Abstract
Intense seizure activity associated with status epilepticus and excitatory amino acid (EAA) imbalance initiates oxidative damage and neuronal injury in CA1 of the ventral hippocampus. We tested the hypothesis that dendritic degeneration of pyramidal neurons in the CA1 hippocampal area resulting from seizure-induced neurotoxicity is modulated by cerebral oxidative damage. Kainic acid (KA, 1 nmol/5 μl) was injected intracerebroventricularly to C57Bl/6 mice. F2-isoprostanes (F2-IsoPs) and F4-neuroprostanes (F4-NeuroPs) were used as surrogate measures of in vivo oxidative stress and biomarkers of lipid peroxidation. Nitric oxide synthase (NOS) activity was quantified by evaluating citrulline level and pyramidal neuron dendrites and spines were evaluated using rapid Golgi stains and a Neurolucida system. KA produced severe seizures in mice immediately after its administration and a significant (p<0.001) increase in F2-IsoPs, F4-NeuroPs and citrulline levels were seen 30 min following treatment. At the same time, hippocampal pyramidal neurons showed significant (p<0.001) reduction in dendritic length and spine density. In contrast, no significant change in neuronal dendrite and spine density or F2-IsoP, F4-NeuroPs and citrulline levels were found in mice pretreated with Vitamin E (α-tocopherol, 100 mg/kg, ip) for 3 days, or with N-tert-butyl-α-phenylnitrone (PBN, 200 mg/kg, ip) or ibuprofen (inhibitors of cyclooxygenase, COX, 14 μg/ml of drinking water) for 2 weeks prior to KA treatment. These findings indicate novel interactions among free radical-induced generation of F2-IsoPs and F4-NeuroPs, nitric oxide and dendritic degeneration, closely associate oxidative damage to neuronal membranes with degeneration of the dendritic system, and point to possible interventions to limit severe damage in acute neurological disorders.
INTRODUCTION
Damage to neuronal cells caused by over-activation of post-synaptic glutamate receptors is considered a key pathological event in stroke, epilepsy, trauma and neurodegenerative disease (Choi, 1988; Macdonald and Stoodley, 1998). Neurochemical changes associated with neuronal injury represent complex and often synergistic mechanisms that include several aspects of the cellular metabolism, including energy metabolism, ion-homeostasis and redox status. Excitotoxicity can be stimulated by intracerebroventricular (icv) injection of kainic acid (KA), the active ingredient originally isolated from sea-weed used as an herbal treatment for ascariasis (Anthony at al., 2001). Kainate is a rigid analog of glutamate, the principal excitatory neurotransmitter in the central nervous system (CNS), and it is a very potent stimulant of a subset of ligand-gated ion channel, called KA receptors (Milatovic et al., 2005). Activation of the KA subtype of ionotropic gluatamate receptors results in sustained epileptic activity in the hippocampus, followed by a selective pattern of neuropathology that is similar to human temporal lobe epilepsy (Ben-Ari, 1985; Ben-Ari and Cossart, 2000; Schwob et al., 1980). Kainate administration and intense seizure activity associated with status epilepticus is sufficient to induce degeneration of cornu ammonis (CA) neurons, and hyper excitability of surviving hippocampal CA neurons (Dudek et al., 1994; Ben-Ari, 1985, 2001; Dong et al., 2003).
Oxidative stress and excessive activation of glutamate receptors represent sequential as well as interacting factors that converge as a final common pathway for neuronal vulnerability (Coyle and Puttfarcken, 1993). While excitotoxic and oxidative injury may occur independently, growing evidence indicates that reactive oxygen (ROS) and nitrogen species (RNS) formation is a consequence of glutamate receptor-mediated neurotoxicity (Dugan et al., 1995). Activation of glutamate receptors and consequent calcium-dependent depolarization of the mitochondrial membrane potential lead to incomplete O2 consumption, reduced production of ATP, overproduction of ROS, nitric oxide and peroxynitrite and consequent damage of cell structures including lipids, proteins and DNA. Thus, impaired mitochondrial respiratory chain function and the ensuing lipid peroxidation that are associated with seizure activity may precede neuronal damage and death in vulnerable brain regions (Kunz et al., 1999; Cheng and Sun, 1994; Frantseva et al., 2000; Milatovic et al., 2002; Bruce and Baudry, 1995). To date, few in vivo studies have directly addressed changes in lipid peroxidation and neurodegeneration of vulnerable neuronal structures in the hippocampal formation.
Free radical damage to the brain can be sensitively and accurately quantified by measuring the chemically stable oxidative damage products of arachidonic acid (AA) and docosahexaenoic acid (DHA), namely, isoprostanes (F2-IsoPs) and neuroprostanes (F4-NeuroPs), respectively. Since AA is relatively evenly distributed in the brain with similar concentrations in gray matter and white matter, and within glia and neurons, measurement of cerebral F2-IsoPs, like all other measures of oxidative damage, reflects damage to brain tissue but not necessary to neurons. F2-IsoPs are also detectable in normal biological fluids and increase dramatically in models of oxidative stress. Unlike AA, DHA is highly concentrated in neuronal membranes to the exclusion of other cell types. Thus, quantification of F4-NeuroPs provides a highly selective, quantitative window into free radical damage to neuronal membranes in vivo (Montine et al., 2004).
Several experimental studies have established that antioxidant therapy affords protection against excitotoxicity-induced by agents such as glutamate. Besides quenching free radicals and blocking the chain-braking reaction, antioxidants may act also as modulators of the inflammatory cascade, shown to play a role in the development of KA-induced neurotoxicity (Marini et al., 2004). However, there is limited data on whether functional changes in pyramidal neurons from the CA1 hippocampal are modulated by lipid peroxidation. To test this hypothesis, we have used a model of kainic acid-induced neurotoxicity and studied whether suppression of lipid peroxidation and neuroinflammation can modulate neuronal damage of hippocampal pyramidal neurons.
