Abstract
There is significant pharmacological and behavioral evidence that group I metabotropic glutamate receptors (mGluR1a and mGluR5) in the nucleus accumbens play an important role in the neurochemical and pathophysiological mechanisms that underlie addiction to psychostimulants. To further address this issue, we undertook a detailed ultrastructural analysis to characterize changes in the subcellular and subsynaptic localization of mGluR1a and mGluR5 in the core and shell of nucleus accumbens following acute or chronic cocaine administration in rats. After a single cocaine injection (30mg/kg) and 45 minutes withdrawal, there was a significant decrease in the proportion of plasma membrane-bound mGluR1a in accumbens shell dendrites. Similarly, the proportion of plasma membrane-bound mGluR1a was decreased in large dendrites of accumbens core neurons following chronic cocaine exposure (i.e. 1 week treatment followed by three weeks withdrawal). However, neither acute nor chronic cocaine treatments induced significant change in the localization of mGluR5 in accumbens core and shell, which is in contrast with the significant reduction of plasma membrane-bound mGluR1a and mGluR5 induced by local intra-accumbens administration of the group I mGluR agonist, DHPG. In conclusion, these findings demonstrate that cocaine-induced glutamate imbalance (Smith et al., 1995; Pierce et al., 1996; Reid et al., 1997) has modest effects on the trafficking of group I mGluRs in the nucleus accumbens. These results provide valuable information on the neuroadaptive mechanisms of accumbens group I mGluRs in response to cocaine administration.
Keywords: immunogold, mGluR1a, mGluR5, nucleus accumbens, cocaine, DHPG
INTRODUCTION
Changes in glutamate neurotransmission in the nucleus accumbens is a key neuroadaptive mechanism in response to acute or chronic cocaine exposure (Robinson and Berridge, 2003). In vivo microdialysis experiments have shown a significant increase in extracellular glutamate levels that peaks approximately 40 minutes following acute systemic cocaine injections in rats (Smith et al., 1995; Reid et al., 1997). In contrast, one week of chronic cocaine exposure, which leads to behavioral sensitization, followed by three weeks withdrawal, reduces basal extracellular glutamate levels by half compared to saline-treated animals (Pierce et al., 1996; Baker et al., 2003). These cocaine-induced effects on extracellular glutamate release lead to rapid α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor subunit internalization after acute cocaine and in contrast, increased AMPA receptor surface expression following chronic cocaine-induced behavioral sensitization (Boudreau and Wolf, 2005; Boudreau et al., 2007).
Metabotropic glutamate receptors (mGluRs) are divided into three classes based on pharmacological and structural properties. Group I mGluRs (mGluR1 and 5) are coupled to Gq and activate phospholipase C, increasing intracellular calcium and activating protein kinase C, while Group II (mGluR2 and 3) and Group III (mGluRs 4, 6, 7, and 8) mGluRs are coupled to Gi and inhibit cAMP formation (for review see (Conn and Pin, 1997)). Both group I mGluRs are widely distributed and partly co-localized in the nucleus accumbens (Mitrano and Smith, 2007) where they likely mediate some of the neuroadaptive changes associated with repeated cocaine administration. First and foremost is the fact that mGluR5 knockout mice do not self-administer cocaine and have decreased locomotor activity in response to cocaine administration despite a significant increase in dopamine release in the nucleus accumbens (Chiamulera et al., 2001). In line with these observations, systemic administration of mGluR5 antagonist reduces cocaine self administration in both rats and monkeys and attenuates the rewarding effects of cocaine in mice (McGeehan and Olive, 2003; Kenny et al., 2005; Lee et al., 2005). On the other hand, pretreatment with mGluR1 antagonist reduces behavioral sensitization to chronic cocaine administration in rats (Dravolina et al., 2006).
At the cellular level, modest, but significant and opposite, changes in mGluR5 protein and mRNA expression have been reported in the rat accumbens after chronic cocaine exposure and three weeks withdrawal (Ghasemzadeh et al., 1999; Swanson et al., 2001). A single in vivo exposure to cocaine abolishes endocannabinoid mGluR5-mediated retrograde long-term depression (LTD) and decreases the surface expression of mGluR5 in the mouse accumbens (Fourgeaud et al., 2004).
Most G-protein coupled receptors (GPCRs), including group I mGluRs, have the ability to travel to and from the plasma membrane in response to changes in extracellular levels of receptor agonists (see Gainetdinov et al., 2004 for review). In cell cultures and mice brain slices, mGluR1a and mGluR5 undergo agonist-stimulated internalization and endocytosis (Dale et al., 2001; Mundell et al., 2001) which, in some cases, was correlated with decreased group I mGluR-mediated physiological effects (Fourgeaud et al., 2004). However, there has been no in vivo study looking at changes in the trafficking of group I mGluRs following glutamate or receptor agonist stimulation in the mammalian brain.
Therefore, to address this issue, we undertook an in-depth ultrastructural analysis of changes in the subcellular and subsynaptic localization of mGluR1a and mGluR5 in the core and shell of the nucleus accumbens in rats acutely or chronically treated with cocaine and in animals that received local intracerebral injection of group I mGluR agonist.
EXPERIMENTAL PROCEDURES
Animals and cocaine treatments
Thirty-three male Sprague Dawley rats weighing 225–250 grams upon arrival were used for the cocaine treatment experiments in this study. All procedures were approved by the animal care and use committee of Emory University and conform to the U.S. National Institutes of Health guidelines. The chronic cocaine administration regimen used in this study was developed by Kalivas and colleagues (Kalivas et al., 1988) and is routinely used to induce behavioral sensitization to psychostimulants in rats. In brief, chronically treated rats were given either an i.p. injection of 0.9% saline or 15mg/kg cocaine on days 1 and 7 of the treatment period and locomotor activity was measured every 5 minutes for 2 hours by an IBM computer using Digipro software in photocell cages (Omnitech Electronics) equipped with 32 photobeams 5 cm above the floor. On days 2–6 of the treatment period, rats were given an i.p. injection of either 0.9% saline or 30mg/kg cocaine. Following the one-week treatment period, rats were left in their home cages for three weeks after the last injection, as a withdrawal period, and then sacrificed. Only rats that showed behavioral sensitization (i.e. animals that displayed a statistically significant increase in total locomotor activity on day 7 compared to day 1, data not shown) were used in the chronically cocaine-treated group.
Two groups of acutely treated rats were given a single i.p. injection of either 0.9% saline or 30mg/kg cocaine, put back in their home cages and then sacrificed either 45 minutes or 24 hours later. These time points were chosen based on previous studies that showed glutamate levels peak at approximately 40 minutes following acute cocaine (Smith et al., 1995; Reid et al., 1997) and changes in mGluR5 expression 24 hours following a cocaine exposure (Fourgeaud et al., 2004).
Tissue preparation
For perfusion, all animals were deeply anesthetized with a cocktail of ketamine (60–100mg/kg, i.p.) and dormitor (0.1mg/kg, i.p.). The animals were then transcardially perfused with cold oxygenated Ringer’s solution followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (0.1M; pH 7.4). Following perfusion, brains were removed from the skull, post-fixed in 4% paraformaldehyde for 24 hours, cut into 60-μm-thick sections using a vibrating microtome and stored in phosphate-buffered saline (PBS; 0.01M, pH 7.4) at 4°C until further processing for immunocytochemistry. Prior to the immunocytochemical reactions, all sections were put into a 1% sodium borohydride solution for 20 minutes and then washed with PBS.
Primary Antibodies
A commercially available monoclonal antibody against calbindin-D28k (Sigma, St. Louis, MO; Cat# C-9848, Lot# 082k4879) was used at a concentration of 1:5000 to distinguish between the accumbens shell and core. The calbindin-D28k antibody is derived from CB-955 hybridomas produced by fusion of mouse myeloma cells and splenocytes from BALB/c mice that were immunized with purified bovine kidney calbindin-D28k. The specificity of this antibody has been demonstrated through preadsorption immunohistochemical assays that abolish calbindin labeling (Celio, 1990), through Western blot analysis of rat brain tissue which shows a distinct band at 28kD (Miyata et al., 2000) and through immunohistochemistry which shows calbindin immunostaining in brain regions known to express a significant level of calbindin-D28k mRNA (Celio et al., 1990; Miyata et al., 2000; Winsky et al., 1989).
To localize mGluR1a, an affinity-purified rabbit polyclonal antibody against the C-terminus of rat mGluR1a (PNVTYASVILRDYKQSSSTL) conjugated to KLH with glutaraldehyde was used at a concentration of 1:1000 (Chemicon, Temecula, CA; Cat# AB1551, Lot# 21100471). In Western blot analysis by the manufacturer, this antibody labels a single band of ~140kD. Previous studies from our lab and others have used a combination of knockout mice, transfected HEK-293 cells, and preadsorption to determine the specificity of this mGluR1a antiserum. These studies showed that brain tissue from mGluR1a knockout mice did not display any specific mGluR1a labeling compared to wild-type. In addition, immunoblotting of cells transfected with mGluR1a, but not mGluR5, labeled a band of 140kD (Kuwajima et al., 2004). Preadsorption studies in rat retina cells abolished mGluR1a labeling (Koulen et al., 1997).
