the contribution to male hormonal balance and physiology is unclear. The identification of a regulatory SNP (rs10835638) in FSHB promoter paves the way to study the effect of constitutively reduced FSH on male health and fertility. Furthermore, as FSH has a well-understood contribution to the regulation of follicular development and sex steroid production in women, the role of the identified FSHB promoter variant in female reproductive success is still to be studied.
Author’s Role
M.G., M.P. and M.L. designed the study; M.P. and K.A. coordinated for the recruitment the study material; M.G. and M.P. contributed to the acquisition of the data; M.G., M.P. and M.L. analyzed and interpreted the data; M.L. drafted the manuscript, M.G. and M.P. revised it critically for substantial intellectual content. All the authors have read and approved the submitted manuscript version.
Funding
The study has been supported by Wellcome Trust International Senior Fellowship (grant no. 070191/Z/03/Z) in Biomedical Science in Central Europe and Howard Hughes Medical Institute International Scholarship (grant no. #55005617) (to M.L.) as well as EU 6th FP project QLRT-2001-02911 (to M.P., K.A.). In part, the work has been supported by the Estonian Science Foundation grants no. 5796 and 7471; Estonian Ministry of Science and Education Core grant no. 018272s06 (to M.L., M.G.) and personal stipend to M.G from Margot M. and Herbert R. Linna Scholarship Fund.
Acknowledgements
We are thankful for the participants of the study, Piret Kelgo for technical assistance, Dr Kristiina Rull for assistance in statistical tests and Dr Robert K Campbell for comments to the early version of the manuscript and editing of the English language.
References
- Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, de Gendt K, Guillou F, O’Shaughnessy PJ. Spermatogenesis and Sertoli cell activity in mice lacking Sertoli cell receptors for follicle stimulating hormone and androgen. Endocrinology. 2008 doi: 10.1210/en.2008-0086. doi: 10.1210/en.2008-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B-S, Choi J-H, Choi K-C, Leung PCK. Differential role of progesterone receptor isoforms in the transcriptional regulation of human gonadotropin-releasing hormone I (GnRH I) receptor, GnRH I, and GnRH II. J Clin Endocrinol Metab. 2005;90:1106–1113. doi: 10.1210/jc.2004-0318. [DOI] [PubMed] [Google Scholar]
- Arslan M, Weinbauer GF, Schlatt S, Shahab M, Nieschlag E. FSH and testosterone, alone or in combination, initiate testicular growth and increase the number of spermatogonia and Sertoli cells in a juvenile non-human primate (Macaca mulatta) J Endocrinol. 1993;136:235–243. doi: 10.1677/joe.0.1360235. [DOI] [PubMed] [Google Scholar]
- Berger K, Souza H, Brito VN, d’Alva CB, Mendonca BB, Latronico AC. Clinical and hormonal features of selective follicle-stimulating hormone (FSH) deficiency due to FSH beta-subunit gene mutations in both sexes. Fertil Steril. 2005;83:466–470. doi: 10.1016/j.fertnstert.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Coss D, Jacobs SBR, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Rull K, Laan M. Haplotype structure of FSHB, the beta-subunit gene for fertility-associated follicle-stimulating hormone: possible influence of balancing selection. Ann Hum Genet. 2007;71:18–28. doi: 10.1111/j.1469-1809.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trends Endocrinol Metab. 2005;16:368–373. doi: 10.1016/j.tem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, O’Donovan MC. Functional analysis of human promoter polymorphisms. Hum Mol Genet. 2003;12:2249–2254. doi: 10.1093/hmg/ddg246. [DOI] [PubMed] [Google Scholar]
- Howles CM. Role of LH and FSH in ovarian function. Mol Cell Endocrinol. 2000;161:25–30. doi: 10.1016/s0303-7207(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Huang H-J, Sebastian J, Strahl BD, Wu JC, Miller WL. The promoter for the ovine follicle-stimulating hormone-β gene (FSHβ) confers FSHβ-like expression on luciferase in transgenic mice: regulatory studies in vivo and in vitro. Endocrinology. 2001;142:2260–2266. doi: 10.1210/endo.142.6.8202. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. Mutations affecting gonadotropin secretion and action. Horm Res. 2003;60:21–30. doi: 10.1159/000074496. [DOI] [PubMed] [Google Scholar]
- Jameson JL, Becker CB, Lindell CM, Habener JF. Human follicle-stimulating hormone beta-subunit gene encodes multiple messenger ribonucleic acids. Mol Endocrinol. 1988;2:806–815. doi: 10.1210/mend-2-9-806. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone β subunit transcription by activin A. J Mol Endocrinol. 2006;36:210–220. doi: 10.1677/jme.1.01961. [DOI] [PubMed] [Google Scholar]
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSH{beta} subunit gene is highly conserved. Mol Hum Reprod. 2005;11:601–605. doi: 10.1093/molehr/gah198. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Miller WL, Shafiee-Kermani F, Strahl BD, Huang H-J. The nature of FSH induction by GnRH. Trends Endocrinol Metab. 2002;13:257–263. doi: 10.1016/s1043-2760(02)00614-8. [DOI] [PubMed] [Google Scholar]
- Moudgal NR, Sairam MR. Is there a true requirement for follicle stimulating hormone in promoting spermatogenesis and fertility in primates? Hum Reprod. 1998;13:916–919. doi: 10.1093/humrep/13.4.916. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Campbell RK. Gonadotropins. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery and Technology. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 684–724. [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med. 1998;338:1729–1732. doi: 10.1056/NEJM199806113382404. [DOI] [PubMed] [Google Scholar]
- Punab M, Zilaitiene B, Jørgensen N, Horte A, Matulevicius V, Peetsalu A, Skakkebaek NE. Regional differences in semen qualities in the Baltic region. Int J Androl. 2002;25:243–252. doi: 10.1046/j.1365-2605.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enchanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang H-J, Pedersen NR, Wu JC, Ghosh BR, Miller WL. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology. 1997;138:2621–2631. doi: 10.1210/endo.138.6.5205. [DOI] [PubMed] [Google Scholar]
- Webster JC, Pedersen NR, Edwards DP, Beck AB, Miller WL. The 5′-flanking region of the ovine follicle-stimulating hormone-β gene contains six progesterone response elements: three proximal elements are sufficient to increase transcription in the presence of progesterone. Endocrinology. 1995;136:1049–1058. doi: 10.1210/endo.136.3.7867558. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory Manual for Examination of Human Semen and Sperm–Cervical Mucus Interaction. 4th edn. New York: Cambridge University Press; 1999. [Google Scholar]
- Yie S-M, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006;21:2538–2544. doi: 10.1093/humrep/del126. [DOI] [PubMed] [Google Scholar]