Abstract
Women are at greater risk than men of sustaining certain kinds of injury and diseases of collagen-rich tissues. To determine whether a high level of oestradiol has an acute influence on collagen synthesis in tendons at rest and in response to exercise, one-legged kicking exercise was performed for 60 min at 67% of maximum power by healthy, young oral contraceptive (OC) users when circulating synthetic (ethinyl) oestradiol was high (n = 11, HE-OC) and compared to similar women who had never used OCs when circulating endogenous oestrogen was low (n = 12, LE-NOC). Interstitial fluid was collected 24 h post-exercise through microdialysis catheters placed anterior to the patellar tendon in both legs and subsequently analysed for the amino-terminal propeptide of type I collagen (PINP), a marker of tendon collagen synthesis. To determine the long-term effect of OC usage, patellar tendon cross-sectional area (CSA) was measured by magnetic resonance imaging (MRI). A lower exercise-induced increase in tendon collagen synthesis was observed in HE-OC than in LE-NOC (ΔPINP (mean ±s.e.m.) 1.5 ± 5.3 versus 24.2 ± 9.4 ng ml−1, P < 0.05). Furthermore, serum and the interstitial peritendinous tissue concentrations of insulin-like growth factor I (IGF-I) and IGF-binding proteins showed a reduced bioavailability in HE-OC compared with results in LE-NOC. No difference in patellar tendon CSA was observed between groups. In conclusion, the selective increase in tendon collagen synthesis in LE-NOC but not HE-OC 24 h post-exercise is consistent with the hypothesis that oestradiol inhibits exercise-induced collagen synthesis in human tendon. The mechanism behind this is either a direct effect of oestradiol, or an indirect effect via a reduction in levels of free IGF-I. However, the data did not indicate any long-term effect on tendon size associated with chronic OC use.
Women are seemingly at a greater risk than men of sustaining certain kinds of sports injury (Engstrom et al. 1991; Arendt & Dick, 1995; Bijur et al. 1997; Bjordal et al. 1997; Bell et al. 2000) and disease (Larsson et al. 1993; Wolfe et al. 1995; Lahita, 1996) in collagen-rich tissues such as bone, ligament, muscle connective tissue and tendon. For instance, women are at a 2–8 times greater risk of anterior cruciate ligament (ACL) ruptures than men when performing similar sports activities (Huston et al. 2000). It has been suggested that part of this discrepancy in injury risk might be explained by differences in sex hormones (Hewett et al. 2006).
The strength and biomechanical properties of tendons play an important role in the force transmission of contractile energy from myofibrillar protein structure to the skeletal bones during locomotion (Kjaer, 2004). Collagen constitutes approximately 90% of the total protein of tendons, or 65–75% of the dry weight of tendons. This collagen is predominantly type I (∼60%) arranged in tensile-resistant fibres (Jozsa & Kannus, 1997). In men it has been shown that an acute bout of exercise increases type I collagen synthesis in tendon (Langberg et al. 1999, 2001; Miller et al. 2005) and resistance training results in patellar tendon hypertrophy (Kongsgaard et al. 2007). In contrast to the findings in men, recent findings from our laboratory have shown a similar tendon CSA (Achilles' and patellar tendon) in experienced female runners and untrained women, while tendon CSA adjusted for body size was larger in equally trained male runners (Westh et al. 2008). This might indicate sex-specific adaptation of tendons to training.
Oestrogen receptors have been localized in synoviocytes in the synovial lining, fibroblasts in the ACL stroma and cells in the blood vessel walls of the ligament (Liu et al. 1996), and animal data have shown that tendons express transcripts for oestrogen receptors (Hart et al. 1998). In relation to this a more than 40% reduction in collagen synthesis and a reduction in fibroblast proliferation have been observed in vitro in tissue samples from the ACL when oestradiol was administered in physiological doses (Liu et al. 1997; Yu et al. 2001). In addition, ovariectomized rabbits exposed to oestradiol for one month have reduced tensile strength of the ACL compared to a control group of ovariectomized rabbits not supplemented with oestradiol (Slauterbeck et al. 1999). In vitro studies and animal studies on bone and ACL tissue have shown an interaction between exercise, the level of oestradiol, and oestrogen receptors on collagen turnover (Chen et al. 2001; Tobias, 2003; Lee et al. 2004; Lee & Lanyon, 2004; Saxon & Turner, 2005). Expression of type I collagen was reduced in fibroblasts when cyclic loading and oestrogen were administered together, but increased when they were administered separately (Lee et al. 2004). A suppressive effect of oestrogen administration on fibroblast response to cyclic loading may be mediated by reduced bioavailability of IGF-I, since oral administration of oestrogens (E2 or ethinyl oestradiol) reduces serum (s-) IGF-I and raises concentrations of IGF-binding proteins (IGFBPs) and s-growth hormone (GH) (Ho & Weissberger, 1992; Campagnoli et al. 1993; Kelly et al. 1993; Bellantoni et al. 1996; Westwood et al. 1999). In cultured tenocytes, IGF-I exerts a potent proliferative and pro-survival effect (Scott et al. 2005). Furthermore, the observed proliferative response of cultured tenocytes to loading (Tsuzaki et al. 2000) and the increase in collagen synthesis in response to exercise in vivo has also been associated with the presence of IGF-I (Olesen et al. 2006b). Therefore, oral administration of oestrogens may reduce the responsiveness of tendons to mechanical loading indirectly by reducing the local level of free IGF-I. A local change in IGF-I in tendon tissue in response to oestrogen administration has to our knowledge not been reported in the literature.
Our primary aim was to investigate whether a high level of oestradiol has an acute influence on collagen synthesis in tendon at rest and in response to exercise. We hypothesized that the response of tendon collagen synthesis to exercise would be lower in women when they were exposed to a high level of oestradiol by OCs compared with women when they were exposed to a low level of endogenous oestradiol. This hypothesis was based on the assumption that synthetic oestradiol (ethinyl oestradiol), like endogenous secreted oestradiol (17-β oestradiol), has an inhibiting effect on collagen synthesis (Liu et al. 1997). In addition, GH, IGF-I and IGFBPs were measured in serum and in the interstitial peritendinous tissue to clarify the effect of oestradiol administration on the level of these growth factors in the peritendinous tissue.
Tendon stress is inversely proportional to tendon CSA during mechanical loading. It has not been reported whether long-term use of OCs has an associated influence on tendon size and occurrence of tendon injury rates. An observed higher risk of lower back pain (Wreje et al. 1997), bone fractures (Cooper et al. 1993) and persistent pelvic pain and pelvic joint instability (Saugstad, 1991) in users of OCs than in controls has been observed. These findings indicate that OC usage could have a long-term negative effect on collagen-rich tissue quality or quantity. In contrast, a prospective study noted fewer non-specific traumatic soccer injuries in women using OCs compared to women who did not use OCs (Moller-Nielsen & Hammar, 1989). Therefore, the epidemiological data are too scarce and inconsistent to firmly establish an association between OC use and the risk of injuries related to the collagen-rich tissue in tendon, ligament and bone.
