Abstract
It was recently shown that the major allele of the SLC30A8 (zinc transporter 8, ZnT8) single nucleotide polymorphism (SNP) rs13266634 was associated with type 2 diabetes and with reduced insulin secretion in non-diabetic relatives. Because of its role in beta-cell function, we hypothesized that this candidate SNP may confer increased susceptibility for beta-cell destruction in type 1 diabetes. We analyzed SLC30A8 genotypes in 874 patients with type 1 diabetes and 1021 control subjects. No difference in allele and genotype frequencies of the SLC30A8 SNP rs13266634 was found between patients and controls. Analysis with respect to age at type 1 diabetes onset, however, showed that patients with a diabetes onset before age 5 years had an increased prevalence of the cytosine (C) allele (risk allele, 82%) and the homozygous CC genotype (65%) compared to patients who developed type 1 diabetes after age 5 years (67% and 49%; p < 0.01) and compared to controls (69% and 48%; p < 0.03). These data suggest that genetic susceptibility for beta-cell dysfunction in the presence of autoimmunity may lead to accelerated progression and early manifestation of the disease.
Keywords: type 1 diabetes, zinc transporter, ZnT-8, SCL30A8, genotype, beta-cell dysfunction, age of onset
Introduction
A non-synonymous polymorphism in the SLC30A8 (solute carrier family 30 (zinc transporter), member 8) gene was recently reported to be more frequent in subjects with type 2 diabetes than in healthy controls [1]. Furthermore, it was shown that the major allele of the SLC30A8 SNP, rs13266634, was associated with reduced insulin secretion after stimulation with intravenous glucose in non-diabetic relatives of subjects with type 2 diabetes [2]. The SLC30A8 gene encodes a zinc transporter protein (ZnT8) which is expressed in pancreatic alpha- and beta-cells [3, 4]. It is localized in the membrane of the insulin secretory granules, facilitates the accumulation of zinc from the cytoplasm in intracellular insulin-containing vesicles and plays a major role in providing zinc for insulin maturation and/or storage processes [3]. Because of its role in beta-cell function, we hypothesized that this candidate SNP may confer increased susceptibility for autoimmune beta-cell destruction in type 1 diabetes. In this research, we analyze SLC30A8 genotypes in patients with type 1 diabetes and control subjects.
Research design and methods
Patients with type 1 diabetes and their spouses were recruited in Germany between 1989 and 2000 as parents of children participating in the prospective cohort studies, BABYDIAB and BABYDIET [5, 6]. Type 1 diabetes was diagnosed by WHO criteria. A total of 874 patients (586 females, 67%) and 1021 non-diabetic spouses (349 females, 34%) were available for genotyping. Patients had a median age at diabetes onset of 17.25 years (interquartile range IQR 11.47-24.86). Written informed consent was obtained from all subjects who participated in the study. The study was approved by the competent ethics committee (Bayerische Landesärztekammer Nr. 95357).
The genotyping of the SLC30A8 SNP rs13266634 was performed with the iPLEX™ (Sequenom San Diego, CA, USA) method by means of matrix-assisted laser desorption-ionisation time-of-flight mass spectrometry (MALDI-TOF MS, Mass Array™, Sequenom, San Diego, CA, USA) according to the manufacturers instructions (www.sequenom.com). The following primers were used: (F) 5'-ACGTTGGATGGCAATTTCTCTCCGAACCAC-3', (R) 5'-ACGTTGGATGGCAATCAGTGCTAATCTCCC-3', and (extension) 5'-TCAACAGCAGCCAGC-3'. Genotype frequencies were determined for patients and the control group. Comparisons of genotype distributions with respect to age were performed using Fisher's exact test by Monte Carlo simulation. Stratification of the results with subject age at type 1 diabetes onset yielded the following groups: 0-4.99, 5-9.99, 10-14.99, 15-19.99, 20-29.99, 30-50 years. Hardy-Weinberg equilibrium was tested using Pearson's χ2 test. Odds ratios and their p-values were calculated by logistic regression. The p-values given are two-sided and the level of significance was set at α ≤ 0.05. The statistical analysis was performed using the software packages SPSS 15.0 (Chicago, IL, USA) and R (version 2.6.1).
