Abstract
Myoblast fusion is essential for muscle development, postnatal growth and muscle repair after injury. Recent studies have demonstrated roles for actin polymerization during myoblast fusion. Dynamic cytoskeletal assemblies directing cell–cell contact, membrane coalescence and ultimately fusion require substantial cellular energy demands. Various energy generating systems exist in cells but the partitioning of energy sources during myoblast fusion is unknown. Here, we demonstrate a novel role for phosphocreatine (PCr) as a spatiotemporal energy buffer during primary mouse myoblast fusion with nascent myotubes. Creatine treatment enhanced cell fusion in a creatine kinase (CK)-dependent manner suggesting that ATP-consuming reactions are replenished through the PCr/CK system. Furthermore, selective inhibition of actin polymerization prevented myonuclear addition following creatine treatment. As myotube formation is dependent on cytoskeletal reorganization, our findings suggest that PCr hydrolysis is coupled to actin dynamics during myoblast fusion. We conclude that myoblast fusion is a high-energy process, and can be enhanced by PCr buffering of energy demands during actin cytoskeletal rearrangements in myoblast fusion. These findings implicate roles for PCr as a high-energy phosphate buffer in the fusion of multiple cell types including sperm/oocyte, trophoblasts and macrophages. Furthermore, our results suggest the observed beneficial effects of oral creatine supplementation in humans may result in part from enhanced myoblast fusion.
Satellite cells present in adult mammalian muscle play a key role in muscle repair and growth. In response to extracellular signals, quiescent satellite cells begin to proliferate and their progeny myoblasts differentiate and fuse to form myotubes, a process called myogenesis. Myotube formation and growth are regulated by two types of fusion events (Horsley & Pavlath, 2004). First, differentiated myoblasts fuse together to form small nascent myotubes containing few nuclei. Subsequently, additional fusion between myoblasts and nascent myotubes results in the formation of large myotubes containing multiple nuclei. The molecular mechanisms of myoblast fusion are incompletely understood.
Cell fusion is an ordered process of migration, cell–cell contact and adhesion followed by plasma membrane alignment and fusion. Extensive cytoskeletal reorganization occurs before and during fusion (Fulton et al. 1981). Dynamic cytoskeletal assemblies regulating cell fusion are dependent upon the hydrolysis of ATP. Although the polymerization and dissociation of actin monomers can account for up to 50% of cellular energy expenditure (Daniel et al. 1986; Bernstein & Bamburg, 2003), the partitioning of energy sources during myoblast fusion has never been studied. Does preferential hydrolysis of a high-energy phosphate buffer occur as cells renounce their individual status and fuse to form multinucleated myotubes?
The universal currency of energy in all cells, ATP, is generated by oxidative phosphorylation in the mitochondria. Two mitochondrial isoforms of creatine kinase (CK), sarcomeric (sMitoCK) expressed in skeletal muscle and ubiquituous (uMitoCK) present in other cell types, catalyse the formation of phosphocreatine (PCr), which transports the high-energy phosphate emanating from mitochondrial sites of ATP production to sites of ATP consumption within cytoplasmic compartments by facilitative diffusion (Meyer et al. 1984). In contrast to ATP, PCr readily diffuses throughout the cell due to favourable physiochemical properties including concentration, size and polarity (Wyss & Kaddurah-Daouk, 2000) and thus can serve as a freely diffusing energy buffer (Wiseman & Kushmerick, 1995). Additionally, PCr buffers intracellular ATP/ADP ratios without adverse affects on pH or the activity of other cellular enzymes. The relative importance of PCr in energy shuttling versus ATP buffering differs among cell types (Wallimann et al. 1992).
Cytosolic isoforms of CK replenish local ATP levels at sites of high ATPase activity by catalysing the transfer of phosphate from PCr to ADP thereby preventing ATP depletion. Cytosolic CK enzymes are composed of both homo- and heterodimeric subunits expressed in a tissue-specific manner. A developmental transition from the brain homodimeric isoform of CK (CK-BB), to a muscle–brain hybrid (CKMB) and finally to a muscle-specific isoform (CK-MM) occurs during myoblast differentiation (Chamberlain et al. 1985). Individual isoforms vary with respect to half-life, kinetic properties and distribution within the cell. While the muscle subtype of creatine kinase (CKM) localizes to the contractile apparatus (Schafer & Perriard, 1988), sequence diversity in the carboxy terminus promotes localization of the brain subtype of creatine kinase (CKB) to the plasma membrane and mitotic spindles (Cande, 1983; Silver et al. 1983).
CK is associated with cytoskeletal rearrangements and cell motility. CKB relays thrombin-mediated signals to the cytoskeleton inducing morphological alterations in astrocyte cell shape (Mahajan et al. 2000). In sea urchin sperm, CKB is concentrated in the distal region of the sperm tail (Tombes et al. 1988) where it binds the cytoskeletal protein dynein at the plasma membrane. Pharmacological inhibition of CK reduces sperm migration suggesting a role for CK in localized ATP replenishment during cell motility. Additionally, treatment of tumour cells with cyclocreatine, a competitive inhibitor of CK, decreases tumour cell motility (Mulvaney et al. 1998). Therefore, CK probably plays a role in localized ATP regeneration in cells where sites of ATP production are separated by large distances from sites of consumption.
We hypothesized that myogenesis is a high-energy process requiring PCr. Our data support a model in which the PCr/CK energy system sustains localized ATP-dependent reactions during actin polymerization in myoblast fusion.
Methods
Ethical approval
All procedures were approved by Emory University's Institutional Animal Care and Use Committee and were in compliance with US National Institutes of Health (NIH) guidelines.
Cell culture
Adult Balb/c mice were killed by cabon dioxide inhalation and hindlimb muscles were collected. Primary myoblasts were isolated as described (Mitchell & Pavlath, 2004) excluding differential centrifugation with Percoll. Myoblast purity was > 90% as confirmed by immunostaining for MyoD. Cells were cultured in growth medium (GM) and differentiation medium (DM) as described (O'Connor et al. 2007).
Cell differentiation and fusion assays
Myoblasts were treated with various concentrations of creatine monohydrate (Sigma) in DM and subsequently immunostained for embryonic myosin heavy chain (eMyHC) as described (Horsley et al. 2003). To analyse differentiation, the number of nuclei in eMyHC+ cells was counted after 24 h in DM and expressed as a percentage of the total nuclei analysed. To analyse fusion, the number of nuclei in myotubes containing two or more nuclei was expressed as a percentage of the total nuclei quantified. The average number of nuclei contained within myotubes was also calculated. Ten random fields containing approximately 1000 nuclei were analysed in each condition. In all assays three independent cell isolates were analysed.
