Summary
The argyrophylic staining of the nucleolar organizer regions (AgNOR positive response) in interphase nuclei is often related directly to the cellular demand for ribosome biogenesis and is considered of relevance in studies of tumor pathology. Transformation of human breast epithelial MCF-10A cells by the c-Ha-ras oncogene results in altered growth, invasiveness and tumorigenicity in nude mice. Since ras transformation may be associated with a more intense nucleolar activity, we examined the influence of transfection by the Ha-ras oncogene on AgNOR staining response in MCF-10A cells. Following assessment of the AgNOR response with video image analysis, the AgNOR-positive areas and the AgNOR area/nuclear area ratio, but not the number of AgNOR aggregates or dots per nucleus, were found to be much higher after ras transformation. A role of the Ha-ras transformation on the nucleolar activity of the MCF-10A is thus suggested as assessed by the AgNOR staining. Based on data of the literature, it is also hypothesized that a decreased wild-type p53 level, possibly promoted by the ras transformation, may be associated with the increased AgNOR response.
Keywords: AgNOR, Ha-ras oncogene, Human breast epithelial cells, in vitro, p53, Image analysis
Introduction
In human cells, the abundance of argyrophylic interphase nucleolar organizer regions (AgNOR) is, in many cases, considered to be of diagnostic and prognostic significance in tumor pathology, because of its direct relationship to the rate of cell proliferation and other demands for ribosome biogenesis (Derenzini and Trere, 1991). The AgNOR technique reveals the presence of nuclear proteins such as phosphoprotein C3 (nucleolin), B23 (numatrin), and factors necessary for rDNA transcription and early rRNA processing (Mehta, 1995). The close functional association between the AgNOR response and the cell cycle, cell proliferation and the rate of rRNA transcription and synthesis means that the number and size of the AgNOR regions may vary with alterations in any of these parameters (Mehta, 1995).
The spontaneously immortalized, non-transformed human breast epithelial cell line, MCF-10A, derived from a human breast epithelial cell strain designated MCF-10M (Soule et al., 1990), has been used as an important model for studies on damage to DNA and on human breast cell transformation in vitro (Ciardiello et al., 1990; Basolo et al., 1991; Bianco et al., 1994; Mello et al., 1994; Yoon et al., 2002; Starcevic et al., 2003; Cuendet et al., 2004; Burdick et al., 2006; Cuendet and Bolton, 2006). These cells are susceptible to in vitro transformation under overexpression of several genes, including the c-Ha-ras oncogene (Ciardiello et al., 1990; Basolo et al., 1991; Mello et al., 1994; Starcevic et al., 2003).
The transformation of MCF-10A cells by transfection with the c-Ha-ras oncogene results in altered growth, invasiveness and tumorigenicity in nude mice (Basolo et al., 1991), and remodeling in chromatin supraorganization (Mello et al., 1994). However, no investigation has yet been performed on intensity of nucleolar activity, as demonstrated with the AgNOR staining, in these cells under ras transformation, when an increased demand for ribosome biogenesis is supposed to occur. Indeed, in a different cell line derived from MCF-10M cells (MCF-10F), the transformation by benzo[a]pyrene followed by transfection with the c-Ha-ras oncogene resulted in increased nucleolar areas and nucleolar/nuclear area ratio, as estimated in toluidine blue-stained preparations (Barbisan et al., 1998).
To test the hypothesis of ras transformation being associated with a more intense nucleolar activity in human breast epithelial cells in vitro, we examined the AgNOR staining profile (Rueschoff et al., 1990; Derenzini and Ploton, 1991; Vidal et al., 1994) of MCF-10A cells transfected with the c-Ha-ras oncogene in comparison with that of nontransformed MCF-10A cells.