MATERIALS AND METHODS
Materials
All chemicals and reagents were obtained from Sigma Chemical Co., St Louis, MO, USA, unless otherwise specified.
Animals
All experiments were performed as approved by the Vanderbilt University and University of Washington Institutional Animal Care and Use Committees. C57Bl/6 female mice (obtained from Charles River Laboratories; Wilmington, MA, USA) between 5 and 7 weeks of age, were housed at 21 ± 1°C, humidity 50 ± 10%, and light/dark cycle 12 h/12 h, and had free access to pelleted food (Rodent Laboratory Chow, Purina Mills Inc., St Louis, MO, USA) and water. Following anesthesia, 5 μl racerebroventricular int (icv) injections were delivered over 1 min into the left lateral ventricle using a 26 gauge Hamilton syringe and the external landmarks of 4 mm lateral to midline and 5 mm posterior from the forward edge of the left ear. Depth of injection was maintained at 3 mm by using a stylet. Kainic acid (KA), obtained from Sigma Chemical Co.(St. Louis, MO), was dissolved in phosphate buffered saline (pH 7.4) at a final concentration of 1 nmol for a total icv dose of 5 μl. Vitamin E (α-tocopherol, 100 mg/kg) was dissolved in mineral oil and administered intraperitoneally (ip) in three doses on days −2, −1 and 0 relative to icv injection. Mice were treated with N-tert-butyl-α-phenylnitrone (PBN) at 200 mg/kg ip at 30 min prior to icv injection. Ibuprofen was administered in the drinking water at 14 μg/mL for 2 weeks prior to icv injection. Mice were euthanized at the indicated times, their brains rapidly removed, the cerebral hemispheres dissected out, flash frozen in liquid nitrogen and stored at −80°C.
Oxidative damage
F2- isoprostanes (F2-IsoPs) and F4-neuroprostanes (F4-NeuroPs) were determined using a stable isotope dilution method with detection by gas chromatography/mass spectrometry (GC/MS) and selective ion monitoring (SIM) as previously described (Milatovic et al., 2003, 2005). Briefly, samples of dissected brain (120−180 mg) were homogenized in Folch solution, chloroform layer evaporated, lipids chemically hydrolyzed using KOH and a stable isotope, 8-iso prostaglandin F2α-d4 internal standard added. Following extraction using C-18 and silica Sep-Pac cartridges, purification by thin layer chromatography, and conversion to O-methyloxime pentafluorobenzyl ester trimethylsilyl derivatives, the compound was dissolved in undecane that is dried over a bed of calcium hydride. Negative ion chemical ionization MS was performed with Hewlett–Packard HP5989A and Model 5973, Agilent Technologies instruments interfaced with monitoring ions for F2-IsoPs (m/z 569), the [2H4]15-F2α-IsoP internal standard (m/z 573) and F4-NeuroPs (m/z 593). The ion source temperature was 250°C, and electron energy was 70 eV.
Quantitation of nitric oxide (NO) as a measure of RNS
The levels of citrulline (the co-product of NO synthesis) were determined using the HPLC method of Bagetta et al. (1995) with minor modifications (Gupta et al., 2001; Gupta and Dettbarn, 2003). Tissues were homogenized in 0.4 M perchloric acid for 1 min using a Brinkman homogenizer, followed by sonication for 10 s with a Biosonic Cell Disruptor coupled with a microprobe. Following centrifugation the resulting supernatants were assayed for citrulline concentrations according to the HPLC system coupled with a fluorescence detector (excitation at 334 nm and emission at 440 nm). The results were expressed as nmol of citrulline/g wet tissue weight.
Dendrites and spine counts
Golgi impregnation of 50-μm-thick sections from paraffin-embedded blocks carried out according to the manufacturer's specifications (FD Rapid GolgiStain Kit). Six or more Golgi-impregnated pyramidal neurons with no breaks in staining along the dendrites from CA1 sector of hippocampus were selected and spines counted according to the methods by Leuner et al., (2003). Tracing and counting were carried out with a Neurolucida system at x100 under oil immersion (MicroBrightField, VT)
Statistics
Data analysis was performed using GraphPad Prism (San Diego, CA, USA). Analyses of variance that showed p < 0.05 were followed with multiple pair comparison post-tests using Bonferroni's correction.
RESULTS
Initial experiments investigated the extent of cerebral oxidative damage and dendritic degeneration in CA1 hippocampal pyramidal neurons of C57Bl/6 mice injected icv with KA. Uniformly, all icv KA treatments led to status epilepticus (seizures >30 min) within a minute of the injection. There was a rapid onset of detectable oxidative damage, with F2-IsoPs levels peaking at 30 min followed icv KA injection, with a subsequent decrease to near basal levels at 60 min following KA injection (Table 1). Similarly, a significant increase in the levels of neuronal markers of oxidative damage, F4-NeuroPs, was noted at 30 min following KA injection, returning to baseline at 60 min post injection. Cerebral F2-IsoPs and F4-NeuroPs were not significantly different between untreated control and icv saline injected mice (data not shown).
Table 1.
Cerebral concentrations of F2-IsoPs and F4-NeuroPs and dendritic degeneration of hippocampal pyramidal neurons following KA-induced seizures in mice.
| F2-IsoPs (ng/g) | F4-NeuroPs (ng/g) | Dendritic length (μm) | Spine density (number/100 μm dendrite) | |
|---|---|---|---|---|
| Control | 3.07 ± 0.05 | 13.89 ± 0.58 | 1032.10 ± 61.41 | 16.45 ± 0.55 |
| KA 30 min | 4.81 ± 0.19* | 34.27 ± 2.71* | 363.44 ± 20.78* | 8.81 ± 0.55* |
| KA 60 min | 3.40 ± 0.18 | 18.55 ± 1.26 | 425.71 ± 23.04* | 7.44 ± 0.56* |
Data from KA exposed mice were collected 30 min or 60 min post injection. One-way ANOVA showed p<0.0001 for each end-point. Bonferroni's multiple comparison test showed significant difference (p<0.001) compared to vehicle-injected control.
