Abstract
The split-ubiquitin technique was used to detect transient protein interactions in living cells. Nub, the N-terminal half of ubiquitin (Ub), was fused to Sec62p, a component of the protein translocation machinery in the endoplasmic reticulum of Saccharomyces cerevisiae. Cub, the C-terminal half of Ub, was fused to the C terminus of a signal sequence. The reconstitution of a quasi-native Ub structure from the two halves of Ub, and the resulting cleavage by Ub-specific proteases at the C terminus of Cub, serve as a gauge of proximity between the two test proteins linked to Nub and Cub. Using this assay, we show that Sec62p is spatially close to the signal sequence of the prepro-α-factor in vivo. This proximity is confined to the nascent polypeptide chain immediately following the signal sequence. In addition, the extent of proximity depends on the nature of the signal sequence. Cub fusions that bore the signal sequence of invertase resulted in a much lower Ub reconstitution with Nub-Sec62p than otherwise identical test proteins bearing the signal sequence of prepro-α-factor. An inactive derivative of Sec62p failed to interact with signal sequences in this assay. These in vivo findings are consistent with Sec62p being part of a signal sequence-binding complex.
INTRODUCTION
A critical step during the translocation of a protein across the membrane of the endoplasmic reticulum (ER) is the interaction between the signal sequence of a nascent polypeptide and its receptors (Walter et al., 1981; Gilmore and Blobel, 1985; Walter and Johnson, 1994). A stretch of 8 to 12 hydrophobic residues, often at the N terminus of a protein, comprises a signal sequence that is sufficient to initiate the protein’s translocation into the endoplasmic reticulum (ER) (Rapoport et al., 1996). To be compatible with a high flux of polypeptides through a limited number of translocation channels in the ER membrane, the interaction between the signal sequence and its receptors has to be short lived. Its transient nature makes such a receptor–ligand interaction difficult to study, especially in living cells. The approaches used for the analysis of protein translocation in cell-free systems circumvent the transience of the signal sequence–receptor interaction by pausing or stopping the synthesis of a nascent polypeptide chain at different stages of its movement to and across the ER membrane (Krieg et al., 1986; Kurzchalia et al., 1986; Connolly et al., 1989). Given these constraints, it is essential to verify in vivo the models derived from in vitro studies. The ability to analyze early translocation events in vivo should also be important for defining the immediate environment of the nascent chain on its path from the ribosome to the ER membrane.
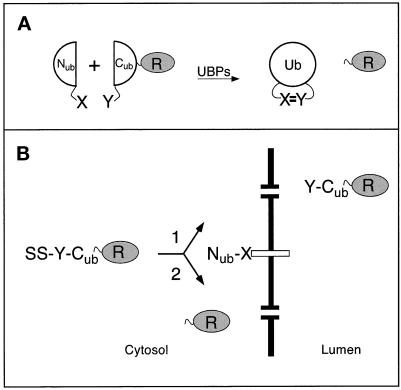
Most of the current methods for detecting protein interactions in vivo either do not operate at the ER membrane or are unable to detect a transient proximity between proteins (Fields and Song, 1989; Aronheim et al., 1997; Rossi et al., 1997; Miyawaki et al., 1997). In the present work, we show that the previously developed split-ubiquitin (split-Ub) technique, also called USPS (Ub/split/protein/sensor) (Johnsson and Varshavsky, 1994a, 1997), is capable of detecting a transient in vivo interaction between polypeptides. The split-Ub method is based on the ability of Nub and Cub, the N- and C-terminal halves of Ub, to assemble into a quasi-native Ub. Ub-specific proteases (UBPs), which are present in all eukaryotic cells, recognize the reconstituted Ub, but not its halves, and cleave the Ub moiety off a reporter protein that had been linked to the C terminus of Cub. The liberation of the reporter serves as a readout indicating the reconstitution of Ub. The assay is designed in a way that prevents efficient association of Nub and Cub by themselves, but allows it if the two Ub halves are separately linked to proteins that interact in vivo (Figure 1A). The split-Ub assay has been shown to detect the in vivo dimerization of a leucine zipper-containing domain of the Gcn4p transcriptional activator, and the in vivo interaction between two subunits of the oligosaccharyltransferase complex (Johnsson and Varshavsky, 1994a; Stagljar et al., 1998).
Figure 1.
The split-Ub technique and its application to the analysis of protein translocation. (A) Nub and Cub are linked to the interacting proteins X and Y. The X–Y complex brings Nub and Cub into close proximity. Nub and Cub reconstitute a quasi-native Ub moiety, which is cleaved by the UBPs, yielding the free reporter R (Johnsson and Varshavsky, 1994a). (B) Using split-Ub to monitor the proximity between a secretory protein and a component of the translocation machinery. A signal sequence-bearing Cub fusion (SS-Y-Cub-R) and a Nub fusion (Nub-X) are coexpressed in a cell. Pathway 1: when Nub is linked to a protein not involved in the targeting for translocation, the uncleaved (except for the signal sequence SS) Y-Cub-R enters the ER. Pathway 2: when Nub is linked to a protein involved in the targeting for translocation the signal sequence of the SS-Y-Cub-R brings Nub and Cub into close contact. As a result, some of the SS-Y-Cub-R and Nub-X molecules interact to form the quasi-native Ub, yielding the free reporter R in the cytosol.
In the present work, we focus on the interaction between Sec62p of the yeast Saccharomyces cerevisiae and proteins bearing two different signal sequences. Extensive evidence indicates that Sec62p is a component of the ER translocation machinery (Deshaies and Schekman, 1989; Rothblatt et al., 1989; Müsch et al., 1992). Sec62p is a part of the tetrameric Sec62/63 complex that also contains Sec71p and Sec72p (Deshaies et al., 1991; Feldheim and Schekman, 1994). Sec62/63p can be isolated as a tetramer, or as a part of a larger assembly, the heptameric Sec complex (Panzner et al., 1995). In addition to the Sec62/63 complex, the heptamer contains the trimer of Sec61p. This trimer (Sec61p, Sss1p, Sbh1p in yeast; Sec61α, Sec61β, Sec61γ in mammals) forms the aqueous channel through which a polypeptide chain is translocated across the ER membrane (Simon and Blobel, 1991; Görlich et al., 1992; Crowley et al., 1993, 1994; Mothes et al., 1994; Hanein et al., 1996; Beckmann et al., 1997).
The role of the Sec62/63 tetramer is less well defined. Cross-linking and reconstitution experiments in vitro have shown that Sec62p is close to the nascent polypeptide chain before the initiation of its translocation (Müsch et al., 1992; Lyman and Schekman, 1997; Matlack et al., 1997). One important role of Sec63p is its ability to recruit the Hsp70-type protein Kar2p of the ER lumen to the vicinity of a translocating polypeptide (Brodsky and Schekman, 1993; Lyman and Schekman, 1997). The Sec62/63 complex is essential for the posttranslational translocation of proteins in reconstituted vesicle preparations (Panzner et al., 1995). Genetic analysis supports this conclusion, by showing that the tetrameric Sec62/63 complex is involved in the translocation of proteins whose targeting to the ER membrane is not abolished by the loss of the signal recognition particle (SRP) (Ng et al., 1996). However, it is less clear whether the Sec62/63 complex is the receptor for the signal sequences of those proteins.
In the present work, we demonstrate the ability of the split-Ub assay to detect transient protein interactions in living cells. We show that the assay can monitor a close proximity between Sec62p and a segment of the nascent chain of a signal sequence-bearing protein. The apparent extent of this proximity is influenced by the nature of the signal sequence and the position of Cub in the nascent polypeptide. Our analysis yields a crude map of the environment of the nascent chain during its targeting to and translocation across the ER membrane. Taken together, these findings are the first in vivo evidence that Sec62p, a component of the translocation machinery in the endoplasmic reticulum, is a part of a signal sequence- binding complex.
MATERIALS AND METHODS
Construction of Test Proteins
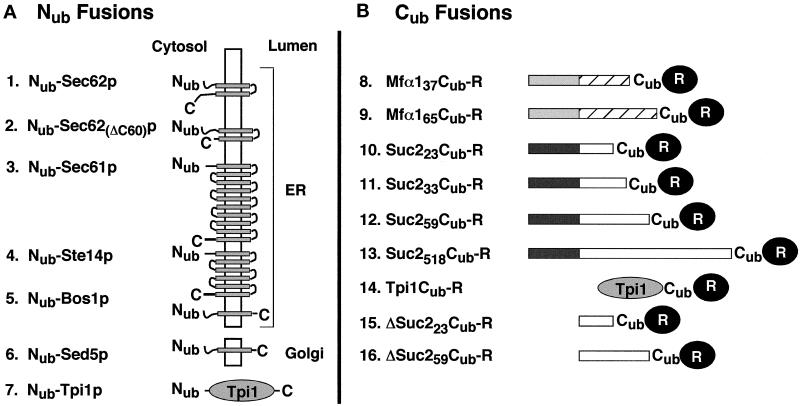
The Cub fusions 8–13 (Figure 2) were derived from the construct I of Johnsson and Varshavsky (1994a), which encoded Ub-DHFR-ha and contained a BamHI site at the amino acid position 36 of Ub, and from the previously described Ub translocation constructs I, VI, IX, X, XXIII, and XXV (Johnsson and Varshavsky, 1994b). The above BamHI site of the Ub-DHFR-ha construct I was fused to a linker sequence in which a 5′-SalI site allowed the in-frame insertion of an EagI–SalI fragment containing the promoter, the signal sequence, and a portion of the mature sequence of the corresponding Ub fusions. The newly introduced sequence was G TCG ACC ATG TCG GGG GGG ATC CCT. The last three triplets encode residues 35, 36, and 37 of Ub (the beginning of Cub). The underlined sequences are the SalI and BamHI sites, respectively. The final constructs were in the single-copy plasmids pRS314 or pRS315 (Sikorski and Hieter, 1989). Expression of the Cub fusions bearing Dha (DHFR-ha) as a reporter was mediated by the PADH1 promoter, except for the Cub fusion 14, which was expressed from the PCUP1 promoter. The same promoter was used for expressing the Ura3p-based Cub fusions.
