Abstract
We evaluated two protocols for treating adults with comorbid asthma and panic disorder. The protocols included elements of Barlow’s “panic control therapy” and several asthma education programs, as well as modules designed to teach participants how to differentiate asthma and panic symptoms, and how to apply specific home management strategies for each. Fifty percent of subjects dropped out of a 14-session protocol by the eighth session; however, 83% of patients were retained in an eight-session protocol. Clinical results were mostly equivalent: significant decreases of >50% in panic symptoms, clinically significant decreases in asthma symptoms, improvement in asthma quality of life, and maintenance of clinical stability in asthma. Albuterol use decreased significantly in the 14-session protocol and at a borderline level in the 8-session protocol, while pulmonary function was maintained. A controlled evaluation of this procedure is warranted.
Keywords: anxiety disorder, albuterol, pulmonary function, severity, cognitive behavior therapy
This paper reports results of a pilot study of a manualized treatment for patients with comorbid asthma and panic disorder (PD). This comorbidity is quite common (Carr et al., 1992, 1994; Goodwin et al., 2003a; Hasler et al., 2005; Lavoie et al., 2005; Nascimento et al., 2002; Shavitt et al., 1992; Yellowlees et al., 1988), and there is reason to believe that the two disorders interact with each other, producing greater morbidity for each. There is a substantial overlap in symptoms (e.g., dyspnea, chest tightness), leading to frequent consequent symptom confusion and errors in self-care (Schmaling & Bell, 1997). The recommended self-care methods for the two disorders sometimes conflict, e.g., exposure to body sensations for PD (Barlow et al, 2005) vs. avoidance of triggers for asthma (Custovic et al, 1998); relaxation to ameliorate anxiety, that could produce bronchoconstriction through a parasympathetic discharge (Lehrer et al 1997), vs. beta-sympathetic agonists for asthma (National Heart Lung and Blood Institute, 1997, 2002; Scalabrin et al., 1996; Shavitt et al., 1993; Rihmer, 1997; Feldman et al., 2000) that could trigger panic; treatment of overperception and catastrophic interpretation of body sensations for PD (Hoehn-Saric et al, 2004) vs. improving poor symptom sensitivity and encouraging appropriate anxiety about symptoms for asthma (Lehrer et al, 2002). Symptom confusion and resulting inappropriate treatment has been implicated in deaths and near deaths from asthma (Tietz et al, 1975).
Presence of asthma may increase the risk of developing PD through a variety of cognitive and behavioral mechanisms, including producing threatening bodily sensations that could trigger panic among susceptible individuals, agoraphobic avoidance, and Treating comorbid panic disorder and asthma aversive conditioning to cues for respiratory impairment (Feldman et al., 2005a). PD patients also may overreact to asthma symptoms, overperceive and overtreat them, experience an exaggerated deterioration in quality of life (Kinsman, Dirks, & Jones, 1982; Feldman et al, 2005a; van Peski-Oosterbaan et al., 1996), and perceive poorer asthma control (Feldman et al., 2005a,b). Asthma patients with frequent panic symptoms also tend to overuse asthma “rescue” medications, particularly beta-2 sympathetic stimulant drugs such as albuterol (Dahlem et al., 1977). Side effects of albuterol include adverse cardiac events (Cazzola et al, 2005) as well as increased anxiety.
Hyperventilation, a common accompaniment of PD, can trigger bronchoconstriction (Kilham, Tooley, & Silverman, 1979) due to airway cooling (Nielsen & Bisgaard, 2005). Symptoms of anxiety and stress can stimulate production of cytokines that lead to airway inflammation (Kang et al., 1997; Liu et al., 2002), and increase vulnerability to upper respiratory infections (Cohen et al., 1998; Frieri, 2003) that can trigger asthma exacerbations (Smith & Nicholson, 2001). Stress also can contribute to asthma exacerbation via parasympathetic rebound after sympathetic activation has subsided (Lehrer et al., 1997).
The growing evidence that asthma and PD may exacerbate each other has led to recent calls for a combined treatment approach (Nardi, 2005; Thomas & Griffiths, 2005). Only one small controlled treatment outcome study (Ross et al., 2005) has been reported, finding that combining cognitive behavioral therapy (CBT) with asthma education was associated with improvements on measures of both panic and asthma in comparison to a wait-list control group (Ross et al., 2005). The current study uses a similar treatment approach and incorporates components of Panic Control Therapy (Barlow & Craske, 2000) and asthma self-management programs (National Heart, Lung, and Blood Institute, 1997; Reynolds, Kotses, Creer, Bruss, & Joyner, 1989). Both programs were adapted for the comorbid group, teaching participants to recognize the differences between asthma and panic symptoms and to engage in appropriate self-care for each. A treatment protocol we proposed previously (see Feldman et al., 2000) was modified to incorporate more recent findings from the literature into the treatment manual. We included a module on assertion and communication training, because asthma presents particular problems requiring a high level of social competence and skill, often lacking in many PD patients, who may have difficulty asking sufficient questions of health care providers to insure proper self-treatment, or and insuring that people around them do not expose them to asthma triggers (allergens from food or animals, tobacco smoke, etc.)
Here we report results of a pilot study designed to explore whether comorbid patients would be amenable to a treatment targeting the comorbidity, and to obtain preliminary evidence for efficacy. We hypothesized that the combined treatment would improve symptoms of both anxiety and asthma, improve quality of life, and reduce the use of albuterol. We began the study using a 14-week treatment protocol However, because of recruitment problems and a high dropout rate, we later implemented an eight-session protocol. Results from both protocols are described in this report.
METHODS
The study was approved by the Institutional Review Board of UMDNJ– Robert Wood Johnson Medical School. In both protocols, each session lasted approximately 60-minutes.
Participants and screening
Participants with comorbid PD and asthma were recruited from local pulmonary and mental health practices, as well as from media advertisements. Participants were initially screened by telephone, followed by a structured clinical interview. DSM-IV diagnosis of PD (American Psychiatric Association, 1994) was made using the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV, Brown, Dinardo, & Barlow, 1994). Diagnosis of asthma was made according to standards recommended by the NHLBI (1977, 2002). At the initial diagnostic session, participants were given a preliminary pulmonary function test using a Koko pneumotach-based spirometer (PDS Instrumentation, Louisville, KY), according to standard American Thoracic Society procedures. They then were screened by a pulmonologist. Where abnormal pulmonary function test results were obtained, presence of asthma was determined by a positive bronchodilator test, which requires an improvement of ≥12% in forced expiratory volume in the first second of a forced expiratory maneuver from maximal vital capacity [FEV1] after administration of albuterol. Where asthma was well controlled (with normal pulmonary function), a positive response to a bronchoprovocation test, or documentation of a prior clinical and pulmonary function response to anti-inflammatory or bronchodilator medication was used as an alternative criterion for presence of asthma.
Exclusion criteria included: psychosis, organic brain syndrome, current alcohol or substance abuse/dependence, or receiving counseling and/or psychotropic medication for less than six months. Longer-term psychotherapy and use of psychotropic medication were allowed to continue, as well as psychotherapy for other kinds of problems, under the assumption that such treatment would not affect results, while a requirement to discontinue therapy might be harmful and/or impede recruitment. Two subjects in each of the two protocols reported having previously or concurrently received cognitive behavior therapy for panic disorder. Ten participants, including six females, were accepted into the 14-session protocol, mean age = 44.1 ± 19.6. The mean age for participants in the eight-session treatment protocol, eight females and four males, was 31 years old (SD=10.8). For the average subject, pulmonary function was close to normal levels before treatment began (percent expected FEV1 = 83.9 ± 13.2 for the 14-session protocol, and 75.6 ± 15.2 for the eight-session protocol, after 12 hours’ deprivation of albuterol, but with usual doses of controller medication). 1 Based on retrospective recall of the two weeks prior to the first training session, average asthma symptomatology and consumption of controller medication were in the range indicating mild persistent asthma (Table 1).
Table 1.