KA-induced seizures also lead to significant (p<0.001) decreases in dendritic length and spine density of pyramidal neurons from CA1 hippocampal area 30 min following the injection. In contrast to the recovery seen at 60 min following the injection with both oxidative damage biomarkers, dendritic length and spine density did not return to control levels and remained indistinguishable from those at 30 min following KA injection (Table 1).
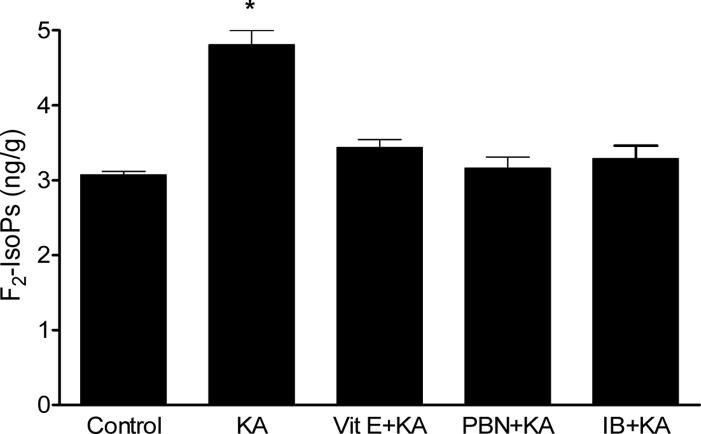
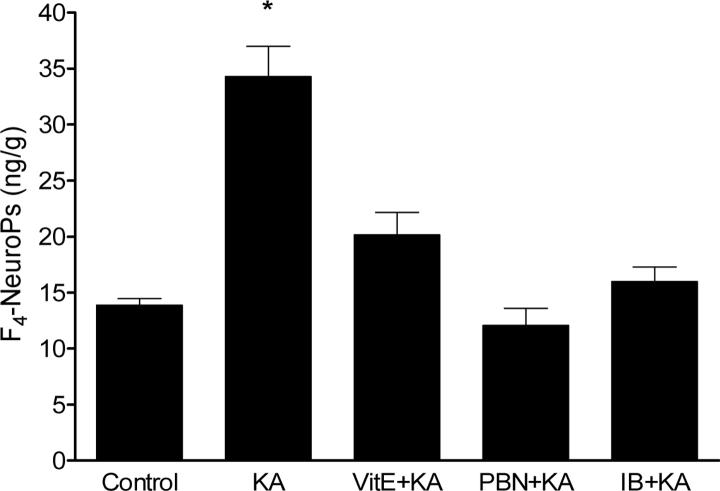
In the next series of experiments, we sought to determine if cerebral lipid peroxidatition associated with KA-induced seizures could be suppressed by pre-treatment with antioxidants and NSAID. Doses of neuroprotectants were based on results from previous research in the model of activated innate immunity (Milatovic et al., 2003; 2004) and excitotoxicity (Gupta et al., 2007). These doses were previously shown to have no effect on seizure severity. We used two different antioxidant agents, vitamin E (α-tocopherol) and PBN. Both significantly suppressed F2-IsoPs and F4-NeuroPs formation following icv KA injections (Figs. 1 and 2) NSAID pretreatment was similarly effective in suppressing oxidative damage in this excitotoxicity model. As shown in Figures 1 and 2, ibuprofen (14 αg/ml) fully suppressed the KA-induced increase in both F2-IsoPs and F4-NeuroPs.
Figure 1.
Ipsilateral cerebral F2-IsoPs concentrations following icv KA with or without vitamin E (Vit E), N-tert-butyl-α-phenylnitrone (PBN) or ibuprofen (IB) pretreatment. Brains from mice exposed to KA were collected 30 minutes post injections (n≥5 for group). One way ANOVA had p<0.0001 with Bonferroni's multiple comparison tests significant for KA vs. control, Vit E+KA, PBN+KA or IB+KA treatment.
Figure 2.
Ipsilateral cerebral F4-NeuroPs concentrations following icv KA with or without vitamin E (Vit E), N-tert-butyl-α-phenylnitrone (PBN) or ibuprofen (IB) pretreatment. Brains from mice exposed to KA were collected 30 minutes post injections (n≥5 for group). One way ANOVA had p<0.0001 with Bonferroni's multiple comparison tests significant for KA vs. control, Vit E+KA, PBN+KA or IB+KA treatment.