A secondary aim in the present study was to evaluate if long-term use of OCs has an influence on patellar tendon CSA and thereby indirectly an effect on the risk of sustaining an injury. This was examined by comparing tendon CSA by magnetic resonance imaging (MRI) in women who had not used OCs with chronic OC users. In OC users, ethinyl oestradiol is elevated for 3 weeks and low for 1 week in each menstrual cycle. In non-users of OCs, exposure to endogenous oestrogens is low in the follicular phase, but similar to OC users, the oestradiol concentration is enhanced during the other phases of the menstrual cycle. Therefore, total exposure to oestrogens might not be greatly different between groups. Based on this, no specific direction of an effect of OC usage on patellar tendon CSA could be assumed.
Methods
Subjects
Twenty-three young, healthy women were recruited for the study. All were non-smokers, nulliparous, non-users of medication (except OCs), and without orthopedic and metabolic disorders, as judged by history and routine medical examination. The subjects gave informed consent to the protocol adhering to the Declaration of Helsinki and the Ethics Committee of Copenhagen and Frederiksberg Communities (KF-01-032/04) approved the study.
Eleven women were long-term users of OCs (7.2 ± 2.1 treatment years (mean ±s.d.). The used types of OCs were Lindynette (n = 7) (30 μg ethinyl oestradiol and gestoden 0.0075 mg per day) or Cilest (n = 4) (35 μg ethinyl oestradiol and 0.25 mg norgestimate per day). The additional 12 women had a normal menstrual cycle and had never used OCs. A cycle length within the range of 21–35 days for at least one year was defined as normal. The two groups were otherwise similar in the following characteristics: age, height, weight, body mass index (BMI), and body composition measured by Dual-Energy x-ray absorptiometry (DEXA). Additionally, the groups were similar with regard to training status as assessed by a questionnaire used to determine frequency and hours spent on regular planned physical training per week. Since cycling is a widely used form of transport for young women in Copenhagen, the participants were also asked to calculate the total time per week they spent cycling (Table 1).
Table 1.
Subject characteristics
| LE-NOC | HE-OC | |
|---|---|---|
| Age (years) | 24 ± 4 | 24 ± 2 |
| Weight (kg) | 65.0 ± 8.4 | 63.7 ± 8.4 |
| Height (m) | 1.67 ± 0.07 | 1.70 ± 0.06 |
| BMI (kg m−2) | 23.3 ± 2.4 | 22.0 ± 2.6 |
| Body fat (%) | 31.1 ± 8.6 | 27.7 ± 7.2 |
| LBM (kg) | 42.6 ± 4.1 | 43.9 ± 4.4 |
| Workload (Wattsubmax) | 44 ± 9 | 37 ± 7 |
| Workload (%Wattmax) | 68 ± 6 | 67 ± 8 |
| PT (times week−1) | 2.2 ± 1.3 | 2.4 ± 1.6 |
| PA (h week−1) | 4.9 ± 2.1 | 5.4 ± 2.0 |
| Bike, transport (% of PA) | 61 ± 30 | 58 ± 23 |
| Patellar tendon, mean CSA (mm2) | 75 ± 7 | 82 ± 10 |
Values are means ±s.d. No significant differences between LE-NOC (women who had never used oral contraceptives tested at low circulating levels of oestradiol) and HE-OC (oral contraceptive users tested in the hours after ingestion of oestradiol) for any of the parameters were observed. Body composition was measured by DEXA. LBM, lean body mass; BMI, body mass index; PT, physical training; PA, physical activity (bike transportation and PT).
Design and methods
Our primary aim was to elucidate whether a differential level of oestradiol had an influence on the response to exercise in tendon collagen synthesis in the in vivo situation in young women. To make the total difference in oestrogens (endogenous secreted and synthetic ethinyl oestradiol) between groups as large as possible, LE-NOC were tested in the early follicular phase of the menstrual cycle when the level of oestrogen is low, whereas HE-NOC were tested on days 18–21 of the pill phase. In the HE-OC group, measurements of tendon synthesis were performed in the hours after the last pill ingestion, when the ethinyl-oestradiol concentration peaks (Jung-Hoffmann et al. 1991).
The effect of exercise on tendon collagen synthesis was determined by using a one-legged kicking exercise model and by measuring tendon collagen synthesis 24 h later in the resting leg and the contralateral leg, which had performed strenuous exercise. The time-point for the measurement of tendon synthesis was based on earlier findings in men showing a peak in tendon synthesis 24 h post-exercise when using a similar exercise protocol (Miller et al. 2005).
Synthesis of collagen was measured by the microdialysis technique (Lonnroth et al. 1987). The procedure is described in more detail below. Dialysate was collected from the peritendinous space anterior to the patellar tendon and analysed for the amino terminal propeptide of type I collagen (PINP) as a marker of collagen synthesis (Langberg et al. 2001). Dialysate from both legs was obtained simultaneously. The remaining dialysate was analysed for IGF-I and IGFBPs (1–4).
Two weeks prior to the study, subjects visited the laboratory for determination of the workload they would use during the experiment when exercising on a one-legged modified Krogh ergometer. After warming up for 5 min without resistance, the subjects began one-legged kicking (35 kicks per minute) for 3 min with a 0.5 kg load; the load was increased by 0.3 kg every 3 min until the subjects could no longer maintain the cadence and this workload was defined as maximum workload (Wmax).
On day 1 of the experiment, the subjects performed the one-legged kicking exercise for 1 h at 67% of each subject's Wmax. The following day subjects arrived at the laboratory at 08.00 h having fasted for the previous 12 h. After baseline urine and blood samples for analysis of sex hormones and growth factors, the subjects received a standardized commercial clinical nutrient drink (Semper, Frederiksberg, DK: 15% protein, 64% carbohydrate and 21% fat) in divided doses every 30 min until the end of the experiment. The drink provided the equivalent of 1.4 × basal metabolic rate per 30 min period, with a double dose at initiation of feeding. Basal metabolic rate was estimated from the subject's fat free mass determined by the skin-fold technique (Durnin & Womersley, 1974; Cunningham, 1982). The reason for the standardization of nutrient intake was that energy and macronutrient intake is known to have an influence on protein metabolism in general. In particular, an increase in synthesis of skeletal muscle proteins in response to provision of essential amino acids is well-documented (Tipton & Sharp, 2005). Knowledge about the effects of food intake on tendon metabolism is limited. New results indicate that collagen synthesis in tendons and muscle connective tissue is not acutely influenced by nutrition (Babraj et al. 2005), but we decided to standardize the nutrient intake to diminish any influence of a potential confounding factor. Furthermore, the subjects completed weighed food records for 3 days prior to the experiment. No difference in energy intake and macronutrient composition between groups was observed (Table 2). The subjects were instructed to avoid strenuous physical activity for at least 2 days prior to and during the experimental days. Isotope data from earlier studies indicate that the fractional patellar tendon collagen synthesis peaks around 24 h postexercise (Miller et al. 2005) and is similar to resting values 72 h post-exercise in young women (Miller et al. 2006).