Figure 2.
Nub and Cub test fusions. (A) Nub (residues 1–36 of Ub) was fused to the N terminus of either a transmembrane protein (constructs 1–6) or a cytosolic protein (construct 7). The N termini of all proteins are located in the cytosol. The orientations and the numbers of membrane-spanning regions (shaded boxes) were derived from the published studies of these proteins, except for Ste14p, for which the exact number of the domains and the localization of the C terminus are not yet known. The Nub fusions 1–5 are located in the membrane of the ER; the Nub fusion 6 resides in the membrane of the early Golgi. The Nub fusion 2 is a Sec62p derivative lacking the C-terminal 60 residues. The Nub fusion 7 contains the full-length triosephosphate isomerase (Nub-Tpi1p). (B) Cub fusions. The Cub fusions 8 and 9 contain the signal sequence of the prepro-α-factor (shaded boxes), followed by either 37 (construct 8) or 65 residues (construct 9) of the mature α-factor sequence (striped boxes) and a 7-residue linker sequence (not shown). Cub fusions 10–13 contain the signal sequence of the Suc2p invertase (dark boxes) followed by 23 (construct 10), 33 (construct 11), 59 (construct 12), or 518 residues (construct 13) of the mature sequence of invertase (open boxes) and a 7-residue linker sequence (not shown). The Cub fusion 14 contains the complete sequence of S. cerevisiae triosephosphate isomerase (Tpi1p) followed by a 17-residue linker peptide and Cub. The Cub fusions 15 and 16 are the signal sequence-lacking counterparts of the fusions 10 and 12. Cub is always followed by a reporter protein. The reporter is DHFR-ha or Ura3p for the Cub fusions 8–13, and DHFR-ha for the Cub fusions 14, 15, and 16.
The Cub fusions 15 and 16 (Figure 2) were derived from constructs 10 and 12 by deleting the HindIII fragment spanning the first four codons of the SUC2 ORF and a short portion of the polylinker sequence between the 3′-end of the PADH1 promoter and the SUC2 ORF. As a result, the translation of Cub fusions 15 and 16 began at the first codon of the mature invertase, skipping its signal sequence. The Cub fusion 14 (Figure 2) was produced through an in-frame fusion of a PCR fragment containing the complete TPI1 coding sequence and Cub-Dha. The sequence between TPI1 and Cub is as follows: AAC GGG TCG ACC GAC TAC AAG GAC GAC GAT GAC AAG GGC TCG ACC ATGTCG GGG GGG ATC CCT. The underlined sequences indicate, respectively, the last codon of TPI1 and the first three codons of Cub.
A fragment encoding Nub-Sec62p was constructed using PCR amplification of a 1050 base pair (bp) fragment containing the SEC62 ORF. PCR introduced a BamHI site and a linker sequence in front of the start codon of SEC62 and an XhoI site 173 bp downstream of the stop codon. The PCUP1-Nub modules were cloned as BamHI fragments in frame with the SEC62 ORF. The sequence between Nub and SEC62 is GGG ATC CCT TCT GGG ATG. The first three codons encode residues 35, 36, and 37 of Nub, followed by the Gly-Ser linker and the start codon of SEC62. The BamHI site is underlined. The final constructs resided in pRS316 or pRS313. Nub-TPI1, Nub-SED5, Nub-STE14 (a gift from N. Lewke), and Nub-Sec62(ΔC60)-Dha were constructed similarly to Nub-SEC62. With the exception of Nub-Sec62(ΔC60)-Dha, which was placed in pRS316 and pRS313, all of these fusions resided in pRS314. The linker connecting codon 35 of Nub and the first codon of a linked gene was GGG ATC CCT GGG GAT ATG for Nub-TPI1 and Nub-SED5, and GGG ATC CCT GGG GAT CAC for Nub-STE14. Underlined are the BamHI site and the first codon of the linked gene. The sequence GGG TCG ACC TTA ATG CAG AGA TCT GGC ATC ATG GTT connected the last codon of SEC62 in Nub-Sec62(ΔC60)-Dha (codon 223, underlined) to the first two codons of DHFR (underlined).
Nub-BOS1 was constructed in part by PCR amplification, with two synthetic oligos and yeast genomic DNA as a template, yielding a 258-bp fragment containing the first 229 bp of the BOS1 ORF. Upstream of the BOS1 ATG was a short linker sequence and a BamHI site, to allow in-frame fusion of the PCUP1 promoter-Nub module. The sequence between Nub and BOS1 reads: GGG ATC CCT CCA GGA ATG. The first four triplets encode residues 35, 36, 37, and 38 of Nub, followed by the Gly codon and the start codon of BOS1. The BamHI site is underlined. The 3′-region of the resulting fragment terminated in a SalI site for insertion into the integrating vectors pRS306 or pRS303. The vector was cut at the unique EcoRI site in the BOS1-containing fragment and transformed into S. cerevisiae strains YPH500 and JD53 to produce, through homologous recombination, the integrated cassette that expressed Nub-Bos1p from the PCUP1 promoter. The presence of the desired gene fusion and the absence of wild-type BOS1 were verified by PCR.
An integrated copy of PCUP1-Nub-SEC62 was produced by amplifying the first 438 bp of the SEC62 ORF, and then cloning it, using the BamHI and EcoRI restriction sites, in frame behind the pRS306-PCUP1-Nub cassette. A unique AflII site in the SEC62 ORF was used to linearize the plasmid for transformation and integration at the S. cerevisiae SEC62 gene, yielding the strain NJY62-I. The N-terminal 147-residue fragment of Sec62p that was coexpressed with Nub-Sec62p in the resulting strain has previously been shown to be inactive in translocation (Deshaies and Schekman, 1990). Nub-SEC61 was constructed by targeted integration of a Nub-SEC61–containing fragment into SEC61 of the S. cerevisiae strain JD53 (Table 1). Specifically, a fragment containing the first 875 bp of the SEC61 ORF was amplified by PCR and inserted downstream of the pRS304- or pRS303-based PCUP1-Nub cassette, using the flanking BamHI and EcoRI sites. The linker sequence between Nub and SEC61 was GGG ATC CCT GGG TCT GGG ATG. Underlined are the BamHI site and the first codon of SEC61. For targeted integration, the plasmid was linearized at the unique StuI site in the SEC61 ORF to create the yeast NJY61-I. A detailed description of the NJY61 strains (Table 1) will be presented elsewhere (Wittke and Johnsson, unpublished data).
Table 1.
Yeast strains
| Strain | Relevant genotype | Source/comment |
|---|---|---|
| YPH500 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 | Sikorski and Hieter (1989) |
| JD53 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Dohmen et al. (1995) |
| RSY529 | MATα his4 leu2-3,112 ura3-52 sec62-1 | Deshaies and Schekman (1989) |
| NJY51 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 | Derivative of YPH500 |
| NUB-BOS1::pRS306 | ||
| NJY62-1 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUB-SEC62::pRS306 | ||
| NJY73-I | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUB-BOS1::pRS303 | ||
| NJY74-I | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUB-BOS1::pRS306 | ||
| NJY61-I | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUB-SEC61::pRS304 | ||
| NJY61-A | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUA-SEC61::pRS304 | ||
| NJY61-G | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 | Derivative of JD53 |
| NUG-SEC61::pRS304 |
All of the PCUP1 promoter-controlled ORFs were expressed under noninducing conditions (no copper added to the medium), except in the experiment shown in Figure 5B, where cells were incubated in the presence of 0.1 mM CuSO4.
Figure 5.