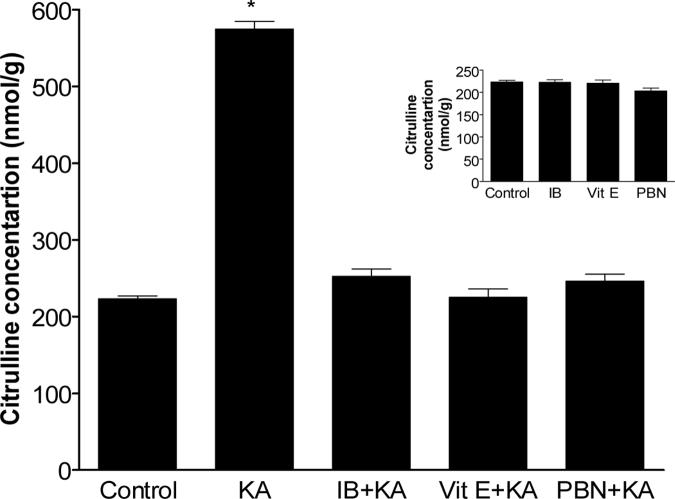
In an attempt to discern additional mediators of oxidative damage in the model of KA-induced neurotoxicity, we determined citrulline concentrations in ipsilateral cerebral hemispheres to KA injection of mice treated similarly to those described above. NOS catalyzes the conversion of arginine to NO and citrulline, and quantification of citrulline is widely employed as an in vivo marker of NOS activity. Basal citrulline concentration was 223.2 ± 3. 93 nmol/g and it was not significantly different in mice treated with saline, IB, Vit E or PBN (Fig. 3). In contrast, significant increase (p<0.001) in citrulline levels were detected in ipsilateral hemispheres of mice 30 min following icv KA injection. As with F2-isoPs and F4-neuroPs, antioxidant pretreatment with Vit E, PBN or ibuprofen fully suppressed KA-induced increase in cerebral citrulline levels (Fig. 3).
Figure 3.
Ipsilateral cerebral citrulline concentrations following icv KA with or without vitamin E (Vit E), N-tert-butyl-α-phenylnitrone (PBN) or ibuprofen (IB) pretreatment. Brains from mice exposed to KA were collected 30 minutes post injections (n≥5 for group). One way ANOVA had p<0.001 with Bonferroni's multiple comparison tests significant for KA vs. control, Vit E+KA, PBN+KA or IB+KA treatment.
Next, we investigated morphological correlates of icv KA injections, determining the structural integrity of the CA1 dendritic system, the neuronal compartment most sensitive to both age-related and disease-related degeneration (Uylings and de Brabander, 2002). Using Golgi impregnation and Neurolucida-assisted morphometry, our results show that oxidative damage is accompanied with reduction in both dendrite length and dendritic spine density in hippocampal CA1 pyramidal neurons post KA injection (Table 1).
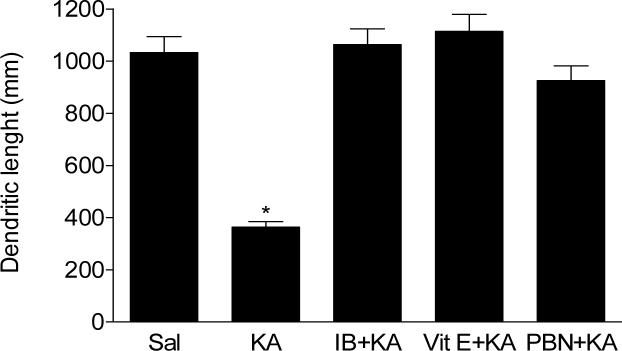
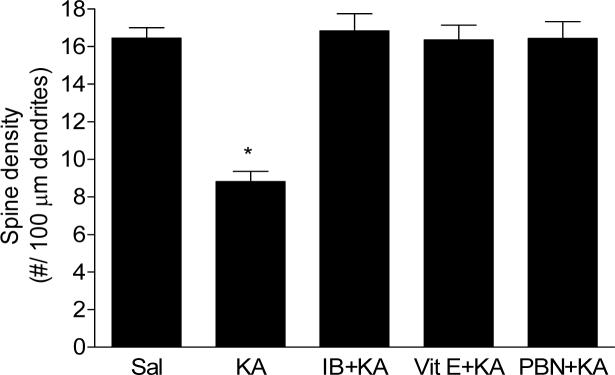
Finally, we directly tested the role of antioxidants and NSAID in preserving the integrity of the dendritic system in the model of KA-induced excitotoxicity. Our results show that Vit E, PBN or ibuprofen pretreatment completely protected both dendrite length (Fig. 4) and dendritic spine density (Fig. 5) from the degenerative consequences of KA at 30 min post icv injection.
Figure 4.
Dendritic length of pyramidal neurons from CA1 hippocampal area of mice following icv KA with or without vitamin E (Vit E), N-tert-butyl-α-phenylnitrone (PBN) or ibuprofen (IB) pretreatment. Brains from mice exposed to KA were collected 30 minutes post injections (n≥5 for each group). One way ANOVA had p<0.001 with Bonferroni's multiple comparison tests significant for KA vs. control, Vit E+KA, PBN+KA or IB+KA treatment.
Figure 5.
Spine density of pyramidal neurons from CA1 hippocampal area of mice following icv KA with or without vitamin E (Vit E), N-tert-butyl-α-phenylnitrone (PBN) or ibuprofen (IB) pretreatment. Brains from mice exposed to KA were collected 30 minutes post injections (n≥5 for each group). One way ANOVA had p<0.001 with Bonferroni's multiple comparison tests significant for KA vs. control, Vit E+KA, PBN+KA or IB+KA treatment.
DISCUSION
Seizure-induced brain injury may contribute to a number of behavioral, cognitive and neuropsychiatric deficits commonly seen in epilepsy patients (Dodrill, 2002; Elger et al., 2004; Zeng et al., 2007). Although seizure-induced neuronal death has been widely documented and studied, especially in animal models, many epilepsy patients have no overt evidence of neuronal death, at least on structural brain imaging, despite suffering from these neurological co-morbidities. Thus, understanding “nonlethal” mechanisms of seizure-induced brain injury, such as oxidative damage and changes in dendritic structures may have more widely applicable clinical relevance and may ultimately lead to novel therapeutic strategies either for treating seizures or preventing neurocognitive deficits in epilepsy. In this study, we tested the hypothesis that seizure-induced cerebral oxidative damage in adult mice is accompanied by alterations in the integrity of the hippocampal CA1 dendritic system. Our results showed that icv KA-induced early increases in biomarkers of global free radical damage (F2-IsoPs) and the selective peroxidation biomarker of neuronal membranes (F4-NeuroPs) was accompanied by dendritic degeneration of pyramidal neurons in the CA1 hippocampal area. Moreover, our results demonstrated that both neuronal oxidative damage and dendritic degeneration of CA1 neurons induced by KA were completely suppressed by the antioxidants Vit E and PBN, as well as the NSAID, ibuprofen.