Table 2.
Energy intake and macronutrient intake
| Habitual diet | LE-NOC | HE-OC |
|---|---|---|
| Energy intake (kJ day−1) | 9033 ± 2032 | 9221 ± 1983 |
| Energy intake RMR−1 | 1.45 ± 0.31 | 1.49 ± 0.35 |
| Protein (g day−1) | 82 ± 29 | 90 ± 17 |
| Protein (g kg−1) | 1.3 ± 0.4 | 1.3 ± 0.2 |
| Protein (g (kg DEXA LBM)−1) | 2.0 ± 0.6 | 2.0 ± 0.3 |
| Protein (E%) | 15 ± 4 | 17 ± 2 |
| Fat (E%) | 23 ± 7 | 24 ± 6 |
| CHO (E%) | 58 ± 6 | 57 ± 7 |
| Alcohol (E%) | 4 ± 6 | 2 ± 5 |
Values are means ±s.d. No significant differences between groups were observed for any of the parameters. Abbreviations: RMR, resting metabolic rate; LBM, lean body mass; CHO, carbohydrate; E%, percentage of daily energy intake.
Microdialysis
Dialysate was sampled from each leg by microdialysis technique (Langberg et al. 1999) on the day following the one-legged exercise bout to detect changes in metabolites in the local tissue fluid surrounding the patellar tendon. After previous preparation of incision sites with local anaesthetic (lidocaine (lignocaine) 1%), ethylene oxide-sterilized catheters with high molecular mass cut-off (3000 kDa, membrane length 30 mm, i.d. 0.50 mm) were inserted under ultrasound guidance as previously described (Langberg et al. 1999) in the peritendinous spaces of patellar tendons. The inflow tube of the microdialysis catheter was connected to a high-precision syringe pump with an infusion rate of 2 μl min−1. The catheters were perfused with a Ringer-acetate solution mixed with radioactive labelled glucose (d-[3-3H]glucose, specific activity 0.25 mCi; 0.25 m) in aqueous solution steri-pack, Perkin Elmer Life and Analytical Sciences, Boston, MA, USA). A sample vial was placed at the end of the outflow tube, and after 30 min perfusion of the catheter, dialysate was collected in three 1 h periods. The relative recovery over the membrane was determined for each dialysate sample (Scheller & Kolb, 1991). The dialysate (3 μl) was pipetted into a counting vial and mixed with 3 ml scintillation fluid (Ultima Gold, Perkin Elmer) and the samples were counted in a β-counter. The mean relative recoveries for the microdialysis catheters did not differ between groups (LE-NOC, 29 ± 12%; HE-OC users, 27 ± 10%).
Magnetic resonance imaging
Patellar tendon CSA was determined by MR axial plane images (General Electric Sigma Horizon LX 1.5 Tesla, T1 weighted S.E.) with the following MRI parameters: TR/TE 400/15 ms, FOV 16, matrix 256 × 256 ms, slice thickness 5.0 mm, spacing 0 mm and 12 slides in total from each tendon (bilateral) starting from apex patellar and ending at the tibial attachment. CSA was measured at the tibial and patellar attachments. All measurements were obtained manually using the software program WEB 1000 in a blinded fashion. The CSA for each anatomical spot was measured three times for each subject. The average intra-individual coefficient of variation (CV%) was 4%, whereas the inter-individual CV was 11%.
Blood, dialysate and urine analysis
All blood samples were collected from a cubital vein into sealed vials. After separation by centrifugation at 4°C the blood samples were either immediately analysed for s-oestradiol and s-progesterone or stored at −80°C until analysis for s-testosterone, s-GH, s-IGF-I, s-IGFBP1 and s-IGFBP3.
Oestradiol was analysed by chemiluminescent competitive immunoassay (Immulite 2500) (NPU 1972, oestradiol; Diagnostic Product, Los Angeles, CA, USA). The detection level for the analysis is 10 nmol l−1. Progesterone was analysed by architect microparticle enzyme immunoassay (Abbott Diagnostics, Wiesbaden, Germany). The analysis for oestradiol and progesterone were performed at Hvidovre Hospital, Denmark. The analysis for testosterone was performed at Statens Serum Institute, Denmark. Prior to analysis, internal standard solution containing deuterated androstenedione and testosterone was added to the sample. The samples were extracted with diethyl ether, evaporated and reconstituted in 50% methanol. Testosterone was measured in the serum extracts by liquid chromatography mass spectrometry (LC-MS) using an atmospheric pressure chemical ionization (APCI) interface (CV < 15%).
Dialysate fluid was analysed for PINP using a sandwich ELISA utilizing purified α1-chain specific rabbit antibodies donated by B. Teisner, Department of Medical Microbiology, University of Odense, Denmark (Jensen et al. 1998). The within- (double determination) and between-assay CVs were on average 2.2% and 4.9%, respectively.
Serum GH was measured using non-competitive time-resolved monoclonal immunofluorometric assay (TR-IFMA; Wallac Oy, Turku, Finland), as previously described (Frystyk et al. 1999). Serum IGF-I and dialysate IGF-I were determined at Aarhus University Hospital by TR-IFMA after acid–ethanol extraction, as previously described (Frystyk et al. 1995). The intra-assay CV for this IGF-I assay is below 5%. Serum IGFBP1 was determined by an in-house radioimmunoassay (RIA) performed as described by Westwood et al. (1994), with modifications as previously described (CV 5%) (Krassas et al. 2003). Serum IGFBP3 was measured by commercially available IRMA (BioSource Europe, Nivelles, Belgium) (CV < 5%). Small volumes of tendon dialysates were analysed for IGFBPs 1–4 by Western ligand blotting, as previously described (Flyvbjerg et al. 1992).
Statistics
To test if oestrogen has an effect on the response of tendon synthesis to exercise, unpaired Student's two-tailed t tests were performed to compare group differences in (A) resting PINP values and (B) ΔPINP values (rest − exercise). The effect of exercise within each group was tested by paired Student's two-tailed t tests. Unpaired Student's two-tailed t tests were used to test for between-group differences in s-testosterone and s-progesterone, tendon CSA, s-GH, s-IGF-I and s-IGFBPs. The results for IGFBPs were only tested for differences between HE-OC and LE-NOC by comparing the resting and post-exercise legs separately since initial paired t tests showed no effect of exercise. The MRI results were tested for a between-group effect in CSA of the patellar tendon both at the proximal and distal level of the tendon. The baseline characteristics of the subject groups are presented as mean ± standard deviation (s.d.), whereas the results are presented as mean ± standard error of the mean (s.e.m.). The level of significance was set at P < 0.05. The statistical analyses were performed using the statistical software package Prism version 4.01 (GraphPad, San Diego, CA, USA) 2004.