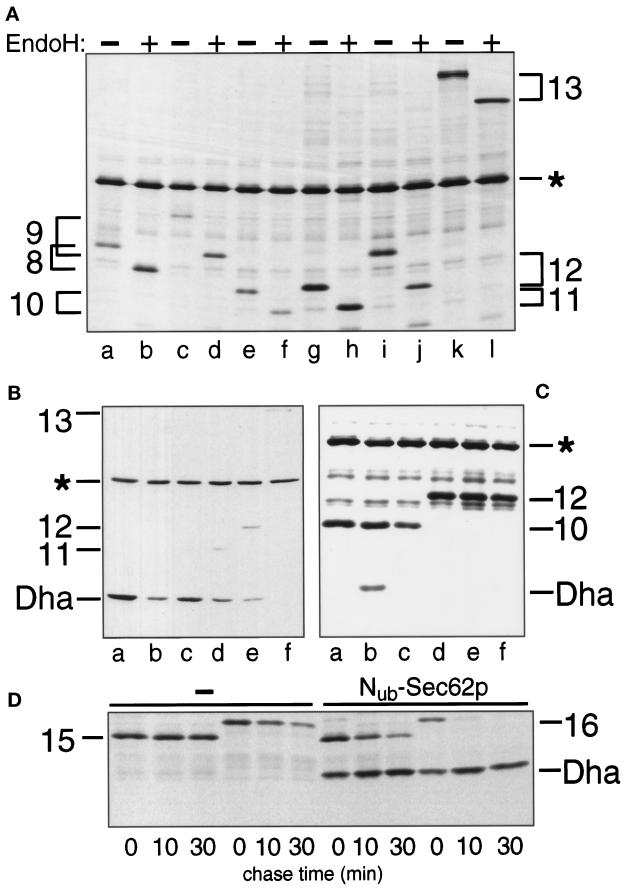
The nature of the signal sequence and its distance from Cub determine the extent of cleavage of Cub-R in the presence of Nub-Sec62p. (A) S. cerevisiae expressing Mfα37-Cub-Dha (construct 8; Figure 2) (lanes a and b), Mfα65-Cub-Dha (construct 9) (lanes c and d), Suc223-Cub-Dha (construct 10) (lanes e and f), Suc233-Cub-Dha (construct 11) (lanes g and h), Suc259-Cub-Dha (construct 12) (lanes i and j) and Suc2518-Cub-Dha (construct 13) (lanes k and l) were labeled with 35S-methionine for 5 min. The extracted proteins were either mock-treated (lanes a, c, e, g, i, and k) or treated with EndoH (lanes b, d, f, h, j, and l), followed by immunoprecipitation with anti-ha antibody and SDS-PAGE. (B) Same as panel A but the cells also contained Nub-Sec62p in addition to the Cub-fusions Mfα37-Cub-Dha, Mfα65-Cub-Dha, Suc223-Cub-Dha, Suc233-Cub-Dha, Suc259-Cub-Dha, Suc2518-Cub-Dha (lanes a–f). The analysis was carried out by immunoblotting whole- cell extracts with the anti-ha antibody. (C) S. cerevisiae cells expressing Suc223-Cub-Dha (construct 10; Figure 2) (lanes a–c) and Suc259-Cub-Dha (construct 12; Figure 2) (lanes d–f) together with either Nub-Sec62p (lanes b and e), Nub-Bos1p (lanes c and f) or the vector (lanes a and d) were labeled for 5 min with 35S-methionine. Whole-cell extracts were immunoprecipitated with anti-ha antibody, followed by SDS-PAGE and autoradiography. (D) S. cerevisiae cells expressing ΔSuc223-Cub-Dha (construct 15; Figure 2) or ΔSuc259-Cub-Dha (construct 16; Figure 2) together with either the vector (first six lanes) or Nub-Sec62p (last six lanes) were labeled for 5 min with 35S-methionine and chased for 10 and 30 min, followed by extraction, immunoprecipitation with anti-ha antibody, and SDS-PAGE. Numbers 15 and 16 indicate the positions of the corresponding (uncleaved) Cub fusions.
Immunoblotting
Proteins fractionated by SDS-12.5% PAGE were electroblotted onto nitrocellulose (Schleicher & Schuell, Dassel, Germany) or polyvinylidene difluoride (Machery-Nagel, Düren, Germany) membranes, using the semidry transfer system (Hoeffer Pharmacia Biotech, San Francisco, CA). Blots were incubated with an anti-ha monoclonal antibody (Babco, Richmond, CA) and visualized using horseradish peroxidase-coupled goat anti-mouse antibody (Bio-Rad, Hercules, CA), the chemiluminescence detection system (Boehringer, Mannheim, Germany), and x-ray films. Where indicated, quantification was performed using the Lumi Imager system (Boehringer).
Pulse-Chase Analysis
Yeast-rich (YPD) and synthetic minimal media with 2% dextrose (SD) were prepared as described previously (Dohmen et al., 1995). S. cerevisiae cells expressing the Nub and Cub fusions were grown at 30°C in 10 ml of SD medium without externally added copper to an OD600 of ∼1 and labeled for 5 min with Redivue Promix-[35S] (Amersham, Buckinghamshire, United Kingdom), followed (either directly or after a chase) by immunoprecipitation with the anti-ha monoclonal antibody, essentially as described by Johnsson and Varshavsky (1994a,b). The EndoH analysis of glycosylated proteins was carried out as described by Orlean et al. (1991). Samples were concentrated before SDS-12.5% PAGE by precipitation with chloroform/methanol. Gels were fixed and enhanced for fluorography. For quantitative analysis, a dried gel was exposed and scanned using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Experimental Strategy
The use of split Ub to monitor the proximity between the proteins X and Y requires the construction of two “complementary” fusion proteins. One fusion bears Nub (see INTRODUCTION) linked to X (Nub-X) and the other bears Cub linked to both Y and a reporter protein R at the C terminus of Cub (Y-Cub-R). The liberation of the reporter through the Ub-dependent cleavage by UBPs indicates the in vivo reconstitution of a quasi-native Ub from Nub and Cub. In the split-Ub assay, the efficiency of cleavage at the C terminus of Cub in Y-Cub-R is measured relative to the efficiency of cleavage observed with selected reference (control) proteins (Figure 1).
To monitor protein interactions during translocation of a protein across the ER membrane, Nub was fused to the N terminus of a membrane protein that is a part of the translocation machinery (Figure 1). Owing to the constraint of the assay, which requires the cytosolic location of the reconstituted Ub, the N terminus of this membrane protein must be located in the cell’s cytosol. Sec62p has an N-terminal cytosolic domain of 158 residues, which is followed by two membrane-spanning segments and a C-terminal segment also facing the cytosol (Deshaies and Schekman, 1990). Nub was therefore fused to the N terminus of Sec62p, yielding Nub-Sec62p. Cub was sandwiched between the 56 N-terminal residues of the precursor of S. cerevisiae α-factor pheromone (prepro-α-factor) and the ha epitope-tagged mouse dihydrofolate reductase (DHFR-ha; denoted as Dha) as a reporter protein, yielding Mfα37-Cub-Dha (Figure 2). The cleavage of the Cub-containing fusion at the Cub–Dha junction was detected with a monoclonal anti-ha antibody.
Split-Ub Detects a Proximity between a Translocating Protein and Sec62p
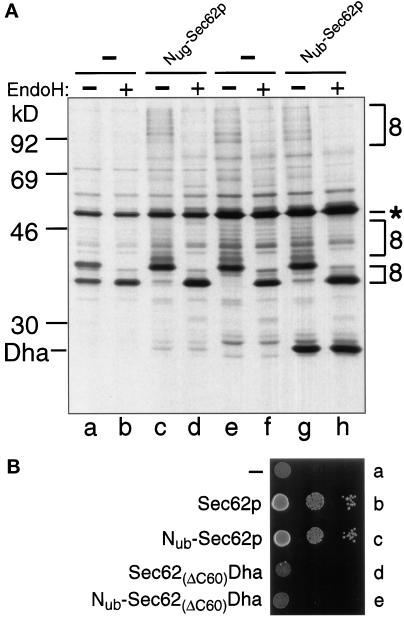
We first verified that Mfα37-Cub-Dha could be translocated across the ER membrane and that the N-terminal extension of Sec62p with Nub did not interfere with the Sec62p function in translocation. After a 5-min pulse of wild-type S. cerevisiae with 35S-methionine, the labeled Mfα37-Cub-Dha was immunoprecipitated as a glycosylated and unclipped fusion (Figure 3A). Thus, Mfα37-Cub-Dha could indeed be translocated into the lumen of ER. Introduction of the same Mfα37-Cub-Dha construct into the yeast strain RSY529, which carries a temperature-sensitive (ts) variant of Sec62p (Rothblatt et al., 1989), confirmed the severe translocation defect of this strain. About 50% of the pulse-labeled Mfα37-Cub-Dha entered the lumen of the ER in this strain at the semipermissive temperature of 30°C, while the rest remained in the cytosol (Figure 3A). Thus, the translocation of Mfα37-Cub-Dha depends on Sec62p. This made it possible to determine whether Nub-Sec62p is functionally active. The test utilized Nug-Sec62p, in which the N-terminal half of Ub contained Gly-13 instead of wild-type Ile-13. This derivative, denoted as Nug, has a lower affinity for Cub than the wild-type Nub (Johnsson and Varshavsky, 1994a). We chose Nug-Sec62p for this experiment to minimize the reconstitution of the Ub moiety through interactions between Nub-Sec62p and potentially arrested molecules of Mfα37-Cub-Dha, which might be localized in the cytosol. Plasmids expressing Nug-Sec62p and Mfα37-Cub-Dha were cotransformed into RSY529 cells and assayed at 30°C. As in wild-type cells, only translocated Mfα37-Cub-Dha, but virtually no free Dha or nontranslocated Mfα37-Cub-Dha, was detected after immunoprecipitation and EndoH treatment of the cells that had been labeled for 5 min with 35S-methionine (Figure 3A).