Icv injection of KA has been used as an experimental model for investigation of cerebral vulnerability, particularly during acute brain disorders (Ben-Ari, 1985) and status epilepticus (Ben-Ari and Cossart, 2000; Schwob et al., 1980). Overstimulation of the KA subtype of ionotropic glutamate receptors results in sustained epileptic activity in the hippocampus, followed by a typical pattern of neuropathologic changes predominantly in the pyramidal neurons. Cell damage is thought to result from intense transient influx of calcium leading to mitochondrial functional impairments characterized by activation of the permeability transition pores in the inner mitochondrial membrane, cytochrome c release, depletion of ATP and simultaneous formation of ROS (Heinemann et al., 2002; Cadenas and Davies, 2000; Patel, 2002; Nicholls and Ward, 2000). In addition, increase in cytoplasmic calcium ions triggers intracellular cascades through stimulation of enzymes, including proteases, phospholipase A2, and nitric oxide synthase, which also lead to increased levels of free radical species and oxidative stress (Lafon-Cazal et al., 1993; Farooqui et al., 2001). Since free radicals are direct inhibitors of the mitochondrial respiratory chain, ROS generation perpetuates a reinforcing cycle, leading to extensive lipid peroxidation and oxidative cell damage (Cock at al., 2002; Cadenas and Davies, 2000).
Here we quantified cerebral lipid peroxidation using F2-IsoPs, and in vivo marker of oxidative damage for neuronal membranes, F4-NeuroPs. Icv KA induced rapid elevation, not only in F2-IsoPs, but also F4-NeuroPs. Our previous study of time-course changes in biomarkers of oxidative damage in the rat model of anticholinesterase induced seizures showed that the highest increase in F2-IsoPs was evaluated at 1 h after the injection of anticholinesterase agent or 40 minutes after beginning of seizure symptoms (Gupta et al., 2007). The earliest time point evaluated in KA-induced excitotoxicity was 30 min since seizures start immediately after the icv KA injection. Elevated levels of these in vivo markers of oxidative damage are in agreement with our previous findings (Montine et al., 2002; Milatovic et al., 2005; Gupta et al., 2007), as well as by others (Patel et al., 2001) and indicate that KA injection leads to profound cerebral and neuronal oxidative damage in mice.
A major goal of this study was to determine whether F2-IsoPs and F4-NeuroPs formation correlated with the vulnerability of pyramidal neurons in the CA1 hippocampal area to KA-induced excitotoxicity. Our results showed that the transient rise in F2-IsoPs and F4-NeuroPs is accompanied by rapid evolution of dendritic abnormalities, apparent in significant decrease in dendritic length and spine density of pyramidal neurons as early as 30 min post KA injection. However, the recovery in both oxidative damage biomarkers at 60 min following the injection was not paralleled by the rescue of damaged neurons from the CA1 hippocampal area. Extended seizures activity (60 min) induced the same level of dendritic length and spine density decrease when compared to 30 min following KA injection (Table 1). Together, this data suggest that both oxidative stress and neurodegeneration occur as an early response to seizures, but do not establish whether oxidative stress is a cause or effect of seizure-induced CA1 cell damage. Neuronal damage processes triggered by sustained seizure activity may occur as a continuum, last longer than formation of oxidative lipids and although not evident by our markers, may already be in progress when the peak increases in F2-IsoPs and F4-NeuroPs occur. Although the purpose of the present study was to investigate dynamic changes in lipid peroxidation and dendritic structures immediately after seizures, future studies over the longer period should be able to determine the long-term time course of these spine and dendritic changes. It is very likely that the spine loss seen in the present study is the initial phase of more chronic spine loss and progressive neurodegeneration reported in other studies (Muller et al., 1993; Multani et al., 1994; Isokawa and Levesque, 1991; Jiang et al., 1998; Zeng et al., 2007).
Another important free radical related to lipid peroxidation is NO. NO is synthesized by hippocampal pyramidal cells from L-arginine by NO synthases (NOS), with the accompanying release of citrulline (Wendland et al., 1994; Burette et al., 2002). There are three different isoenzymes of NOS, neuronal (nNOS), inducible (iNOS), and endothelial (eNOS). A major stimulus for NO production is elevation in intracellular Ca2+, which binds to calmodulin, resulting in the activation of constitutive NOS (nNOS and eNOS). The neurotoxicity data suggest that NOS activity increases during kainic acid (KA)-induced excitotoxicity (Parathath et al., 2006). It has been suggested that NO is involved in glutamate receptor-mediated neurotoxicity by decreasing intracellular ATP levels. There are two possible mechanisms which might be responsible for energy depletion caused by NO in neuronal cells: one is the prolonged activation of poly-(ADP ribose) polymerase (PARP), which can be activated by NO (Zhang et al., 1994), leading to depletion of ATP. The other mechanism is the inhibition of the mitochondrial complexes, leading to diminished ATP production. NO was reported to inhibit complexes II and III (Bolanos et al., 1994), as well as complex IV (Lizasoain et al., 1996) in neuron-derived mitochondria. It was also reported that NO inhibits neuronal energy production in cultured hippocampal neurons (Brorson et al., 1999), leading to rapid ATP depletion. In addition, increased production of NO in the presence of the superoxide anion radical (O2−) may generate peroxynitrite radical (OONO−) (Montine et al., 2002; Milatovic et al., 2002), a potent inducer of lipid peroxidation.