Materials and methods
Cell lines and culture conditions
MCF-10A cells in passage 98 and MCF-10A cells transfected with the plasmid Homer 6 alone, containing the neomycin-resistance gene (MCF-10Aneo cell line), or with this vector plus a construct containing the c-Ha-ras oncogene (MCF-10AneoT cell line), as described previously (Basolo et al., 1991), were used. The cells were grown in Dulbecco’s minimal essential medium/F-12 medium (1:1) (Grand Island Biological Co.(GIBCO), Long Island, New York, USA) supplemented with 5% equine serum (GIBCO), 0.1 µg/mL cholera-toxin (ICN Biomedicals, Cleveland, OH, USA), 10 µg/mL insulin (GIBCO), 100 U/mL penicillin (GIBCO), 100 µg/mL streptomycin (GIBCO), 2.5 µg/mL amphotericin B (GIBCO), 0.5 µg/mL hydrocortisone (Sigma Chemical Co., St. Louis, MO, USA), and 0.02 µg/mL epidermal growth factor (Collaborative Research Inc., Palo Alto, USA), as reported previously (Soule et al., 1990; Basolo et al., 1991). The transfected cells were selected by their ability to grow and form colonies after three weeks at 37°C in a 5% CO2 atmosphere in the medium described above but containing 750 µg/mL geneticin (GIBCO)(Basolo et al., 1991).
Cell preparation and staining
Cells grown on coverslips were fixed in an absolute ethanol-acetic acid (3:1, v/v) mixture for 1 min, rinsed in 70% ethanol for 3–5 min, and air-dried. Three coverslips of each cell line were used. AgNOR staning was done as described by Rueschoff et al (1990) and Derenzini and Ploton (1991), preceded by treatment with a 1% Triton X-100 solution in the presence of 4 M glycerol for 15 min at 37°C (Vidal and Mello, 1995). Briefly, the cells were treated with a solution containing 2 volumes 50% aqueous silver nitrate (Merck, USA) and 1 volume 2% gelatin in 1% aqueous formic acid (v/v). The aqueous solutions were prepared with deionized water. The optimal staining time was found to be 10 min at 37°C. Immediately after the silver impregnation, the material was rinsed in deionized water, air dried, cleared in xylene and mounted in Canada balsam.
Image analysis
Zeiss-Kontron equipment (Oberkochen/Eching-Munich, Germany) was used for image acquisition, segmentation and featuring using a Kontron-IPS system and appropriate programs, according to the Kontron-IBAS 2000 instruction manual. The preparations were observed with a Zeiss Universal N microscope equipped with a Neofluar 100/1.30 objective, optovar 1.25, 1.3 condenser, and λ = 546 nm obtained with a Schott monochromator filter ruler. The images to be processed were fed from the microscope into a computer via a Hamamatsu C3077 (Hamamatsu Photonics, Japan) monochrome CCD video camera. In this investigation, 1 µm = 11.3 pixels. Different grey levels were converted to pseudocolorized images to facilitate the identification and discrimination of different areas of the image.
A macro developed by one of us (WP) provided quantitative morphological information on nuclear size and on the number and area of AgNOR-positive aggregates or dots. Since densitometric features were derived from whole nuclei as well as from distinct AgNOR-positive regions, two segmentation levels were used. The threshold levels for whole nuclear images and for AgNOR-positive areas were established based on previously determined grey level histograms of all image pixels of the MCF-10A cell nuclei. The same threshold levels were automatically used to analyze all the nuclei. Details of the image analysis procedure have been described elsewhere (Mello et al., 1994; Vidal et al., 1994). Although interactive image editing was possible while running the program, it was rarely required for the nuclei analyzed here.
Statistical analysis
All calculations and statistical analyses were done using the Minitab 12™ software (State College, PA, USA). For comparisons in which Anova was not applicable, nonparametric tests (Kruskal-Wallis, Mann-Whitney) for medians were used.
Results
Well-defined nuclear contours and conspicuous AgNOR-stained structures were detected in all of the cell nuclei. The nuclei stained pale yellow whereas the AgNOR dots or aggregates stained dark brown, as illustrated in Fig. 1a–c; 2a. Examples of nuclei that showed some of the steps of the Kontron-IPS system segmentation process were photographed from the color monitor, illustrated in Fig. 2b–g. Some AgNOR responsive dots outside the nucleoli and probably corresponding to Cajal bodies (Bilinski and Kloc, 2002; Niedojadlo and Gorska-Brylass, 2003) were occasionally found and also considered as positive regions. These are shown in Fig. 2c,f.