Figure 3.
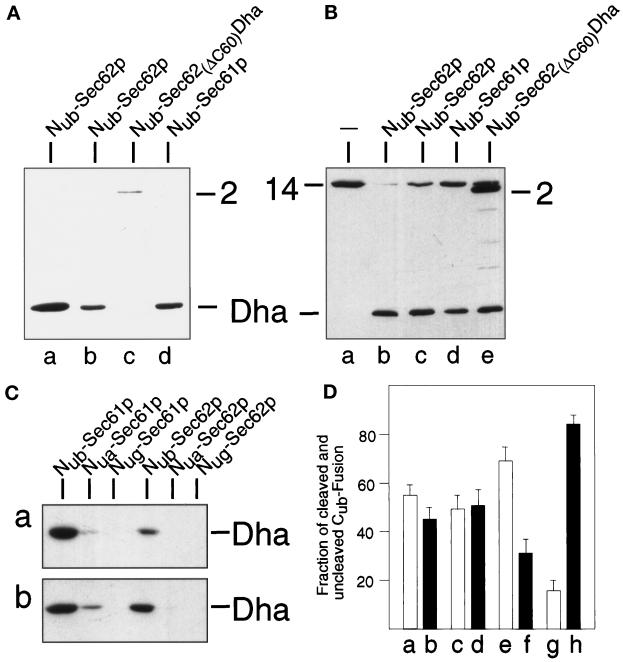
Sec62p is close to the signal sequence of the α-factor precursor. (A) S. cerevisiae cells expressing Mfα37-Cub-Dha (construct 8; Figure 2) were labeled for 5 min with 35S-methionine. The extracted proteins were immunoprecipitated with anti-ha antibody, followed by a mock treatment (lanes a, c, e, and g) or the treatment with EndoH (lanes b, d, f, and h), and SDS-PAGE. The results with cells coexpressing Nug- or Nub-Sec62p are shown in lanes c and d and g and h, respectively. The analysis was performed with Nug-Sec62p in the S. cerevisiae mutant RSY529 carrying a ts allele of SEC62 (lanes a–d) or with Nub-Sec62p in the wild-type yeast (lanes e–h). Number 8 (following the numbering of the constructs in Figure 2) on the right indicates the positions of uncleaved Mfα37-Cub-Dha and its glycosylated forms. An asterisk denotes an unrelated yeast protein that cross-reacts with the anti-ha antibody. (B) Nub-Sec62p encodes a functionally active protein. RSY529 cells carrying an empty plasmid (a), Sec62p (b), Nub-Sec62p (c), Sec62(ΔC60)Dha (d), or Nub-Sec62(ΔC60)Dha (e) were spotted on minimal media and grown for 2 d at 30°C (semipermissive temperature for unmodified RSY529).
To test Nub-Sec62p directly (Nub is the wild-type half of Ub, containing Ile at position 13), we examined its ability to complement the growth defect of RSY529 cells. RSY529 cells expressing Nub-Sec62p were found to grow at the semipermissive temperature of 30°C, in contrast to congenic cells carrying a control plasmid (Figure 3B). To verify that the suppression of the ts phenotype was not due to the initiation of translation from the first (internal) ATG codon of Sec62p within the Nub-Sec62p fusion, the rescue experiment was successfully repeated with the otherwise identical derivative of Nub-Sec62p that lacked the first ATG of SEC62 (our unpublished results).
A significant amount of free Dha was generated when Mfα37-Cub-Dha was expressed (in either wild-type or RSY529 cells) together with Nub-Sec62p, which contained the wild-type half of Ub (Figure 3A and our unpublished results). We concluded that Sec62p is close to the nascent polypeptide chain during its translocation into the ER. The cleavage at the C terminus of Cub requires its interaction with Nub and depends on the presence of UBPs (Johnsson and Varshavsky, 1994a). Since UBPs have previously been shown to be absent from the ER (Johnsson and Varshavsky, 1994b), the free Dha moiety had to be produced in the cytosol. Fractionation experiments confirmed that free Dha was absent from membrane-enclosed compartments in whole-cell extracts (our unpublished results). An entirely independent evidence for this conclusion was produced by replacing Dha in Mfα37-Cub-Dha with Ura3p as the reporter moiety. Ura3p confers the Ura+ phenotype on ura3Δ cells only if Ura3p has access to the cytosol (Johnsson and Varshavsky, 1994b). In our tests, the cytosolic Ura3p was produced only if Mfα37-Cub-Ura3p was coexpressed with Nub-Sec62p (compare A and B in Figure 7), in agreement with the other evidence (see above) that the cleavage at the Cub–protein junction takes place exclusively in the cytosol.
Figure 7.
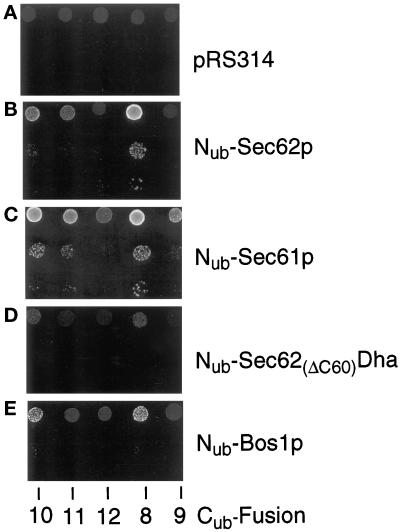
The use of a metabolic marker to assess the proximity between a component of the translocation machinery and a translocated protein. S. cerevisiae expressing the Cub fusions 8–12 (Figure 2) that contained Ura3p instead of Dha (see the main text) were transformed with the vector (A) or plasmids expressing Nub-Sec62p (B), Nub-Sec61p (C), Nub-Sec62(ΔC60)Dha (D), and Nub-Bos1p (E). Cells were grown in a liquid uracil-containing SD medium, and ∼105, 103, and 102 cells were spotted onto uracil-lacking SD medium. Plates were examined after 18 h at 30°C.
The transient nature of the proximity between Sec62p and the nascent chain of a translocated protein was indicated by the near-absence of the released Dha moiety if Mfα37-Cub-Dha was coexpressed with either Nug-Sec62p or Nua-Sec62p instead of Nub-Sec62p (Nua denotes Ala at position 13 of Nub); by contrast, the same experiment with Nub-Sec62p resulted in a significant cleavage of Mfα37-Cub-Dha (Figures 3A and 6C). Previous work (Johnsson and Varshavsky, 1994a) has shown that Nua and Nug can induce significant Ub reconstitution when either of them and Cub are linked to polypeptides that form a stable (long-lived) complex in a cell. In summary, the observed absence of significant Ub reconstitution with Nua and Nug (in contrast to Nub) was interpreted to signify a close but transient (short-lived) proximity between Sec62p and Mfα37-Cub-Dha.
Figure 6.
Sec61p, but not a mutant of Sec62p, are close to the nascent chain of a translocated protein. (A) These assays employed S. cerevisiae expressing Mfα37-Cub-Dha (construct 8, Figure 2) and one of the following Nub fusions (Figure 2): Nub-Sec62p, either integrated (lane a) or plasmid borne (lane b); Nub-Sec62(ΔC60)Dha (lane c); and Nub-Sec61p (lane d). Whole-cell extracts from these strains were subjected to immunoblot analysis with anti-ha antibody. (B) Same as panel A but the same Nub fusions were coexpressed with Tpi1-Cub-Dha (construct 14; Figure 2). Numbers 2 and 14 indicate the positions of the corresponding (uncleaved) fusions. (C) Lane a: S. cerevisiae expressing Suc223-Cub-Dha (construct 10; Figure 2) together with either Nub-Sec61p, Nua-Sec61p, Nug-Sec61p, Nub-Sec62p, Nua-Sec62p, or Nug-Sec62p; lane b: same as lane a but cells expressed Mfα37-Cub-Dha (construct 8; Figure 2) instead of Suc223-Cub-Dha. (D) S. cerevisiae cells expressing Mfα37-Cub-Dha together with Nub-Sec61p (a and b) or Nub-Sec62p (e and f), and cells expressing Suc223-Cub-Dha together with Nub-Sec61p (c and d) or Nub-Sec62p (g and h) were labeled for 5 min with 35S-methionine. Whole-cell extracts were immunoprecipitated with anti-ha antibody, followed by SDS-PAGE, and quantitation of the cleaved and uncleaved Cub fusions using PhosphorImager. Shown are the relative amounts of the cleaved (white bars: a, c, e, and g) and uncleaved (black bars: b, d, f, and h) Cub fusions. The sum of a cleaved and uncleaved fusion was set at 100 in each of the three independent experiments. SDs are also indicated.