Our in vivo data established that KA-induced a significant increase in citrulline concentrations 30 min following the injection (Fig. 3). Although we did not determine whether increased citrulline originated from a combination of NOS isozymes or one in particular, our data are in agreement with the results from the models of anticholinesterase toxicity and activated innate immunity (Gupta et al., 2007; Milatovic et al., 2003; 2004) and indicate that a subset of NOS activity also contribute to cerebral oxidative damage in the model of KA-induced excitotoxicity.
An additional goal of this study was to determine whether suppression of lipid peroxidation prevents neurodegeneration of pyramidal neurons in CA1 hippocampal area in the model of KA-induced excitotoxicity. We tested the efficacy of the spin trapping agent, PBN and the antioxidant tocopherol. Protective effects of these agents are described in experimental models of brain ischemia/reperfusion (Phillis and Clough-Helfman, 1990; Carney and Floyd,1991; Gido et al., 1997; Fetcher et al., 1997), excitotoxicity (Cheng and Sun,1994; Lancelot et al., 1997; Milatovic et al., 2002), inhibition of nitric oxide synthase induction (Krishna et al., 1996; Miyajima and Kotake, 1995), and in different models of seizures (He et al., 1997; Thomas et al., 1997). Additional findings also corroborate that PBN effectively prevents neurodegeneration in Parkinson's (Fallon et al., 1997; Frederiksson et al., 1997; Sack et al., 1996), Alzheimer's disease and anticholinesterase neurotoxicity (Sack et al., 1996; Gupta et al., 2001a; Gupta et al., 2001b). Thus, PBN has been proven to rescue neurons in multiple experimental injury models. Alpha-tocopherol or PBN alone did not alter basal F4-NeuroPs levels or dendritic arborization (not shown). However, Vit E effectively reduced KA-induced lipid peroxidation and PBN suppressed KA-induced increases in cerebral and neuronal markers of oxidative damage, F2-IsoPs and F4-NeuroPs, respectively (Figs. 1 and 2). Importantly, we showed that Vit E and PBN completely suppressed both reduction in dendrite length and reduction in spine density of pyramidal neurons from CA hippocampal area from KA exposed mice (Figs. 4 and 5). We observed close concordance between these results showing that protected cerebrum from neuronal oxidative damage also protected hippocampal CA1 pyramidal neurons from dendritic degeneration. These agents did not alter kainate seizure severity, indicating that the protective effect of Vit E and PBN is most likely mediated by scavenging ROS and thus preventing lipid peroxidation and consequent neuronal damage, and not by a specific effect on seizures per se. One limitation to the potential therapeutic application is the drugs need to be administered prophylactically before the onset of seizures to be effective. Future research should address the efficacy of these agents when administered at higher concentrations either during or possibly even after seizures, in preventing seizure-induced oxidative and dendritic changes and potentially reducing resultant neurocognitive deficits. Next, we extended our studies to ibuprofen, a NSAID with some COX-independent actions (Hamburger and McCay, 1990; Asanuma et al., 2001; Weggen et al., 2001). We have previously showed that ibuprofen is at least an order of magnitude more potent than naproxen or acetylsalicylic acid in suppressing neuronal oxidative damage and degeneration of the dendritic system in the model of directly activated glial innate immunity (Milatovic et al., 2004). Analogous to the tested antioxidants, ibuprofen completely suppressed lipid peroxidation and protected both dendrite length and spine density (Figs. 4 and 5) from the degenerative consequences of icv KA. Several studies have found a marked expression of cerebral cyclooxygenase mRNA and protein following the systemic administration of KA (Sanz et al., 1997; Sandhya et al., 1998; Hashimoto et al., 1998). While both COX-1 and COX-2 immunoreactivities are present in hippocampal neuronal populations (Breder et al., 1995, 1992), it has been shown that neuronal overexpression of inducible COX-2 potentiate KA-induced seizures in human COX-2 transgenic mice (Kelley et al., 1999) and stimulate neuronal excitability immediately after seizures by synthesizing PGE2. These findings suggest that COX inhibitors could prevent seizure-induced neuronal damage by fully blocking PGE2 synthesis (Takemya et al., 2006). In addition, another study showed that induction of COX expression paralleled formation of ROS indicating that COX may be involved in pathways leading to neuronal damage (Tocco et al., 1997). Since ibuprofen did not alter kainate seizure severity in our study, its neuroprotective effect is likely to be due to a decreased susceptibility of neurons to the effects of seizures. While the mechanisms associated with NSAID suppression of oxidative damage and neurodegeneration need to be further probed, it is possible that its neuroprotective effects are COX independent, residing in its antioxidant activity (Asanuma et al., 2001; Hamburger et al., 1990).
In summary, our data demonstrate that lipid peroxidation is closely associated with dendritic degeneration following KA-induced excitotoxic injury. Furthermore, the data indicate that neuronal oxidative damage is an early event in seizure activity. Suppression of oxidative damage and dendritic degeneration by PBN, Vit E and the NSAID, ibuprofen merits further evaluation of their therapeutic efficacy in preventing severe damage in acute neurological disorders.