Results
The women performed the strenuous exercise bout at an average of 67 ± 2% of Wmax without any difference between the two groups (Table 1).
Sex hormones
On the day of the experiment, the concentration of s-17-β oestradiol was very low in both groups. In the LE-NOC tested in the early follicular phase s-17-β oestradiol was in the very low end of the range during a normal menstruation period (follicular phase < 0.60 nmol l−1, reference values from Hvidovre Hospital, Denmark). One subject reached a concentration of 0.16 nmol l−1, whereas the others were at or below the analytical detection level (≤ 10 nmol l−1). Within the HE-OC-group s-17-β oestradiol was below analytical detection level in all except one (0.26 nmol l−1). Serum progesterone was within the low end of normal range in both groups (follicular phase ≤ 4 nmol l−1, luteal phase ≥ 25 nmol l−1, reference values from Hvidovre Hospital, Denmark). Although statistically significant (LE-NOC: 0.9 ± 0.0 nmol l−1versus HE-OC: 0.7 ± 0.0 nmol l−1, P < 0.05), the difference between groups in s-progesterone was so small compared to the normal range over the menstrual cycle (5.5–33 nmol l−1) that it was physiologically negligible. Fasting level in testosterone did not differ between LE-NOC (n = 10) (0.96 ± 0.09 nmol l−1) and HE-OC (n = 11) (0.9 ± 0.09 nmol l−1) (P = 0.82).
Tendon collagen synthesis
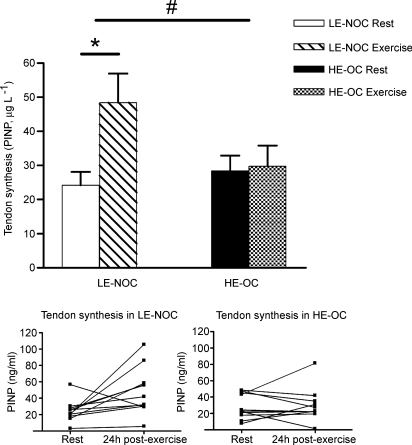
Because of technical problems with the flow through the catheters, no PINP values were available from any of one subject's legs (LE-NOC). In two HE-OC subjects, dialysate from either the resting leg or the post-exercise leg was missing and a value corresponding to the mean Δ response in PINP (+1.5 ± 5.3 μg l−1) was inserted to increase the statistical power for detecting a response to exercise.
At rest no difference in interstitial peritendinous PINP between groups was observed (P = 0.50). However, the response to exercise in tendon collagen synthesis was lower in HE-OC than in LE-NOC (ΔPINP (exercise − rest): 1.5 ± 5.3 versus 24.2 ± 9.4 μg l−1 in HE-OC and LE-NOC, P < 0.05) (Fig. 1). The increase in tendon synthesis was significant compared with resting values in LE-NOC (P < 0.05). Only in one subject in the LE-NOC group was tendon synthesis lower post-exercise compared with resting values. In contrast, only in three subjects in the HE-OC group was a higher tendon synthesis measured 24 h post-exercise compared with resting values, and the average tendon synthesis was not significantly enhanced by exercise (P = 0.79).
Figure 1.
N-terminal propeptide of human collagen type I (PINP, μg l−1) measured at rest and after exercise in LE-NOC and HE-OC in the peritendinous tissue in front of the patellar tendon. Values are mean ±s.e.m.#P < 0.05: increase in tendon collagen synthesis (PINP) in response to exercise was significant higher in LE-NOC than HE-OC. *P < 0.05: tendon PINP higher 24 h post-exercise than at rest in LE-NOC.
Tendon CSA
One LE-NOC was excluded from MR scanning due to safety reasons (metal device in the body). The proximal CSA of the patellar tendon was significantly smaller than the distal one (P < 0.001). Nevertheless, no difference in CSA between groups was observed either at the proximal or distal level of the patellar tendon (proximal: OC-users (n = 11) 65.8 ± 5.2; never-OC-users (n = 11) 60.4 ± 3.6 mm, P = 0.40) (distal: HE-OC 97.3 ± 4.4; LE-NOC 93.4 ± 5.6 mm, P = 0.21). Mean CSA (mm2) for each group is shown in Table 1.
GH, IGF-I, IGFBPs
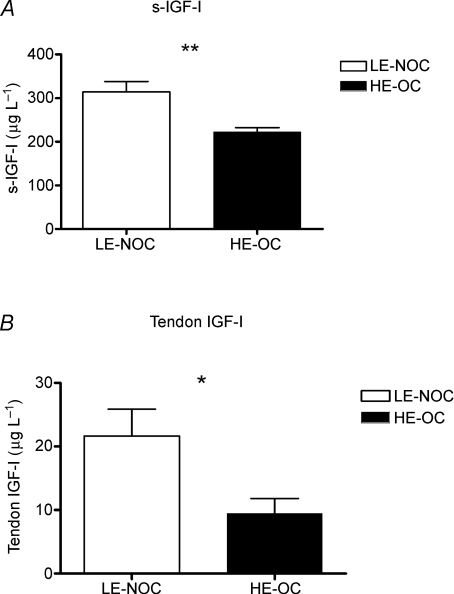
Overnight fasting s-GH levels did not differ between groups (LE-NOC (n = 8) 11 ± 3 μIU ml−1; HE-OC (n = 8) 15 ± 2 μIU ml−1, P = 0.36), whereas s-IGF-I was lower in HE-OC than in LE-NOC (Fig. 2A). Serum IGFBP1 was significant higher in HE-OC (n = 11) (121 ± 14 μg l−1) than in LE-NOC (n = 12) (57 ± 7 μg l−1) (P < 0.001), whereas no group difference was observed in IGFBP3 (HE-OC (n = 8) 5289 ± 180 μg l−1; LE-NOC (n = 9) 5166 ± 373 μg l−1, P = 0.78).
Figure 2.
A, concentration of insulin-like growth factor I (IGF-I) in serum (μg l−1) in LE-NOC (n = 12) and HE-OC (n = 11). B, IGF-I in interstitial tissue fluid surrounding the patellar tendon in LE-NOC (n = 11) and HE-OC (n = 7) collected as paired samples from the resting leg and the contralateral leg which had performed exercise the previous day. *P < 0.05; **P < 0.01: IGF-I higher in LE-NOC than HE-OC.