Criteria for assessing asthma severity (from NHLBI, 1997)
| SEVERITY CLASS | SYMPTOM CLASS (scored 1–4 for the four severity categories) | PULMONARY FUNCTION CLASS |
|---|---|---|
| Mild Intermittent | • Symptoms (wheeze/cough/dyspnea) 2 times a week | • FEV1 or PEF 80% predicted |
| • Asymptomatic between exacerbations | ||
| • Nighttime asthma symptoms ≤2 times a month | ||
| Mild Persistent | • Symptoms > 2 times a week but < 1 time a day | • FEV1 or PEF 80% predicted |
| • Exacerbations may affect activity | ||
| • Nighttime asthma symptoms > 2 times a month (3–4/month) | ||
| Moderate Persistent | • Daily symptoms | • FEV1 or PEF > 60% 80% predicted |
| • Daily use of inhaled short-acting β2-agonist | ||
| • Exacerbations: ≥ 2 times a week; may last days; affect activity | ||
| • Nighttime asthma symptoms > 1 time a week (≥ 5/month) | ||
| Severe Persistent | • Continuous symptoms | • FEV1 or PEF ≤60% predicted |
| • Limited physical activity | ||
| • Frequent exacerbations | ||
| • Frequent nighttime asthma symptoms |
FEV1 = expiratory volume in the first second of a forced expiratory maneuver from maximum vital capacity
PEF = peak expiratory flow during a forced expiratory maneuver
Fourteen-session protocol
Evaluation data, as described below, were collected at an orientation session, at treatment Sessions 5, 10, 14, and at two follow-up sessions, one and two months after the last treatment session, respectively. Participants were paid $25 for each testing session, for a total of $150.
The protocol was based on our previous clinical experience with the comorbid population, suggesting that a lengthy protocol would evoke resistance, despite Barlow’s experience that a 16-session protocol was acceptable to a large proportion of PD patients. We spent more time than in Barlow’s protocol training subjects to relax and breathe slowly, because of evidence that these methods are helpful for treating asthma (Lehrer et al, 1986, 1994, 2004), and we combined materials on cognitive restructuring into fewer sessions. We eliminated hyperventilation as a method of exposing patients to panicogenic cognitions because hyperventilation may induce bronchoconstriction (Kilham et al, 1979).
Session content was as follows:
Orientation session: Use of peak flow meters; record keeping (NHLBI, 1997, 2002; various patient education materials from NHLBI and the American Lung Association)
Sessions 1–2: Anatomy & physiology of asthma; proper use of asthma medications (ibid.); differentiation between asthma & panic symptoms (Feldman et al, 2000)
Sessions 3–5: an intensive version Jacobson’s progressive relaxation method (as described by Lehrer & Carr, 1996); and breathing training (Barlow & Craske, 2000)
Sessions 6–7: cognitive restructuring (Barlow & Craske, 2000); asthma problem solving (Reynolds et al, 1988, 1989).
Sessions 8–10: exposure (Barlow & Craske, 2000).
Session 11: asthma and medications (NHLBI, 1997, 2002; various patient education materials on asthma and panic disorder from NHLBI and NIMH, effective communication with doctors (Reynolds et al, 1988, 1989).
Session 12: smoking reduction (Reynolds et al, 1988, 1989), treatment of agoraphobic symptoms (Barlow & Craske, 2000)
Session 13: assertiveness training (Alberti & Emmons, 1995), with particular reference to managing asthma (Reynolds et al, 1988, 1989).
Session 14: relapse prevention (Barlow & Craske, 2000).
Eight-session protocol
Evaluation data, as described below, were collected at an orientation session, at Treatment Sessions 4 and 8, and at two follow-up sessions, scheduled as in the 14-session protocol. As in the first protocol, participants were paid $25 for each testing session, for a total of $125. Session content was as follows:
Orientation session: Use of peak flow meters; record keeping.
Session 1: Anatomy & physiology of asthma; proper use of asthma medications; differentiation between asthma and panic.
Session 2: Jacobson’s progressive relaxation training of the arms and trunk; breathing training
Sessions 3 & 4: cognitive restructuring; problem solving
Session 5 & 6: exposure
Session 7: medications, effective communication with doctors
Session 8: relapse prevention
Evaluation procedures
Measures, taken at the orientation session and at each of the testing sessions included: pulmonary function testing, assessment of asthma and panic symptoms, and medication consumption. Patients also logged their daily average asthma and panic symptoms and included such illness behavior events as staying home from work or school, calling a doctor, taking extra medication. This material was used to rate asthma symptoms according to NHLBI (1997, 2002) criteria. Patients also completed an “event” record for each asthma or panic attack, with a check list that included presence and severity of all of DSM-IV panic symptoms, as well as asthma symptoms of wheezing, chest tightness, coughing, mucous congestion, and difficulty breathing (dyspnea). They reported daily consumption of medication for both disorders.
Evaluation of panic
The primary panic outcome measure was the Panic Disorder Severity Scale (PDSS; Shear, Pilkonis, Cloitre, & Leon, 1997), a well-standardized therapist rating scale. As secondary measures, participants were also asked to complete the following questionnaires at each evaluation: the Anxiety Sensitivity Index (ASI) (Peterson et al., 1987, 1992), the Agoraphobic Cognitions Questionnaire and the Body Sensations Questionnaire (Chambless et al., 1984), and the Beck Anxiety Inventory Beck et al., 1988).
Evaluation of asthma
We primarily expected changes in symptoms of asthma, as well as appropriateness of medication use, as indicated by decreased reliance on albuterol, and improved quality of life. We also monitored pulmonary function and use of anti-inflammatory medication. We analyzed asthma symptoms scored according to four levels of asthma severity, as standardized by the National Heart Lung and Blood Institute (1 = mild intermittent, 2= mild persistent, 3= moderate, 4= severe). Severity was based on a combination of symptoms and pulmonary function measures (Table 1), which, in turn, were derived from home questionnaires completed daily. Symptom severity was rated from questionnaire data by an undergraduate research assistant who had no contact with the patients. Medication was scored by a student in the School of Pharmacy at Rutgers University (S.A.). Pulmonary function was evaluated from spirometry tests given by the therapist in each evaluation session. The severity for each criterion class (symptom, medication, or pulmonary function) was determined by the sign or symptom with the highest “severity category” in Table 1.
Secondary measures of asthma condition
Asthma Quality of Life
Juniper et al’s (1992) Asthma Quality of Life questionnaire was given, inquiring about limitations in daily self-care, recreational, and vocational activities due to asthma.
Patient ratings of asthma severity
At each evaluation session patients rated the severity of their own asthma symptoms of the past two weeks, using a five-item scale derived from NHLBI criteria for asthma exacerbations: wheezing, shortness of breath, coughing, and tightness in the chest. Each symptom was rated on a four-point scale, derived from a previous study of asthma symptom descriptors (0=none, 1=mild (just noticeable), 2 = moderate (annoying), and 3=severe (distressing). The approximate numerical equidistance of these descriptors has been assessed in our previous research (Lehrer, et al., 1993).
Asthma Symptom Check List (ASC, Kinsman et al., 1973; Brooks et al., 1989)
The ASC contains 36 items and describes the subjective symptomatology of asthma. The patient indicates on a five-point scale the frequency of a symptom experienced during an asthma exacerbation (1=never, 5=always). Reliability has been calculated between .84–.94 (Brooks et al, 1989).