Acknowledgment
This study was supported by grants from NIH: NS057223 (DM), NIEHS07331 (MA) and AG05136, AG05144 (TM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anthony DC, Montine TJ, Graham DG. In: Casarett and Doull's Toxicology: The Basic Science of Poisons. Klaasen CD, editor. McGraw-Hill; New York: 2001. p. 535. [Google Scholar]
- Asanuma M, Nishibayashi-Asanuma S, Miyazaki I, Kohno M. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001;76:1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Rodino P, Paoletti M, Arabia A, Massoud R, Nistico G. Systemic administration of lithium chloride and tacrine but not kainic acid augments citrulline content of rat brain. Eur J Pharmacol. 1995;294:341–344. doi: 10.1016/0014-2999(95)00689-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42:5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- Burette A, Zabel U, Weinberg RJ, Schmidt HH, Valtschanoff JG. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J Neurosci. 2002;22:8961–8970. doi: 10.1523/JNEUROSCI.22-20-08961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neuro. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol. 1992;322:409–438. doi: 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Manzolillo PA, Miller RJ. Ca2+ entry via AMPA/KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J Neurosci. 1994;14:187–197. doi: 10.1523/JNEUROSCI.14-01-00187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic Biol Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Carney JM, Floyd RA. Protection against oxidative damage to CNS by alpha-phenyl-tert-butylnitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavengers. J Mol Neurosci. 1991;3:47–57. doi: 10.1007/BF02896848. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Sun AY. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem Res. 1994;19:1557–1564. doi: 10.1007/BF00969006. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock HR, Tong X, Hargreaves IP. Mitochondrial dysfunction associated with neuronal death following status epilepticus in rat. Epilepsy Res. 2002;48:157–168. doi: 10.1016/s0920-1211(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. The effects of oxidative stress on the brain. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Progressive cognitive decline in adolescents and adults with epilepsy. Prog Brain Res. 2002;135:399–407. doi: 10.1016/S0079-6123(02)35037-4. [DOI] [PubMed] [Google Scholar]
- Dong H, Csernansky CA, Goico B, Csernansky JG. Hippocampal neurogenesis following kainic acid-induced apoptosis in neonatal rats. J Neurosci. 2003;23:1742–1749. doi: 10.1523/JNEUROSCI.23-05-01742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F, Obenaus A, Schweitzer J, Wuarin J. Functional significance of hippocampal plasticity in epileptic brain: electrophysiological changes of the dentate granule cells associated with mossy fiber sprouting. Hippocampus. 1994;4:259–265. doi: 10.1002/hipo.450040306. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LMT, Handran SD, Rothman SM, Lin TS, Goldberg MP, Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurology. 1995;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Fallon J, Mathews RT, Hyman BT, Beal MF. MPP+ produces progressive neuronal degeneration which is mediated by oxidative stress. Exp Neurol. 1997;144:193–198. doi: 10.1006/exnr.1997.6416. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Yi Ong W, Lu XR, Halliwell B, Horrocks LA. Neurochemical consequences of kainate-induced toxicity in brain: involvement of arachidonic acid release and prevention of toxicity by phospholipase A(2) inhibitors. Brain Res Rev. 2001;38:61–78. doi: 10.1016/s0169-328x(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Fetcher LD, Liu Y, Pearce TA. Cochlear protection from carbon monoxide exposure by free radical blockers in the guinea pig. Toxicol Appl Pharmacol. 1997;142:47–55. doi: 10.1006/taap.1996.8027. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Velazquez JLP, Hwang PA. Free radical production correlates with cell death in an in vitro model of epilepsy. Eur J Neurosci. 2000;12:1431–1439. doi: 10.1046/j.1460-9568.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- Frederiksson A, Eriksson P, Archer T. MPTP-induced deficits in motor activity: neuroprotective effects of the spin trapping agents, alpha-phenyl-tert-butylnitrone (PBN). J Neural Trans. 1997;104:579–592. doi: 10.1007/BF01291877. [DOI] [PubMed] [Google Scholar]
- Gido G, Kristian T, Siesjo BK. Extracellular potassium in a neocortical cone area after transient focal ischemia. Stroke. 1997;28:206–210. doi: 10.1161/01.str.28.1.206. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, Dettbarn W-D. Nitric oxide (NO) modulates high-energy phosphates in brain regions of rats intoxicated with DFP or carbofuran: prevention by PBN or vitamin E. Archives of Toxicology. 2001;75:346–356. doi: 10.1007/s002040100249. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, Dettbarn W-D. Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: Protection by antioxidants. Neurotoxicology. 2001a;22:271–282. doi: 10.1016/s0161-813x(01)00013-4. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, Zivin M, Dettbarn W-D. Seizure-induced changes in energy metabolites and effects of N- tert-buty-α-phenylnitrone (PBN) and vitamin E. Pfluger's Arch Eur J Physiol. 2001b;440:R160–R162. doi: 10.1007/s004240000047. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Dettbarn W-D. Prevention of kainic acid seizures-induced changes in levels of nitric oxide and high-energy phosphates by 7-nitroindazole in rat brain regions. Brain Res. 2003;981:184–192. doi: 10.1016/s0006-8993(03)03034-8. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic S, Dettbarn W-D, Aschner M, Milatovic D. Neuronal oxidative injury and dendritic damage induced by carbofuran: Protection by memantine. Toxico Appl Pharmacol. 2007;219:97–105. doi: 10.1016/j.taap.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Hamburger SA, McCay PB. Spin trapping of ibuprofen radicals: evidence that ibuprofen is a hydroxyl radical scavenger. Free Rad Res. 1990;9:337–342. doi: 10.3109/10715769009145692. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Watanabe K, Nishimura T, Iyo M, Shirayama Y, Minabe Y. Behavioral changes and expression of heat shock protein HSP-70 mRNA, brain-derived neurotrophic factor mRNA, and cyclooxygenase-2 mRNA in rat brain following seizures induced by systemic administration of kainic acid. Brain Res. 1998;804:212–223. doi: 10.1016/s0006-8993(98)00708-2. [DOI] [PubMed] [Google Scholar]