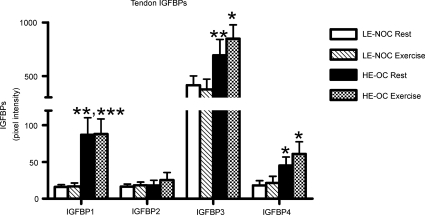
Dialysate samples were missing for IGF-I analysis in 4 out of 11 subjects in the HE-OC group and 1 out of 12 subjects in the LE-NOC group caused by problems with the flow through the catheters or cracking of the catheters caused by movements of the leg. For similar reasons paired dialysate samples from the resting leg and the leg that had performed exercise the previous day were only available for IGFBP analysis from 9 subjects in each group. In the tendon dialysate a significantly lower concentration of IGF-I was observed in HE-OC compared with LE-NOC (Fig. 2B), whereas a significantly higher level of IGFBP1, -3 and -4 was observed in HE-OC compared with LE-NOC. No effect of exercise on IGFBPs was observed in a subset of paired samples from each group (Fig. 3).
Figure 3.
Insulin-like binding proteins (IGFBPs) 1–4 (pixel intensity) in the interstitial tissue fluid surrounding the patellar tendon in LE-NOC (n = 9) and HE-OC (n = 9) collected as paired samples from the resting leg and the leg which had performed exercise the previous day. *P < 0.05; **P < 0.01; ***P < 0.001: IGFBP higher in LE-NOC than HE-OC at rest and/or post-exercise.
Discussion
The main finding in the present study was a significantly higher increase in tendon collagen synthesis in LE-NOC compared with HE-OC. The increase in tendon synthesis 24 h post-exercise was significant compared to resting values in LE-NOC, whereas no difference in tendon synthesis was observed in HE-OC in response to exercise (Fig. 1). A secondary finding was that no long-term effect of OC usage on tendon CSA was observed.
Sex steroid exposure
The concentration of endogenous 17-β-oestradiol was low in both groups due to the chosen time period of the menstruation cycle in the LE-NOC and because endogenous oestradiol production was suppressed by ethinyl oestradiol (Arden et al. 1998). Thus, the two groups of subjects had contrasting exposure to ethinyl oestradiol with minimal levels of endogenous oestrogen.
Total circulating oestrogen concentration was not measured, because of the low sensitivity of our method to synthetic ethinyl oestradiol. However, it is known that in the hours following ingestion of OC, ethinyl oestradiol may reach levels comparable to the concentrations of 17-β-oestradiol experienced in the luteal phase of a normal menstruation cycle (Jung-Hoffmann et al. 1991). In the present study, we started the 4 h collection period of dialysate one hour after ingestion of OC when others have shown a 100-fold increase in circulating ethinyl oestradiol in users of OCs after ingestion of 30 μg ethinyl oestradiol (Jung-Hoffmann et al. 1991).
In vitro and animal data support a direct effect of oestradiol on collagen tissue turnover (Liu et al. 1997; Slauterbeck et al. 1999; Yu et al. 1999, 2001). Nevertheless, based on present findings and cross-sectional experimental design, we cannot exclude that the observed differences between groups might be due to other hormonal differences between groups such as testosterone, progesterone, IGF-I or progestogens. Testosterone has been shown to increase collagen content and fibril diameter in the hip joint capsule in rats (Hama et al. 1976), but no difference in s-testosterone was observed between groups in the present study. The difference in endogenous progesterone between LE-NOC and HE-OC was physiologically negligible, but we cannot rule out that the observed difference in tendon synthesis between groups was due to exogenous progestogens in OCs. The different types of synthetic progesterone are known to vary in androgenic effects (Sulak, 2004). Our results do not support a stimulating androgenic effect on tendon collagen synthesis of OCs. At least, the potentially stimulating effect of the synthetic progesterone on collagen turnover does not overrule the inhibiting effect of oestradiol, which has been reported in the literature (Fischer, 1973; Liu et al. 1997; Yu et al. 2001) and is supported by the findings in the present study. The lower IGF-I level in HE-OC further underlines the dominating effect of oestradiol, since exogenous progestogens, in contrast to ethinyl oestradiol, have been shown to enhance the IGF-I level (Nugent et al. 2003). However, we cannot rule out that the inhibiting effect of oestradiol is underestimated in the present study caused by a stimulating effect of exogenous progestogens.
Effects on resting and exercise-stimulated collagen synthesis
To our knowledge this is the first time the effect of an enhanced level of oestradiol introduced by OCs on tendon collagen synthesis has been measured in vivo. By simultaneously collecting tendon dialysate from the resting and exercise leg 24 h post-exercise it was possible to document that a local exercise-induced change in tendon collagen formation was taking place rather than a general effect on bone collagen formation. This is supported by earlier observations (Langberg et al. 2001; Miller et al. 2005).
The findings suggest that synthetic oestradiol in OCs inhibits the exercise-induced increases in tendon collagen synthesis. In an earlier study a lower fractional tendon collagen synthesis rate was observed in young eumenorrhoeic women compared to young men, both at rest and in response to an exercise bout similar to the exercise protocol in the present study (Miller et al. 2006). The findings by Miller et al. (2006) combined with those of the present study support the hypothesis that female sex hormones may inhibit adaptability to an increased training load, which may require repair of microdamage if the tissue is overloaded. In support of this, increases in collagen content and fibril diameter in the hip capsules have been observed in ovariectomized rats (Hama et al. 1976). These increases were shown to be inhibited by oestrogen treatment, or oestrogen combined with progesterone (Hama et al. 1976). A change in tendon collagen structure, introduced by oestradiol, may affect the strength of the tissue and partly explain why individual patellar tendon fascicles from women have been shown to be less resistant to rupture than fascicles from men (Haraldsson et al. 2006) and why greater knee and ankle joint laxities are observed in women compared to men (Beynnon et al. 2005; Shultz et al. 2005). However, the effect of oestradiol on the biomechanical properties of tendons is not convincingly clarified in vivo either in animals (Wentorf et al. 2006; Slauterbeck et al. 1999) or in humans (Griffin et al. 2006).
Effects on resting and exercise-stimulated growth factors
IGF-I and IGFBPs were markedly influenced by OCs in the present study. The results indicate that OCs reduce the bioavailability of IGF-I in the blood, as have been shown by others (Garnero et al. 1995; Wreje et al. 2000; van Rooigen et al. 2006), and in the interstitial fluid surrounding the patellar tendon, which to our knowledge is a novel finding. Earlier findings have shown that the exercise-induced increase in IGF-I is only transient and returns to baseline within 1 h (Berg & Bang, 2004). Therefore the dialysate was not analysed for changes in IGF-I in response to exercise.
IGF-I is expressed in tendons in animals and humans (Abrahamsson et al. 1991; Olesen et al. 2006c) and has been shown to stimulate collagen production by fibroblasts in animals (Abrahamsson et al. 1991). Based on our findings we cannot rule out that the difference in local IGF-I was due to spillover from the blood. Nevertheless, as a growth factor, the consequential local group difference in the bioavailability of IGF-I is of importance per se since new findings indicate that IGF-I and exercise-induced changes in IGFBPs seem to be involved in the observed increase in tendon collagen synthesis in response to exercise. Therefore, we suggest that the higher level of free IGF-I at rest in LE-NOC, potentially combined with a further transient increase introduced by exercise (Berg & Bang, 2004), may at least partly explain the increase in tendon collagen synthesis in response to exercise in LE-NOC, whereas HE-OC may have experienced a direct inhibition of ethinyl oestradiol combined with an indirect effect by a low IGF-I bioavailability.