An affinity-purified synthetic rabbit polyclonal antibody against the C-terminus of mGluR5 with a lysine added to the N-terminus (KSSPKYDTLIIRDYTNSSSSL) in a concentration of 1:5000 (Upstate Biotechnology, Lake Placid, NY; Cat# 06-451, Lot# 27884) was used to label mGluR5. According to the manufacturer’s immunoblot analysis, the mGluR5 antibody labels a band of ~130kD. Specificity of the mGluR5 antibody has been shown in previous studies from our laboratory using knockout mice, transfected cells and homogenates of rat brain. These studies showed that brain tissue from mGluR5 knockout mice do not stain for mGluR5 and HEK-293 cells transfected with mGluR5 label a band of the correct molecular weight (Kuwajima et al., 2004). Furthermore, immunoblot analysis on proteins isolated from various brain regions labels a band that corresponds to the size of mGluR5 in regions known to express mGluR5 protein and mRNA (Mannaioni et al., 2001).
Immunoperoxidase labeling for light microscopy
Following sodium borohydride treatment, sections were incubated for 1 hour at room temperature (RT) in PBS containing either 10% normal goat serum (NGS; for group I mGluRs), or normal horse serum (NHS; for calbindin-D28k), 1% BSA, and 0.3% Triton X-100, followed by the primary antibody solution containing 1% NGS or NHS, 1% BSA, and 0.3% Triton X-100 in PBS for 24 hours at RT. After three rinses in PBS, sections were incubated in secondary biotinylated goat anti-rabbit or horse anti-mouse IgGs at a concentration of 1:200 (Vector Laboratories, Burlingame, CA) for 90 minutes. The sections were rinsed again in PBS and then incubated another 90 minutes with the avidin-biotin peroxidase complex (ABC) at a dilution of 1:100 (Vector Laboratories). Finally, the sections were washed in PBS and TRIS buffer (50mM; pH 7.6) and transferred to a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St Louis, MO), 10mM imidazole, and 0.005% hydrogen peroxide in TRIS buffer for 10 minutes. Sections were rinsed in PBS, mounted onto gelatin-coated slides, dehydrated and then coverslipped with Permount. Tissue was examined with a Leica DMRB microscope (Leica Microsystems, Inc., Bannockburn, IL) and images were taken using a CCD camera (Leica DC500) which was controlled by Leica IM50 software.
Preembedding Immunoperoxidase labeling for electron microscopy
Following sodium borohydride treatment, sections were placed in a cryoprotectant solution for 20 minutes (PB 0.05M, pH 7.4, 25% sucrose, and 10% glycerol), frozen at −80°C for 20 minutes, returned to a decreasing gradient of cryoprotectant solutions, and rinsed in PBS. Sections were then incubated in primary and secondary antibody solutions, identical to those used for light microscopy; with two exceptions: 1) the omission of Triton X-100 and 2) incubation in primary antibody for 48 hours at 4°C.
After the DAB reaction, the tissue was rinsed in PB (0.1M, pH 7.4) and treated with 1% OsO4 for 20 minutes. It was then returned to PB and dehydrated with increasing concentrations of ethanol. When exposed to 70% ETOH, 1% uranyl acetate was added to the solution for 35 minutes to increase the contrast of the tissue at the electron microscope. Following dehydration, sections were treated with propylene oxide and embedded in epoxy resin for 12 hours (Durcupan ACM, Fluka, Buchs, Switzerland), mounted onto slides and placed in a 60°C oven for 48 hours. Separate samples of the nucleus accumbens core and medial shell were cut out of the larger sections, mounted onto resin blocks and cut into 60-nm sections using an ultramicrotome (Leica Ultracut T2). The 60-nm sections were collected on Pioloform-coated copper grids, stained with lead citrate for 5 minutes to enhance tissue contrast and examined on the Zeiss EM-10C electron microscope. Electron micrographs were taken and saved with a CCD camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by DigitalMicrograph software (version 3.10.1, Gatan, Inc.). Some of the digitally acquired electron micrographs were adjusted only for brightness or contrast using either the DigitalMicrograph software or Adobe Photoshop software (version 8.0, Adobe Systems Inc.). Micrographs were then compiled into figures using Adobe Illustrator (version 11.0, Adobe Systems Inc.).
Preembedding Immunogold labeling for electron microscopy
Following sodium borohydride and cryoprotectant treatments, sections were incubated for 30 minutes in PBS containing 5% dry milk at RT and then rinsed in TBS-gelatin buffer (0.02M and pH 7.6). Sections were then transferred to primary antibody solutions with 1% dry milk in TBS-gelatin buffer for 24 hours at room temperature and then rinsed again in TBS-gelatin. After rinses, sections were treated for 2 hours at RT with secondary goat anti-rabbit IgGs conjugated with 1.4nm gold particles at a concentration of 1:100 (Nanoprobes, Yaphank, NY) diluted with 1% dry milk in TBS-gelatin. Sections were rinsed in TBS-gelatin and 2% sodium acetate buffer before gold particles were silver intensified to 30–50nm using the HQ silver kit (Nanoprobes) for approximately 10 minutes. The sections were then treated according to the same protocol of osmification, dehydration, embedding, and tissue selection described above for the immunoperoxidase procedure including the following changes: 1) the tissue was kept in 0.5% OsO4 for 10 minutes instead of 20 and 2) the tissue was stained with 1% uranyl acetate for 10 minutes instead of 35.
DHPG Injections
In order to ensure that the immunogold method used in our study was sensitive enough to detect changes in plasma membrane-bound group I mGluRs immunoreactivity in response to direct receptor agonist exposure in vivo, we examined changes in the subcellular localization of mGluR1a and mGluR5 in the accumbens of rats following local administration of group I mGluRs agonist. Six male Sprague-Dawley rats were anesthetized with isoflurane and fixed in a stereotaxic frame (Kopf; Tugunga, CA). Using a 10μl Hamilton syringe (Hamilton; Reno, NV), 2μl of either 1μmol (RS)-3,5-dihydroxyphenylglycine (DHPG; Tocris, Ballwin, MO) dissolved in 0.9% saline or 0.9% saline was injected over a 5 minute period into the nucleus accumbens core and medial shell based on coordinates from Paxinos and Watson (1998; +1.7mm AP, +1.3mm ML, −7.4mm DV). Rats were sacrificed 45 minutes after injections. This time point was chosen based on previous internalization studies of other GPCRs in response to agonist stimulation (Bernard et al., 1998; Dumartin et al., 1998; Bernard et al., 1999; Csaba et al., 2001). To assess the accuracy of the DHPG injection, the striatal tissue from these animals was serially cut, and 1 out of 4 sections were Nissl-stained to reveal the exact placement of the syringe. Tissue from animals in which the injection site was in the core and/or medial shell of the accumbens, was prepared for single preembedding immunogold labeling as described above.
Analysis of Material
Immunoperoxidase labeling for group I mGluRs
Data for single immunoperoxidase labeling were collected from a total of 132 blocks of tissue, 1 block/animal in the medial shell (referred to as shell in the following sections) and 1 block/animal in core immunostained for either mGluR1a and mGluR5 from 7 rats chronically treated with cocaine, 7 rats chronically treated with saline, 10 rats acutely treated with cocaine, and 10 rats acutely treated with saline. Serial ultrathin sections taken from each of the blocks were examined and 35–40 electron micrographs of randomly selected immunoreactive elements were digitized at 25,000X. This resulted in a total surface of 18,027μm2 of accumbens tissue to be examined for mGluR1a in saline-treated rats and 15,700μm2 in cocaine-treated rats; 14,305μm2 for mGluR5 in saline-treated rats and 15,235μm2 in cocaine-treated rats. Labeled elements were categorized as dendrites, spines, unmyelinated axons, myelinated axons, and axon terminals on the basis of ultrastructural features described by Peters et al.(1991). The density of labeled elements for each receptor subtype was calculated in the shell and core by dividing the number of labeled elements by the area of tissue examined. The means +/− SEM of labeled elements for the different animal groups were calculated and presented as bar histograms. Significant differences were assessed using Sigma stat software (version 2.03, SPSS Inc.) for one-way ANOVAs and Tukey’s post-hoc test. The density of labeled elements was compared across each neuronal element between the cocaine- and saline-treated animals within each receptor subtype.