Specificity of the Spatial Proximity between a Signal Sequence-bearing Nascent Polypeptide and Sec62p
A commonly used negative control in a translocation assay is a protein with a defective or absent signal sequence (Allison and Young, 1988; Müsch et al., 1992). Such a control is not entirely compatible with spatio-temporal aspects of the split-Ub assay. Specifically, a Cub-fusion protein lacking a signal sequence accumulates in the cytosol (where the split-Ub assay operates), whereas an analogous signal sequence-bearing protein is continuously removed from this compartment. A direct comparison between reactions that involve a signal sequence-bearing polypeptide and its signal sequence-lacking counterpart requires the ability to compare the local concentrations of the two polypeptides at the site of translocation. We are not aware of an in vivo technique that would be independent of the split-Ub assay and at the same time would allow a measurement of these parameters. Therefore, we devised an alternative control. The extent of cleavage of Mfα37-Cub-Dha at the Cub–Dha junction should reflect the time-averaged spatial proximity between the nascent Mfα-chain and a coexpressed Nub-containing fusion. By comparing the extent of cleavage of Mfα37-Cub-Dha in the presence of Nub-Sec62p (Figure 3A) with the analogous activity of Nub-fusion proteins that are not involved in the ER targeting and translocation, we could assess the specificity of the reaction between Nub-Sec62p and Mfα37-Cub-Dha.
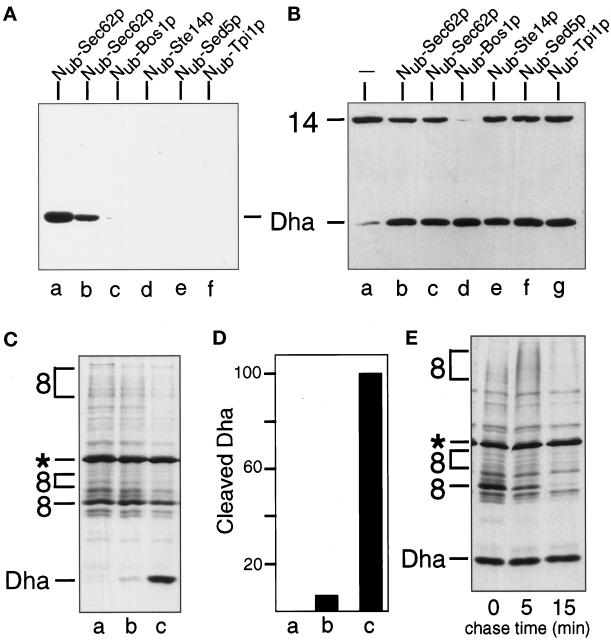
Four Nub-fusion proteins, Nub-Bos1p, Nub-Ste14p, Nub-Sed5p, and Nub-Tpi1p were tested in the split-Ub assay with Mfα37-Cub-Dha. The expected intracellular locations of these Nub fusions, and their predicted topologies in the membrane are shown in Figure 2A (Shim et al., 1991; Banfield et al., 1994; Sapperstein et al., 1994; Lewke and Johnsson, unpublished data). We found that, in contrast to Nub-Sec62p, none of the four tested Nub fusions induced a significant cleavage of Mfα37-Cub-Dha (Figure 4A). A small amount of free Dha could be detected in the immunoblots when Nub-Bos1p was overexpressed. The lack of significant Ub reconstitution from Nub and Cub upon coexpression of Mfα37-Cub-Dha and the Nub-modified ER membrane proteins, Bos1p, Ste14p (Figure 4A), and Sec12p (Nakano et al., 1988; our unpublished results), confirmed that the steady-state concentration of Mfα37-Cub-Dha in the cytosol was extremely low.
Figure 4.
The in vivo proximity between Sec62p and Mfα37-Cub-Dha is transient and specific. (A) Immunoblot analysis of extracts of S. cerevisiae coexpressing the Mfα37-Cub-Dha (construct 8; Figure 2) and one of the following constructs: Nub-Sec62p, integrated (lane a) or plasmid-borne (lane b); Nub-Bos1p (lane c); Nub-Ste14p (lane d); Nub-Sed5p(lane e); and Nub-Tpi1p (lane f). (B) Immunoblot analysis of extracts of S. cerevisiae expressing the Tpi1p-Cub-Dha fusion (construct 14; Figure 2) alone (lane a) or together with one of the following constructs: Nub-Sec62p, either integrated (lane b) or plasmid-borne (lane c); Nub-Bos1p (lane d); Nub-Ste14p (lane e); Nub-Sed5p(lane f); and Nub-Tpi1p (lane g). Number 14 on the left indicates the position of uncleaved Tpi1p-Cub-Dha. (C) S. cerevisiae cells expressing Mfα37-Cub-Dha (construct 8; Figure 2) together with either the vector (lane a), Nub-Bos1p (lane b), or Nub-Sec62p (lane c) were labeled for 5 min with 35S-methionine. The extracted proteins were immunoprecipitated with anti-ha antibody and analyzed by SDS-PAGE. (D) Quantitation of the pulse-labeling experiment (C) using Phosphor-Imager. The extent of Dha release in the presence of Nub-Sec62p was arbitrarily set at 100. The averages of three experiments are shown. Lanes a, b, and c are the same as in panel C. (E) S. cerevisiae cells expressing Mfα37-Cub-Dha together with Nub-Sec62p were labeled for 5 min with 35S-methionine and chased for 5 and 15 min, followed by extraction of proteins, immunoprecipitation with anti-ha antibody, and SDS-PAGE.
To verify that the observed absence of Ub reconstitution (Figure 4A) was not due to either low concentrations of the tested fusion proteins or reduced accessibility of their linked Nub moieties, we compared the activity of these Nub fusions toward a cytosolic Cub-fusion protein. Cub-Dha was fused to the C terminus of the cytosolic enzyme triosephosphate isomerase (Tpi1p), yielding Tpi1-Cub-Dha (Figure 2B). All of the Nub-fusion proteins in Figure 2A induced a significant release of Dha from the test protein Tpi1-Cub-Dha (Figure 4B). This analysis also suggested that Nub-Bos1p was expressed to higher levels than other Nub fusions.
To quantify the relative proximities of Nub-Bos1p and Nub-Sec62p to Mfα37-Cub-Dha, yeast cells were labeled for 5 min with 35S-methionine, and the released Dha was determined as described in the legend to Figure 4. Coexpression of Nub-Sec62p and Mfα37-Cub-Dha yielded ∼15 times more of the free Dha than coexpression of Nub-Bos1p and Mfα37-Cub-Dha (Figure 4, C and D). Assuming that the Nub moieties in Nub-Sec62p and Nub-Bos1p were equally accessible to the cytosol (Figure 4B), we concluded that the time-averaged proximity between the nascent chain of Mfα37-Cub-Dha and the Nub-bearing transmembrane proteins was much higher for Sec62p than for the ER membrane proteins that are not involved in targeting or translocation. Note that this analysis may actually underestimate the proximity of Sec62p to the nascent chain, because we invariably observed a more efficient cleavage of Mfα37-Cub-Dha when Nub-Sec62p was the only form of Sec62p in the cell (Figures 4A and 6A). Therefore we interpret the reduced cleavage of Mfα37-Cub-Dha in the presence of both Nub-Sec62p and the native Sec62p as the consequence of competition between those two Sec62p-containing species for either the signal sequences of translocated proteins or the ligands of Sec62p in the complex of Sec proteins.
Recent evidence indicates that misfolded or otherwise abnormal proteins in the lumen of the ER can be retrotransported across the ER membrane back into the cytosol, where they are degraded by the Ub system (Biederer et al., 1996; Hiller et al., 1996; Wiertz et al., 1996). This retrotransport involves at least some of the components of the known ER translocation machinery (Plemper et al., 1997). To determine whether the cleavage of Mfα37-Cub-Dha at the Cub-Dha junction occurs during translocation into the ER or during (in this case) a hypothetical retrotransport from the ER, cells coexpressing Nub-Sec62p and Mfα37-Cub-Dha were labeled for 5 min with 35S-methionine, and then chased for 15 min (Figure 4E). Although the translocated Mfα37-Cub-Dha disappeared rapidly during the chase, the amount of free Dha that accumulated during the pulse remained constant.
We conclude that the in vivo proximity between Sec62p and Mfα37-Cub-Dha that is detected by the split-Ub assay occurs either during or very shortly after the synthesis of Mfα37-Cub-Dha. The apparent disappearance of the pulse-labeled, translocated Mfα37-Cub-Dha during the chase accounts for the difficulty in detecting this species by a steady-state assay such as immunoblotting (Figures 4A, 5B, and 6A). The likely cause of the disappearance of translocated Mfα37-Cub-Dha is its molecular mass heterogeneity, owing to its glycosylation, which results in a smear upon SDS-PAGE (Figures 3A, 4C, and 4E).