Results
The SLC30A8 SNP rs13266634 genotype distribution was consistent with Hardy-Weinberg equilibrium in the whole cohort and in the age sub-categories (p < 0.1 in all cases). There was no difference in allele and genotype frequencies of the SLC30A8 SNP rs13266634 between patients with type 1 diabetes and control subjects. Analysis with respect to age of type 1 diabetes onset, however, showed that patients with diabetes onset before age 5 years had an increased prevalence of the C allele (risk allele, 82%) compared to patients who developed type 1 diabetes after age 5 years (67%; p = 0.0018) and compared to control subjects (69%; p = 0.0084). Moreover, genotype frequencies were also different in the young-onset patients with an increase in the CC genotype as compared to older-onset patients and control subjects (p = 0.008 compared to older-onset age; 0.027 compared to controls; Table 1). The differences were most apparent in patients who developed diabetes in the first two years of life (C allele frequency of 88%). Typical of type 1 diabetes patients, these very young-onset patients also had a high prevalence of the HLA DR3-DQ2/DR4-DQ8 genotype (60%).
Table 1. Association of SLC30A8 SNP rs13266634 with age at type 1 diabetes onset in 874 type 1 diabetic patients.
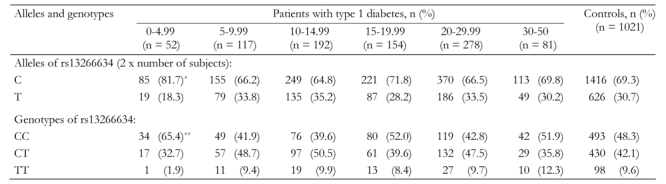
Data are absolute numbers of subjects in age at onset groups (percentages of age groups in parentheses). Fisher’s exact test p-value was calculated by Monte Carlo simulation. * p = 0.0018 (odds ratio 2.2, 95%CI 1.3-3.6) vs. patients with diabetes onset < age 5 years; p = 0.0084 (odds ratio 2.0, 95%CI 1.19-3.28) vs. control subjects. ** p = 0.008 (odds ratio 2.4, 95%CI 1.31-4.24) vs. patients with diabetes onset < age 5 years; p = 0.027 (odds ratio 2.0, 95%CI 1.13-3.63) vs. control subjects.
Conclusion
It has been previously proposed that impaired insulin action (insulin resistance) as well as a priori impaired beta-cell function may contribute to the development of islet autoimmunity and type 1 diabetes [7]. Our data support part of this assumption by suggesting that genetic susceptibility for beta-cell dysfunction (through SLC30A8 SNP) in the presence of autoimmunity leads to accelerated progression of beta-cell destruction and early manifestation of the disease. Moreover, an association that is limited to young-onset diabetes may further suggest that the polymorphic variants have their greatest effect on beta-cell function during early life when beta-cell mass is still small. It will also be of interest to determine whether early manifestation of autoimmunity to ZnT8, recently described as an autoantigen target in type 1 diabetes [8], is related to the SLC30A8 genotype. In conclusion, this analysis suggests that type 1 and type 2 diabetes may indirectly share some mechanisms of pathogenesis.
Acknowledgments
We thank A. Knopff for data collection and expert technical assistance. This work was supported by grants from the Juvenile Diabetes Research Foundation (JDRF #1-2006-665), the German Research Foundation (Deutsche Forschungsgemeinschaft ZI 310/14-1 and 14-2), the German National Genome Research Network (NGFN), and the Munich Center of Health Sciences (MC Health) initiative as part of LMUinnovativ.
References
- 1.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 2.Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, Schäfer SA, Kirchhoff K, Fritsche A, Häring HU. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS ONE. 2007;2(9):e832. doi: 10.1371/journal.pone.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53(9):2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 4.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283(15):10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler AG, Hillebrand B, Rabl W, Mayrhofer M, Hummel M, Mollenhauer U, Vordemann J, Lenz A, Standl E. On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia. 1993;36(5):402–408. doi: 10.1007/BF00402275. [DOI] [PubMed] [Google Scholar]
- 6.Schmid S, Buuck D, Knopff A, Bonifacio E, Ziegler AG. BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with Type 1 diabetes by delaying exposure to gluten. Diabetologia. 2004;47(6):1130–1131. doi: 10.1007/s00125-004-1420-9. [DOI] [PubMed] [Google Scholar]
- 7.Gale EA. To boldly go - or to go too boldly? The accelerator hypothesis revisited. Diabetologia. 2007;50(8):1571–1575. doi: 10.1007/s00125-007-0726-9. [DOI] [PubMed] [Google Scholar]
- 8.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]