Preembedding immunogold labeling for group I mGluRs
Immunogold data were collected from 125 blocks of mGluR1a- and mGluR5-immunostained shell and core tissue as described above. Serial ultrathin sections from the surface of the blocks were collected and 35–40 electron micrographs of randomly selected immunoreactive elements were taken at 25,000X, for a total tissue surface area of 13,316μm2 of accumbens tissue to be examined for mGluR1a in saline-treated rats and 13,840μm2 in cocaine-treated rats; 12,502μm2 for mGluR5 in saline-treated rats and 14,247μm2 in cocaine-treated rats. Labeled elements were classified as described above with the addition that dendrites were further categorized arbitrarily as large (cross-sectional diameter greater than 0.75μm) or small (cross-sectional diameter equal to or less than 0.75μm). Gold particles were classified as either intracellular or plasma membrane-bound depending on their localization relative to the plasma membrane. To be categorized as plasma membrane-bound, gold particles had to be in contact with the membrane; all other particles were considered intracellular. The total number (i.e. 100%) of gold particles for each animal is equal to the number of intracellular + plasma membrane-bound gold particles examined. The mean (+/−SEM) percent of plasma membrane-bound gold particles was then calculated across all animals and presented as a bar histogram; the remaining gold particles were found in the intracellular compartment. Data were analyzed for significant differences between saline- and cocaine-treated animals using Sigma Stat software by one-way ANOVAs and Tukey’s post-hoc test. Plasma membrane-bound gold particles were further classified into three categories; perisynaptic (touching or within a 20 nm range of the edges of postsynaptic specializations); synaptic (in contact with the main body of postsynaptic specializations); or extrasynaptic (on the plasma membrane but not associated with synapses).
Preembedding immunogold labeling for group I mGluRs following DHPG Injections
Immunogold labeling data were collected from 24 blocks of mGluR1a- and mGluR5-immunostained shell and core tissue as described above. To make sure the tissue examined had been exposed to DHPG, all blocks were taken within a range of 0.2 to 0.5mm from the tip of the injection syringe and from sections no more than 120μm away in the rostral/caudal plane. Serial ultrathin sections from the surface of the blocks were collected and 35 electron micrographs of randomly selected immunoreactive elements were taken at 25,000X, for a total tissue surface area of 2,440μm2 of accumbens tissue to be examined for mGluR1a in saline-treated rats and 2,440μm2 in DHPG-treated rats; 2,440μm2 for mGluR5 in saline-treated rats and 2,440μm2 in DHPG-treated rats. The quantitative analysis of gold labeling distribution was the same as described in the previous section.
RESULTS
Light Microscopic Observations
Tissue containing the nucleus accumbens was stained separately for calbindin D28k, mGluR1a or mGluR5. As shown in our previous study (Mitrano and Smith, 2007) and others (Meredith et al., 1996), calbindin immunoreactivity clearly delineated the boundaries of the shell and core of the nucleus accumbens, hence serving as a guide for the selection of blocks of tissue in subsequent electron microscopic experiments. Tissue from all three cocaine treatment groups and saline-treated animals were immunostained for the two group I mGluRs. In line with our previous study, the immunoreactivity for mGluR1a and mGluR5 was distributed heavily in the neuropil of the shell and core of the accumbens, with very light labeling in cell bodies (Mitrano and Smith, 2007). No obvious difference in the overall distribution of mGluR1a and mGluR5 immunoreactivity was found in the nucleus accumbens between cocaine- and saline-treated rats (data not shown).
Electron Microscopic Observations
Cellular and Subcellular Localization of mGluR1a and mGluR5 in Cocaine-treated Groups
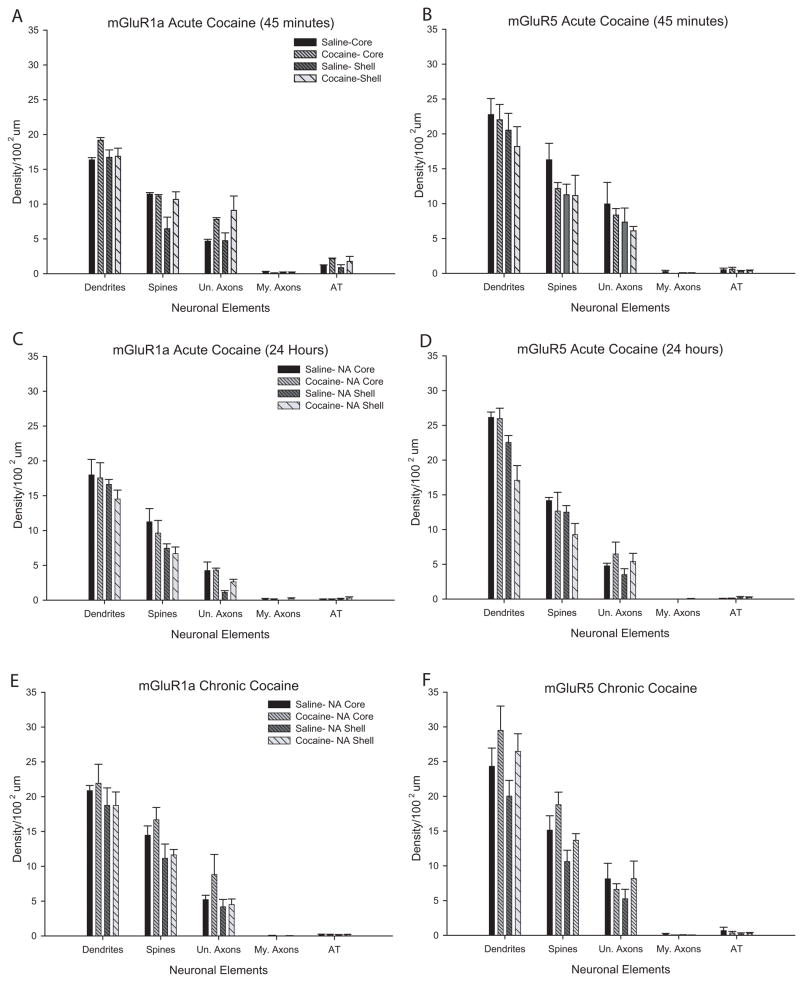
Because both mGluR1a and mGluR5 immunoreactivity is found in the neuropil of accumbens tissue, high resolution electron microscopy is essential to assess potential changes in the cellular, subcellular and subsynaptic localization of these receptors between normal and cocaine-treated rats. First, we looked at the subcellular localization of both group I mGluRs in the three groups of cocaine-treated rats and control saline-treated animals using immunoperoxidase labeling for mGluR1a or mGluR5. The majority of labeling for both receptor subtypes in each experimental group was found in dendrites and spines, and less frequently in unmyelinated axons. Electron micrographs of immunoreactive elements from each treatment group are shown in figure 1, while quantitative data for these observations are depicted in figure 2 as the average density of labeled elements per 100 square microns (±SEM) of accumbens shell or core tissue. Overall, there was no significant difference in the distribution of mGluR1a- or mGluR5-immunoreactive neuronal elements between the different cocaine treatment groups and controls, regardless of receptor type, time point, or region of the accumbens examined using a series of one-way ANOVAs (Fig. 2).
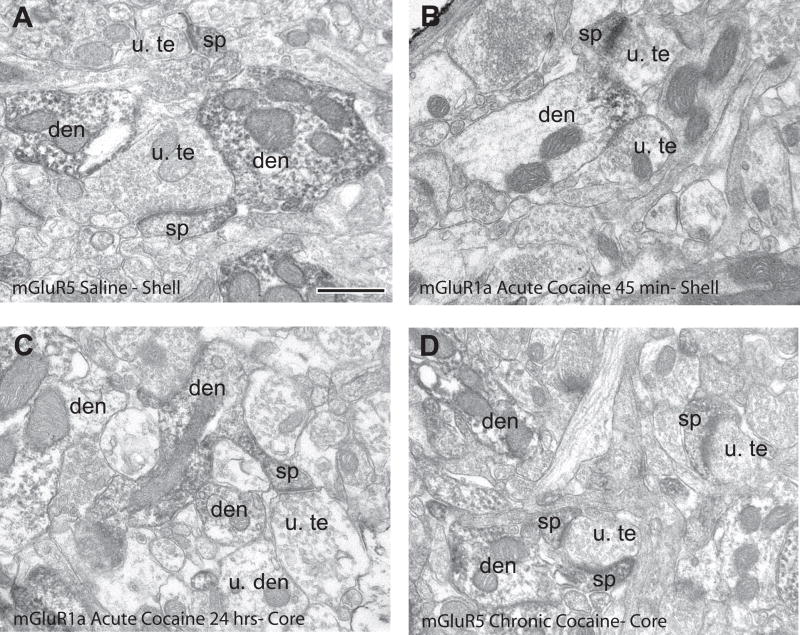
Figure 1.
Immunoperoxidase labeling of mGluR1a and mGluR5 in saline- and cocaine-treated rats. (A) mGluR5-labeled elements in the nucleus accumbens shell of a saline-treated rat. (B) mGluR1a-labeled elements in the nucleus accumbens shell of a rat treated with cocaine, 45 minutes withdrawal. (C) mGluR1a-labeled elements in the nucleus accumbens core of a rat treated with cocaine, 24 hours withdrawal. (D) mGluR5-labeled elements in the nucleus accumbens core of animals treated chronically with cocaine for one week. Note the bulk of postsynaptic labeling in dendrites (den) and spines (sp). Abbreviations: u. te, unlabeled terminal; u. den, unlabeled dendrite; u. sp, unlabeled spine. Scale bar = 0.5 μm.