RESULTS
In the 14-session protocol, of the 54 participants who met criteria for PD in the initial screening session and appeared to have a positive history of asthma (prior to the pulmonologist visit), 31 (57%) said that they were not interested in treatment or did not return telephone calls. Thirteen did not meet either DSM-IV criteria for panic disorder or pulmonologist screening criteria for asthma. Of ten participants who began, only five (50%) completed all 14 sessions. These subjects also completed the two follow-up sessions. One participant completed six sessions, one completed 10 sessions while three participants dropped out after the eighth session. Recruitment difficulties and the high drop out rate after the eighth session persuaded us to reduce the length of the protocol to eight sessions and two follow-up sessions. When we changed to an eight-session protocol, the frequency of people not interested or who could not be contacted dropped modestly to 37 of 79 (47%), but the dropout rate decreased drastically. Ten of 12 patients attended all eight training sessions (83%), while eight also attended the first of the two followup sessions, and seven attended all sessions. Thirty volunteers for the eight-session protocol failed screening criteria either for PD or asthma. We detected no differences in staff rated asthma severity and/or total PDSS scores (initial scores and changes across sessions) between subjects who dropped out and those who remained in the study until the final follow-up session, and therefore assumed that dropout status was not informative.
For each measure, we also computed the effect size (Cohen, 1988), calculated as follows for PDSS scores for panic disorder and asthma symptoms and frequency of albuterol use for asthma. For estimates of placebo response sizes, we used placebo response from two large multicenter trials, Barlow et al (2000) for panic disorder and O’Byrne et al (2001) for asthma. We computed the effect size as
d = [(treatment posttest − pretest) − (placebo posttest − pretest)] / s.d. of placebo pretest
For calculating the “usual medical care” treatment effect size for asthma quality of life, we used a study of asthma education by Marabini et al (2005), which used the same outcome measure we did. Because asthma severity (and, hence, albuterol use) was lower at baseline in our study than in O’Byrne et al’s, such that even a fall from baseline to zero albuterol use in the current study would be smaller than the decrease in O’Byrne et al’s study, we computed asthma effect sizes as follows: We first subtracted the mean value from our study at post-test from the mean value in the first session, divided by the standard deviation in the first session. This estimated the combined effect of our treatment and placebo components in our treatment. We then subtracted the placebo effect size (computed the same way from data by O’Byrne et al’s study) from the treatment effect size in our study.
Statistical model
We used a repeated measures (Sessions) mixed models analysis to test the effects of the two protocols. The alpha level was set at p < 0.05 for the main time (session) effect, and p < 0.01/0.0125 for the 14 and eight-session protocols respectively, according to Bonferroni criteria, to evaluate differences between values in the first treatment session and those obtained in later sessions.
Measures of panic
Panic Disorder Severity Scale (PDSS)
The therapy produced major decreases in PDSS scores in both protocols. The scores decreased faster in the eight-session than in the 14-session protocol, although the effect sizes were larger in the longer protocol. All PDSS scales were reduced by more than 50% in both protocols, and results were maintained for the two-month followup (Table 3 and Fig. 1). Significant decreases occurred in the tenth and last sessions in the 14-session protocol and during followup in the total PDSS score, whereas decreases were significant from the fourth session onwards in the eight-session protocol. In the eight-session protocol, decreases were significant by the fourth treatment session, and remained so through the followup period. Effect sizes were medium to large for mean PDSS scores at the end of treatment, but fell to the upper levels of “small” by the second follow-up period (Table 2). For the 14-session protocol the effect size was similar to that in Barlow et al’s (2000) multicenter trial, which obtained d = 1.53. For the 8-session protocol the effect size was about one third this level.
Table 3.
Main Effect for Session
| Measure | Protocol | df | F | p | Model** |
|---|---|---|---|---|---|
| Panic Disorder Severity Scale (PDSS) | |||||
| PDSS total score | 14-ses | 5,24 | 4.90 | <0.004 | AR1 |
| 8-ses | 4,28 | 10.61 | <0.0001 | AR1 | |
| PDSS # of attacks | 14-ses | 5,24 | 3.99 | <0.009 | AR1 |
| 8-ses | 4,28 | 3.57 | <0.02 | AR1 | |
| PDSS Panic severity | 14-ses | 5,22 | 4.40 | <0.007 | AR1 |
| 8-ses | 4,28 | 5.74 | <0.002 | AR1 | |
| PDSS Fear of next attack | 14-ses | 5,24 | 2.42 | <0.07 (n.s.) | AR1 |
| 8-ses | 4,28 | 4.98 | <0.004 | AR1 | |
| Panicogenic Cognitions | |||||
| Anxiety Sensitivity Index | 14-ses | 5,12 | 2.21 | n.s. | AR1 |
| 8-ses | 4,24 | 13.40 | <0.0001 | CS | |
| Agoraphobic Cognitions | 14-ses | 5,13 | 77.50 | <0.0001 | HCS |
| Quest | 8-ses | 4,24 | 4.08 | <0.02 | HCS |
| Body Sensations Quest | 14-ses | 5,13 | 4.43 | <0.02 | HAR1 |
| 8-ses | 4,24 | 3.67 | <0.02 | HAR1 | |
| Beck Anxiety Inventory | 14-ses | 5,19 | 3.45 | <0.03 | CS |
| 8-ses | 4,25 | 13.05 | <0.0001 | CS | |
| Asthma severity | |||||
| Symptom class | 14-ses | 5,15 | 5.59 | <0.005 | AR1 |
| (Scored by staff) | 8-ses | 4,25 | 11.10 | <0.0001 | AR1 |
| Medication class | 14-ses | 5,15 | 2.79 | <0.06 (ns) | HAR1 |
| 8-ses | 4,23 | 0.45 | n.s. | HAR1 | |
| Self-ratings of severity | 14-ses | 5,14 | 9.63 | 0.0004 | CS |
| 8-ses | 4,25 | 4.26 | <0.01 | CS | |
| Albuterol use* | 14-ses | 3,11 | 3.99 | <0.04 | CS |
| 8-ses | 2,10 | 3.66 | <0.07 (ns) | CS | |
| Asthma quality of life | 14-ses | 5 | 7.45 | 0.0007 | CS |
| 8-ses | 4 | 11.87 | <0.0001 | CS | |
| Asthma Symptom Check List | |||||
| Total score | 14-ses | 5,22 | 1.92 | n.s. | CS |
| 8-ses | 4,25 | 12.41 | <0.0001 | CS | |
| Panic-fear | 14-ses | 5,22 | 2.29 | <0.09 (ns) | CS |
| 8-ses | 4,25 | 12.92 | <0.0001 | CS | |
| Airways obstruction | 14-ses | 5,22 | 3.12 | <0.03 | CS |
| 8-ses | 4,25 | 3.38 | <0.03 | CS | |
| Hyperventilation | 14-ses | 5,21 | 0.76 | n.s. | CS |
| 8-ses | 4,25 | 3.41 | <0.03 | CS | |
| Irritability | 14-ses | 5,22 | 0.70 | n.s. | CS |
| 8-ses | 4,25 | 3.53 | <0.03 | CS | |
| Fatigue | 14-ses | 5,22 | 0.75 | n.s. | CS |
| 8-ses | 4,25 | 5.89 | <0.002 | CS |
Albuterol use was not scored for the second follow-up session due to missing data and scoring difficulties.
AR1 = autoregressive model order = 1
CS = compound symmetry model
HCS = heterogeneous compound symmetry model
HAR1 = heterogeneous autoregressive model order = 1
Note. The alpha level of statistical significance for the overall Session effects was set at p<0.05. It was set at 0.01/0.0125 for the comparison between first and last sessions in the 14 and 8 session protocols, respectively.
Fig. 1.