Differences in patellar tendon CSA
A secondary aim in the present study was to clarify if long-term use of OCs has an effect on tendon size. No group difference in tendon size (CSA) measured by MRI was detected in the present study, even though HE-OC had been exposed to OCs for more than 7 years, but this may have been a Type 2 error (P = 0.07). To increase the statistical power, we included data from eight OC users from an earlier study. These women were very similar to our subjects (age: 25 ± 1 years; height: 1.68 ± 0.03 m; weight: 60 ± 2 kg; BMI: 21 ± 1 kg m−2; physical activity 5.0 ± 2.8 h week−1). The same scientist, who was blinded for the OC status of the subjects, analysed the data. The increase in group size did not change the conclusion about no difference in tendon CSA between groups (LE-NOC (n = 11) 75 ± 2 mm2; all OC users (n = 19) 76 ± 3 mm2, P = 0.32).
A greater tendon size has been observed in postmenopausal women compared with young women, which was suggested to be due to lowered inhibition of tendon collagen synthesis after the menopause, when the level of oestradiol is persistently low (Magnusson et al. 2003). Furthermore, results have shown that men both acutely (Miller et al. 2006) and in response to strength training (Kongsgaard et al. 2007) increase tendon collagen synthesis and tendon CSA. In addition, tendons in young women exposed to a generally higher level of oestrogen do not seem to adapt to training in a similar manner to tendons in men (Miller et al. 2006; Westh et al. 2008; Magnusson et al. 2007). No difference in tendon CSA was observed between experienced female runners and untrained women in a cross-sectional study (Magnusson et al. 2007; Westh et al. 2008). This observation is in contrast to men, where a larger tendon CSA (Achilles' and patellar tendon) has been observed in male runners compared to untrained men (Magnusson et al. 2007).
An obvious explanation for the similar CSA in the present study is that the difference in exposure to total bioactive oestradiol (endogenous and synthetic) is quite small. Still, it should be kept in mind that the sensitivity for detecting a small difference in tendon CSA is quite low. Therefore, statistical Type 2 error cannot be excluded. Furthermore, there might be a difference in tendon collagen content (fibril diameter, fibril density) and collagen cross-links, which cannot be detected by measuring tendon CSA.
Limitations of the experiment
A potential limitation to the study could be the uncertainty of the difference in sensitivity for the different types of oestrogen existing in tendon tissue. Though, in vitro studies have shown that sensitivity for ethinyl oestradiol is comparable to the response seen when oestrogen receptor-positive cells (Prifti et al. 2003) and rat uterus (Hyder et al. 1999) are exposed to endogenously secreted 17-β-oestradiol. Whether there are differences in sensitivity for ethinyl oestradiol between the different types of oestrogen receptors in tendon tissue needs to be elucidated in order to understand the influences of endogenous and synthetic oestradiol on tendon structure and function.
The collagen content is a result of the balance between collagen synthesis and breakdown. In the present study we only assessed collagen synthesis. Oestradiol may also have an influence on tendon collagen breakdown. This view is supported in a study by Chen et al. (2001), which showed that exercise and oestrogen both had an anti-resorption effect in bone tissue in rats. In addition, a consistently lower bone degradation and bone turnover has been observed in postmenopausal women using hormone replacement therapy containing oestrogen (Burkman et al. 2004). OC use has also been associated with a lower bone turnover (Garnero et al. 1995; Wreje et al. 2000). It is tempting to hypothesize that OC use will reduce the tendon collagen turnover and thereby influence the tissue composition and biomechanical properties of tendons. Unfortunately, good markers of tendon collagen degradation in dialysate are not available, which precludes measurements of the net turnover of collagen in tendons.
Another way of examining the effect of oestradiol on tendon tissue in vivo would be to measure collagen synthesis in different phases of the menstrual cycle. In an earlier study, we measured fractional tendon collagen synthesis in a group of women and men after an acute exercise bout similar to the exercise protocol in the present study (Miller et al. 2006). Half of the women were tested in the early part of the follicular phase and the others in the mid-luteal phase of the menstrual cycle. In the four women tested in the follicular phase we observed a tendency towards an increase in fractional collagen synthesis in response to exercise (P = 0.09), which was not seen in the three women tested in the luteal phase (P = 0.31). Because of the limited numbers of samples in each phase of the menstrual cycle, we cannot rule out that the latter observation was due to a Type 2 error. This was not investigated any further with this model for two reasons. Firstly, hormone profiles (e.g. cycle length, hormone phasing, and hormone concentration changes) vary considerably between normal menstruating women (Landgren et al. 1980). In the study by Miller et al. (2006) phases was established using an ovulation kit and by measuring progesterone, luteinizing hormone and follicle-stimulating hormone besides the oestradiol level. Yet, there was quite a large overlap in the oestradiol level between the two groups tested in the follicular phase (n = 8; oestradiol: 0.07–0.42 nmol l−1) and luteal phase (n = 7; oestradiol: 0.24–0.79 nmol l−1) separately. At the same time a 15-fold higher level of progesterone was measured in the luteal phase compared to the follicular phase (Miller et al. 2006). The cross-sectional design of the present study provided a clearer difference in sex hormones. Secondly, we have noticed that mere repeated insertion of a needle in the microdialysis technique increases collagen synthesis (authors' unpublished observations).
Since fractional tendon collagen synthesis has been shown to peak 24 h post-exercise in men (Miller et al. 2005), we also chose to measure collagen synthesis 24 h post-exercise. We cannot exclude that the time frame for the response to exercise is different in women than in men, especially at a high level of oestradiol. An exercise-induced increase in collagen synthesis may have been present in HE-OC before or after the time we measured. If the time pattern for the adaptive response in connective tissue to exercise is dependent upon the level of oestrogen, as suggested in the present study, more sex-specific training protocols are warranted.
Conclusions
In conclusion, we can report that the stimulating effect of strenuous exercise upon tendon collagen synthesis was higher 24 h post-exercise during low exposure to oestradiol compared to a situation where synthetic oestradiol was administered. This indicates that oestradiol may have an inhibiting effect on collagen synthesis in vivo. The mechanism behind the inhibiting effect of oestradiol upon collagen synthesis could be either direct or indirect suppression of IGF-I. No differences in patellar tendon CSA between users and non-users of OCs were observed, which may be because exposure to total bioactive oestradiol not differ much over a menstrual cycle. Since no difference in tendon CSA was observed, future training studies will be needed to clarify if chronic use of OCs has an influence on the ability to adapt to an increased training load.