Figure 2.
Histograms summarizing the distribution of mGluR1a and mGluR5 immunoperoxidase labeling in saline- and cocaine-treated rats, expressed as mean density of labeled elements per 100μm2 (±SEM). (A–B) Immunoperoxidase labeling for mGluR1a and mGluR5 in the core and shell of the nucleus accumbens 45 minutes following a saline or cocaine injection. Total number of animals and labeled elements: mGluR1a: saline, core=4, 552; saline, shell=4, 470; cocaine, core=5, 822; cocaine, shell=5, 784. mGluR5: saline, core=5, 1012; saline, shell=5, 807; cocaine, core=5, 882; cocaine, shell=5, 729. (C–D) Immunoperoxidase labeling for mGluR1a and mGluR5 24 hours following saline or cocaine. Total number of animals and labeled elements: mGluR1a: saline, core=5, 786; saline, shell=4, 473; cocaine, core=5, 672; cocaine, shell=5, 643. mGluR5: saline, core=4, 831; saline, shell=4,710; cocaine, core=5, 1053; cocaine, shell=5, 694. (E–F) Immunoperoxidase labeling for mGluR1a and mGluR5 3 weeks following one-week chronic saline or cocaine. Total number of animals and labeled elements: mGluR1a: saline, core=7, 1682; saline, shell=7, 1381; cocaine, core=7, 1770; cocaine, shell=7, 1365. mGluR5: saline, core=7, 1897; saline, shell=7, 1471; cocaine, core=7, 1939; cocaine, shell=7, 1882. Abbreviations: Un. Axons: Unmyelinated axons; My. Axons: Myelinated axons; AT: Axon terminals.
Subsynaptic Localization of mGluR1a and mGluR5 in Cocaine-treated Groups
Because the immunoperoxidase method results in an amorphous diffuse reaction product within labeled elements, this method is not suitable to determine the exact subsynaptic localization of group I mGluRs in accumbens neurons. We therefore used the preembedding immunogold method that allows for a higher level of spatial resolution to detect changes in the subsynaptic localization of mGluR1a and mGluR5 immunoreactivity in response to chronic and acute cocaine treatment regimens. Examples of immunogold-labeled elements analyzed for this part of the study are illustrated in figure 3. The quantitative data, which provide the percentage of plasma membrane-bound gold particles (±SEM) labeling for mGluR1a or mGluR5 on dendrites and spines for the different treatment groups are presented in figure 4. Because the majority of gold labeling for mGluR1a and mGluR5 was found postsynaptically in dendrites and spines, the quantitative analysis of immunoreactivity was focused on these elements.
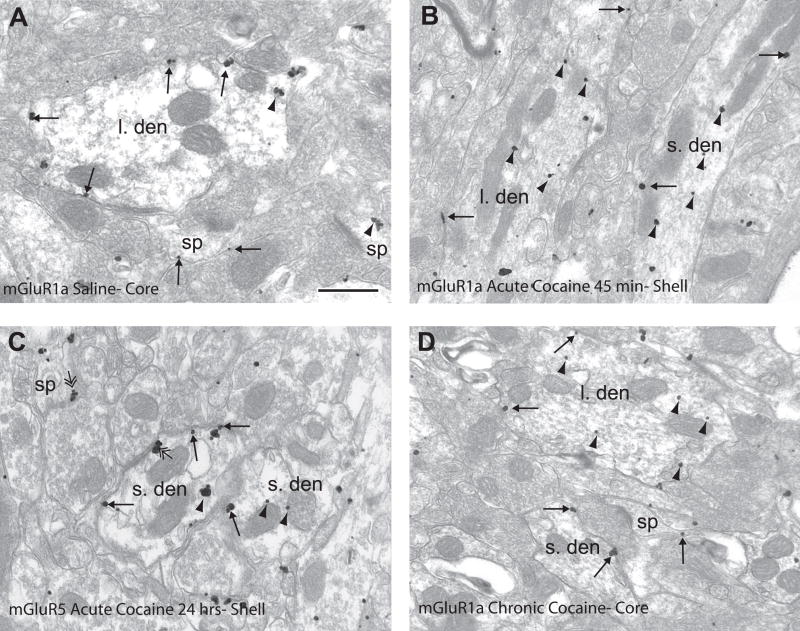
Figure 3.
mGluR1a and mGluR5 immunogold labeling in saline- and cocaine-treated rats. (A) mGluR1a immunogold labeling in the nucleus accumbens core of a saline-treated rat. Note the extrasynaptic (single-headed arrows) labeling on the plasma membrane of the large dendrite (l. den) and spine (sp). (B) mGluR1a immunogold labeling in nucleus accumbens shell 45 minutes following cocaine treatment. Note the increased intracellular labeling (arrowheads) in large and small dendrites (s. den). (C) mGluR5 immunogold labeling in the nucleus accumbens shell 24 hours following cocaine injection. mGluR5 is distributed extrasynaptically (single-headed arrows) and intracellularly (arrowheads) in the dendrites. Also note the perisynaptic labeling to an asymmetric axodendritic and axospinous synapse (double-headed arrow). (D) mGluR1a immunogold labeling in the nucleus accumbens core of a rat chronically treated with cocaine followed by 3 weeks withdrawal. Note the larger amount of intracellular labeling in the large dendrite and extrasynaptic plasma membrane labeling in the small dendrite and spine. Scale bar=0.5μm.
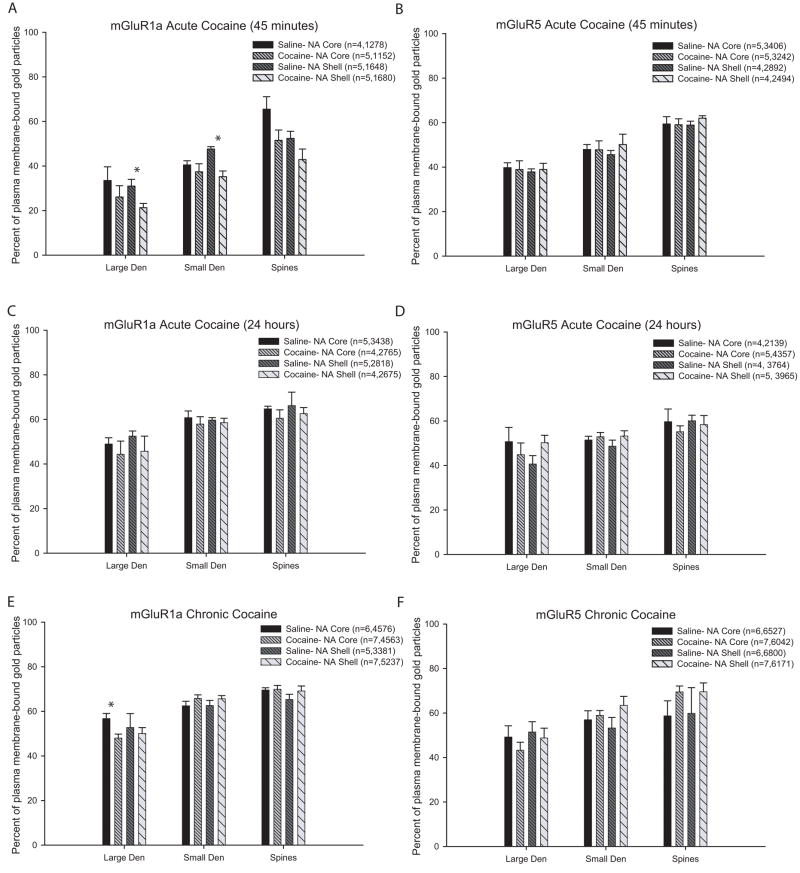
Figure 4.