Measurement of intracellular phosphocreatine by HPLC
Myoblasts were plated and several hours later, the medium was replaced with GM containing creatine at either 12.5 mm or 50 mm. Following 24 h, the medium was replaced with fresh creatine-containing medium for an additional 24 h. Cells were harvested in 0.5 m perchloric acid and 5 mm EDTA and subsequently lysed by repeated cycles of freeze/thawing. The samples were then centrifuged at 10 000 g for 10 min and the pellet was resuspended in 1 m NaOH for protein assay using the Bradford method (Bradford, 1976). The supernatant was neutralized to pH 7.0 with a solution of 2 n KOH, 150 mm TES and 0.3 m KCl and then centrifuged for 20 min at 20 200 g. The resulting supernatant was rapidly frozen in liquid nitrogen and stored at −80°C for HPLC analyses.
HPLC analysis was performed as described (Wiseman et al. 1992). Briefly, PCr was eluted from an anion-exchange column using a linear phosphate gradient (KH2PO4) from 50 mm (pH 4.5) to 500 nm (pH 2.7) at a flow rate of 2.0 ml min−1 at room temperature. PCr was quantified by comparing the area under the peaks of sample metabolites against known PCr standards that were calibrated enzymatically. Three independent experiments were analysed. In each experiment, all measurements were performed in duplicate to determine the intracellular concentration of PCr.
Cell migration assays
To evaluate the effect of creatine on cell motility, primary myoblasts were plated and switched to either DM or DM containing 12.5 mm creatine after 1 h. After 24 h, the dishes were transferred to a thermoregulated microscope stage maintained at 37°C and 5% CO2. Cells were visualized using a Zeiss Axiovert 200M microscope with a 0.3 NA 10× Zeiss Plan-Neofluar objective and images recorded (QImaging camera and Openlab version 5.0.2 software, Improvision) every 6 min for 80 min. The movement of individual cells was tracked using ImageJ software (version 1.36) with manual tracking plug-ins. The initial point of each migratory path was mathematically set at 0,0 and subsequent displacement and velocity were calculated using Excel (version 5.0). Cell migration was quantified from triplicate independent experiments, scoring 15–18 cells per condition in each experiment.
RNA interference
An oligo encoding small interfering RNA for mouse CKB (5′-GGAAGTGTTCACCCGATTC-3′) was cloned into the retroviral plasmid pSuperRetro (Oligoengine Inc., Seattle, WA, USA). This oligo was specific for its target gene by BLAST sequence analyses. As a control, an oligo containing scrambled small interfering RNA (siRNA) sequence from the mouse β-actin gene was also cloned. Retroviral infections were performed as described (Abbott et al. 1998). Three to five days following retroviral infection, myoblasts were transiently transfected with 80 nm double stranded ribonucleotides (dsRNA) against CKM (5′-GGCUACAAACCCACAGACAAGCAUA-3′) using lipofectamine 2000 (Invitrogen). Alternatively, myoblasts containing control retrovirus were transiently transfected with control ribonucleotides containing scrambled sequence. Transfections were performed as described (Horsley and Pavlath, 2003).
Creatine kinase assays
Cells expressing either control siRNA or siRNA for both CKB and CKM were differentiated for 42–46 h and subsequently lysed in glycylglycine buffer (0.05 m glycylglycine, 1% ipegal and 0.1%β-mercaptoethanol). Cell lysates were incubated with creatine kinase assay reagent (Pointe Scientific) at 37°C for 5 min. As creatine kinase activity is directly proportional to the reduction of NAD+ at 340 nm, the average absorbance per minute was calculated. Creatine kinase activity was measured in triplicate using two independent cell isolates.
CK-BB immunocytochemistry
For detection of CK-BB in differentiating/fusing myoblasts, cells were fixed in ice-cold 100% methanol and incubated in blocking buffer (phosphate-buffered saline (PBS) containing 5% donkey serum, 0.5% BSA, 0.25% Triton X-100) followed by overnight 4°C incubation with either rabbit anti-human CK-BB (Abcam) or control rabbit IgG diluted 1: 100 in blocking buffer. Cells were incubated with biotin-conjugated donkey anti-rabbit IgG diluted 1: 250 in PBS + 0.2% Tween-20, then with streptavidin–horseradish peroxidase (HRP) diluted 1: 200 in TNB [0.1 M Tris-HC1, pH 7.5, 0.15 M NaCl, and 0.5% blocking reagent (Perkin Elmer, Waltham, MA, USA)]. The tyramide signal amplification green reagent (Perkin Elmer) diluted 1: 250 in amplification reagent was used to visualize antibody binding. No staining was observed with control antibody. Images were acquired using an Axiovert 200M microscope with a 0.3 NA 20× LD A-Plan objective (Carl Ziess MicroImaging, Inc.), and images were recorded (QImaging Camera and OpenLab 5.0.2 software). Images were assembled using Illustrator CS (Adobe) software and were not modified with the exception of equal adjustments in size, brightness and contrast.
Muscle injury and histological analyses
Male and female C57BL/6 mice (n = 9), of between 8 and 12 weeks of age were used. Mice were housed under a 12 h dark–light cycle and received food and water ad libitum. Mice were anaesthetized by intraperitoneal injection of a mixture of xylazine (15 mg kg−1) and ketamine (100 mg kg−1). Local muscle injury was induced by injection of 50 μl of 1.2% BaCl2 in PBS using a Hamilton syringe and a 27 G needle into both tibialis anterior (TA) muscles. A 70 mm creatine solution in PBS (50 μl) was injected intramuscularly at 24 and 48 h after injury. The contralateral muscle received PBS alone. Mice were killed by cabon dioxide inhalation 5 days following injury. TA muscles were collected, embedded in mounting medium, and frozen in isopentane cooled in liquid nitrogen. Cyrosections from the belly of the TA muscle were analysed by quantifying myofibre cross-sectional area (XSA) for 100–250 myofibres in a 307 200 μm2 field in the core of the regenerating muscle (Horsley et al. 2003). All analyses were performed double-blinded.
Image preparation and statistical analyses
Unless stated, images were obtained using a Zeiss Axioplan microscope with a 0.3 NA 10× Zeiss Plan-Neofluar objective. Images were captured using Scion Image (version 1.63) and globally processed for brightness and contrast using Adobe Photoshop (version 7.0).
To determine statistical significance, the mean data obtained from two groups were analysed using Student's t test for unpaired data. Data from multiple groups were analysed by ANOVA with Neuman–Keuls post hoc test using GraphPad Prism version 4.0a (GraphPad Software Inc., San Diego, CA, USA). For all tests, the significance of the difference between means was accepted at the 0.05 level.