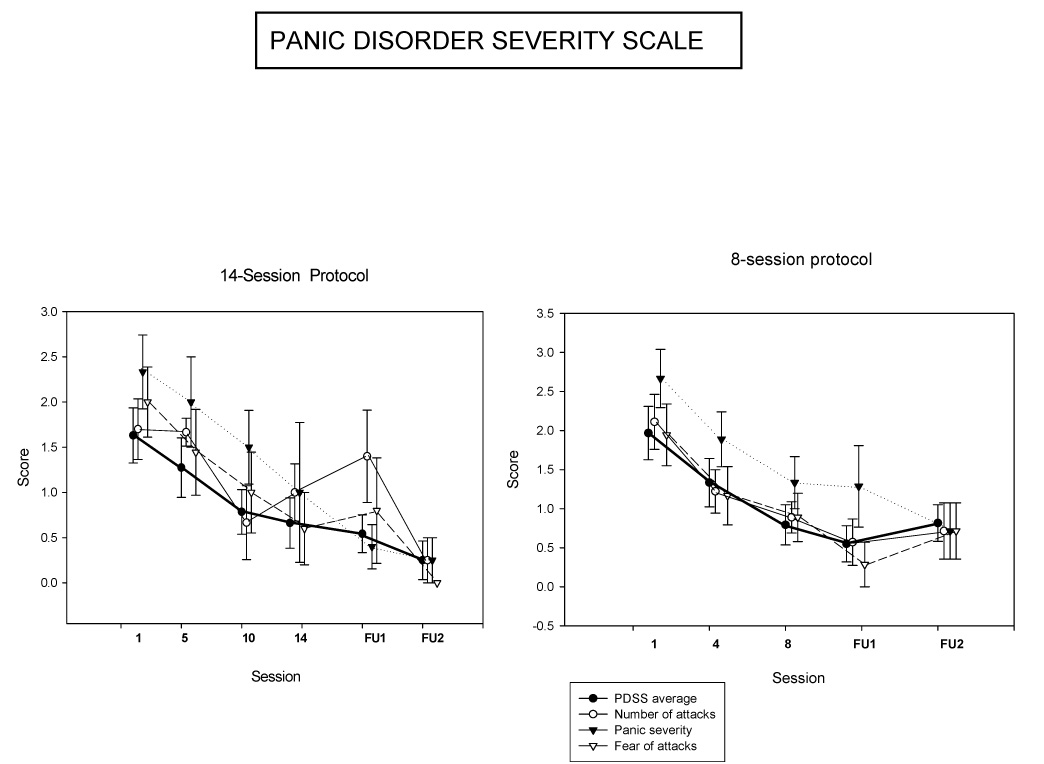
Panic Disorder Severity Scale
Note: Error bars represent standard errors.
Table 2.
Effect sizes (Cohen’s d)
| Measure | Pretest to last treatment session | Pretest to second follow up session | ||
|---|---|---|---|---|
| 14-session | 8-session | 14-session | 8-session | |
| PDSS average score | 1.01 | 1.16 | 1.43 | 1.12 |
| PDSS # Symptoms | 0.66 | 1.33 | 1.37 | 1.16 |
| PDSS Severity of attacks | 1.09 | 1.19 | 1.70 | 1.75 |
| PDSS Fear of next attack | 1.14 | 0.57 | 1.63 | 1.04 |
| Beck Anxiety Inventory | 1.21 | 0.48 | 1.59 | 0.74 |
| Agorophob Cognit Quest. | 0.65 | 0.33 | 0.99 | 1.51 |
| Anxiety Sensitivity Index | 0.19 | 0.68 | 1.84 | 0.61 |
| Body Sensations Quest. | 0.72 | 0.60 | 1.11 | 0.57 |
| Asthma severity: symptoms | 1.67 | 1.49 | 1.42 | 1.92 |
| Asthma severity: medications | 0.10 | 0.47 | 0.91 | 0.64 |
| Asthma symptoms self-rated | 2.34 | 2.77 | 1.05 | 1.07 |
| Asthma Quality of Life | 1.63 | 1.04 | 4.71 | 2.34 |
| Asthma Symptom Check List | 0.39 | 1.49 | 1.50 | 1.61 |
| Albuterol use* | 0.31 | 0.56 | 1.03 | 1.24 |
For albuterol use, we compare the baseline with the period before the first followup session rather than the first, because of missing data.
Cohen (1988) defined effect sizes as "small, d = .2," "medium, d = .5," and "large, d = 8"(p. 24).
Self-reported anxiety: Panicogenic Cognitions (ASI, ACQ, BSQ) and the Beck Anxiety Inventory (BAI)
(See Fig. 2 and Table 3.). Because these secondary measures all assessed aspects of anxiety cognitions, we analyzed them with a single mixed models analysis. In both protocols we found a significant decrease across sessions (main effect for Session), but no Measure × Session interaction, for both protocols. For the 14-session protocol the decrease was significant from the last treatment session through the two followup sessions; for the 8-session protocol it was significant beginning in the fourth session. The effect sizes were medium to large. BAI scores were in the pathological range at the first session (M = 25.6 ± 11.0 for the 14-session protocol, M = 26.0 ± 14.9 for the eight-session protocol). The decrease was at the clinically significant level of 50% in the 14-session protocol, but fell slightly short of this in the eight-session protocol. In both protocols, scores during the follow-up period tended to be in the normal range (Table 3, Fig 3).
Fig. 2.
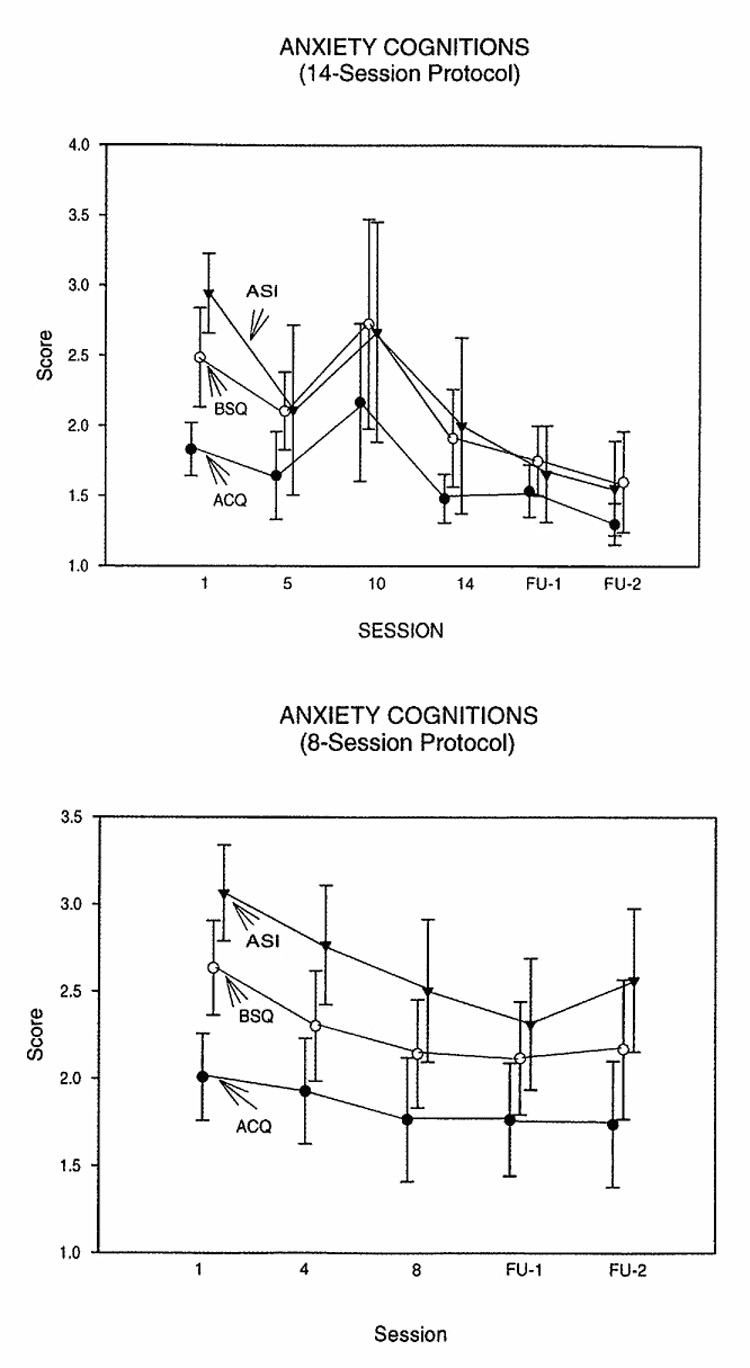
Panicogenic Cognitions: Anxiety Sensitivity Index, Agoraphobia Cognitions Questionnaire, Body Sensations Questionnaire, and Beck Anxiety Inventory
Fig. 3.