Histograms showing the percentage of plasma membrane labeling of mGluR1a and mGluR5 in the core and shell of the nucleus accumbens of saline- and cocaine-treated rats. Data is presented as the mean percentage (±SEM) of gold particles on the plasma membrane of large or small dendrites and spines; in parentheses n= number of animals used followed by the total number of gold particles counted in each experimental group. (A–B) Mean percentage of plasma membrane-bound mGluR1a (A) and mGluR5 (B) labeling in rats sacrificed 45 minutes following cocaine treatment. One-way ANOVA with Tukey’s post-hoc test revealed a lower proportion of plasma membrane-bound mGluR1a in large and small dendrites in the shell of cocaine-treated rats (p<0.05, single asterisks). Total number of elements examined: mGluR1a: Saline, Core: large den=38, small den=233, spines=117; Cocaine, Core: large den=55, small den=198, spines=114; Saline, Shell: large den=64, small den=236, spines=116; Cocaine, Shell: large den=52, small den=260, spines=140. mGluR5: Saline core: large den=64, small den=365, spines=248; Cocaine, Core: large den=44, small den=365, spines=248; Saline, Shell: large den=65, small den=260, spines=171; Cocaine, Shell: large den=47, small den=292, spines=170. (C–D) Percentage of plasma membrane-bound mGluR1a (C) and mGluR5 (D) in rats sacrificed 24 hours following cocaine treatment. No significant difference was found between the saline- and cocaine-treated animals. Total number of elements examined: mGluR1a: Saline, Core: large den=53, small den=432, spines=263; Cocaine, Core: large den=19, small den=342, spines=210; Saline, Shell: large den=34, small den=368, spines=216; Cocaine, Shell: large den=36, small den=311, spines=179. mGluR5: Saline, core: large den=20, small den=270, spines=165; Cocaine, Core: large den=40, small den=464, spines=315; Saline, Shell: large den=34, small den=416, spines=256; Cocaine, Shell: large den=39, small den=446, spines=263. (E–F) Percentage of plasma membrane-bound mGluR1a (E) and mGluR5 (F) in rats chronically treated with cocaine and sacrificed 3 weeks later. One-way ANOVA and Tukey’s post-hoc tests revealed that the there is significantly lower percentage of plasma membrane-bound mGluR1a in the accumbens core of rats treated chronically with cocaine compared to saline (p<0.05, single asterisk). Total number of elements examined: mGluR1a: Saline, Core: large den=53, small den=575, spines=380; Cocaine, Core: large den=76, small den=667, spines=531; Saline, Shell: large den=53, small den=427, spines=264; Cocaine, Shell: large den=83, small den=732, spines=427. mGluR5: Saline, core: large den=78, small den=717, spines=430; Cocaine, Core: large den=85, small den=748, spines=471; Saline, Shell: large den=104, small den=678, spines=372; Cocaine, Shell: large den=71, small den=783, spines=427.
In the 45 minute acute treatment group, a significant decrease in the percent of plasma membrane-bound mGluR1a in large (30.9±3.0% saline vs. 21.3±1.9% cocaine, F(3,15)=3.3, n=5, p<0.05) and small (47.6±1.0% saline vs. 35.2±2.6% cocaine, F(3,15)=4.9, n=5, p<0.05) dendrites in the accumbens shell was found in cocaine-treated rats compared to controls, but no noticeable change in mGluR5 localization was found in these animals (Figs 3A,B; 4 A,B). In the 24 hours post-treatment group, neither mGluR1a or mGluR5 displayed any significant change in their pattern of subsynaptic localization compared to controls (Figs 3C; 4 C, D). Finally, a significant decrease in the percent of plasma membrane-bound mGluR1a immunoreactivity was found in large dendrites in the accumbens core of chronically cocaine-treated rats (48.0±1.8%, n=7) compared to controls (56.7±2.3%, F(3,21)=3.1, n=6; p<0.05), but no significant change in the subsynaptic distribution of either group I mGluR subtypes was found in spines or small dendrites in these animals.
The pattern of subsynaptic labeling on the plasma membrane was further classified into extrasynaptic, perisynaptic and synaptic. As shown in tables 1 and 2, there was no significant difference in the pattern of subsynaptic labeling between all treatment groups. In each group, more than 80% plasma membrane labeling in spines and 96–98% labeling in dendrites was extrasynaptic. A slightly larger incidence of perisynaptic labeling to asymmetric synapses was seen in spines compared to dendrites.
Table 1.
Subsynaptic group I mGluRs labeling after cocaine treatment in the accumbens core. Data are presented as percent of gold particles ±SEM for each subsynaptic domain in Spines, Sm. Den (small dendrites) and Lg. Den (large dendrites). The different categories of subsynaptic domains are abbreviated as follows: Extra= extrasynaptic; Peri-Asym=perisynaptic labeling to asymmetric synapses; Syn-Asym=synaptic labeling of asymmetric synapses; Peri-Symm=perisynaptic labeling at symmetric synapses; Syn-Symm=synaptic labeling at symmetric synapses. Data for mGluR1a are in the top row of each box, while mGluR5 data are in the bottom row of each box. The number of animals used, elements examined and gold particles counted is presented in the legend of figure 4.
| Acute Cocaine-
45 MINUTES |
Acute Cocaine-
24 HOURS |
Chronic Cocaine | ||||
|---|---|---|---|---|---|---|
| MGluR1a | Saline-Core | Cocaine-Core | Saline-Core | Cocaine-Core | Saline-Core | Cocaine-Core |
| mGluR5 | ||||||
| SPINES Extra | 78.7±4.5 | 79.8±6.1 | 85.3±2.3 | 81.0±1.2 | 82.6±1.3 | 86.8±1.5 |
| 84.3±3.5 | 83.8±2.1 | 82.7±7.4 | 83.2±2.2 | 83.1±1.3 | 82.2±1.3 | |
| Peri-Asym | 18.3±3.4 | 20.2±6.1 | 14.5±2.1 | 18.2±1.4 | 14.8±0.7 | 12.5±1.4 |
| 14.9±1.7 | 16.0±2.1 | 18.9±5.5 | 14.8±2.3 | 14.6±1.9 | 16.2±1.7 | |
| Syn-Asym | 2.9±1.4 | 0 | 0.3±0.3 | 0.2±0.2 | 1.6±0.7 | 0.7±0.3 |
| 0.8±0.6 | 0.2±0.2 | 1.4±0.7 | 1.7±0.5 | 1.1±0.3 | 1.1±0.4 | |
| Peri-Symm | 0 | 0 | 0 | 0.6±0.6 | 1.3±0.6 | 0 |
| 0 | 0 | 0.4±0.4 | 0.3±0.3 | 1.1±0.4 | 0 | |
| Syn-Symm | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0.2±0.2 | 0.3±0.3 | |
| SM. DEN Extra | 98.1±1.2 | 96.3±1.7 | 97.6±0.8 | 96.2±0.7 | 97.3±0.9 | 98.5±1.0 |
| 98.5±0.5 | 97.3±1.4 | 97.9±0.7 | 98.3±0.6 | 98.5±0.6 | 98.0±0.4 | |
| Peri-Asym | 0.3±0.3 | 1.8±1.2 | 1.1±0.5 | 0.7±0.3 | 0.9±0.2 | 1.5±0.2 |
| 0.4±0.2 | 0.7±0.3 | 1.3±0.7 | 0.4±0.2 | 0.4±0.2 | 0.9±0.4 | |
| Syn-Asym | 0.3±0.3 | 0 | 0.2±0.1 | 0.3±0.2 | 0.1±0.1 | 0±0.1 |
| 0.1±0.1 | 0.3±0.3 | 0.4±0.4 | 0 | 0.2±0.2 | 0.1±0.1 | |
| Peri-Symm | 1.4±0.8 | 1.9±1.1 | 0.7±0.3 | 1.3±0.4 | 1.7±0.9 | 0±0.6 |
| 0.8±0.3 | 1.3±1.0 | 0.2±0.1 | 0.7±0.3 | 0.7±0.2 | 1.0±0.3 | |
| Syn-Symm | 0 | 0 | 0.5±0.2 | 1.6±0.6 | 0.1±0.1 | 1.0±0.5 |
| 0.2±0.1 | 0.5±0.5 | 0.1±0.1 | 0.7±0.3 | 0.1±0.1 | 0.1±0.0 | |
| LG. DEN Extra | 97.5±1.6 | 96.1±2.9 | 96.4±1.5 | 94.9±3.4 | 96.0±1.5 | 96.5±1.7 |
| 94.2±3.3 | 97.3±1.7 | 100±0 | 96.4±2.3 | 96.7±2.3 | 96.1±1.5 | |
| Peri-Asym | 0 | 0 | 1.9±1.2 | 2.4±2.4 | 0 | 0 |
| 1.4±1.4 | 0.8±0.8 | 0 | 1.4±0.9 | 0.9±0.9 | 2.2±1.3 | |
| Syn-Asym | 0 | 0 | 0.3±0.3 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | |
| Peri-Symm | 2.4±1.5 | 2.2±2.2 | 0.8±0.8 | 1.6±1.6 | 4.0±1.5 | 3.0±1.8 |
| 1.8±1.1 | 0.8±0.8 | 0 | 0.4±0.4 | 2.3±2.3 | 1.8±1.0 | |
| Syn-Symm | 0 | 1.7±1.0 | 0.7±0.4 | 1.2±1.2 | 0 | 0.5±0.5 |
| 1.8±1.4 | 1.2±1.2 | 0 | 1.9±1.9 | 0 | 0 | |
Table 2.