Results
Creatine enhances myonuclear addition and myotube growth
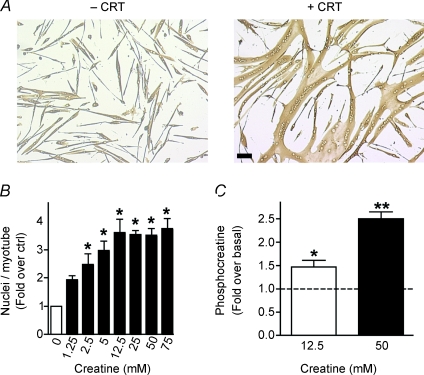
To test the hypothesis that myogenesis relies on intracellular stores of the high-energy phosphate buffer PCr, differentiating cultures were treated with creatine for 48 h. Creatine treatment greatly enhanced both myotube growth and myonuclear addition (Fig. 1A). Skeletal muscle is enriched with 30–40 mm of intracellular creatine (Snow & Murphy, 2001); 66% of this pool is phosphorylated, acting as a high-energy phosphate buffer. To determine the effective range of creatine doses on myonuclear addition, differentiating myoblasts were treated with various concentrations of creatine (1.25–75 mm). These concentrations were similar in magnitude to other studies (Ingwall et al. 1972; Pulido et al. 1998; Vierck et al. 2003). A dose-dependent effect was observed up to 12.5 mm creatine after which a plateau in myonuclear addition was obtained (Fig. 1B).
Figure 1. Creatine enhances myonuclear addition in a dose-dependent manner.
A, representative images of primary mouse muscle cells differentiated for 48 h in the absence (–) or presence (+) of 75 mm creatine (CRT) are shown. Bar: 100 μm. B, a dose-dependent increase occurred in myonuclear number with creatine treatment for 48 h with evidence of saturation at higher concentrations. Data are means ±s.e.m. from three independent experiments. (*P < 0.05 relative to control.) C, primary myoblasts were treated with either 12.5 or 50 mm creatine. Creatine enhanced intracellular PCr accumulation as measured by HPLC analysis. All data were normalized to protein content and values are means ±s.e.m. from three independent experiments. (*P < 0.05 relative to control.)
In line with previous studies (Ceddia & Sweeney, 2004; Alfieri et al. 2006), increasing extracellular levels of creatine to 12.5 mm enhanced intracellular PCr stores from 53.9 to 78.5 mmol (mg protein)−1 (Fig. 1C), a 1.5-fold increase without any effect on ATP levels (data not shown). Although creatine uptake into cells is regulated by a transport-mediated saturable process (Wyss & Kaddurah-Daouk, 2000), increasing the concentration of exogenous creatine to 50 mm further enhanced the intracellular accumulation of PCr to 134.4 mmol (mg protein)−1 (Fig. 1C), a 2.5-fold increase relative to basal without any effect on ATP levels (data not shown). Thus, the plateau in myonuclear addition at 12.5 mm creatine suggests that myonuclear addition is limiting rather than the expression and/or activity of the creatine transporter. These results indicate that myogenesis is enhanced in the presence of increased PCr.
Creatine mediated-growth is dependent on actin polymerization
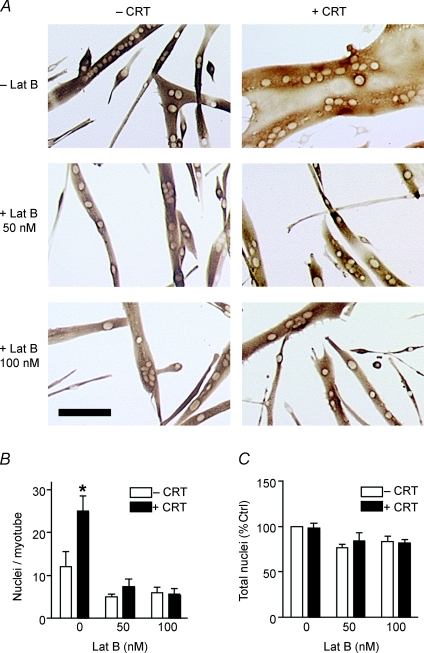
As myoblasts renounce their individual status, extensive cytoskeletal rearrangements direct membrane coalescence, fusion pore formation and cytoplasmic continuity (Fulton et al. 1981). In Drosophila myogenesis actin polymerization regulates the fusion process between fusion competent myoblasts and founder cells (Kim et al. 2007; Massarwa et al. 2007; Richardson et al. 2007). The polymerization and dissociation of actin filaments is ATP dependent with actin dynamics accounting for up to 50% of cellular energy expenditure (Daniel et al. 1986; Bernstein & Bamburg, 2003). Thus, as actin polymerization is required during multiple phases of the fusion process and the high-energy phosphate buffer PCr enhances myoblast fusion, we hypothesized that creatine-mediated myotube growth occurs in an actin-dependent manner. We differentiated mouse myoblasts in the presence of creatine for the initial 24 h of myogenesis and subsequently treated them with either 50 or 100 nm latrunculin B (Lat B) for the final 24 h. Although the equilibration dissociation constant for the interaction of Lat B and actin monomers is 200 nm (Coue et al. 1987; Yarmola et al. 2000), most studies use a range of doses from 1 to 5 μm Lat B (Ivanov et al. 2004; Murakoshi et al. 2004). Thus, as the dose used in our study is an order of magnitude less than those in other studies, both drug-induced toxicity and non-specific side-effects should be minimal. Either 50 or 100 nm Lat B was sufficient to inhibit creatine-mediated growth (Fig. 2A and B). Inhibition of actin polymerization with Lat B resulted in minor deficits in myoblast fusion in the absence of creatine (Fig. 2B). No difference existed in the total number of nuclei per field following Lat B treatment suggesting that the effects of Lat B are not due to loss of cells (Fig. 2C). As Lat B binds to actin through its nucleotide binding domain (Belmont et al. 1999) and inhibits ATP turnover (Ayscough et al. 1997; Yarmola et al. 2000), our data are consistent with a role for PCr as a high-energy phosphate buffer, enhancing ATP repletion and subsequent actin polymerization.
Figure 2. Actin polymerization is required for creatine-mediated effects.
A, myoblasts were differentiated in the presence or absence of 12.5 mm creatine (CRT) for 24 h. This medium was subsequently replaced with DM containing vehicle, 50 or 100 nm latrunculin B (Lat B) for the remainder of myogenesis. Representative images following 48 h of differentiation are shown. Bar: 100 μm. B, myonuclear number was reduced to control levels in cells that received either 50 or 100 nm Lat B following creatine treatment. C, no significant difference in the total number of nuclei per field occurred with either creatine or Lat B treatment. Data are means ±s.e.m. from three independent experiments. (P < 0.05.)