The Efficiency of Ub Reconstitution Mediated by Nub-Sec62p Depends on Both the Identity of a Signal Sequence and the Position of Cub in the Nascent Polypeptide Chain
The proximity of Sec62p to the signal sequence of Mfα37-Cub-Dha is detected, in the split-Ub assay, through the ability of Nub-Sec62p to induce the cleavage of Mfα37-Cub-Dha at the Cub–Dha junction (Figure 3A). If this cleavage reflects the physical proximity between Sec62p and a signal sequence, the efficiency of cleavage should decrease if the Cub moiety is moved closer to the C terminus of the nascent polypeptide chain. However, this purely spatial consideration neglects the temporal aspect of the translocation process (Walter and Johnson, 1994). The targeting and the actual translocation are initiated during or shortly after the synthesis of a signal sequence-bearing protein. Consequently, the C-terminal parts of the nascent chain may still be synthesized, or at least associated with the ribosome, at the time when Sec62p and the signal sequence have already become spatially close. Extending the spacer would increase the distance between Cub and the signal sequence of Mfα37-Cub-Dha. This would be expected to decrease the time window available for the interaction between the Cub moiety of Mfα37-Cub-Dha and the Nub moiety of Nub-Sec62p. Therefore, a test of this kind cannot deconvolute the contribution of each of the two parameters (increased spatial distance along the chain between Sec62p and Cub and decreased time window for the Nub–Cub interaction) to the overall effect of extending the length of the polypeptide between the signal sequence and Cub. These constraints notwithstanding, moving the Cub moiety of Mfα37-Cub-Dha further away from its signal sequence makes it possible to gauge the accessibility of Sec62p to specific regions of the nascent polypeptide chain in vivo.
In the actual experiment, the distance between the signal sequence of Mfα37-Cub-Dha and its Cub moiety was increased from 37 to 65 residues (Mfα65-Cub-Dha; Figure 2B, construct 9). The results of EndoH treatment of Mfα65-Cub-Dha immunoprecipitated from pulse-labeled wild-type cells confirmed that Mfα65-Cub-Dha was efficiently translocated into the ER (Figure 5A). However, the efficiency of the Dha-yielding cleavage of Mfα65-Cub-Dha upon coexpression of Nub-Sec62p was clearly reduced in comparison to the same cleavage with Mfα37-Cub-Dha and Nub-Sec62p (Figure 5B).
Both the kinetics and the mode of targeting for translocation are influenced by the identity of a signal sequence (Bird et al., 1987; Johnsson and Varshavsky, 1994b; Ng et al., 1996). For example, the efficient translocation of invertase (Suc2p) requires the SRP, in contrast to a much weaker requirement for SRP in the case of the prepro-α-factor’s signal sequence (Hann and Walter, 1991; Ogg et al., 1992; Johnsson and Varshavsky, 1994b). Consequently, the coupling between translation and translocation is tighter for proteins bearing the invertase signal sequence than for proteins carrying the signal sequence of the α-factor.
We assessed the in vivo proximity of the invertase signal sequence to Sec62p by measuring the reconstitution of Ub from Nub-Sec62p and Suc2-Cub-Dha, where the Suc2p moiety was linked to Cub through a spacer of increasing length (Figure 2B). The expression and efficient translocation of different Suc2-Cub-Dha constructs were assayed by immunoprecipitation and subsequent EndoH treatment (Figure 5A). The proximity of Cub in Suc2-Cub-Dha to Nub of Nub-Sec62p was assayed by immunoblot detection of the cleavage-derived free Dha in whole-cell extracts. The pattern already observed for the Mfα-Cub-Dha constructs recurred with the constructs bearing the invertase signal sequence (Figure 5B). Moreover, coexpression of Nub-Sec62p with either Suc223-Cub-Dha or Suc233-Cub-Dha yielded lower amounts of free Dha than the analogous assays with Nub-Sec62p and Mfα37-Cub-Dha, which bears a spacer of comparable length (Figure 5B).
Pulse-chase analyses with cells expressing Nub-Sec62p (or Nub-Bos1p) and either Suc223-Cub-Dha or Suc259-Cub-Dha confirmed the immunoblot data. Specifically, a significant release of free Dha was observed only for the pair of Nub-Sec62p and Suc223-Cub-Dha (Figure 5C). Our previous work has shown that the segment of the nascent polypeptide chain where the Cub moiety was inserted in either the Suc223-Cub-Dha or the Mfα37-Cub-Dha fusion is transiently exposed to the cytosol—until the initiation of ER translocation (Johnsson and Varshavsky, 1994b). Therefore, we compared the ratios of cleaved to uncleaved Suc223-Cub-Dha and Mfα37-Cub-Dha. Cells expressing Nub-Sec62p and either the Cub fusion 8 or 10 (Figure 2B) were labeled for 5 min with 35S-methionine and processed for immunoprecipitation with anti-ha antibody, followed by determination of the cleaved-to-uncleaved ratio (Figure 6D). This ratio, a measure of the time-averaged proximity of Sec62p to a translocating protein, was ∼eightfold higher for a nascent polypeptide bearing the signal sequence of α-factor than for a nascent polypeptide bearing the invertase signal sequence (Figure 6D; compare Figures 4C and 5C).
Spacer sequences of different length or composition upstream of the Cub moiety might nonspecifically influence the interaction between Nub and Cub. To assess this potential spacer effect, we constructed signal sequence-lacking versions of Suc223-Cub-Dha and Suc259-Cub-Dha (Figure 2B, Cub fusions 15 and 16), and compared their ability to reconstitute Ub in the presence of coexpressed Nub-Sec62p. Both of these Cub fusions were cleaved at the Cub-Dha junction at approximately the same rate in the presence of Nub-Sec62p (Figure 5D), in contrast to the marked difference in the rate of cleavage observed for their signal sequence-bearing counterparts (Figure 5, B and C). This control experiment further emphasized the effect of distance between a signal sequence and the Cub moiety on the efficiency of Ub reconstitution in the presence of Nub-Sec62p. We conclude that the accessibility of Sec62p in vivo to a specific region of the nascent polypeptide chain is influenced by both the nature of a signal sequence and its distance from that region.
Sec61p Is Equidistant from Two Different Signal Sequences
A direct comparison between two different signal sequences upstream of the Cub moiety presumes approximately equal residence times of the corresponding Cub moieties in the cytosol. It is also essential to know that the influence of the identity of a signal sequence on the rate of Ub reconstitution is not due to a nonspecific intramolecular interaction. One way to address these issues involves measuring the reconstitution of Ub from the Cub moieties of the fusions 8 and 10 (Figure 2B) and a Nub-containing fusion that is not involved in translocation. As illustrated in Figure 4, this test is not feasible because of the rapid translocation of Cub-containing constructs into the ER. Note, however, that since Sec61p is the central component of the translocation pore, proteins that utilize different targeting pathways will converge at Sec61p shortly before their translocation (Jungnickel and Rapoport, 1995). Taking advantage of this property of Sec61p, we assayed the proximity of Mfα37-Cub-Dha and Suc223-Cub-Dha to Nub-Sec61p. If Mfα37-Cub-Dha and Suc223-Cub-Dha are cleaved at the Cub–Dha junction equally well in the presence of Nub-Sec61p, the above interpretation of the observed selectivity of Nub-Sec62p toward Mfα37-Cub-Dha (Figures 5 and 6) would be confirmed.
To carry out this test, Nub was fused to the cytosolic N terminus of Sec61p (Figure 2A) (Wilkinson et al., 1996). Nub-Sec61p is functionally active (Wittke and Johnsson, unpublished data). It induced the release of free Dha from of Mfα37-Cub-Dha and Tpi1p-Cub-Dha with efficiency similar to that of Nub-Sec62p (Figure 6, A and B). Thus, the split-Ub assay independently confirmed that Sec61p is close to the nascent polypeptide chain during its translocation. To compare the in vivo interactions of Sec61p with the Cub fusions 8 and 10, which bore different signal sequences (Figure 2B), the amount of free Dha was determined by immunoblotting of whole-cell extracts. It was found that in the presence of Nub-Sec61p, similar amounts of Dha were released from Mfα37-Cub-Dha and Suc223-Cub-Dha, whereas in the presence of Nub-Sec62p twice as much Dha was released from Mfα37-Cub-Dha than from Suc223-Cub-Dha (Figure 6C).
This result was confirmed and extended by labeling the cotransformed cells for 5 min with 35S-methionine and quantifying the ratio of cleaved-to-uncleaved Cub fusions (Figure 6D). As was already observed by the immunoblot analysis, the above ratio was ∼1 for both Mfα37-Cub-Dha and Suc223-Cub-Dha in the presence of Nub-Sec61p, but ∼2 for Mfα37-Cub-Dha, and ∼0.25 for Suc223-Cub-Dha in the presence of Nub-Sec62p (Figure 6D). The difference revealed by the pulse-immunoprecipitation analysis is higher than the estimate obtained by the immunoblot analysis, most likely because of the continuous accumulation of cleaved (and long-lived) Dha before the processing of cells for immunoblotting.