Figure 1.
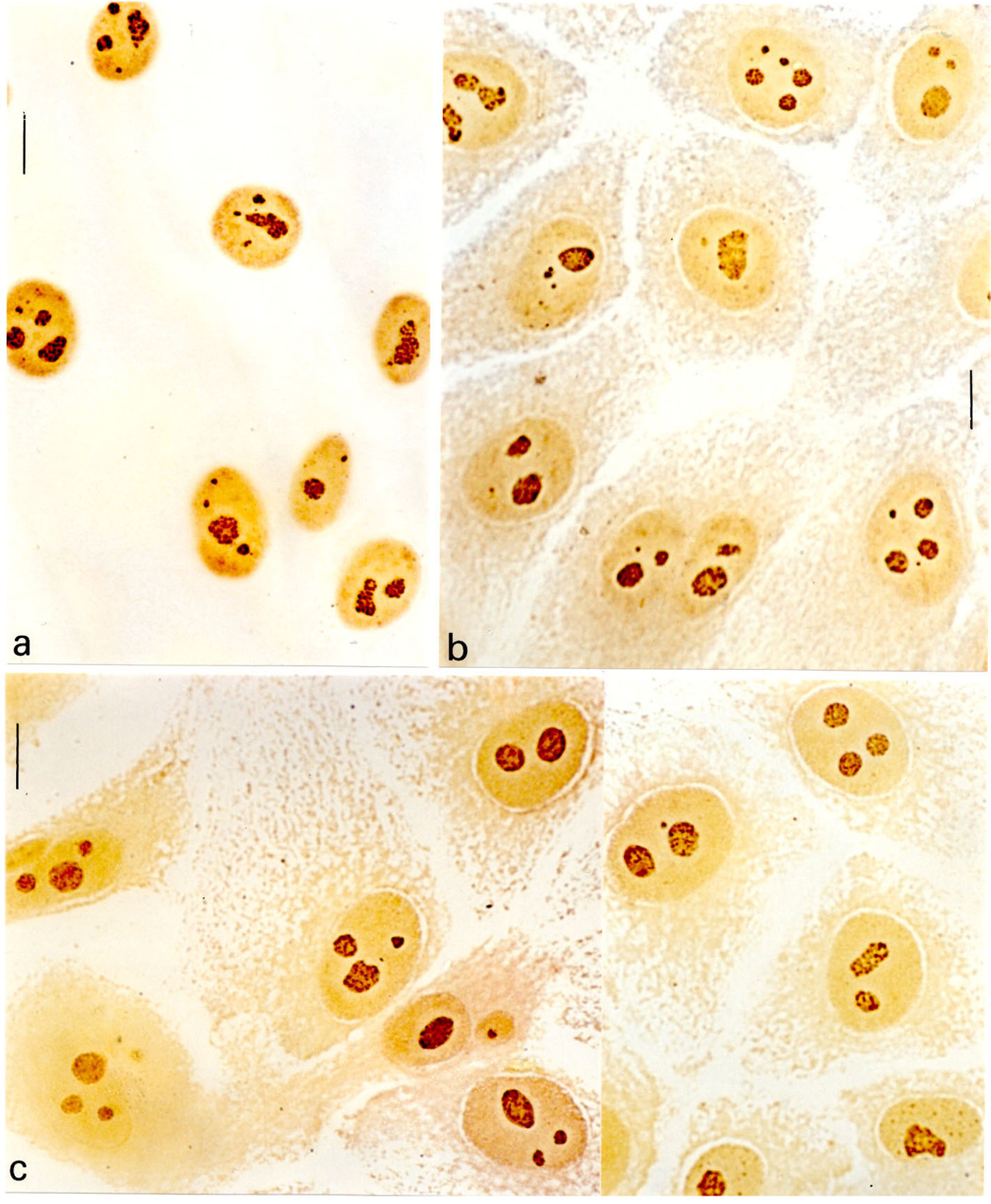
AgNOR staining in nucleoli (nu) of MCF-10A (a), MCF-10Aneo (b) and MCF-10AneoT (c) cells. Bar, 10 µm.
Figure 2.