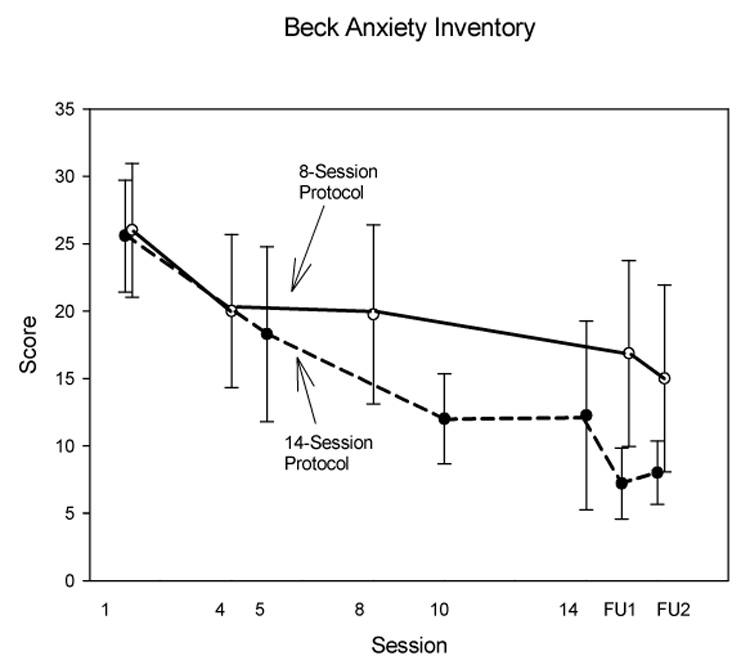
Beck Anxiety Inventory
Effects on asthma
Asthma severity: symptom class (staff ratings)
Asthma symptoms changed significantly (Table 3) in both protocols (main effect). They decreased significantly and remained significantly lower than Session 1 levels by and after Session 10 in the 14-session protocol and Session 4 in the eight-session protocol, with significant decreases persisting into the followup periods for both protocols (Table 3 and Fig 4). The effect size was large in both protocols (Table 2), higher in the 14-session protocol. In asthma education programs, improvement in this measure has tended to be minimal (Marabini, 2002, 2005).
Fig 4.
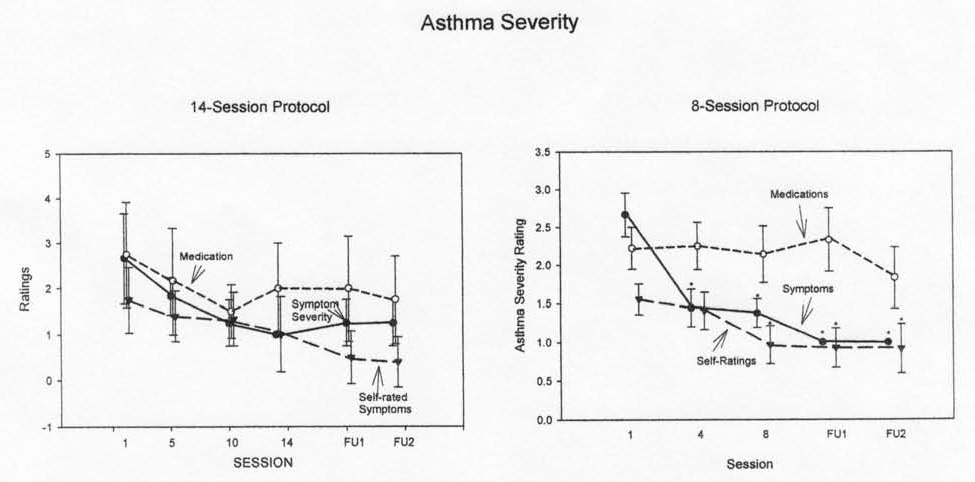
Asthma severity: Symptom and Medication Classes
Symptoms are classified using NHLBI criteria as 1 = mild intermittent, 2 = mild persistent, 3 = moderate, 4 = severe. An asterisk (*) denotes a significant change from baseline.
Asthma severity: medication class
We scored asthma medication severity class according to the level of asthma severity appropriate for the patient’s medication consumption. Levels of asthma medication consumption did not change significantly (Table 3 and Fig 4). The effect size for medication decreases tended to be small to moderate, although it was large at the second followup session for the 14-session protocol.
Asthma severity: pulmonary function class (spirometry)
The therapy had few significant effects on spirometry (Table 3). A nonsignificant tendency toward improvement in peak expiratory flow and FEV1 was noted (for the 14-session protocol, p < .06 for changes from the first to the 10th training session and first follow-up session; for the 8-session protocol, p < .03 for the 4th training session).
Patient self-ratings of asthma symptom severity
Levels of patient-rated asthma symptom severity decreased significantly across groups in both protocols. In both protocols the decrease was significant for the last treatment session and the two follow-up sessions (Table 3, Fig. 4).
Albuterol use
Data on albuterol use were taken from the Daily Questionnaires. Because of large amounts of missing data for the period between the 1 and 2-month follow-up periods, we dropped the two-month follow-up period from the analysis. We examined albuterol use as the percent of days between assessment sessions in which albuterol was taken. For this measure, we did not rely on post-hoc interviews, thus yielding data that were more accurate, but with more missing values. (It was our impression that participants had better memory for asthma symptoms than for the details of albuterol use.) For the 14- session protocol, we found that percent of days with albuterol fell significantly across sessions. Although the decrease was not significant during the treatment period, it became significant for the period between the last treatment session and the followup session. For the eight-session protocol, the overall between-session effect only approached significance (Table 3, Fig 5). Although effect sizes were small to moderate for the period preceding the last training session, they were large for the period preceding the first followup session. The effect size for the follow-up assessment (0.9 in the 14-session protocol and 1.1 in the eight-session protocol) was smaller than that reported by O’Byrne et al (d = 2.2) in a multi-center trial of formeterol treatment among asthma patients already taking inhaled corticosteroid therapy, a drug therapy taken by almost all patients in our study. However, no decreases at all are found for albuterol use in several asthma education programs (Grampian Asthma Study of Integrated Care, 1994; Marabini et al, 2002, 2005; Perneger et al, 2002), (where most asthma patients did not have panic disorder, nor did they necessarily overuse bronchodilators), although one study did report a significant decrease in abluterol use (Gani et al, 2001).
Fig. 5.
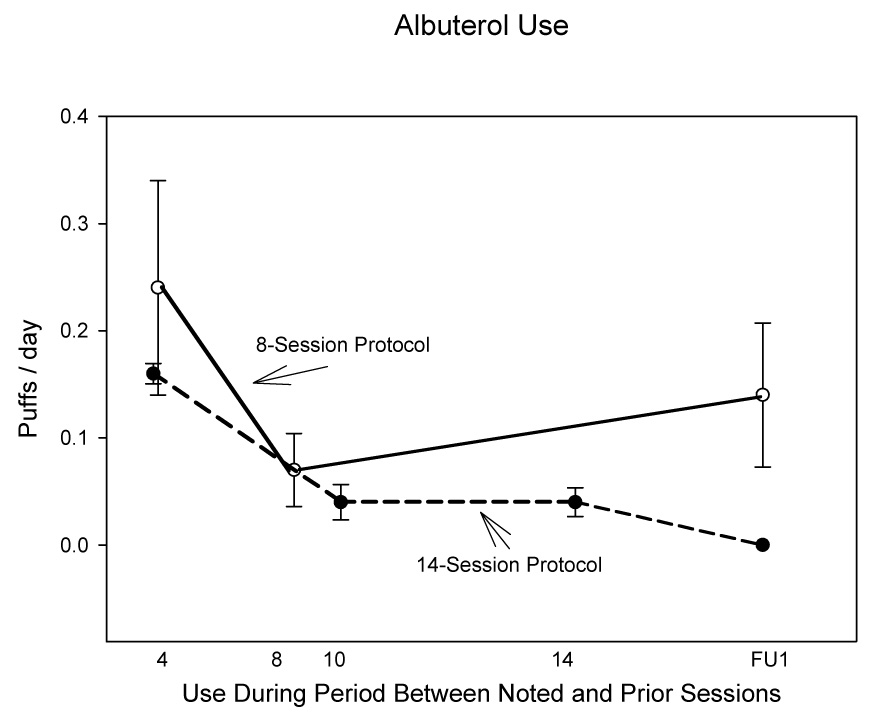
Albuterol use
Note: Data for the period between the first and second followup sessions are omitted because of data recording difficulties.