Cell motility is unaltered following creatine treatment
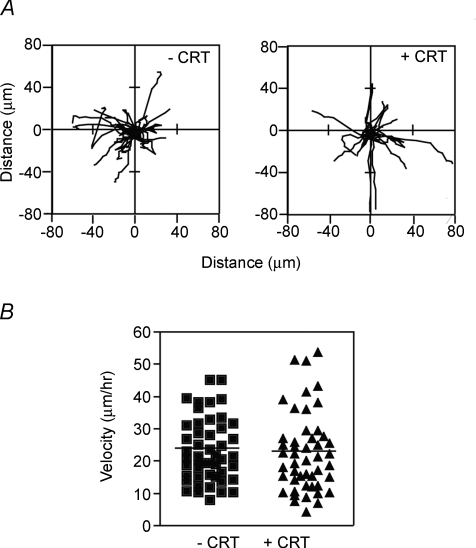
Cell fusion involves a multistep process of cell recognition, alignment, adhesion and membrane coalescence. Cell migration is indispensable for the alignment of fusion competent myoblasts. Previous studies have demonstrated a role for PCr/CK in both spermatocyte and glial cell motility (Tombes et al. 1988; Mulvaney et al. 1998; Wang et al. 1998). CKB is located at the plasma membrane (Cande, 1983; Silver et al. 1983) suggesting potential roles in membrane dynamics during both migration and fusion. However, analysing the motility of differentiated myoblasts by time-lapse microscopy for 80 min revealed that creatine had no effect on either the migratory paths of individual cells (Fig. 3A) or the average velocity of these cells (Fig. 3B). These results suggest that creatine modulates cell fusion independent of changes in cell motility.
Figure 3. Myoblast motility is unaffected by creatine.
A, myoblasts were differentiated in the presence and absence of 12.5 mm creatine (CRT) for approximately 24 h and then time-lapse photographs were recorded every 6 min for 80 min. The migratory paths of individual mononucleated cells are shown. Paths of 8–10 cells from each of three independent experiments were pooled for a combined total of 26 paths. B, the mean velocity was similar in control and creatine-treated cells. Data are scatter plots of 48 cells (approximately 15 cells per experiment).
Creatine enhances myonuclear addition independent of either proliferation or differentiation
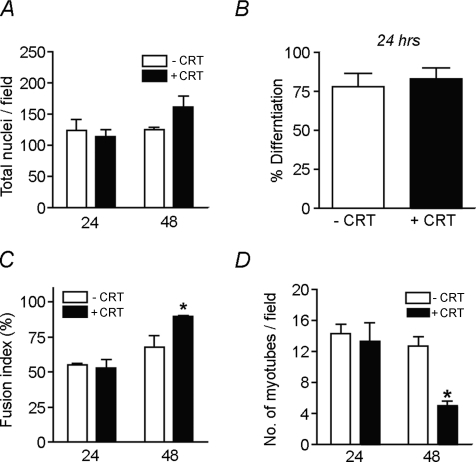
Myogenesis involves an ordered progression of myoblast proliferation, differentiation and fusion. Therefore, we analysed whether creatine modulated cell fusion independent of effects on earlier stages of myogenesis. Creatine had no effect on myoblast proliferation/survival (Fig. 4A) or differentiation (Fig. 4B). Similarly, creatine had no effect on the initial fusion of myoblasts to form nascent myotubes as the fusion index was similar in control and experimental groups after 24 h in DM (Fig. 4C). However, the enhanced fusion index at 48 h suggests creatine promoted myoblast fusion with nascent myotubes at later stages of myogenesis (Fig. 4C). Additionally, the decline in the number of myotubes per field (Fig. 4D) suggests creatine may also promote myotube–myotube fusion during these later times. Taken together, creatine enhances myonuclear addition to nascent myotubes independent of either myoblast proliferation or differentiation.
Figure 4. Creatine enhances myoblast fusion independent of proliferation or differentiation.
Myoblasts were differentiated in the presence and absence of 75 mm creatine (CRT) for either 24 or 48 h and subsequently immunostained for eMyHC. A, no significant difference in the total number of nuclei per field occurred with creatine treatment. B, creatine did not effect the percentage of nuclei in eMyHC+ cells at 24 h. C, the fusion index was significantly increased in cells treated with creatine for 48 h. D, the number of myotubes per field was significantly decreased following 48 h of creatine treatment. Data are means ±s.e.m. from three independent experiments. (*P < 0.05.)
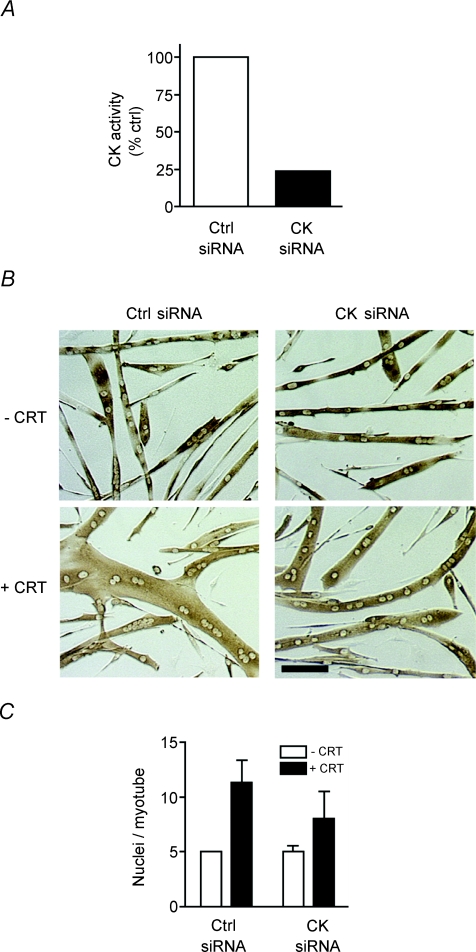
CK activity is necessary for creatine-mediated myonuclear addition
PCr enhances ATP repletion in a CK-dependent manner in multiple cells with high-energy demands including neurons, skeletal muscle and spermatozoa (Wyss & Kaddurah-Daouk, 2000). To determine if CK plays a role in myonuclear addition, myoblasts were transduced with siRNA for CKB and CKM and differentiated into myotubes. As functional redundancy exists between CKB and CKM (Renema et al. 2007), we targeted both isoforms of CK, decreasing enzymatic activity by 70% (Fig. 5A). In the absence of creatine no difference was observed in the average number of nuclei per myotube in cells expressing either control siRNA or siRNA for CKB and CKM (Fig. 5B and C), suggesting residual CK activity may be sufficient for myoblast differentiation and myotube formation. However, in the presence of creatine, myonuclear addition was diminished on average by 27% in cells containing siRNA against CKB and CKM (Fig. 5C). These data demonstrate that creatine-induced myonuclear addition to nascent myotubes is partially dependent upon CK activity.
Figure 5. Creatine enhances myonuclear addition through a CK-dependent mechanism.