A C-terminally Truncated Sec62p Is No Longer Proximal to the Signal Sequence
Does the proximity of Sec62p to a nascent polypeptide chain that is detected by the split-Ub assay reflect the physical binding of the signal sequence to this protein? We constructed a derivative of Nub-Sec62p in which the C-terminal 60 residues of Sec62p were replaced by the DHFR-ha (Dha) moiety, yielding Sec62(ΔC60)-Dha. A similar Sec62p-invertase fusion was described by Deshaies and Schekman (1990) and shown to be nonfunctional. As expected, neither Sec62(ΔC60)-Dha nor Nub-Sec62(ΔC60)-Dha complemented the ts phenotype of RSY529 cells (Figure 3B).
The Ub-reconstitution activity of Nub-Sec62(ΔC60)-Dha in the presence of either Mfα37-Cub-Dha or Tpi1-Cub-Dha (Figure 6, A and B) was compared with the activity of Nub-Sec62p and Nub-Sec61p in the presence of the same Cub-containing fusions. Remarkably, no cleavage of Mfα37-Cub-Dha was observed in the presence of Nub-Sec62(ΔC60)-Dha, whereas the cytosolic Tpi1-Cub-Dha was cleaved. This result (Figure 6, A and B) indicated that the concentration and accessibility of Nub were comparable for the functionally inactive Nub-Sec62(ΔC60)-Dha and the functionally active Nub-Sec62p. In these experiments, Nub-Sec62(ΔC60)-Dha, which could be detected with the anti-ha antibody (Figure 6, A and B), was expressed from the uninduced PCUP1 promoter. Strikingly, even overexpression of Nub-Sec62(ΔC60)-Dha, from the copper-induced PCUP1, did not result in a significant cleavage of Mfα37-Cub-Dha (our unpublished results). These control experiments with the inactive derivative of Sec62p indicated that the proximity signal in the split-Ub assay with Sec62p requires the functional activity of Sec62p.
Using Ura3p Reporter to Detect the In Vivo Proximity between Sec62p and Signal Sequences
The DHFR-ha (Dha) reporter moiety of Mfα37-Cub-Dha was replaced by S. cerevisiae Ura3p (orotidine-5′-phosphate decarboxylase), yielding Mfα37-Cub-Ura3p. The use of cytosolic Ura3p as a reporter for translocation across membranes is well documented (Maarse et al., 1992; Johnsson and Varshavsky, 1994b; Ng et al., 1996). The high sensitivity of Ura3p-based assays (cells become Ura+ if a threshold amount of Ura3p is present in the cytosol) allowed us to express the Nub and Cub fusions from the uninduced PCUP1 promoter. Since the efficient translocation of Mfα37-Cub-Ura3p sequesters the Ura3p activity in the ER, a ura3Δ strain of S. cerevisiae that expressed Mfα37-Cub-Ura3p remained Ura− (Figure 7A). Nub-Sec62p, which, as shown above, is close to the nascent chain of Mfα37-Cub-Dha during its translocation, induced enough cleavage of Mfα37-Cub-Ura3p at the Cub–Ura3p junction to render cells Ura+ (Figure 7B). Cells were transformed with either Nub-Sec62p, Nub-Sec61p, Nub-Sec62(ΔC60)-Dha, or Nub-Bos1p to compare relative proximities of these Nub-containing proteins to Cub fusions bearing the Ura3p reporter moiety and either the invertase-derived or the α-factor-derived signal sequence (Figure 7, B–E). The cells were spotted on plates lacking uracil and incubated at 30°C for 18 h. The growth patterns of strains that expressed different combinations of Nub- and Cub-containing fusions confirmed the results of analyses with analogous (but more highly expressed) Dha-based constructs.
In particular, the interaction of Sec62p with the signal sequence of prepro-α-factor was stronger than with the signal sequence of invertase. This proximity was not detectable when the distance between a signal sequence and the Cub moiety of a fusion was increased (Figure 7B). Sec61p appears to be equally close to both of the signal sequences tested. Again, the proximity signal was gradually lost when the distance between the signal sequence and the Cub moiety was increased (Figure 7C). Cells acquired a weak Ura+ phenotype in the presence of Nub-Bos1p and the Cub–Ura3p fusions 8 and 10 (Figure 7E). If used as a reference to discriminate between specific and nonspecific signals in this assay, Sec62p, under these conditions, appears to interact only with the α-factor signal sequence. No interaction with any of the tested Cub constructs was detectable with the functionally inactive Nub-Sec62(ΔC60)-Dha (Figure 7D).
DISCUSSION
The new application of the split-Ub technique (Johnsson and Varshavsky, 1994a, 1997) described in the present work introduces a tool for the analysis of transient (short-lived) protein interactions in living cells. A split-Ub assay involves the tagging of two (presumably) interacting proteins with the N- and C-terminal halves of Ub, Nub and Cub, and monitoring, in a variety of ways, the release of the reporter protein fused to the C terminus of Cub. The reporter release, through the cleavage by Ub-specific processing proteases (UBPs), takes place in the cytosol if the two halves of Ub interact in vivo to form a quasi-native Ub moiety upstream of the reporter (Figure 1). Among the advantages of this method are its applicability either in living cells or in vitro and its sensitivity to kinetic aspects of a protein interaction.
In the present work, we applied the split-Ub technique to the problem of protein translocation across membranes. We showed that Sec62p of S. cerevisiae is spatially close to the signal sequence of the nascent α-factor polypeptide in vivo. This proximity is confined to the nascent polypeptide chain immediately following the signal sequence. In addition, the extent of proximity depends on the nature of the signal sequence. Specifically, Cub-containing test proteins that bore the signal sequence of invertase resulted in a much lower Ub reconstitution with Nub-Sec62p than the same Cub-containing proteins bearing the signal sequence of α-factor. An inactive derivative of Sec62p failed to interact with signal sequences in the split-Ub assay. Taken together, these findings are the first in vivo evidence that S. cerevisiae Sec62p, a component of the ER translocation machinery, is a part of a signal sequence-binding complex.
In Vivo Proximity between Sec62p and the Signal Sequence of α-Factor
We have previously shown that a region of the nascent polypeptide chain that lies close to the signal sequence of invertase or the prepro-α-factor is briefly exposed to the cytosol before its translocation into the ER (Johnsson and Varshavsky, 1994b). This feature of translocation enabled us, in the present work, to use the split-Ub assay for monitoring the proximity between a secretory protein and components of the translocation machinery. The Cub moiety was placed 37 residues downstream from the signal sequence of the α-factor precursor, and the N terminus of Sec62p (see INTRODUCTION) was extended with Nub. Using this version of the split-Ub assay, we could demonstrate that Sec62p is close to the nascent chain of the α-factor during its translocation. By moving the Cub moiety farther downstream from the signal sequence of the α-factor precursor, we obtained “snapshots” of the relative proximity between the nascent polypeptide chain and Sec62p in vivo. The proximity thus detected was considerably reduced once the spacer sequence between the signal sequence of the α-factor precursor and Cub was increased from 37 to 65 residues (Figures 5B and 7B). The data strongly suggest that the access of Sec62p to the nascent chain of α-factor is confined to a region of the nascent chain that is very close to the signal sequence. Similar results were obtained with the signal sequence of invertase as well. This property of Sec62p is the expected feature of a component of a signal sequence receptor.
Our interpretation, supported by several control experiments, is in agreement with the results produced by cross-linking and binding studies in cell-free systems (Müsch et al., 1992; Lyman and Schekman, 1997; Matlack et al., 1997). Sec62p could be cross-linked to the α-factor precursor in vitro, but only when ATP was omitted and the initiation of translocation of α-factor was halted. Upon the addition of ATP, the translocation resumed and cross-linking was no longer possible (Müsch et al., 1992; Lyman and Schekman, 1997). The cross-linking between Sec62p and the nascent polypeptide chain was not observed when the translocating chain was halted in the ER channel (Müsch et al., 1992; Sanders et al., 1992). It was therefore assumed that Sec62p is not a part of the channel and that it functions in the early steps of substrate recognition and initiation of translocation. The split-Ub assay, in its current form, depends on both halves of Ub being in the cell’s cytosol. Therefore, the absence of the diagnostic cleavage (Figures 5B, 5C, and 7) when the Cub moiety was placed farther downstream from the signal sequence (see RESULTS), while consistent with the absence of interactions between Sec62p and the nascent chain after the initiation of its translocation, does not address this issue directly. The Cub moiety that emerges from the ribosome after it has docked at the ER channel is not accessible to the cytosolic Nub moiety even if an Nub-linked protein is spatially close to the translocation pore. This also explains the inability of Nub-Sec61p to induce the cleavage of Cub-containing translocation substrates bearing long spacer sequences between the signal sequence and Cub, although the in vitro cross-linking studies have shown Sec61p to be in constant contact with the translocating polypeptide (Mothes et al., 1994) (Figure 7).