Asthma quality of life
In the 14-session protocol, scores improved significantly by the last session, and remained significantly better than baseline during two followup assessments. In the eight-session protocol, significant improvement occurred by the 4th session, and also remained significant through the followup period (Table 3, Fig 6). Comparing results in this study to the results of the treatment group in the representative asthma education study using the same outcome measure by Marabani et al (2005), our effect size was large (Table 2).
Fig. 6.
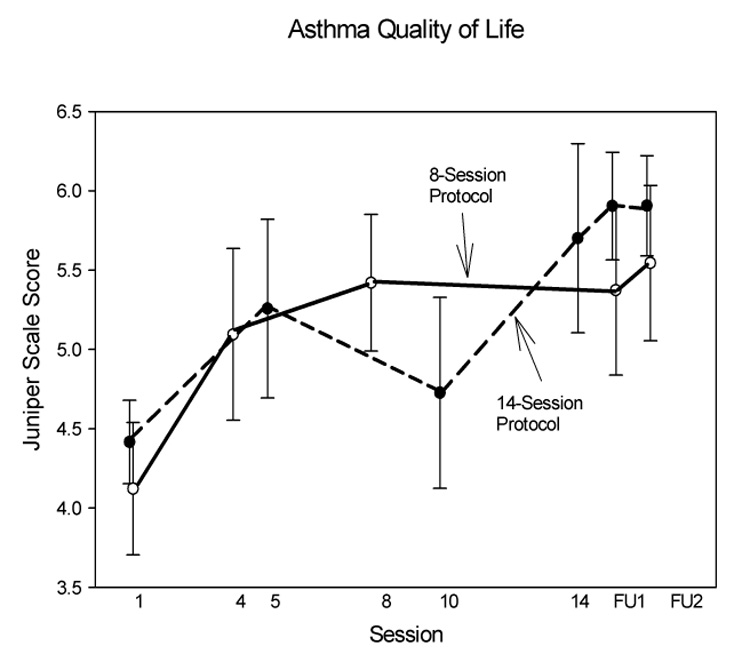
Asthma Quality of Life
Asthma Symptom Check List (ASCL)
Total score on the ASCL was analyzed as the mean for the 36 scale items. Scores did not differ significantly among sessions for the 14-session protocol, but decreased significantly in the short protocol values by the last treatment session and during the followup period (Table 3, Fig 7). Most subscales of the ASCL also showed significant symptom decreases (main Session effect) in the 8-session protocol, but only airway obstruction symptoms improved in the 14-session protocol.
Fig. 7.
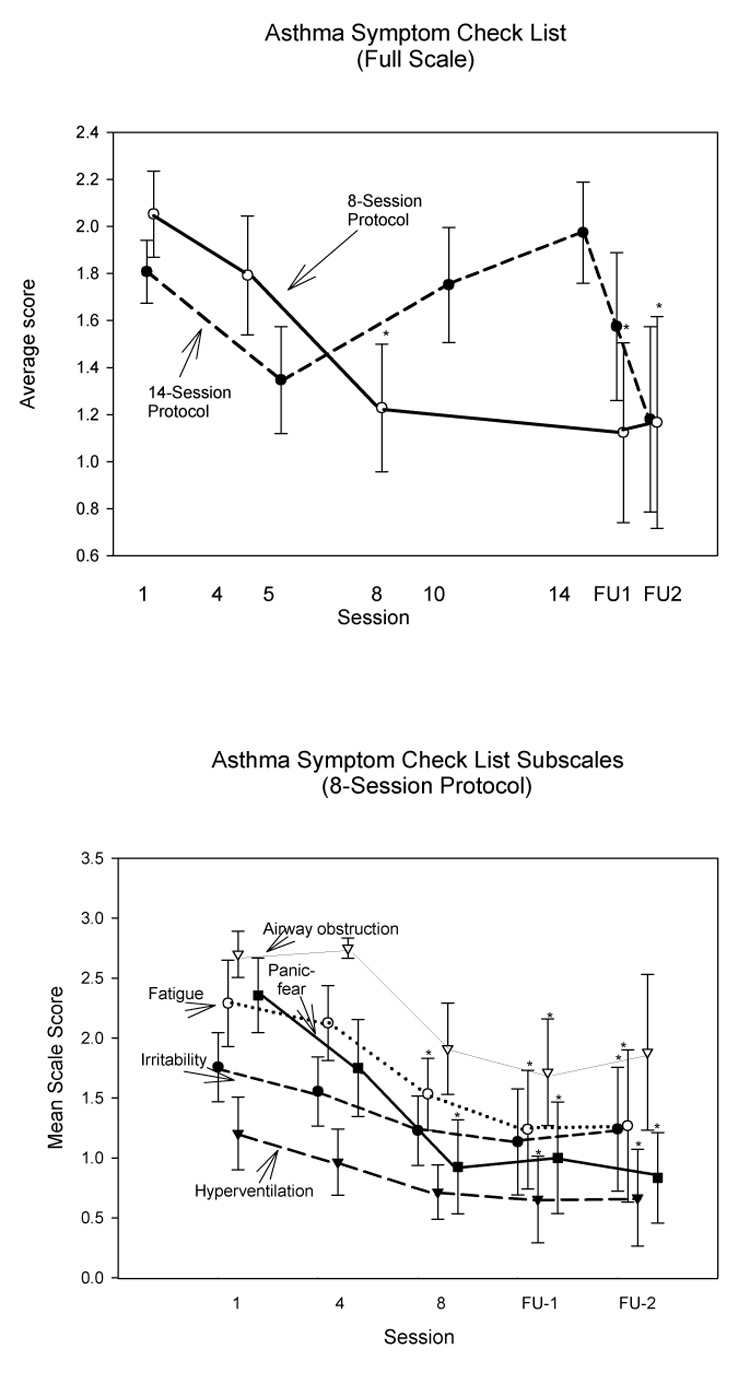
Asthma Symptom Check List
DISCUSSION
The effects of both protocols were large and clinically meaningful. Panic symptomatology, as reflected in PDSS scores, dropped by more than 50% and remained low during the follow-up period. Asthma symptoms also decreased during treatment, along with decreases in albuterol use in the 14-session protocol, consistent with better and more appropriate asthma control (NHLBI, 1997, 2002). This decrease did not compromise pulmonary function or lead to more asthma exacerbations, although a small decrease in pulmonary function was observed, suggesting that medication consumption and pulmonary function should be closely followed among comorbid patients receiving this treatment. The observed improvement in asthma quality of life suggests appropriate use of asthma medication, perhaps with fewer side effects. Because level of asthma symptomatology tends to be exaggerated in the comorbid group compared with pulmonary function, an improvement in symptoms and reported quality of life are a clinically relevant outcome. In general, the effects of PD were similar to those of standard behavioral treatments of the disorder, although somewhat smaller in the eight-session protocol. The effects on asthma were larger than usually obtained in asthma education programs.
Although the more lengthy 14-session treatment for the comorbidity had marginally greater effect sizes, the large dropout rate in the longer protocol indicates that the shorter one has greater patient acceptance. The average treatment length of cognitive behavior therapy for PD alone in private practice ranges between 12–84 sessions (Biondi & Picardi, 2003), and Barlow and Craske’s manual for PD treatment prescribes 15 sessions. Although the briefer protocol did have significant effects, presumably from early attention to teaching participants to distinguish asthma from panic symptoms treating each disorder appropriately, it nevertheless may be useful to evaluate motivational interventions to keep patients in therapy for a longer time. The highest dropout rates occurred before and during sessions devoted to exposure therapy. Perhaps reframing these techniques or introducing them more gradually may improve patient acceptance.