A, myoblasts containing siRNA for CKB and CKM or a control siRNA were differentiated for 42–46 h. Myotube lysates were analysed for creatine kinase (CK) activity. Data are means from two independent experiments. B, representative images of siRNA containing cells following differentiation for 48 h in the absence (–CRT) and presence of 12.5 mm creatine (+CRT) are shown. Bar: 100 μm. C, following creatine treatment myonuclear number was decreased in cells containing siRNA for CK. Data are means ±s.e.m. from three independent experiments.
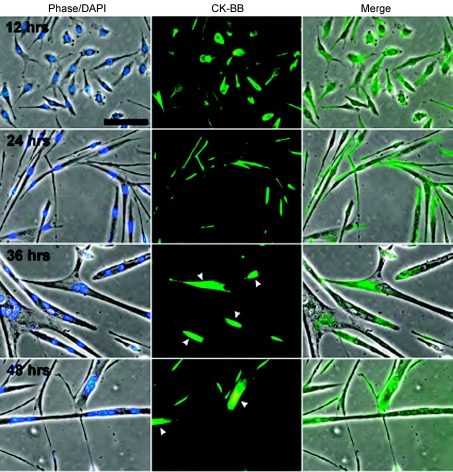
CKB becomes highly localized during muscle differentiation
We hypothesized that CK may be localized in differentiating/fusing muscle cells at potential sites of high ATP turnover. CKB in particular has been shown to relay signals to the cytoskeleton (Mahajan et al. 2000), and thus, to determine the localization of CK-BB during differentiation and fusion, immunocytochemistry was performed at different times during myogenesis (Fig. 6). After 12 h in DM, CK-BB was relatively evenly distributed throughout the cell cytoplasm, whereas at 24 h when myoblasts were beginning to fuse, CK-BB became more localized. At 36 and 48 h in DM as myoblast fusion progressed and mature myotubes formed, CK-BB became prominently localized to the ends of myotubes (arrowheads). The progression of CK-BB polarization during myogenesis suggests that CKB might be playing a role in the cytoskeletal rearrangements required for fusion and is consistent with the hypothesis that PCr provides energy for the actin-mediated fusion process.
Figure 6. CK-BB becomes highly localized as myoblast differentiation and fusion progresses.
Representative images obtained from myoblasts differentiated for 12, 24, 36 and 48 h and immunostained with a CK-BB antibody. The nuclei were visualized with DAPI. After 12 h in DM, CK-BB was fairly regularly distributed throughout the cytoplasm but became prominently localized to the ends of myotubes after 36–48 h (arrowheads). Bar = 40 μm.
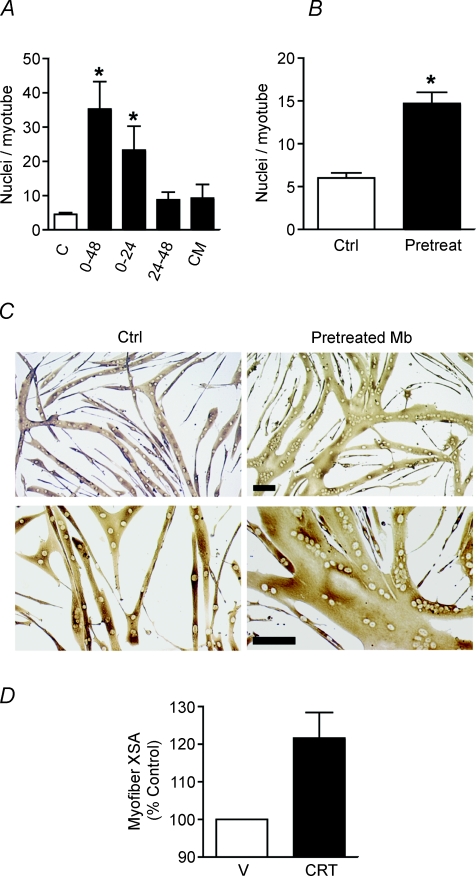
Myotube growth requires creatine during the initial 24 h of myogenesis
Due to the observed affects of creatine on cell fusion only after 48 h of myogenesis (Fig. 4), we hypothesized that PCr accumulates early in myogenesis and may act as a spatiotemporal energy buffer supplying ATP in later stages. Experiments were performed varying the timing of creatine addition during differentiation. Creatine treatment greatly increased myonuclear addition over 48 h (Fig. 7A), but supplementing muscle cultures with creatine during 24–48 h of myogenesis did not enhance fusion. In contrast, the presence of creatine during the initial 24 h of myogenesis was both necessary and sufficient for enhanced fusion during later stages of myogenesis also (Fig. 7A).
Figure 7. Pretreating proliferating myoblasts with creatine increases myonuclear addition to nascent myotubes.
A, myoblasts were differentiated for 48 h in the presence or absence (C) of 75 mm creatine in DM. 0–48: cells received creatine throughout myogenesis. 0–24: cells received creatine during the first 24 h of differentiation only. This medium was subsequently replaced with DM for the later 24 h of myogenesis. 24–48: these cells received DM for the initial 24 h of differentiation. These cells were supplemented with 75 mm creatine without a media change from 24 to 48 h. CM: cells received DM for the initial 24 h of differentiation. This was replaced with conditioned medium (CM) collected from a parallel dish of differentiating myoblasts treated with creatine during the initial 24 h of myogenesis. Data are means ±s.e.m. from three independent experiments. (*P < 0.05 relative to control.) No statistical difference was observed between 0–24 h and 0–48 h of creatine treatment. B, proliferating myoblasts were cultured in GM containing 12.5 mm creatine for 2 days and then switched to DM in the absence of creatine for 48 h. Myonuclear number was significantly increased in cells that received creatine prior to the induction of differentiation. Data are means ±s.e.m. from three independent experiments. (*P < 0.05 relative to control.) C, representative images of cells pretreated with creatine as myoblasts (Mb) are shown. Bar: 100 μm. D, the XSA of regenerating TA myofibres 5 days following injury was increased in creatine-treated mice. Values are means ±s.e.m., n = 9 per treatment group.
Secreted proteins such as growth hormone (Sotiropoulos et al. 2006), IGF-1 (Jacquemin et al. 2004) and the cytokines IL-4 and IL-13 (Horsley et al. 2003; Jacquemin et al. 2007) all contribute to myonuclear addition to nascent myotubes. To determine if secreted factors are induced in a creatine-dependent manner and may contribute to its growth-inducing affects, medium was collected from differentiating myoblasts treated with creatine for the initial 24 h of myogenesis and substituted for DM in parallel fusing cultures completing the final 24 h of myogenesis. Conditioned medium (CM) did not increase myonuclear addition demonstrating that creatine did not induce the secretion of a stable soluble factor that acts during the second phase of myoblast fusion (Fig. 7A).