Acknowledgments
We thank the subjects for their time and devotion to the study. Additionally, we thank Peter Butty, Lise Ejlertsen, Birgitte Lillethorup, Karen Mathiassen, Merete Møller, Kirsten Nyborg and Ann-Christina R. Reimann for their technical assistance, and Statens Serum Institute for analysing serum samples for oestradiol and testosterone. This work was supported by the Danish Rheumatism Association, the Danish Research Council, the Danish Health Science Research Board, the Lundbeck Foundation and the HS Foundation.
References
- Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- Arden U, Jung-Hoffmann C, Kuhl H. A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception. 1998;58:75–81. doi: 10.1016/s0010-7824(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller BF, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005;289:E864–E869. doi: 10.1152/ajpendo.00243.2005. [DOI] [PubMed] [Google Scholar]
- Bell NS, Mangione TW, Hemenway D, Amoroso PJ, Jones BH. High injury rates among female army trainees: a function of gender? Am J Prev Med. 2000;18:141–146. doi: 10.1016/s0749-3797(99)00173-7. [DOI] [PubMed] [Google Scholar]
- Bellantoni MF, Vittone J, Campfield AT, Bass KM, Harman SM, Blackman MR. Effects of oral versus transdermal estrogen on the growth hormone/insulin-like growth factor I axis in younger and older postmenopausal women: a clinical research center study. J Clin Endocrinol Metab. 1996;81:2848–2853. doi: 10.1210/jcem.81.8.8768841. [DOI] [PubMed] [Google Scholar]
- Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Horm Res. 2004;62(Suppl. 1):50–58. doi: 10.1159/000080759. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Bernstein IM, Belisle A, Brattbakk B, Devanny P, Risinger R, Durant D. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33:1298–1304. doi: 10.1177/0363546505275149. [DOI] [PubMed] [Google Scholar]
- Bijur PE, Horodyski M, Egerton W, Kurzon M, Lifrak S, Friedman S. Comparison of injury during cadet basic training by gender. Arch Pediatr Adolesc Med. 1997;151:456–461. doi: 10.1001/archpedi.1997.02170420026004. [DOI] [PubMed] [Google Scholar]
- Bjordal JM, Arnly F, Hannestad B, Strand T. Epidemiology of anterior cruciate ligament injuries in soccer. Am J Sports Med. 1997;25:341–345. doi: 10.1177/036354659702500312. [DOI] [PubMed] [Google Scholar]
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190:S5–S22. doi: 10.1016/j.ajog.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Campagnoli C, Biglia N, Altare F, Lanza MG, Lesca L, Cantamessa C, Peris C, Fiorucci GC, Sismondi P. Differential effects of oral conjugated estrogens and transdermal estradiol on insulin-like growth factor 1, growth hormone and sex hormone binding globulin serum levels. Gynecol Endocrinol. 1993;7:251–258. doi: 10.3109/09513599309152509. [DOI] [PubMed] [Google Scholar]
- Chen JL, Yao W, Frost HM, Li CY, Setterberg RB, Jee WS. Bipedal stance exercise enhances antiresorption effects of estrogen and counteracts its inhibitory effect on bone formation in sham and ovariectomized rats. Bone. 2001;29:126–133. doi: 10.1016/s8756-3282(01)00496-3. [DOI] [PubMed] [Google Scholar]
- Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: a prospective study. Bone. 1993;14:41–45. doi: 10.1016/8756-3282(93)90254-8. [DOI] [PubMed] [Google Scholar]
- Cunningham JJ. Body composition and resting metabolic rate: the myth of feminine metabolism. Am J Clin Nutr. 1982;36:721–726. doi: 10.1093/ajcn/36.4.721. [DOI] [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Engstrom B, Johansson C, Tornkvist H. Soccer injuries among elite female players. Am J Sports Med. 1991;19:372–375. doi: 10.1177/036354659101900408. [DOI] [PubMed] [Google Scholar]
- Fischer GM. Comparison of collagen dynamics in different tissues under the influence of estradiol. Endocrinology. 1973;93:1216–1218. doi: 10.1210/endo-93-5-1216. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A, Kessler U, Dorka B, Funk B, Ørskov H, Kiess W. Transient increase in renal insulin-like growth factor binding proteins during initial kidney hypertrophy in experimental diabetes in rats. Diabetologia. 1992;35:589–593. doi: 10.1007/BF00400489. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Dinesen B, Ørskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5:169–176. [PubMed] [Google Scholar]
- Frystyk J, Ivarsen P, Skjaerbaek C, Flyvbjerg A, Pedersen EB, Orskov H. Serum-free insulin-like growth factor I correlates with clearance in patients with chronic renal failure. Kidney Int. 1999;56:2076–2084. doi: 10.1046/j.1523-1755.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Delmas PD. Decreased bone turnover in oral contraceptive users. Bone. 1995;16:499–503. doi: 10.1016/8756-3282(95)00075-o. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- Hama H, Yamamuro T, Takeda T. Experimental studies on connective tissue of the capsular ligament. Influences of aging and sex hormones. Acta Orthop Scand. 1976;47:473–479. doi: 10.3109/17453677608988723. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Sex specific differences in mechanical properties of isolated collagen fascicles from the human patellar tendon (Abstract) 2006 European Congress of Sports Science Louisanne, Portugal 2006. [Google Scholar]
- Hart DA, Archambault JM, Kydd A, Reno C, Frank CB, Herzog W. Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin Orthop Relat Res. 1998:44–56. [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- Ho KK, Weissberger AJ. Impact of short-term estrogen administration on growth hormone secretion and action: distinct route-dependent effects on connective and bone tissue metabolism. J Bone Miner Res. 1992;7:821–827. doi: 10.1002/jbmr.5650070711. [DOI] [PubMed] [Google Scholar]
- Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop. 2000;372:50–63. doi: 10.1097/00003086-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Chiappetta C, Stancel GM. Synthetic estrogen 17α-ethinyl estradiol induces pattern of uterine gene expression similar to endogenous estrogen 17β-estradiol. J Pharmacol Exp Ther. 1999;290:740–747. [PubMed] [Google Scholar]
- Jensen CH, Hansen M, Brandt J, Rasmussen HB, Jensen PB, Teisner B. Quantification of the N-terminal propeptide of human procollagen type I (PINP): comparison of ELISA and RIA with respect to different molecular forms. Clin Chim Acta. 1998;269:31–41. doi: 10.1016/s0009-8981(97)00182-4. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P. Structure and metabolism of normal tendons. In: Jozsa L, Kannus P, editors. Human Tendons–Anatomy, Physiology, and Pathology. Champagne, IL, USA: Human Kinetics; 1997. pp. 46–96. [Google Scholar]