We emphasize that our results are preliminary. The sample size was small, with consequent limitations in power and generalizability. Dropout rate, particularly from the 14-session protocol, was high, and lack of control group leaves the possibility that results were due to a host of factors unrelated to the study’s hypotheses. Nonetheless, the large effects we found strongly support the value of continued research on this topic.
Acknowledgements
This work was supported by Grant #R21MH58196 from the National Institute for Mental Health. The authors are indebted to Anthony Scardella, M.D., Mahmood Siddique, M.D., and Stuart Hockron, M.D., for evaluation of asthma, and to Jim Lewellis for help in developing the treatment manual.
Footnotes
Values of FEV1 < 80% expected are considered abnormal.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Paul M. Lehrer, UMDNJ - Robert Wood Johnson Medical School, Piscataway, NJ
Maria Katsamanis Karavidas, UMDNJ - Robert Wood Johnson Medical School, Piscataway, NJ.
Shou-En Lu, UMDNJ-School of Public Health, Piscataway, NJ
Jonathan Feldman, Ferkauf Graduate School of Psychology, Yeshiva University, Bronx, NY
Linda Kranitz, Graduate School of Arts and Sciences, Rutgers University, Piscataway, NJ
Smrithy Abraham, School of Pharmacy, Rutgers University, Piscataway, NJ
William Sanderson, Hofstra University, Hempstead, NY
Russ Reynolds, Knoxville, TN
REFERENCES
- Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Budapest: Academiai Kiado; 1973. pp. 267–281. [Google Scholar]
- Alberti RE, Emmons ML. Your perfect right. Champaign, IL: Research Press; 1995. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barlow D, Craske M. Mastery of your anxiety and panic (MAP-3): Client workbook for anxiety and panic. Oxford University Press; 2000. [Google Scholar]
- Barlow JH, Ellard DR, Hainsworth JM, Jones FR, Fisher A. A review of self-management interventions for panic disorders, phobias and obsessive-compulsive disorders. Acta Psychiatrica Scandinavica. 2005;111:272–285. doi: 10.1111/j.1600-0447.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring anxiety, Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Biondi M, Picardi A. Increased probability of remaining in remission from panic disorder with agoraphobia after drug treatments in patients who received concurrent cognitive behavioural therapy: A follow-up study. Psychotherapy and Psychosomatics. 2003;72:34–42. doi: 10.1159/000067186. [DOI] [PubMed] [Google Scholar]
- Brooks CM, Richards JM, Bailey WC, Martin B, Windsor RA, Soong SJ. Subjective symptomatology of asthma in an outpatient population. Psychosomatic Medicine. 1989;51:102–108. doi: 10.1097/00006842-198901000-00010. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety disorders interview schedule for DSM-IV. Albany, NY: Graywind Publications; 1994. [Google Scholar]
- Carr RE, Lehrer PM, Rausch LL, Hochron SM. Anxiety sensitivity and panic attacks in an asthmatic population. Behaviour Research and Therapy. 1994;32:411–418. doi: 10.1016/0005-7967(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Carr RE, Lehrer PM, Hochron SM. Panic symptoms in asthma and panic disorder: A preliminary test of the dyspnea-fear theory. Behaviour Research and Therapy. 1992;30:251–261. doi: 10.1016/0005-7967(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of fear in agoraphobics: The Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Matera MG, Donner CF. Inhaled beta2-adrenoceptor agonists: cardiovascular safety in patients with obstructive lung disease. Drugs. 2005;65:1595–1610. doi: 10.2165/00003495-200565120-00001. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cohen S, Tyrell DA, Smith AP. Psychological stress in humans and susceptibility to the common cold. New England Journal of Medicine. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson A, Chapman MD, Woodcock A. Allergen avoidance in the treatment of asthma and atopic disorders. Thorax. 1998:63–72. doi: 10.1136/thx.53.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem NW, Kinsman RA, Horton DJ. Panic-fear in asthma: requests for as-needed medications in relation to pulmonary function measurements. Journal of Allergy and Clinical Immunology. 1977;60:295–300. doi: 10.1016/0091-6749(77)90108-7. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Kessler RC, Wittchen HU, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry. 1994;151:413–420. doi: 10.1176/ajp.151.3.413. [DOI] [PubMed] [Google Scholar]
- Feldman JM, Giardino ND, Lehrer PM. Asthma and panic disorder. In: Mostofsky DI, Barlow DH, editors. The Management of Stress and Anxiety in Medical Disorders. Needham Heights, MA: Allyn & Bacon; 2000. pp. 220–239. [Google Scholar]
- Feldman JM, Lehrer PM, Borson S, Hallstrand TS, Siddique MI. Health care use and quality of life among patients with asthma and panic disorder. Journal of Asthma. 2005a;42:179–184. doi: 10.1081/JAS-200054633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JM, Siddique MI, Morales E, Kaminski B, Lu SE, Lehrer PM. Psychiatric disorders and asthma outcomes among high-risk inner-city patients. Psychosomatic Medicine. 2005b;67:989–996. doi: 10.1097/01.psy.0000188556.97979.13. [DOI] [PubMed] [Google Scholar]
- Foa EB, Steketee G, Young MC. Agoraphobia: Phenomenological aspects, associated characteristics, and theoretical considerations. Clinical Psychology Review. 1984;4:431–457. [Google Scholar]
- Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Annals of Allergy, Asthma, and Immunology. 2003;90 Suppl 3:34–40. doi: 10.1016/s1081-1206(10)61658-4. [DOI] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. Journal of the Anxiety Disorders. 1992;6:55–61. [Google Scholar]
- Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Archives of General Psychiatry. 2003;60:1125–1130. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Wamboldt MZ, Pine DS. Lung disease and internalizing disorders. Is childhood abuse a shared etiologic factor? Journal of Psychosomatic. Research. 2003;55(3):215–219. doi: 10.1016/s0022-3999(02)00497-x. [DOI] [PubMed] [Google Scholar]
- Drummond N, Abdalla M, Buckingham JK, Beattie JAG, Lindsay T, Osman LM, Ross SJ, Roy-Chaudhury A, Russell I, Turner M, Douglass JG, Legge JS, Friend JAR GRASSIC. Integrated care for asthma: a clinical, social, and economic evaluation. BMJ. 1994;308:559–564. [PMC free article] [PubMed] [Google Scholar]
- Gani F, Pozzi E, Crivellaro MA, Senna G, Landi M, Lombardi C, Canonica GW, Passalacqua G. The role of patient training in the management of seasona rhinitis and asthma: clinicalimplications. Allergy. 2001;56:65–68. doi: 10.1034/j.1398-9995.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- Hasler G, Gergen PJ, Kleinbaum DG, Ajdacic V, Gamma A, Eich D, Rossler W, Angst J. Asthma and Panic in Young Adults. A Twenty Year Prospective Community Study. American Journal of Respiratory and Critical Care Medicine. 2005;171(11):1224–1230. doi: 10.1164/rccm.200412-1669OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Archives of General Psychiatry. 2004;61:913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- Jacobson E. Progressive relaxation. Chicago: University of Chicago Press; 1938. [Google Scholar]
- Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Coe CL, McCarthy DO, Jarjour NN, Kelly EA, Rodriguez RR, Busse WW. Cytokine profiles of stimulated blood lymphocytes in asthmatic and healthy adolescents across the school year. Journal of Interferon and Cytokine Research. 1997;17:481–487. doi: 10.1089/jir.1997.17.481. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonable KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the national comorbidity survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kilham H, Tooley M, Silverman M. Running, walking and hyperventilation causing asthma in children. Thorax. 1979;34:582–586. doi: 10.1136/thx.34.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman RA, Dirks JF, Jones NF. Psychomaintenance of chronic physical illness: clinical assessment of personal styles affecting medical management. In: Millon T, Green C, Meagher R, editors. Handbook of Clinical Health Psychology. New York: Plenum Press; 1982. pp. 435–466. [Google Scholar]