Pretreating proliferating myoblasts with creatine enhances myonuclear addition during differentiation in the absence of creatine
To analyse further if creatine is a spatiotemporal energy buffer, we treated proliferating myoblasts over two consecutive days with 12.5 mm creatine and subsequently differentiated them for 48 h in the absence of creatine. The addition of creatine to proliferating myoblasts promoted myonuclear addition during myoblast differentiation without any exogenous creatine (Fig. 7B and C). As myoblasts express the creatine transporter (data not shown) these data are consistent with an accumulation of PCr in myogenic progenitor cells promoting enhanced fusion events during later stages of myogenesis.
Creatine increases growth of regenerating myofibres in vivo
Skeletal muscle regeneration is dependent upon a local source of myogenic stem cells that fuse together to form nascent myofibres in a temporally regulated manner. Satellite cells undergo an ordered progression involving release from quiescence, proliferation, expression of muscle-specific genes and finally fusion together to form terminally differentiated myofibres. Based on our in vitro results, we hypothesized that enhancing localized stores of creatine in differentiating myoblasts in vivo would enhance skeletal muscle repair following injury. To determine if exogenous creatine promotes muscle regeneration, we injured the tibialis anterior muscles of mice by intramuscular injection of BaCl2 and then locally injected either 50 μl vehicle or 70 mm creatine into the injured muscles on the following two days, a time of extensive myoblast proliferation. The XSA of regenerating myofibres was enhanced 20% in muscles injected with creatine (Fig. 7D) 5 days following injury. Collectively, these findings suggest that the accumulation of a spatiotemporal energy buffer in myoblasts enhances later stages of muscle growth both in vitro and in vivo.
Discussion
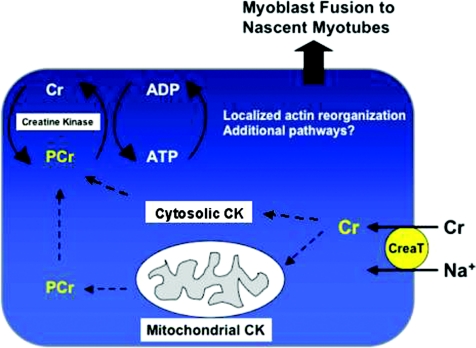
We uncovered a role for PCr as a spatiotemporal energy buffer during myoblast fusion independent from contractile activity suggesting that myogenesis is a high-energy process. Creatine selectively enhanced myonuclear addition to nascent myotubes suggesting the energy cost of cell fusion with multinucleated myotubes may be higher than the fusion of two mononucleated cells. Our data support a model in which the PCr/CK energy system sustains localized ATP-dependent reactions during actin polymerization in myoblast fusion (Fig. 8).
Figure 8. Working model for enhanced myonuclear addition following creatine treatment.
Similar to other cells, creatine is transported into muscle cells through the creatine transporter (CreaT). Subsequently, creatine is converted to PCr by mitochondrial and cytosolic CK. We propose that PCr diffuses to sites of high-energy demands where it acts as a spatiotemporal energy buffer during myoblast fusion. PCr hydrolysis by local CKB or CKM provides dynamic bursts of energy during actin cytoskeletal rearrangements associated with the fusion of myoblasts to nascent myotubes.
Our findings are complemented by multiple studies demonstrating increasing energy demands during myogenesis (Den et al. 1975; Plaghki & Marechal, 1976; Fulco et al. 2003). Although energy is supplied through the cooperative interplay of multiple systems, the accumulation of PCr in proliferating and differentiating cultures may facilitate maximal rates of ATP replenishment during myoblast fusion. While the oxidation of glucose may provide energy for myoblast fusion, anerobic glycolysis promotes intracellular acidification through proton accumulation. This may be detrimental to proteins actively engaging in the fusion process. Thus, fusing myoblast cultures may be predisposed to PCr hydrolysis during signal transduction in myogenesis. Furthermore, during muscle regeneration, a reduction in localized oxygen tension and pH favour the enzyme kinetics of creatine kinase-mediated ATP repletion.
Creatine enhances myonuclear addition in a CK-dependent manner
Creatine increased myoblast fusion in a CK-dependent manner indicating that PCr hydrolysis is necessary for myonuclear addition. Interestingly, partial (70%) inhibition of CK activity had no effect on myoblast fusion in the absence of creatine treatment. As ATP is replenished from the interplay of multiple enzymatic activities, functional redundancy between glycolytic, oxidative and phosphagen-directed energy sources may occur. An alternative explanation for not observing an effect of CK siRNA on basal levels of myoblast fusion is that residual CK activity is sufficient for the terminal differentiation of myoblasts into myotubes. More thorough inhibition of CK could inhibit myoblast fusion during basal myogenesis. Of caution is the observation that addition of cyclocreatine, a competitive inhibitor of CK enzymes, to myoblasts at the time of switching cells to DM severely decreased differentiation and, consequently, myotube formation (data not shown). The effect of cyclocreatine may be explained by a requirement for CK/PCr in terminal differentiation during myogenesis. Thus, more efficient siRNA-mediated knockdown of CK may block differentiation and not allow studies of downstream myoblast fusion events.
Generation of null mice for the four cytosolic and mitochondrial CK isoforms has revealed much about the role of specific isoforms in skeletal muscle contractile function and brain physiology (Renema et al. 2007) but key experiments on myoblast differentiation and fusion in CKB and CKM null mice have not been performed to date. Potentially confounding analyses of myoblast fusion in these mice is the functional equivalence of CKB and CKM isoforms in vivo (Renema et al. 2007). However, generation of mice deficient in both CKB and CKM has not been reported and in fact, these mice may not be viable given the number of tissues that express these two isoforms (Wyss & Kaddurah-Daouk, 2000). Muscle mass is decreased in CKM null mice (Momken et al. 2005) but whether this decrease is an indirect result of their impaired exercise tolerance (Momken et al. 2005) or tendency towards decreases in basal activity during the dark cycle (Steeghs et al. 1998) is unknown. Other studies demonstrating recriprocal relationships between intramuscular PCr content and myofibre cross-sectional area (Wyss & Kaddurah-Daouk, 2000) also suffer from the inability to separate out effects on muscle growth due to changes in muscle use.
Actin rearrangements are energy dependent
The ability of Lat B to prevent myonuclear addition following creatine treatment (Fig. 2) suggests that actin dynamics are coupled to PCr hydrolysis during myogenesis. The molecular events regulating actin polymerization have been well defined in multiple cell types with a critical involvement of ATP. Both the addition of actin monomers to the growing ends of filaments and the dissociation of monomers at the tail end require ATP hydrolysis. Thus, our findings support a role for PCr as a localized energy buffer for actin cytoskeleton remodelling during myonuclear addition.