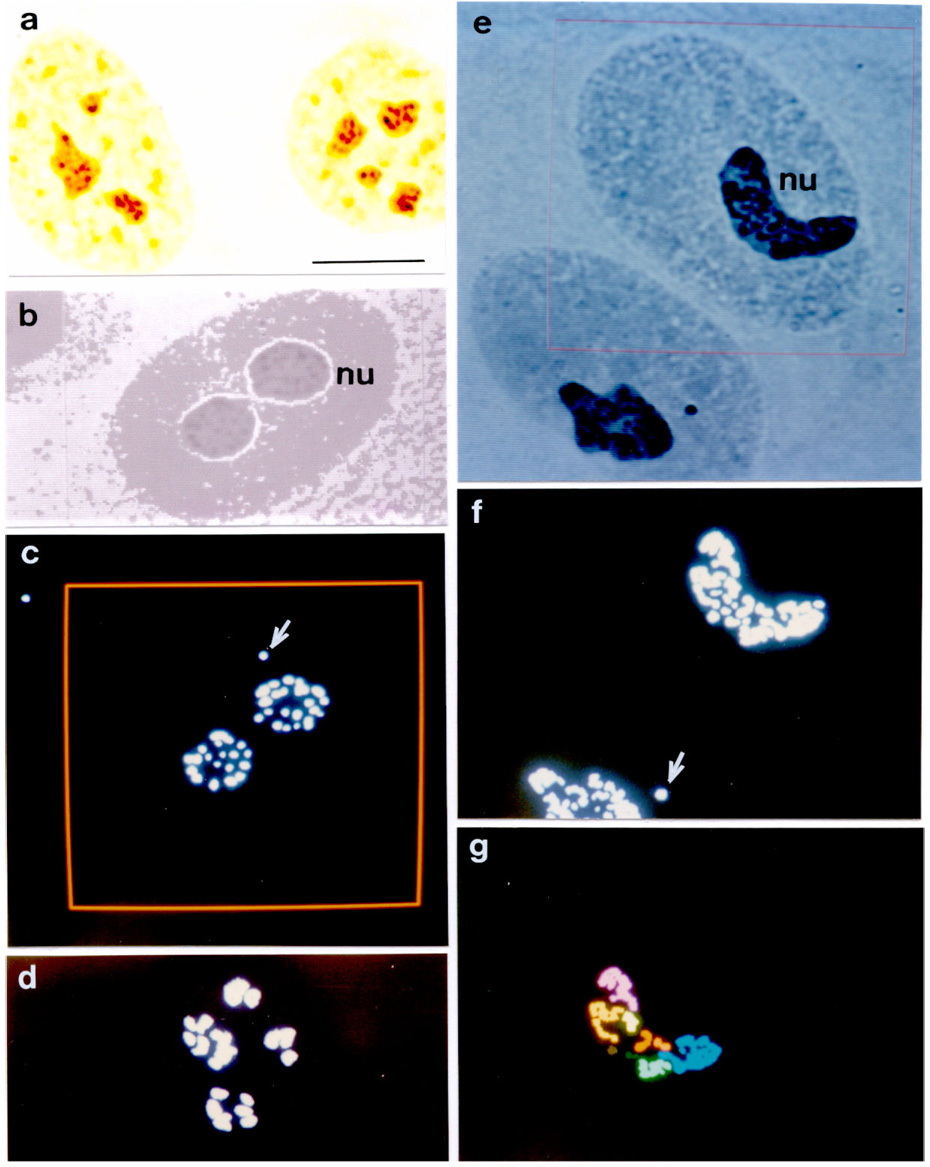
AgNOR staining in nucleoli (nu) of nontransformed and transformed MCF-10A cells. a. AgNOR response (black dots) in control MCF-10A cells: photomicrograph obtained directly from the microscope. Bar, 10 µm. b–g. Examples of some steps of the segmentation procedure and feature evaluation of the image analysis by Kontron-IPS system of AgNOR-stained particles or aggregates as photographed from the color monitor. b. Original image (TV on) of one MCF-10A cell nucleus. c. Grey level segmentation of the AgNOR-stained dots of the nucleus shown in b. The arrow indicates a positive dot in a putative Cajal body. The area under analysis is delimited by the red frame. d. Grey level segmentation of the AgNOR-stained dots of an MCF-10Aneo cell nucleus. e. Original image (TV on) of two MCF-10AneoT cell nuclei. The nucleus to be analyzed is inside the area delimited by the red frame. f. Grey level segmentation of the AgNOR-stained particles of the image shown in e. The arrow indicates a positive dot in a putative Cajal body. g. Identification of the AgNOR-stained image of the nucleus shown inside the frame area in e. Different pseudocolors identify aggregates of dots not in contact with each other.