- He QP, Smith ML, Li PA, Siesjo BK. Necrosis of the substantia nigra, pars reticulate, influorothyl-induced status epilepticus is ameliorated by the spin trap alpha-phenyl-N-tertbutylnitrone. Free Rad Biol Med. 1997;22:917–922. doi: 10.1016/s0891-5849(96)00478-9. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Buchheim K, Gabriel S. Cell death and metabolic activity during epileptiform discharges and status epilepticus in the hippocampus. Prog Brain Res. 2002;135:197–210. doi: 10.1016/S0079-6123(02)35019-2. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991;132:212–216. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna MC, Russo A, Mitchell JB. Do nitroxide antioxidants act as scavengers of O2 or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Goussakov IV, Beck H. Altered mitochondrial oxidative phosphorylation in hippocampal slices of kainate-treated rats. Brain Res. 1999;826:236–242. doi: 10.1016/s0006-8993(99)01279-2. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lancelot E, Revaud ML, Boulee RG. Alpha-N-tert-butylnitone attenuates excitotoxicity in rat striatum by preventing hydroxyl radical accumulation. Free Rad Biol Med. 1997;23:1031–1034. doi: 10.1016/s0891-5849(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors T. Associative memory mormation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Stoodley M. Pathophysiology of cerebral ischaemia. Neurol Med Chirurg (Tokyo) 1998;38:1–11. doi: 10.2176/nmc.38.1. [DOI] [PubMed] [Google Scholar]
- Marini H, Altavilla D, Bellomo M, Adamo EB, Marini R, Laureanti RF, Bonaccorso MC, Seminara P, Passaniti M, Minutoli L, Bitto A, Calapai G, Squadrito F. Modulation of IL-1 beta gene expression by lipid peroxidation inhibition after kainic acid-induced rat brain injury. Exp Neuro. 2004;188:178–186. doi: 10.1016/j.expneurol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Dettbarn W-D. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Research. 2002;957:330–337. doi: 10.1016/s0006-8993(02)03669-7. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem. 2003;87:1518–1526. doi: 10.1046/j.1471-4159.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine K, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immunity response in vivo. J Neuroinflamm. 2004;1:1–20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of cerebral oxidative damage from excitotoxicity and innate immune response in vivo by α- or γ- tocopherol. Journal of Chromatography B. 2005;827:88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Miyajima T, Kotake Y. Spin trap phenyl-N-tert-butylnitone (PBN) inhibits induction of nitric oxide synthase in endotoxin-induction in mice. Free Rad Biol Med. 1995;22:463–470. doi: 10.1016/s0891-5849(96)00391-7. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Milatovic D, Gupta RC, Morrow JD, Breyer R. Neuronal oxidative damage from activated innate immunity in EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ, II, Morrow JD, Montine TJ. Chem Phys Lipids. 2004;128:117. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Muller M, Gahwiler BH, Rietschin L, Thompson SM. Reversible loss of dendritic spines and altered excitability after chronic epilepsy in hippocampal slice cultures. Proc Natl Acad Sci USA. 1993;90:257–261. doi: 10.1073/pnas.90.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani P, Myers RH, Blume HW, Schomer DL, Sotrel A. Neocortical dendritic pathology in human partial epilepsy: a quantitative Golgi study. Epilepsia. 1994;35:728–736. doi: 10.1111/j.1528-1157.1994.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Butler T, Kang DE, Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic A[beta]42 independently of cyclo-oxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–74. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- Parathath S, Parathath S, Tsirka S. Nitric oxide mediates neurodegeneration and breakdown of the blood brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Patel MN. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res. 2002;36:1139–1146. doi: 10.1080/1071576021000016391. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang LP, Roberts LJ. Enhanced hippocampal F2-isoprostane formation following kainate-induced seizures. J Neurochem. 2001;79:1065–1069. doi: 10.1046/j.1471-4159.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Clough-Helfman C. Protection from cerebral ischemic injury in gerbils with the spin trap agent N-tert-butyl-alpha-phenylnitrone (PBN). Neurosci Lett. 1990;116:315–319. doi: 10.1016/0304-3940(90)90093-o. [DOI] [PubMed] [Google Scholar]
- Sack CA, Socci DJ, Crandall BM, Arendash GW. Antioxidant treatment with phenyl-alpha-tert-butylnitrone (PBN) improves the cognitive performance and survival of aging rats. Neurosci Lett. 1996;205:181–184. doi: 10.1016/0304-3940(96)12417-4. [DOI] [PubMed] [Google Scholar]
- Sandhya TL, Ong WY, Horrocks LA, Farooqui AA. A light and electron microscopic study of cytoplasmic phospholipase A2 and cyclooxygenase-2 in the hippocampus after kainate lesions. Brain Res. 1998;788:223–231. doi: 10.1016/s0006-8993(97)01552-7. [DOI] [PubMed] [Google Scholar]
- Sanz O, Estrada A, Ferrer I, Planas AM. Differential cellular distribution and dynamics of HSP70, cyclooxygenase-2, and c-Fos in the rat brain after transient focal ischemia or kainic acid. Neuroscience. 1997;80:221–232. doi: 10.1016/s0306-4522(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ohlweiler DF, Taylor VL, Schmidt CJ. Radical trapping and inhibition of iron-dependent CNS damage by cyclic nitrone spin traps. J Neurochem. 1997;68:1173–1182. doi: 10.1046/j.1471-4159.1997.68031173.x. [DOI] [PubMed] [Google Scholar]
- Tocco G, Freire-Moar J, Schreiber SS, Sakhi SH, Aisen PS, Pasinetti GM. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: implications for Alzheimer's disease. Exp Neurol. 1997;144:339–349. doi: 10.1006/exnr.1997.6429. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietzik CU, Findlay KA, Smith TE, Murphy MP, Butler T, Kang DE, Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic A[beta]42 independently of cyclo-oxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Wendland B, Schweize FE, Ryan TA, Nakane M, Murad F, Scheller RH, Tsien RW. Existence of nitric oxide synthase in rat hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1994;91:2151–2155. doi: 10.1073/pnas.91.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly (ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J Neurosci. 2007;27:11604–11613. doi: 10.1523/JNEUROSCI.0983-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]