Subsynaptic group I mGluRs labeling after cocaine treatment in the accumbens shell. Data are presented as percent of gold particles ±SEM for each subsynaptic domain in Spines, Sm. Den (small dendrites) and Lg. Den (large dendrites). The different categories of subsynaptic domains are abbreviated as follows: Extra= extrasynaptic; Peri-Asym=perisynaptic labeling to asymmetric synapses; Syn-Asym=synaptic labeling of asymmetric synapses; Peri-Symm=perisynaptic labeling at symmetric synapses; Syn-Symm=synaptic labeling at symmetric synapses. Data for mGluR1a are in the top row of each box, while mGluR5 data are in the bottom row of each box. The number of animals used, elements examined and gold particles counted is presented in the legend of figure 4.
| Acute Cocaine-
45 MINUTES |
Acute Cocaine-
24 HOURS |
Chronic Cocaine | ||||
|---|---|---|---|---|---|---|
| mGluR1a | Saline-Shell | Cocaine-Shell | Saline-Shell | Cocaine-Shell | Saline-Shell | Cocaine-Shell |
| mGluR5 | ||||||
| SPINES Extra | 79.5±4.1 | 73.8±1.7 | 83.7±2.8 | 85.6±1.9 | 82.2±3.3 | 85.5±2.4 |
| 82.6±3.5 | 85.4±3.6 | 88.0±1.7 | 85.3±2.5 | 80.6±1.3 | 84.3±1.4 | |
| Peri-Asym | 16.6±4.5 | 21.0±1.2 | 13.3±1.3 | 13.5±1.5 | 14.7±2.4 | 13.0±2.2 |
| 17.1±3.4 | 13.6±3.2 | 10.9±1.4 | 13.0±2.0 | 17.7±1.5 | 13.6±1.6 | |
| Syn-Asym | 3.9±1.1 | 5.2±2.6 | 2.0±1.1 | 0.5±0.5 | 1.0±0.6 | 0.9±0.3 |
| 0.3±0.3 | 1.0±0.6 | 1.1±0.7 | 1.3±0.8 | 1.3±0.5 | 1.6±0.6 | |
| Peri-Symm | 0 | 0 | 0.8±0.8 | 0.5±0.5 | 1.7±1.4 | 0.6±0.5 |
| 0 | 0 | 0 | 0.2±0.2 | 0.4±0.4 | 0.6±0.4 | |
| Syn-Symm | 0 | 0 | 0.3±0.3 | 0 | 0.5±0.3 | 0 |
| 0 | 0 | 0 | 0.2±0.2 | 0 | 0 | |
| SM. DEN Extra | 94.7±1.7 | 93.0±1.5 | 96.8±0.5 | 96.3±0.4 | 96.4±1.2 | 95.4±0.9 |
| 97.0±1.0 | 98.0±1.0 | 98.3±0.5 | 97.9±0.5 | 97.9±0.6 | 95.9±0.9 | |
| Peri-Asym | 2.3±1.0 | 2.6±0.9 | 1.6±0.5 | 0.7±0.4 | 1.2±0.5 | 2.0±0.6 |
| 1.9±0.5 | 0.6±0.4 | 0.5±0.3 | 0.6±0.2 | 0.6±0.2 | 1.1±0.4 | |
| Syn-Asym | 0.4±0.4 | 0 | 0.2±0.2 | 0.1±0.1 | 0 | 0 |
| 0.1±0.1 | 0.3±0.2 | 0 | 0.2±0.1 | 0.1±0.1 | 0 | |
| Peri-Symm | 1.4±0.6 | 2.1±0.9 | 0.1±0.1 | 1.9±0.4 | 1.5±0.8 | 2.3±0.6 |
| 0.7±0.4 | 0.5±0.5 | 0.9±0.4 | 0.9±0.4 | 1.0±0.6 | 2.1±0.6 | |
| Syn-Symm | 1.2±0.5 | 2.2±1.0 | 1.4±0.5 | 1.1±0.3 | 0.8±0.2 | 0.3±0.2 |
| 0.2±0.2 | 0.7±0.4 | 0.3±0.1 | 0.5±0.2 | 0.4±0.1 | 0.7±0.1 | |
| LG. DEN Extra | 100±0 | 100±0 | 98.2±1.1 | 93.2±1.9 | 88.8±4.4 | 96.9±1.8 |
| 98.0±1.2 | 99.5±0.5 | 97.4±2.6 | 98.5±1.5 | 98.4±1.0 | 93.6±2.1 | |
| Peri-Asym | 0 | 0 | 0 | 1.6±1.6 | 4.9±1.8 | 0.1±0.1 |
| 0.6±0.6 | 0 | 0 | 0 | 0.8±0.6 | 0.3±0.3 | |
| Syn-Asym | 0 | 0 | 0.9±0.9 | 0 | 2.2±2.2 | 0.3±0.3 |
| 0 | 0 | 0.5±0.5 | 0 | 0 | 0 | |
| Peri-Symm | 0 | 0 | 0.6±0.6 | 5.3±0.5 | 2.5±1.6 | 2.4±1.6 |
| 1.3±0.8 | 0 | 1.6±1.6 | 1.0±1.0 | 0.7±0.7 | 2.7±1.4 | |
| Syn-Symm | 0 | 0 | 0.3±0.3 | 0 | 1.6±1.6 | 0.2±0.2 |
| 0 | 0.5±0.5 | 0.5±0.5 | 0.5±0.5 | 0.1±0.1 | 3.4±2.4 | |
Agonist-induced changes in the subsynaptic localization of group I mGluRs in the nucleus accumbens
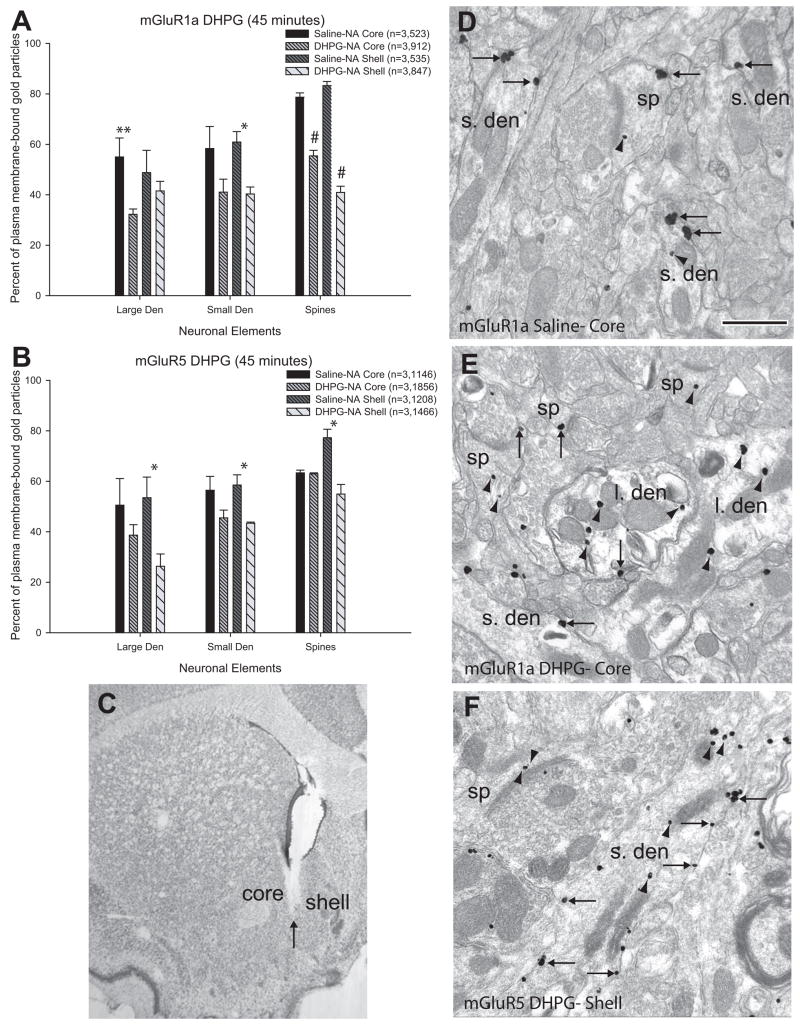
Despite the well-established increases or decreases in extracellular glutamate in the nucleus accumbens (Smith et al., 1995; Pierce et al., 1996; Reid et al., 1997; Baker et al., 2003), only modest changes in group I mGluRs distribution were induced following acute or chronic cocaine treatment (see above). A potential interpretation for these observations might be that the pre-embedding immunogold method is not sensitive enough to detect changes in the subcellular and subsynaptic localization of group I mGluRs induced by changes in extracellular glutamate in cocaine-treated rats. In order to validate our approach, we assessed changes in the distribution of the two group I mGluRs in the rat accumbens forty-five minutes after intra-accumbal injection of the group I mGluR agonist, DHPG (Fig. 5C). Consistent with previous studies that showed internalization of various GPCRs following agonist stimulation (Bernard et al., 1998; Dumartin et al., 1998; Bernard et al., 1999; Csaba et al., 2001), local DHPG application induced significant decreases in the percentage of plasma membrane-bound labeling for mGluR1a or mGluR5 in specific neural elements in the shell or core of the rat accumbens (Fig. 5). In the shell, a significant decrease in plasma membrane-bound mGluR1a immunoreactivity was found in small dendrites (61.0±4.1% saline vs. 40.3±2.8% DHPG, F(3,8)=11.2, n=3, p<0.05) and spines (83.3±1.6% saline vs. 40.9±2.4% DHPG, F(3,8)=96.5, n=3, p<0.001), while plasma membrane-bound labeling for mGluR5 was significantly reduced in all neural compartments examined (large dendrites: 53.5±8.2% saline vs. 26.3±4.9% DHPG, F(3,8)=7.0, n=3, p<0.05; small dendrites: 58.5±4.1% saline vs. 43.4±0.5% DHPG, F(3,8)=12.0, n=3, p<0.05; spines: 77.2±3.4% saline vs. 54.9±3.8% DHPG, F(3,8)=12.4, n=3, p<0.01). The pattern was quite different in the accumbens core where a significant decrease in the percent of plasma membrane-bound mGluR1a was found in large dendrites (55.0±7.5% saline vs. 32.3±2.1% DHPG, F(3,8)=5.8, n=3, p<0.05) and spines (78.7±1.7% saline vs. 55.4±2.2% DHPG, F(3,8)=96.5, n=3, p<0.001), but not in small dendrites. As for mGluR5, no significant change in the percentage of plasma membrane-bound labeling was found in the core.