Nuclei of different sizes were observed. Details are given in Table 1. In control MCF-10A cells, the smaller nuclei generally showed smaller AgNOR areas whereas larger nuclei presented larger AgNOR aggregates. In the other cells no correlation between the nuclear and AgNOR areas was detected. The abundance of AgNOR aggregates or dots was not correlated with the nuclear size or the AgNOR total area in any of the cells analyzed here. It is worth mentioning that the number of points considered as AgNOR dots or aggregates do not coincide with the number of nucleoli detected visually.
Table 1.
Correlation coefficient r for the total area (A) and number (B) of AgNOR-stained aggregates or dots in the nuclear area (C), and for the number of AgNOR-stained aggregates or dots (B) in the total AgNOR area (A) in the MCF-10A cell lines.
| Cells | n | Variable series | rand z(r) |
|---|---|---|---|
| MCF-10A | 111 | A × C | 0.24* |
| B × C | 0.06 | ||
| B × A | 0.02 | ||
| MCF-10Aneo | 91 | A × C | 0.16 |
| B × C | 0.13 | ||
| B × A | 0.00 | ||
| MCF-10neoT | 96 | A × C | 0.10 |
| B × C | 0.11 | ||
| B × A | 0.00 | ||
P < 0.05. In all cases, a significance at the P0.05 level required r values > 0.19.
The nuclear areas of cells transfected with the plasmid alone or with the plasmid plus the Ha-ras oncogene did not differ significantly from each other, but were larger than the nuclear areas of the nontransformed controls, detailed in Table 2. The AgNOR-positive areas and the AgNOR area/nuclear area ratio of MCF-10AneoT cells were significantly greater than those of the controls and of cells transfected with the plasmid alone. The frequency of AgNOR aggregates or dots counted in Ha-ras-transformed cells did not differ from that of control cells, but was smaller than in cells transfected with the plasmid alone.
Table 2.
Image analysis parameters for AgNOR-stained MCF-10A cell lines
| Cells | n | Nuclear areas (pixels2*) | AgNOR total areas (pixels2*) | AgNOR dots (no.) | AgNOR area/nuclear area ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | S | X | S | Median | X | S | Median | X | S | Median | ||
| MCF-10A | 111 | 20444a | 5307 | 1232a | 567 | 1269a | 5.3 | 2.8 | 4a | 6.1 | 2.8 | 6a |
| MCF-10Aneo | 91 | 22500b | 6706 | 1280a | 609 | 1214a | 6.4 | 2.5 | 6b | 5.9 | 3.0 | 6a |
| MCF-10AneoT | 96 | 22783b | 5967 | 2616 | 1319 | 2534b | 5.2 | 3.4 | 4a | 11.8 | 6.1 | 11b |
1 µm2 = 127.70 pixels2. Significant differences (P0.05) are indicated by different letters in a column (Anova for arithmetic means and Kruskal-Wallis or Mann-Whitney tests for medians. S, standard deviation; X, arithmetic mean.
Discussion
The results of this study indicate that Ha-ras transformation induces enhancement in the nucleolar activity of human breast epithelial MCF-10A cells, as measured by increased AgNOR-stained areas and AgNOR area/nuclear area ratios. The enhanced metabolic activity involved is certainly related to an increased demand for a rapid supply of ribosomes. The present findings also agree with the increase in nucleolar areas reported for toluidine blue-stained preparations of MCF-10F cells transformed by benzo[a]pyrene and transfected with the Ha-ras oncogene (Barbisan et al., 1998), and in nucleolar function as expressed in breast neoplasms reported in several studies (Helpap, 1989; van Diest et al., 1989, 1990; Derenzini and Trere, 1991; Belotti et al., 1997; Kruger et al., 2000; Kanna et al., 2001).