Actin rearrangements and myogenesis
Actin polymerization is critical for myoblast fusion in Drosophila (Kim et al. 2007; Massarwa et al. 2007; Richardson et al. 2007). Several cellular processes that occur during myogenesis are dependent on actin arrangements and could be enhanced by PCr hydrolysis. Muscle cell fusion requires cell migration and cell–cell contact; actin rearrangements figure prominently in cell migration (Pollard & Borisy, 2003). However, enhancing stores of PCr during myoblast differentiation had no effect on myoblast motility (Fig. 3). In Drosophila myogenesis the requirement for actin polymerization is confined to the fusion of myoblasts with nascent myotubes (Kim et al. 2007; Massarwa et al. 2007). This is the same stage of myoblast fusion that was enhanced by creatine treatment in our studies with primary mouse myoblasts. In Drosophila, multinucleated muscle cells are formed by the fusion of two distinct myoblast populations (Chen & Olson, 2004). Microscopic analyses revealed actin-rich foci localized to sites of contact between both founder cells and fusion-competent myoblasts(Kim et al. 2007; Richardson et al. 2007). The recruitment of multiple actin nucleation promoting factors including rolling pebbles (rols), kette, WASp and Wip to sites of cell–cell contact is indispensable to the fusion process (Chen et al. 2007). Loss of function mutations in any one of these proteins selectively inhibits the second phase of fusion. Although cell migration, recognition, adhesion and membrane fusion are involved in the fusion of founder myoblasts with fusion-competent myoblasts, the role of nucleation-promoting factors is limited to fusion of nascent myotubes with fusion-competent myoblasts for unknown reasons.
Potential roles for PCr besides actin rearrangements
Although we demonstrate that actin is required for creatine-mediated effects on cell fusion, additional mechanisms may also be involved. Multiple chromatin-remodelling enzymes regulate myogenic gene expression in an ATP-dependent manner. For example, sirtuins are a class of ATP-sensitive histone deacetylases involved in myogenic gene repression (Fulco et al. 2003). In addition, SWI/SNF complexes are chromatin-remodelling ATPases required for myoblast differentiation (Ohkawa et al. 2007). Other putative targets for PCr include signalling kinases. Protein kinases relay signals from the membrane to the nucleus through sequential phosphorylation of downstream effectors using ATP as a phosphate donor. Elevating PCr stores may also direct myonuclear addition through alterations in transcriptional activation and repression or protein kinase activity.
Implications of PCr for regulation of satellite cell dynamics
Muscle regeneration in vivo results from the fusion of satellite cells to form new myofibres. As 95% of all physiologicalal creatine is contained within skeletal muscle (30–40 mm) (Snow & Murphy, 2001) sarcolemmal disruption may promote the release and diffusion of PCr into the satellite cell niche. Since creatine regulates myoblast fusion with nascent myotubes in vitro, the release of intramuscular creatine during injury may lead to an increase in PCr in satellite cells during regeneration and promote satellite cell fusion with newly forming myofibres in vivo. Creatine may also promote muscle regeneration through additional mechanisms including enhanced satellite cell proliferation (Dangott et al. 2000; Olsen et al. 2006) and/or survival (Passaquin et al. 2002). Thus, creatine may contribute to the observed enhancement of muscle growth during regeneration both by increasing the number of satellite cells and by enhancing cell fusion.
Therapeutic uses of creatine supplementation
Oral creatine supplementation is popular among athletes as it increases the levels of force production during high intensity exercise of limited duration (Brosnan & Brosnan, 2007). In addition, creatine supplementation can increase muscle mass particularly when combined with resistance exercise (Branch, 2003; Olsen et al. 2006). The cellular and molecular mechanisms responsible for enhanced performance and muscle growth are under investigation. Creatine supplementation is also associated with enhanced muscle growth in several pathological situations. For example, oral creatine can enhance the growth of atrophied myofibres following muscle unloading (Hespel et al. 2001). Creatine supplementation also demonstrated a beneficial role in Duchenne muscular dystrophy, a degenerative neuromuscular disease characterized by persistent muscle degeneration and regeneration (Pearlman & Fielding, 2006). Our results suggest that creatine supplementation may lead to changes in muscle composition in part by enhancing the fusion of satellite cells. In addition, oral creatine may also be beneficial in rehabilitation from muscle injuries induced by intense exercise, surgery or trauma.
The ability to increase PCr in proliferating myoblasts and then enhance myoblast fusion during later stages of myogenesis in the absence of creatine (Fig. 7) has important implications for muscle stem cell biology. Creatine may enhance the therapeutic potential of transplanted stem cells in muscular dystrophy as the efficacy of treatment is often limited by poor cell engraftment within the host tissue. Treatment of an exogenous population of stem cells with creatine may enhance fusion of these muscle precursor cells with the host myofibre following transplantation. Furthermore, as creatine is normally absent from standard culture media our findings raise important questions – Are current in vitro conditions suboptimal for studies of myoblast fusion? Are differentiating myoblast cultures energetically hindered? While much insight has been gleaned from studies of myoblast fusion in vitro to date, our findings may accelerate future progress by accurately complementing the experimental and physiological environments.
Summary
In summary, we identified a novel role for PCr in mammalian myoblast fusion. Our work suggests localized ATP-dependent reactions are necessary for cytoskeletal dynamics during myoblast fusion and the PCr/creatine kinase system is coupled to actin polymerization during myogenesis. Increasingly, the importance of localized PCr within multiple cell types to supply energy for specific cellular responses is being recognized (Mahajan et al. 2000; Burklen et al. 2007). Future work will be directed toward identifying and characterizing putative binding partners of CKB and CKM during myoblast fusion. Such studies will shed further light on the mechanisms regulating myoblast fusion, a process crucial for muscle growth and regeneration.
Acknowledgments
We thank K. Jansen and K. Kafadar for helpful discussions and K. Brown for technical assistance with in vivo experiments. This work was supported by grants AR052730, AR047314, AR051372 from the National Institutes of Health and the Muscular Dystrophy Association to G.K.P., and MA 00210 from the National Space Biomedical Research Institute to RWW.