Figure 5.
Histograms and immunogold labeling for mGluR1a and mGluR5 in saline- and DHPG-treated rats. (A) and (B) are summary histograms showing the percentage of plasma membrane-bound labeling for mGluR1a (A) and mGluR5 (B) in the core and shell of the nucleus accumbens of saline- and DHPG-treated rats. Data are presented as mean percentages (±SEM) of gold particles on the plasma membrane of large or small dendrites and spines; in parentheses n= number of animals used, followed by the total number of gold particles counted in each experimental group. One-way ANOVAs and Tukey’s post-hoc tests reveal that there is a lower percentage of plasma membrane-bound mGluR1a in large dendrites of the core (p<0.05, double asterisk), in small dendrites of the shell (p<0.05, single asterisk), and in spines of both shell and core of the accumbens (p<0.001, number signs) in DHPG-treated animals. For mGluR5, there is a significantly lower percentage of labeling on the plasma membrane in the shell of the accumbens on large and small dendrites (p<0.05, single asterisk) and spines (p<0.01, number sign). (C) Light micrograph of a sample DHPG injection into the core and medial shell of the nucleus accumbens. The arrow indicates the tip of the syringe. (D–F) show examples of labeled elements from saline- treated (D) and DHPG-treated animals (E–F). (D) mGluR1a labeling in the core of the accumbens of a saline-treated animal. Note the majority of extrasynaptic labeling (single-headed arrows) on the plasma membrane of small dendrites (s.den) and spines (sp). (E) mGluR1a labeling in the core of the accumbens of a DHPG-treated animal. Notice the increase in intracellular (arrowheads) labeling in both dendrites and spines. (F) mGluR5 labeling in the shell of the accumbens of a DHPG-treated animal. Note the large pool of intracellular labeling in dendrites and spines. Scale bar=0.5μm. Total number of elements examined: mGluR1a: Saline, Core: large den=15, small den=137, spines=72; DHPG, Core: large den=57, small den=170, spines=91; Saline, Shell: large den=24, small den=94, spines=40; DHPG, Shell: large den=50, small den=134, spines=60. mGluR5: Saline core: large den=36, small den=115, spines=111; DHPG, Core: large den=38, small den=190, spines=105; Saline, Shell: large den=34, small den=147, spines=47; DHPG, Shell: large den=32, small den=157, spines=57.
DISCUSSION
Two main conclusions can be drawn from this study. First, in contrast to the strong response of ionotropic AMPA receptors (Boudreau and Wolf, 2005; Boudreau et al., 2007), group I mGluRs in the nucleus accumbens display modest changes in their subcellular and subsynaptic localization after chronic or acute cocaine administration. Second, both mGluR1a and mGluR5 show a significant degree of internalization following local extracellular application of the group I mGluR agonist DHPG in both the core and shell of the nucleus accumbens. Together, these observations suggest that the trafficking of AMPA and group I mGluRs is differently affected by acute or chronic cocaine-induced changes in extracellular glutamate in the nucleus accumbens (Smith et al., 1995; Pierce et al., 1996; Reid et al., 1997). Furthermore, in line with other GPCRs (Bernard et al., 1998; Dumartin et al., 1998; Bernard et al., 1999; Csaba et al., 2001), our data provide the first in vivo evidence, in the brain, that group I mGluRs in the nucleus accumbens are endowed with trafficking properties that allow for rapid internalization in response to strong agonist stimulation.
One explanation for the minimal effect of cocaine administration on group I mGluRs trafficking could be that the cocaine administration regimen used in our study did not induce changes in extracellular glutamate in the nucleus accumbens. However, this is unlikely based on many previous studies showing rapid changes in glutamate release in the rat accumbens following administration regimens of acute or chronic cocaine similar to those used in the present study. For instance, extracellular glutamate levels in the accumbens are increased by as much as 300% over baseline approximately 40 minutes after a single injection of 30 mg/kg cocaine (Smith et al., 1995; Reid et al., 1997). In contrast, the behavioral sensitization paradigm of 7 days of chronic cocaine exposure followed by three weeks withdrawal results in almost 50% reduction in extracellular glutamate compared to controls (Pierce et al., 1996; Hotsenpiller et al., 2001; Baker et al., 2003). Although not fully understood, there is good evidence that increased synaptic release of neurotransmitter from prefrontal cortical afferents may be the main source of extracellular glutamate buildup following acute cocaine administration (McFarland et al., 2003), while behavioral sensitization to chronic cocaine exposure decreases the activity of the cystine/glutamate exchanger, thereby, lowering extracellular glutamate levels in sensitized rats (Baker et al., 2003). In light of these findings, we can, therefore, assume that the levels of glutamate in the nucleus accumbens of rats used in the present study have been altered in the same manner by acute and chronic cocaine exposure.
A significant 15–20% increase in mGluR5 mRNA, but 10% decrease in mGluR5 protein expression along with blunted functioning of mGluR1 in the medial accumbens was reported in chronically cocaine-treated rats (Ghasemzadeh et al., 1999, Swanson 2001). On the other hand, 24 hours following a single cocaine injection, endocannabinoid-mediated LTD, regulated by mGluR5, is abolished and accompanied by a 50% reduction in surface expression of mGluR5 in mice accumbal medium spiny neurons (Fourgeaud et al., 2004). Furthermore, Homer 1b/c, one of the scaffolding proteins that regulates the trafficking and plasma membrane expression of group I mGluRs in vitro (Roche et al., 1999; Ango et al., 2002), is decreased by about 20% in the accumbens of behaviorally sensitized rats three weeks following chronic cocaine exposure (Swanson et al., 2001).
Apart from minor changes of dendritic mGluR1a distribution in the 45 minutes acutely cocaine-treated group and the chronically cocaine-treated animals, our electron microscopic study did not reveal any significant change in the overall pattern of subcellular and subsynaptic localization of mGluR1a or mGluR5 in response to either regimens of cocaine administration. Because the regulation of extracellular glutamate that reaches extrasynaptic group I mGluRs may be very complex and rely on many factors including the amount of glutamate released from axon terminals, amount of glutamate released by astrocytes and amount of glutamate reuptake by glial and neuronal glutamate transporters (Brasnjo and Otis, 2001; Oliet et al., 2001; Reichelt and Knopfel, 2002; Rusakov and Lehre, 2002), it is hard to predict how much glutamate, indeed, reaches these mGluRs. As shown in the cerebellum, there could be very subtle subsynaptic regulation of glutamate by neuronal transporters that strongly modulate the activity of group I mGluRs (Brasnjo and Otis, 2001). If such regulatory processes are in place in the nucleus accumbens, changes in extracellular glutamate levels measured with microdialysis do not provide an accurate index of the exact concentration of transmitter individual group I mGluRs may be exposed to following cocaine administration.
Our results are different from those of Fourgeaud et al. (2004) showing a dramatic reduction in mGluR5 plasma membrane expression 24 hours post-cocaine injection in mouse accumbal neurons. The use of different species, approaches and experimental conditions between this study and ours may account for these divergent results. Although the limited sensitivity of the immunogold method to localize small amount of protein cannot be ruled out (Galvan et al., 2006), it is noteworthy that the same method was sensitive enough to detect changes in both group I mGluRs plasma membrane expression following intra-accumbens DHPG administration, and was successfully used by different groups to demonstrate internalization of various GPCRs in response to systemic or local agonist application (Bernard et al., 1998; Dumartin et al., 1998; Bernard et al., 1999; Csaba et al., 2001).