The image analysis parameters were more useful for discriminating MCF-10AneoT cells from MCF-10neo or MCF-10A cells than was the number of AgNOR aggregates or dots per nucleus. Indeed, there was considerable variability in the AgNOR areas relative to the nuclear size in MCF-10AneoT and MCF-10Aneo cells, in contrast to the significant correlation between AgNOR and nuclear areas in MCF-10A cells (control). The larger variability in the AgNOR areas of MCF-10AneoT cells may be related to that in the Feulgen-DNA content induced by the c-Ha-ras oncogene in these cells (Mello et al., 1994), probably as an effect of the genomic instability created by transfection with the mentioned oncogene. Indeed, nuclear polymorphism has been observed during malignant progression of primary human breast cancer (van Diest et al., 1989; Tajima et al., 1991).
The AgNOR response in human breast cancer has been suggested to be affected by the status of the oncosuppressor proteins p53 and pRb (Derenzini et al., 2004; Trere et al., 2004). In 71 human primary breast carcinomas with a mutated p53, as studied by Trere and co-workers (2004), the mean AgNOR area was greater than in 272 tumors with a normal (wild-type) p53. Presence of a wild-type p53 has been reported for nontransformed MCF-10A cells cultivated in several other laboratories (Bianco et al., 1994; Merlo et al., 1995; Cuendet et al., 2006). However, with progressively elevated Ras protein and increased cellular resistance to experimental oxidative DNA damage, a tumorigenic MCF-10A cell line (MCF10ATG3B) has shown a marked decrease (58%) in wild-type p53 protein levels (Starcevic et al., 2003). Although the p53 status for the MCF-10A cells used in this study has not been estimated, based on Starcevic and co-workers’ (2003) report, it is suggested that a decrease in the wild-type p53 levels may also have occurred under the Ha-ras oncogene transformation analyzed here. In addition, present results on increased AgNOR areas and AgNOR area/nuclear area ratios in Ha-ras-transformed MCF-10A cells would thus not be in disagreement with Trere et al (2004) consideration of an increase in AgNOR area response related to increase in levels of mutated p53 in human breast tumors.
The inclusion of the AgNOR-positive dots assumed to represent Cajal bodies in the evaluation of the overall nuclear silver-staining response in a few nuclei of control and transformed cells does not overlook the association of the AgNOR response and RNA metabolism, since these bodies have been considered as sites for the concentration of RNA-processing factors and elements of the splicing system (Bilinski and Kloc, 2002; Niedojadlo and Gorska-Brylass, 2003).
The transfection of the MCF-10A cells with the c-Ha-ras oncogene or the Homer 6 plasmid alone increased the nuclear area. A similar finding has been reported for these cells after the Feulgen reaction, and associated with a certain chromatin unraveling in response to transfection of the plasmid, an event which was more prominent in the MCF-10Aneo cells (Mello et al., 1994). It is thus possible that with the induced chromatin loosening in the MCF-10Aneo cell nuclei, their nucleolar organizer regions would become more separated from each other in comparison to those of the MCF-10A cells. Consequently, the accumulation of the proteins reactive to the silver staining in the NOR regions of the MCF-10Aneo cells, as discriminated by the image analysis software, would reveal a number of AgNOR-stained dots larger than that in MCF-10A cells. The same may have occurred in MCF-10AneoT cells. However, in this case there was an increase in AgNOR-reactive proteins resulting in a larger area being occupied and in the AgNOR-positive sites being discriminated individually as aggregates (Fig. 2 – different pseudocolors) and not as dots, on account of a more intense nucleolar activity. The usefulness of counting the number of AgNOR dots for this model should thus be disregarded.
Acknowledgments
This research was supported by grants from the Brazilian National Research and Development Council (CNPq), the São Paulo State Research Foundation (FAPESP), the Alexander von Humboldt Foundation, and the National Institutes of Health (grant RO1 CA67238).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maria Luiza S. Mello, Email: mlsmello@unicamp.br.