- Jung-Hoffmann C, Fitzner M, Kuhl H. Oral contraceptives containing 20 or 30 micrograms ethinylestradiol and 150 micrograms desogestrel: pharmacokinetics and pharmacodynamic parameters. Horm Res. 1991;36:238–246. doi: 10.1159/000182172. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, Rajkovic IA, O'Sullivan AJ, Sernia C, Ho KK. Effects of different oral oestrogen formulations on insulin-like growth factor-I, growth hormone and growth hormone binding protein in post-menopausal women. Clin Endocrinol (Oxf) 1993;39:561–567. doi: 10.1111/j.1365-2265.1993.tb02410.x. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 2007;191:111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A. Free and total insulin-like growth factor (IGF)-I-II, and IGF binding protein-1-2, and - 3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab. 2003;88:132–135. doi: 10.1210/jc.2002-021349. [DOI] [PubMed] [Google Scholar]
- Lahita RG. The connective tissue diseases and the overall influence of gender. Int J Fertil Menopausal Stud. 1996;41:156–165. [PubMed] [Google Scholar]
- Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bülow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LG, Baum J, Mudholkar GS, Srivastava DK. Hypermobility: prevalence and features in a Swedish population. Br J Rheumatol. 1993;32:116–119. doi: 10.1093/rheumatology/32.2.116. [DOI] [PubMed] [Google Scholar]
- Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev. 2004;32:64–68. doi: 10.1097/00003677-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Lee CY, Liu X, Smith CL, Zhang X, Hsu HC, Wang DY, Luo ZP. The combined regulation of estrogen and cyclic tension on fibroblast biosynthesis derived from anterior cruciate ligament. Matrix Biol. 2004;23:323–329. doi: 10.1016/j.matbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Liu SH, Al Shaikh RA, Panossian V, Finerman GA, Lane JM. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. Am J Sports Med. 1997;25:704–709. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- Liu SH, al Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, Finerman GA, Lane JM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14:526–533. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol Endocrinol Metab. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci. 2003;58:123–127. doi: 10.1093/gerona/58.2.b123. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol. 2007;88:237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2006;102:541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Nielsen J, Hammar M. Women's soccer injuries in relation to the menstrual cycle and oral contraceptive use. Med Sci Sports Exerc. 1989;21:126–129. [PubMed] [Google Scholar]
- Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK. Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women. Clin Endocrinol (Oxf) 2003;59:690–698. doi: 10.1046/j.1365-2265.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol. 2006a;101:183–188. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Langberg H, Magnusson SP, Kjaer M, Flyvbjerg A. Expression, content, and localization of insulin-like growth factor I in human Achilles tendon. Connect Tissue Res. 2006b;47:200–206. doi: 10.1080/03008200600809893. [DOI] [PubMed] [Google Scholar]
- Prifti S, Mall P, Rabe T. Synthetic estrogen-mediated activation of ERK 2 intracellular signaling molecule. Gynecol Endocrinol. 2003;17:423–428. doi: 10.1080/09513590312331290338. [DOI] [PubMed] [Google Scholar]
- Saugstad LF. Is persistent pelvic pain and pelvic joint instability associated with early menarche and with oral contraceptives? Eur J Obstet Gynecol Reprod Biol. 1991;41:203–206. doi: 10.1016/0028-2243(91)90025-g. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Turner CH. Estrogen receptor β: the antimechanostat? Bone. 2005;36:185–192. doi: 10.1016/j.bone.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Scott A, Khan KM, Duronio V. IGF-I activates PKB and prevents anoxic apoptosis in Achilles tendon cells. J Orthop Res. 2005;23:1219–1225. doi: 10.1016/j.orthres.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45:594–603. [PMC free article] [PubMed] [Google Scholar]
- Slauterbeck J, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17:405–408. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- Sulak PJ. Oral contraceptive update: new agents and regimens. J Fam Pract. 2004;(Suppl.):S5–12. [PubMed] [Google Scholar]
- Tipton KD, Sharp NC. The response of intracellular signaling and muscle-protein metabolism to nutrition and exercise. Eur J Sports Sci. 2005;5:107–121. [Google Scholar]
- Tobias JH. At the crossroads of skeletal responses to estrogen and exercise. Trends Endocrinol Metab. 2003;14:441–443. doi: 10.1016/j.tem.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Brigman BE, Yamamoto J, Lawrence WT, Simmons JG, Mohapatra NK, Lund PK, Van Wyk J, Hannafin JA, Bhargava MM, Banes AJ. Insulin-like growth factor-I is expressed by avian flexor tendon cells. J Orthop Res. 2000;18:546–556. doi: 10.1002/jor.1100180406. [DOI] [PubMed] [Google Scholar]
- van Rooigen M, Hansson LO, Frostegard J, Silveira A, Hamsten A, Bremme K. Treatment with combined oral contraceptives induces a rise in serum C-reactive protein in the absence of a general inflammatory response. J Thromb Haemost. 2006;4:77–82. doi: 10.1111/j.1538-7836.2005.01690.x. [DOI] [PubMed] [Google Scholar]
- Wentorf FA, Sudoh K, Moses C, Arendt EA, Carlson CS. The effects of estrogen on material and mechanical properties of the intra- and extra-articular knee structures. Am J Sports Med. 2006;34:1948–1952. doi: 10.1177/0363546506290060. [DOI] [PubMed] [Google Scholar]
- Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports. 2008;18:23–30. doi: 10.1111/j.1600-0838.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Davies AJ, Young RJ, White A. The phosphorylation pattern of insulin-like growth factor-binding protein-1 in normal plasma is different from that in amniotic fluid and changes during pregnancy. J Clin Endocrinol Metab. 1994;79:1735–1741. doi: 10.1210/jcem.79.6.7527409. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Pennells LA, White A. Modification of plasma insulin-like growth factors and binding proteins during oral contraceptive use and the normal menstrual cycle. Am J Obstet Gynecol. 1999;180:530–536. doi: 10.1016/s0002-9378(99)70249-8. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- Wreje U, Brynhildsen J, Aberg H, Bystrom B, Hammar M, von Schoultz B. Collagen metabolism markers as a reflection of bone and soft tissue turnover during the menstrual cycle and oral contraceptive use. Contraception. 2000;61:265–270. doi: 10.1016/s0010-7824(00)00106-2. [DOI] [PubMed] [Google Scholar]
- Wreje U, Isacsson D, Aberg H. Oral contraceptives and back pain in women in a Swedish community. Int J Epidemiol. 1997;26:71–74. doi: 10.1093/ije/26.1.71. [DOI] [PubMed] [Google Scholar]
- Yu WD, Liu SH, Hatch JD, Panossian V, Finerman GA. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop Relat Res. 1999:229–238. doi: 10.1097/00003086-199909000-00030. [DOI] [PubMed] [Google Scholar]
- Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001:268–281. doi: 10.1097/00003086-200102000-00031. [DOI] [PubMed] [Google Scholar]