In striking contrast with group I mGluRs, AMPA receptor subunits display significant and opposite changes in their subsynaptic distribution after acute or chronic cocaine exposures (Boudreau and Wolf, 2005; Boudreau et al., 2007). Following the chronic behavioral sensitization paradigm, known to decrease extracellular glutamate (Pierce et al., 1996; Baker et al., 2003), an increased surface expression of synaptic AMPA receptors was found in the rat accumbens compared to saline-treated animals (Boudreau and Wolf, 2005). In contrast, acute cocaine exposure, which results in a significant raise in accumbens glutamate (Smith et al., 1995; Reid et al., 1997), lead to the internalization of AMPA GluR1- and GluR2-containing receptors (Boudreau and Wolf, 2005; Boudreau et al., 2007). Although these findings likely illustrate genuine differences in the trafficking of AMPA versus group I mGluRs in response to cocaine-induced changes in extracellular glutamate, the use of different techniques measure the surface expression of the receptors, may also account for these differential responses between ionotropic and metabotropic receptors after cocaine challenges. An important issue to consider is the exact localization of these glutamate receptors in relation to the release sites of glutamate. In contrast to AMPA receptor subunits, which are mainly concentrated in the main body of asymmetric glutamatergic synapses (Petralia et al., 1992; Baude et al., 1995), both mGluR1a and mGluR5 are largely extrasynaptic or perisynaptic to glutamatergic synapses (Baude et al., 1993; Ottersen and Landsend, 1997; Galvan et al., 2006). In light of data showing that increased glutamatergic transmission at cortical synapses may account for the raise in extracellular glutamate measured in the accumbens after acute cocaine administration (McFarland et al., 2003), the rapid internalization of AMPA receptors following such treatment is predictable because of the increase of glutamate in the synaptic cleft. On the other hand, group I mGluRs, located further away from these synapses may be less affected by this synaptically regulated change in glutamate release. Although this may partly account for the differential responses of these receptors after acute cocaine, it is unlikely to be the case in chronically treated animals which display a significant reduction in extracellular glutamate mainly due to functional changes in the cystine/glutamate exchangers predominantly located on glial cells (Baker et al., 2003). Overall, these findings indicate that cocaine can induce significant changes in AMPA receptor subsynaptic localization, but not as much in group I mGluRs. The mechanisms underlying these differential effects may be complex and involve a multitude of factors including the localization, trafficking as well as the pharmacological and physiological properties of these receptors.
A few in vivo studies demonstrated significant changes in the ultrastructural localization of GPCRs in response to modulation of extracellular activating neurotransmitter (Dumartin et al., 1998; Dumartin et al., 2000; Bernard et al., 2003; Toda et al., 2003; Stanwood and Levitt, 2007). However, in MPTP-treated mice, an experimental condition characterized by a significant increase in glutamatergic transmission in the subthalamic nucleus and globus pallidus (Mally et al., 1997; Fedele et al., 2001; Wichmann and DeLong, 2003), very little, if any, changes were induced in the subsynaptic and subcellular distribution of group I mGluRs in these nuclei (Kuwajima et al., 2007). Together, these findings highlight the unique intrinsic properties of group I mGluRs compared to other GPCRs in their response to changes in ambient activating transmitter levels. Receptor phosphorylation, lateral trafficking and receptor-receptor interactions are additional mechanisms, not examined in this study, that should be considered as playing major roles in regulating group I mGluRs responses to agonist exposure (Bernard et al., 2006; Dhami et al., 2006).
In contrast to systemic cocaine, intracerebral injections of group I mGluR agonist significantly decreased the percentage of plasma membrane-bound mGluR1a and mGluR5 in the accumbens. These observations are consistent with previous studies of other GPCRs known to internalize following direct agonist application (Bernard et al., 1998; ; Dumartin et al., 1998; Bernard et al., 1999; Csaba et al., 2001). Together, these in vivo data demonstrate that group I mGluRs share common internalization properties with other GPCRs when directly exposed to high dose of receptor agonists, but not in response to physiological or pathological changes in the level of ambient activating neurotransmitter. One possible explanation might be that the magnitude of cocaine-induced changes in glutamate release is not high enough to affect group I mGluRs trafficking. Another possibility is that the mechanism underlying the internalization of group I mGluRs after DHPG is different from that following glutamate-mediated receptor activation. For instance, in vitro data have shown that application of glutamate versus the agonist quisqualate attract different arrestin proteins that aid in internalization of mGluR1a (Dale et al., 2001; Mundell et al., 2001). Thus, the mechanisms underlying group I mGluRs internalization appear to be quite complex and highly dependent on various factors including the source and pharmacological properties of the activating agonist.
Acknowledgments
The authors would like to thank Susan Jenkins and Jean-Francois Pare for their valuable technical assistance. This work was supported by NIH grants RR 00165 to the Yerkes National Primate Research Center, R01NS037423 to YS and 5F31DA020301-02 to DM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20(2):323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11(4):771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnár E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69(4):1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Bernard V, Laribi O, Levey AI, Bloch B. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18(23):10207–18. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Levey AI, Bloch B. Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J Neurosci. 1999;19(23):10237–49. doi: 10.1523/JNEUROSCI.19-23-10237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Brana C, Liste I, Lockridge O, Bloch B. Dramatic depletion of cell surface m2 muscarinic receptor due to limited delivery from intracytoplasmic stores in neurons of acetylcholinesterase-deficient mice. Mol Cell Neurosci. 2003;23(1):121–133. doi: 10.1016/s1044-7431(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Bernard V, Decossas M, Liste I, Bloch B. Intraneuronal trafficking of G-protein-coupled receptors in vivo. Trends in Neurosci. 2006;29(3):140–147. doi: 10.1016/j.tins.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27(39):10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25(40):9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Celio MR, Baier W, Scharer L, Gregersen HJ, de Viragh PA, Norman AW. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium. 1990;11(9):599–602. doi: 10.1016/0143-4160(90)90014-l. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4(9):873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Csaba Z, Bernard V, Helboe L, Bluet-Pajot MT, Bloch B, Epelbaum J, Dournaud P. In vivo internalization of the somatostatin sst2A receptor in rat brain: evidence for translocation of cell-surface receptors into the endosomal recycling pathway. Mol Cell Neurosci. 2001;17(4):646–661. doi: 10.1006/mcne.2000.0958. [DOI] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol Pharmacol. 2001;60(6):1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- Dhami G, Ferguson S. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacology & Therapeutics. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187(4):397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci. 1998;18(5):1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumartin B, Jaber M, Gonon F, Caron MG, Giros B, Bloch B. Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice. Proc Natl Acad Sci U S A. 2000;97(4):1879–1884. doi: 10.1073/pnas.97.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele E, Mazzone P, Stefani A, Bassi A, Ansaldo MA, Raiteri M, Altibrandi MG, Pierantozzi M, Giacomini P, Bernardi G, Stanzione P. Microdialysis in Parkinsonian patient basal ganglia: acute apomorphine-induced clinical and elctrophysiological effects not paralleled by changes in the release of neuroactive amino acids. Exp Neurol. 2001;167:356–365. doi: 10.1006/exnr.2000.7568. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24(31):6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143(2):351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G Protein-Coupled Receptors and Neuronal Functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Nelson LC, Lu XY, Kalivas PW. Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J Neurochem. 1999;72(1):157–165. doi: 10.1046/j.1471-4159.1999.0720157.x. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14(11):1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther. 1988;245(2):485–492. [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179(1):247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Koulen P, Kuhn R, Wassle H, Brandstatter JH. Group I metabotropic glutamate receptors mGluR1alpha and mGluR5a: localization in both synaptic layers of the rat retina. J Neurosci. 1997;17(6):2200–2211. doi: 10.1523/JNEUROSCI.17-06-02200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004;474(4):589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci. 2007;27(23):6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312(3):1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Mally J, Szalai G, Stone TW. Changes in the concentration of amino acids in serum and cerebrospinal fluid of patients with Parkinson’s disease. J Neurological Sci. 1997;151:159–162. doi: 10.1016/s0022-510x(97)00119-6. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21(16):5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47(3):240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Pattiselanno A, Groenewegen HJ, Haber SN. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol. 1996;365(4):628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500(4):788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Miyata S, Nakai S, Kiyohara T, Hatton GI. Calbindin-D28k and calretinin in the rat posterior pituitary; light and electron microscopic localization and upregulation with dehydration. J Neurocytol. 2000;29(1):5–17. doi: 10.1023/a:1007180328597. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78(3):546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292(5518):923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Landsend AS. Organization of glutamate receptors at the synapse. Eur J Neurosci. 1997;9(11):2219–2224. doi: 10.1111/j.1460-9568.1997.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. London: Academic Press Limited; 1998. [Google Scholar]
- Peters A, Palay S, Webster HD. The fine structure of the nervous system: Neurons and their supporting cells. New York: Oxford University Press; 1991. p. 494. [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16(4):1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt W, Knöpfel T. Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophysiol. 2002;87(4):1974–1980. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- Reid M, Hsu K, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system. Synapse. 1997;27(2):95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274(36):25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Lehre KP. Perisynaptic asymmetry of glia: new insights into glutamate signalling. Trends Neurosci. 2002;25(10):492–494. doi: 10.1016/s0166-2236(02)02230-0. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683(2):264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27(1):152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21(22):9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Alguacil LF, Kalivas PW. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem. 2003;87(6):1478–1484. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong M. Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Ann NY Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Winsky L, Nakata H, Martin BM, Jacobowitz DM. Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci U S A. 1989;86(24):10139–10143. doi: 10.1073/pnas.86.24.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]