References
- Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG. Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol. 2006;576:391–401. doi: 10.1113/jphysiol.2006.115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont LD, Patterson GM, Drubin DG. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J Cell Sci. 1999;112:1325–1336. doi: 10.1242/jcs.112.9.1325. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13:198–226. doi: 10.1123/ijsnem.13.2.198. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- Burklen TS, Hirschy A, Wallimann T. Brain-type creatine kinase BB-CK interacts with the Golgi matrix protein GM130 in early prophase. Mol Cell Biochem. 2007;297:53–64. doi: 10.1007/s11010-006-9322-4. [DOI] [PubMed] [Google Scholar]
- Cande WZ. Creatine kinase role in anaphase chromosome movement. Nature. 1983;304:557–558. doi: 10.1038/304557a0. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol. 2004;555:409–421. doi: 10.1113/jphysiol.2003.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JS, Jaynes JB, Hauschka SD. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985;5:484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Chen EH, Olson EN. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004;14:452–460. doi: 10.1016/j.tcb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Dangott B, Schultz E, Mozdziak PE. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. Int J Sports Med. 2000;21:13–16. doi: 10.1055/s-2000-8848. [DOI] [PubMed] [Google Scholar]
- Daniel JL, Molish IR, Robkin L, Holmsen H. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem. 1986;156:677–684. doi: 10.1111/j.1432-1033.1986.tb09631.x. [DOI] [PubMed] [Google Scholar]
- Den H, Malinzak DA, Keating HJ, Rosenberg A. Influence of Concanavalin A, wheat germ agglutinin, and soybean agglutinin on the fusion of myoblasts in vitro. J Cell Biol. 1975;67:826–834. doi: 10.1083/jcb.67.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Prives J, Farmer SR, Penman S. Developmental reorganization of the skeletal framework and its surface lamina in fusing muscle cells. J Cell Biol. 1981;91:103–112. doi: 10.1083/jcb.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536:625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Prostaglandin F2 (alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J Cell Biol. 2003;161:111–118. doi: 10.1083/jcb.200208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- Ingwall JS, Morales MF, Stockdale FE. Creatine and the control of myosin synthesis in differentiating skeletal muscle. Proc Natl Acad Sci U S A. 1972;69:2250–2253. doi: 10.1073/pnas.69.8.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007;120:670–681. doi: 10.1242/jcs.03371. [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Furling D, Bigot A, Butler-Browne GS, Mouly V. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res. 2004;299:148–158. doi: 10.1016/j.yexcr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Mahajan VB, Pai KS, Lau A, Cunningham DD. Creatine kinase, an ATP-generating enzyme, is required for thrombin receptor signaling to the cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:12062–12067. doi: 10.1073/pnas.97.22.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the ‘phosphocreatine shuttle’. Am J Physiol Cell Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol. 2004;287:C1753–C1762. doi: 10.1152/ajpcell.00292.2004. [DOI] [PubMed] [Google Scholar]
- Momken I, Lechene P, Koulmann N, Fortin D, Mateo P, Doan BT, Hoerter J, Bigard X, Veksler V, Ventura-Clapier R. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol. 2005;565:951–964. doi: 10.1113/jphysiol.2005.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney PT, Stracke ML, Nam SW, Woodhouse E, O'Keefe M, Clair T, Liotta LA, Khaddurah-Daouk R, Schiffmann E. Cyclocreatine inhibits stimulated motility in tumor cells possessing creatine kinase. Int J Cancer. 1998;78:46–52. doi: 10.1002/(sici)1097-0215(19980925)78:1<46::aid-ijc9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Iino R, Kobayashi T, Fujiwara T, Ohshima C, Yoshimura A, Kusumi A. Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci U S A. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RS, Mills ST, Jones KA, Ho SN, Pavlath GK. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J Cell Sci. 2007;120:149–159. doi: 10.1242/jcs.03307. [DOI] [PubMed] [Google Scholar]
- Ohkawa Y, Yoshimura S, Higashi C, Marfella CG, Dacwag CS, Tachibana T, Imbalzano AN. Myogenin and the SWI/SNF ATPase Brg1 maintain myogenic gene expression at different stages of skeletal myogenesis. J Biol Chem. 2007;282:6564–6570. doi: 10.1074/jbc.M608898200. [DOI] [PubMed] [Google Scholar]
- Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573:525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord. 2002;12:174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Pearlman JP, Fielding RA. Creatine monohydrate as a therapeutic aid in muscular dystrophy. Nutr Rev. 2006;64:80–88. doi: 10.1301/nr.2006.feb.80-88. [DOI] [PubMed] [Google Scholar]
- Plaghki L, Marechal G. Time course of regeneration of minced frog muscles estimated by the level of energetic substrates. Pflugers Arch. 1976;361:135–143. doi: 10.1007/BF00583457. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Ruegg UT. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett. 1998;439:357–362. doi: 10.1016/s0014-5793(98)01399-4. [DOI] [PubMed] [Google Scholar]
- Renema WK, Kan HE, Wieringa B, Heerschap A. In vivo magnetic resonance spectroscopy of transgenic mouse models with altered high-energy phosphoryl transfer metabolism. NMR Biomed. 2007;20:448–467. doi: 10.1002/nbm.1117. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer BW, Perriard JC. Intracellular targeting of isoproteins in muscle cytoarchitecture. J Cell Biol. 1988;106:1161–1170. doi: 10.1083/jcb.106.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RB, Saft MS, Taylor AR, Cole RD. Identification of nonmitochondrial creatine kinase enzymatic activity in isolated sea urchin mitotic apparatus. J Biol Chem. 1983;258:13287–13291. [PubMed] [Google Scholar]
- Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224:169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, Pende M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci U S A. 2006;103:7315–7320. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs K, Oerlemans F, de Haan A, Heerschap A, Verdoodt L, de Bie M, Ruitenbeek W, Benders A, Jost C, van Deursen J, Tullson P, Terjung R, Jap P, Jacob W, Pette D, Wieringa B. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem. 1998;184:183–194. [PubMed] [Google Scholar]
- Tombes RM, Farr A, Shapiro BM. Sea urchin sperm creatine kinase: the flagellar isozyme is a microtubule-associated protein. Exp Cell Res. 1988;178:307–317. doi: 10.1016/0014-4827(88)90401-6. [DOI] [PubMed] [Google Scholar]
- Vierck JL, Icenoggle DL, Bucci L, Dodson MV. The effects of ergogenic compounds on myogenic satellite cells. Med Sci Sports Exerc. 2003;35:769–776. doi: 10.1249/01.MSS.0000065005.96298.01. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YE, Esbensen P, Bentley D. Arginine kinase expression and localization in growth cone migration. J Neurosci. 1998;18:987–998. doi: 10.1523/JNEUROSCI.18-03-00987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RW, Kushmerick MJ. Creatine kinase equilibration follows solution thermodynamics in skeletal muscle. 31P NMR studies using creatine analogs. J Biol Chem. 1995;270:12428–12438. doi: 10.1074/jbc.270.21.12428. [DOI] [PubMed] [Google Scholar]
- Wiseman RW, Moerland TS, Chase PB, Stuppard R, Kushmerick MJ. High-performance liquid chromatographic assays for free and phosphorylated derivatives of the creatine analogues b-guanidopropionic acid and 1-carboxy-methyl-2-iminoimidazolidine (cyclocreatine) Anal Biochem. 1992;204:383–389. doi: 10.1016/0003-2697(92)90255-6. [DOI] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yarmola EG, Somasundaram T, Boring TA, Spector I, Bubb MR. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem. 2000;275:28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]