Benedicto C. Vidal, Email: camposvi@unicamp.br.
Jose Russo, Email: J_Russo@fccc.edu.
Wolfgang Planding, Email: ulrich@schenck.de.
Ulrich Schenck, Email: ulrich@schenck.de.
References
- Barbisan LF, Russo J, Mello MLS. Nuclear and nucleolar image analysis of human breast epithelial cells transformed by benzo[a]pyrene and transfected with the c-Ha-ras oncogene. Anal Cell Path. 1998;16:193–199. doi: 10.1155/1998/832901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, Pauley R, Momiki S, Caamano J, Klein-Szanto AJP, Koszalka M, Russo J. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- Bellotti M, Elsner B, Kahn A, Bezodnick L, Pisilli L, Greco P. Morphometric determination of AgNORs in breast carcinoma – Correlation with flow cytometry and proliferating cell nuclear antigen. Anal Quant Cytol Histol. 1997;19:139–144. [PubMed] [Google Scholar]
- Bianco C, Tortora G, Basolo F, Fiore L, Fontaninin G, Merlo G, Salomon DS, Bianco AR, Ciardiello F. Effects of mutant p53 genes on transformation of human mammary epithelial cells. Int J Oncol. 1994;4:1077–1082. [PubMed] [Google Scholar]
- Bilinski SM, Kloc M. Accessory nuclei revisited: the translocation of snRNPs from the germinal vesicle to the periphery of the future embryo. Chromosoma. 2002;111:62–68. doi: 10.1007/s00412-002-0186-4. [DOI] [PubMed] [Google Scholar]
- Burdick AD, Ivnitski-Steele ID, Lauer FT, Burchiel SW. PYK2 mediates anti-apoptotic AKT signaling in response to benzo[a]pyrene diol epoxide in mammary epithelial cells. Carcinogen. 2006;27:2331–2340. doi: 10.1093/carcin/bgl083. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, McGeady ML, Kim N, Basolo F, Hynes N, Langton BC, Yokozaki H, Saeki T, Elliott JW, Masui H. Transforming growth factor-alpha expression in human mammary epithelial cells transformed by an activated c-Ha-ras protooncogene but not by the c-neu protooncogene, and overexpression of the transforming growth factor-alpha complementary DNA leads to transformation. Cell Growth & Differ. 1990;1:407–420. [PubMed] [Google Scholar]
- Cuendet M, Bolton JL. Response of human mammary epithelial cells to DNA damage induced by 4-hydroxyequilenin: lack of p53-mediated G1 arrest. Chemico-Biol Interact. 2006:271–278. doi: 10.1016/j.cbi.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet M, Liu X, Pisha E, Li Y, Yao J, Yu L, Bolton JL. Equine estrogen metabolite 4-hydroxyequilenin induces anchorage-independent growth of human mammary epithelial MCF-10A cells: differential gene expression. Mut Res (Fund Mol Mech Mutagen) 2004;550:109–121. doi: 10.1016/j.mrfmmm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Derenzini M, Ploton D. Interphase nuclear regions in cancer cells. Int Rev Exp Path. 1991;32:149–192. doi: 10.1016/b978-0-12-364932-4.50008-3. [DOI] [PubMed] [Google Scholar]
- Derenzini M, Trere D. Importance of interphase nucleolar organizer regions in tumor pathology. Virchows Arch B- Cell Path. 1991;61:1–8. doi: 10.1007/BF02890399. [DOI] [PubMed] [Google Scholar]
- Derenzini M, Ceccarelli C, Santini D, Taffurelli M, Trere D. The prognostic value of the AgNOR parameter in human breast cancer depends on the pRb and p53 status. J Clin Path. 2004;57:755–761. doi: 10.1136/jcp.2003.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpap B. Nucleolar grading of breast cancer. Comparative studies on frequency and localization of nucleoli and histological stage, hormonal receptor status and lectin histochemistry. Virchows Arch A - Pathol Anat. 1989;415:501–508. doi: 10.1007/BF00718643. [DOI] [PubMed] [Google Scholar]