- Kinsman RA, Luparello T, O'Banion K, Spector S. Multidimensional analysis of the subjective symptomatology of asthma. Psychosomatic Medicine. 1973;35:250–267. doi: 10.1097/00006842-197305000-00008. [DOI] [PubMed] [Google Scholar]
- Lavoie KL, Cartier A, Labrecque M, Bacon SL, Lemiere C, Malo JL, Lacoste G, Barone S, Verrier P, Ditto B. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respiratory Medicine. 2005;99:1249–1257. doi: 10.1016/j.rmed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Carr R. Jacobson's method of progressive relaxation. In: Roth WT, editor. Treating the anxiety disorders. San Francisco, CA: Jossey-Bass Publishers; 1996. [Google Scholar]
- Lehrer P, Feldman J, Giardino N, Song HS, Schmaling K. Psychological aspects of asthma. Journal of Consulting & Clinical Psychology. 2002;70:691–711. doi: 10.1037//0022-006x.70.3.691. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Hochron SM, Isenberg S, Rausch L, Carr R. The Asthma Symptom Profile: a psychophysically based scale for assessment of asthma symptoms. Journal of Psychosomatic Research. 1993;37:515–521. doi: 10.1016/0022-3999(93)90007-3. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Hochron S, Mayne TM, Isenberg S, Lasoski AM, Carlson V, Gilchrist J, Porges S. Relationship between changes in EMG and respiratory sinus arrhythmia in a study of relaxation therapy for asthma. Applied Psychophysiology and Biofeedback. 1997;22:183–191. doi: 10.1023/a:1026263826106. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. American Journal of Respiratory and Critical Care Medicine. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Marabini A, Brugnami G, Curradi F, Casciola G, Stopponi R, Pettinari L, Siracusa A. Short-term effectiveness of an asthma educational program: results of a randomized controlled trial. Respiratory Medicine. 2002;96:993–998. doi: 10.1053/rmed.2002.1394. [DOI] [PubMed] [Google Scholar]
- Marabini A, Brugnami G, Curradi F, Siracusa A. Does an asthma education program improve quality of life? A two-year randomized trial. Journal of Asthma. 2005;42:577–581. doi: 10.1080/02770900500216101. [DOI] [PubMed] [Google Scholar]
- Nardi AE. Where are the guidelines for the treatment of asthma with panic spectrum symptoms? American Journal of Respiratory and Critical Care Medicine. 2005;172:1055–1056. doi: 10.1164/ajrccm.172.8.952. [DOI] [PubMed] [Google Scholar]
- Nascimento I, Nardi AE, Valenca AM, Lopes FL, Mezzasalma MA, Nascentes R, Zin WA. Psychiatric disorders in asthmatic outpatients. Psychiatry Research. 2002;110:73–80. doi: 10.1016/s0165-1781(02)00029-x. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Asthma prevalence, health care use and mortality. Hyattsville, MD: 2002. Centers for Disease Control and Prevention (2005) www.cdc.gov/nchs/products/pubs/pubd/hestats/asthma/asthma.htm. [Google Scholar]
- National Heart Lung and Blood Institute. Expert panel report 2: Guidelines for the diagnosis and management of asthma. Bethesda MD: U.S. Department of Health and Human Services; National Asthma Education and Prevention Program. Publication No. 97-4051. 1997
- National Heart Lung and Blood Institute (NHLBI) Expert panel report: Guidelines for the diagnosis and management of asthma: Update on Selected Topics. Washington DC: U.S. Department of Health and Human Services; National Asthma Education and Prevention Program. 2002 [PubMed]
- Nielsen KG, Bisgaard H. Hyperventilation with cold versus dry air in 2- to 5-year-old children with asthma. American Journal of Respiratory and Critical Care Medicine. 2004:238–241. doi: 10.1164/rccm.200404-528OC. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. American Journal of Respiratory and Critical Care Medicine. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- Perneger TV, Sudre P, Muntner P, Uldry C, Courteheuse C, Naef A, Jacquemet S, Nicod L, Rochat T, Assal J. Effect of patient education on self-management skills and health status in patients with asthma: a randomized trial. The American Journal of Medicine. 2002;113:7–14. doi: 10.1016/s0002-9343(02)01136-1. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Heibronner R. The anxiety sensitivity index: construct validity and factor analytic structure. Journal of the Anxiety Disorders. 1987;1:117–121. [Google Scholar]
- Peterson RA, Reiss S. Test manual for the ASI. Orland Park, IL: International Diagnostic Systems; 1992. [Google Scholar]
- Reynolds RV, Kotses H, Creer TL. Living with asthma: help for adults with asthma. Department of Psychology, University of Ohio; 1988. [Google Scholar]
- Reynolds RV, Kotses H, Creer TL, Bruss G, Joyner CA. Living with asthma: Help for adults with asthma. Athens: Unpublished manual, Ohio University, Department of Psychology; 1989. [Google Scholar]
- Rihmer Z. Successful treatment of salbutamol-induced panic disorder with citalopram. European Neuropsychopharmacoligy. 1997;7:241–242. doi: 10.1016/s0924-977x(97)00408-2. [DOI] [PubMed] [Google Scholar]
- Ross CJ, Davis TM, MacDonald GF. Cognitive-behavioral treatment combined with asthma education for adults with asthma and coexisting panic disorder. Clinical Nursing Research. 2005;14:131–157. doi: 10.1177/1054773804273863. [DOI] [PubMed] [Google Scholar]
- Scalabrin DMF, Sole D, Naspitz CK. Efficacy and side effects of beta2-agonists by inhaled route in acute asthma in children: comparison of salbutamol, terbutaline, and fenoterol. Journal of Asthma. 1996;33:407–415. doi: 10.3109/02770909609068185. [DOI] [PubMed] [Google Scholar]
- Schmaling KB, Bell J. Asthma and panic disorder. Archives of Family Medicine. 1997;6:20–23. doi: 10.1001/archfami.6.1.20. [DOI] [PubMed] [Google Scholar]
- Shavitt RG, Gentil V, Croce J. Panic and asthma: a dangerous mislabeling. European Psychiatry. 1993;8:41–43. [Google Scholar]
- Shavitt RG, Gentil V, Mandetta R. The association of panic/agoraphobia and asthma: Contributing factors and clinical implications. General Hospital Psychiatry. 1992;14:420–423. doi: 10.1016/0163-8343(92)90010-8. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative panic disorder severity scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Smith A, Nicholson K. Psychosocial factors, respiratory viruses and exacerbation of asthma. Psychoneuroendocrinology. 2001;26:411–420. doi: 10.1016/S0306-4530(00)00063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Griffiths C. Asthma and panic: scope for intervention? American Journal of Respiratory and Critical Care Medicine. 171:1197–1198. doi: 10.1164/rccm.2503005. [DOI] [PubMed] [Google Scholar]
- Tietz W, Kahlstrom E, Cardiff M. Relationship of psychopathology to death in asthmatic adolescents. The Journal of Asthma Research. 1975;12:199–206. doi: 10.3109/02770907509098947. [DOI] [PubMed] [Google Scholar]
- Van Peski-Oosterbaan AS, Spinhoven P, Van der Does AJ, Willems LN, Sterk PJ. Is there a specific relationship between asthma and panic disorder? Behaviour Research and Therapy. 1996;34:333–340. doi: 10.1016/0005-7967(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Yellowlees PM, Haynes S, Potts N, Ruffin RE. Psychiatric morbidity in patients with life-threatening asthma. Medical Journal of Australia. 1988;149:246–249. doi: 10.5694/j.1326-5377.1988.tb120596.x. [DOI] [PubMed] [Google Scholar]