- Khanna AK, Ansari MA, Kumar M, Khanna A. Correlation between AgNOR count and subjective AgNOR pattern assessment score in cytology and histology of breast lumps. Anal Quant Cytol Histol. 2001;23:388–394. [PubMed] [Google Scholar]
- Kruger S, Stahlhut M, Muller H. Cell cycle-dependent AgNOR analysis in invasive breast cancer. Anal Quant Cytol Histol. 2000;22:358–363. [PubMed] [Google Scholar]
- Mehta R. The potential for the use of cell-proliferation and oncogene expression as intermediate markers during liver carcinogenesis. Cancer Lett. 1995;93:85–102. doi: 10.1016/0304-3835(95)03790-4. [DOI] [PubMed] [Google Scholar]
- Mello MLS, Lin TY, Russo J. Scanning microphotometry image analysis in breast epithelial cells. Anal Cell Path. 1994;7:301–319. [PubMed] [Google Scholar]
- Mello MLS, Vidal BC, Planding W, Schenck U. Image analysis: video system adequacy for the assortment of nuclear phenotypes based on chromatin texture evaluation. Acta Histochem Cytochem. 1994;27:23–31. [Google Scholar]
- Merlo GR, Basolo F, Fiore L, Duboc L, Hynes NE. P53-dependent and p53-independent activation of apoptosis in mammary epithelial cells reveals a survival function of EGF and insulin. J Cell Biol. 1995;128:1185–1196. doi: 10.1083/jcb.128.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedojadlo J, Gorska-Brylass A. New type of snRNP containing nuclear bodies in plant cells. Biol Cell. 2003;95:303–310. doi: 10.1016/s0248-4900(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Rueschoff J, Plate KH, Contractor H, Stern S, Timmermann R, Thomas C. Evaluation of nucleolus organizer regions (NORs) by automatic image analysis: a contribution to standardization. J Path. 1990;161:113–118. doi: 10.1002/path.1711610205. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of an immortalized human breast epithelial cell line MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Starcevic SL, Diotte NM, Zukowski KL, Cameron MJ, Novak RF. Oxidative DNA damage and repair in a cell lineage model of human proliferative breast disease (PBD) Toxicol Sci. 2003;75:74–81. doi: 10.1093/toxsci/kfg154. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Ishige H, Kondo Y. Morphometric studies for objective diagnosis of intraductal carcinoma of the breast. Acta Pathol Jpn. 1991;41:604–609. doi: 10.1111/j.1440-1827.1991.tb02528.x. [DOI] [PubMed] [Google Scholar]
- Trere D, Ceccarelli C, Montanaro L, Tosti E, Derenzini M. Nucleolar size and activity are related to pRB and p53 status in human breast cancer. J Histochem Cytochem. 2004;52:1601–1607. doi: 10.1369/jhc.4A6454.2004. [DOI] [PubMed] [Google Scholar]
- van Diest PJ, Mouriquand J, Schipper NW, Baak JPA. Prognostic value of nucleolar morphometric variables in cytological breast cancer specimens. J Clin Path. 1990;43:157–159. doi: 10.1136/jcp.43.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diest PJ, Risse KJ, Schipper NW, Baak JPA. Comparison of light microscopic grading and morphometric features in cytological breast cancer specimens. Path Res Pract. 1989;185:612–616. doi: 10.1016/s0344-0338(89)80204-3. [DOI] [PubMed] [Google Scholar]
- Vidal BC, Mello MLS. Re-evaluating the AgNOR staining response in Triton X-100-treated liver cells by image analysis. Anal Cell Path. 1995;9:39–43. [PubMed] [Google Scholar]
- Vidal BC, Planding W, Mello MLS, Schenck U. Quantitative evaluation of AgNOR in liver cells by high-resolution image cytometry. Anal Cell Path. 1994;7:27–41. [PubMed] [Google Scholar]
- Yoon DS, Wersto RP, Zhou WB, Chrest FJ, Garrett ES, Kwon TK, Gabrielson E. Variable levels of chromosomal instability and mitotic spindle checkpoint defects in breast cancer. Am J Path. 2002;161:391–397. doi: 10.1016/S0002-9440(10)64194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