The proximity between Sec62p (or Sec61p) and a translocating polypeptide is short lived. The rapid transfer of the Cub moiety into the lumen of the ER was shown to either prevent or strongly inhibit its interaction with the Nub moiety of Nub-Sec62p and Nub-Sec61p (Figures 3A and 6C). In these experiments, the Nub moieties bore either the glycine (Nug) or the alanine (Nua) residue at position 13 of Nub. These modifications decrease the affinity between the two halves of Ub (see INTRODUCTION), thereby making the reconstitution of a quasi-native Ub moiety more dependent on the stability (half-life) of interactions between the proteins linked to Nub and Cub. These assays clearly distinguished the Sec62p–Sec61p signal sequence interactions from those that underlie the better understood, longer-lived protein complexes. For example, when linked to homodimerizing leucine zippers, the Nua moiety, and even the Nug moiety, is sufficient for reconstitution of the Ub (Johnsson and Varshavsky, 1994a).
Is Sec62p Part of a Signal Sequence Receptor?
The split-Ub assay measures the concentrations of the protein-coupled Nub and Cub moieties in the immediate vicinity of each other. Therefore, a positive result of a split-Ub assay signifies a spatial proximity between the two proteins but cannot, by itself, prove their physical interaction or address the functional significance of this proximity. Nub-Sec62(ΔC60)Dha is functionally inactive and was shown to be not close to the translocating Mfα37-Cub-Dha (Figures 6 and 7). Since the sequence between Nub and the first membrane-spanning region of Sec62 was retained in the C-terminally truncated Sec62(ΔC60)Dha, the distance between Nub and the ER membrane was, most probably, not altered relative to wild-type Sec62p. The lack of significant cleavage of Mfα37-Cub-Dha in the presence of Nub-Sec62(ΔC60)Dha must therefore result from the increased distance between Sec62(ΔC60)p and the translocating polypeptide chain. The C-terminal domain of intact Sec62p may contact other components of the translocation complex; alternatively, it may contribute to a binding site for the signal sequence or the nascent chain. These and related uncertainties notwithstanding, our results (Figures 6 and 7) provide the first in vivo evidence to support the view that Sec62p is part of a signal sequence-binding complex.
Sec62p Discriminates between Different Signal Sequences
The split-Ub assay has made it possible to show that Sec62p discriminates, in living cells, between two distinct signal sequences in otherwise identical fusion proteins. In the presence of Nub-Sec62p, more of the free Dha reporter protein was produced in vivo from Mfα37-Cub-Dha than from Suc223-Cub-Dha (Figures 5 and 6). This selectivity is a property of Sec62p and not a feature of the assay used, since approximately equal amounts of the cleaved reporter were produced with different signal sequences if the Nub moiety was present as the Nub–Sec61p fusion (Figures 6 and 7). This result confirmed, in vivo, that different targeting pathways converge at the Sec61-containing complex to initiate translocation. The existence of at least two different targeting pathways to the translocation pore was suggested by Walter and co-workers on the basis of the properties of yeast mutants that lacked either SRP or its receptor (Hann and Walter, 1991; Ogg et al., 1992). One possibility is that the targeting via SRP operates cotranslationally, whereas the targeting via the Sec62/63 complex is predominantly posttranslational. The bifurcation between the cotranslational and posttranslational targeting is expected to be stochastic for many translocated proteins. Nonetheless, certain signal sequences do prefer SRP, while some of the other signal sequences are targeted by the Sec62/Sec63 complex (Ng et al., 1996). Genetic studies have shown that the translocation of invertase continues in the presence of mutations in either the SRP or the Sec62/63 complex (Deshaies and Schekman, 1989; Ogg et al., 1992). However, the kinetics and efficiency of invertase translocation are altered in the absence of SRP (Hann and Walter, 1991; Johnsson and Varshavsky, 1994b).
To explain the different efficiencies of cleavage of the two signal sequence-bearing Cub fusions, we propose the following model. The signal sequence of invertase is recognized primarily by SRP and then transferred to the trimeric Sec61p complex, which completes the protein’s translocation across the ER membrane. Under conditions that result in a shortage of SRP or a competition among different signal sequences, the Sec62p/Sec63p-containing complex, being a part of the alternative targeting pathway, would recognize an increasing fraction of invertase. This would explain why specific interactions between the invertase signal sequence-bearing proteins and Sec62p can only be observed for the more highly expressed Cub–Dha fusions (Figure 5C). By contrast, the targeting of proteins bearing the signal sequence of the α-factor precursor is mediated, in vivo, predominantly by the Sec62p/Sec63p-containing complex. This would account for the observed close proximity of these test proteins to Sec62p (Figures 5–7). If so, the split-Ub technique makes it possible to estimate the flux of two different secretory proteins through the targeting pathways to the ER membrane without the necessity of deleting or otherwise inactivating specific components of the targeting complex.
Further Applications of the Split-Ub Technique
The split-Ub sensor should also be applicable to other settings that involve short-lived protein interactions that occur in the cytosol and are freely accessible to the Ub-specific proteases. The advantage of using this method for the analysis of protein translocation stems, in part, from the fast and irreversible removal of the translocated chain from the location (cytosol) where the Nub/Cub interaction is monitored. Similar situations are expected for the translocation of proteins into other organelles such as the mitochondrion, the nucleus, and the peroxisome.
The demonstration, in the present work, that Ura3p can serve as a reporter in a split-Ub assay (Figure 7) opens the way to genetic screens based on this assay. For example, introducing a DNA library consisting of random Nub–gene fusions into a strain expressing a signal sequence-bearing Cub-Ura3p protein should allow the identification of genes involved in targeting or translocation by enabling the cells to form colonies on media lacking uracil. This selection for protein ligands that interact transiently in the vicinity of a membrane complements the recent split-Ub–based screen for ligands that form relatively stable complexes (Stagljar et al., 1998).
ACKNOWLEDGMENTS
We thank Ray Deshaies, Jürgen Dohmen, Nicole Lewke, Randy Schekman, and Sandra Wittke for the gifts of yeast strains and plasmids. M.D. and N.J. thank Silke Müller for excellent technical assistance. This work was supported by a grant to N.J. from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (0311107), and a grant to A.V. from the National Institutes of Health (GM-31530).
REFERENCES
- Allison DS, Young ET. Single-amino-acid substitutions within the signal sequence of yeast prepro-α-factor affect membrane translocation. Mol Cell Biol. 1988;8:1915–1922. doi: 10.1128/mcb.8.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein- protein interactions. Mol Cell Biol. 1997;17:3094–3102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Rabouille C, Warren G, Pelham HR. Localization of Sed5, a putative vesicle targeting molecule, to the cis- Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol. 1994;127:357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R, Bubeck D, Grassucci R, Penczek P, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 1997;278:2123–2126. doi: 10.1126/science.278.5346.2123. [DOI] [PubMed] [Google Scholar]
- Biederer T, Volkwein C, Sommer T. Degradation of subunits of the Sec61p complex, an integral component of the ER membrane, by the ubiquitin-proteasome pathway. EMBO J. 1996;15:2069–2076. [PMC free article] [PubMed] [Google Scholar]
- Bird P, Gething MJ, Sambrook J. Translocation in yeast and mammalian cells: not all signal sequences are functionally equivalent. J Cell Biol. 1987;105:2905–2914. doi: 10.1083/jcb.105.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T, Collins P, Gilmore R. Access of proteinase K to partially translocated nascent polypeptides in intact and detergent-solubilized membranes. J Cell Biol. 1989;108:299–307. doi: 10.1083/jcb.108.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. doi: 10.1016/0092-8674(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Crowley KS, Reinhart GD, Johnson AE. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell. 1993;73:1101–1115. doi: 10.1016/0092-8674(93)90640-c. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 1991;349:806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989;109:2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R. Structural and functional dissection of Sec62p, a membrane-bound component of the yeast endoplasmic reticulum protein import machinery. Mol Cell Biol. 1990;10:6024–6035. doi: 10.1128/mcb.10.11.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen RJ, Stappen R, McGrath JP, Forrova H, Kolarov J, Goffeau A, Varshavsky A. An essential yeast gene encoding a homolog of ubiquitin-activating enzyme. J Biol Chem. 1995;270:18099–18109. doi: 10.1074/jbc.270.30.18099. [DOI] [PubMed] [Google Scholar]
- Feldheim D, Schekman R. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol. 1994;126:935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R, Blobel G. Translocation of secretory proteins across the microsomal membrane occurs through an environment accessible to aqueous perturbants. Cell. 1985;42:497–505. doi: 10.1016/0092-8674(85)90107-2. [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Hartmann E, Kalies KU, Rapoport TA. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992;71:489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Hanein D, Matlack KE, Jungnickel B, Plath K, Kalies KU, Miller KR, Rapoport TA, Akey CW. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- Hann BC, Walter P. The signal recognition particle in S. cerevisiae. Cell. 1991;67:131–144. doi: 10.1016/0092-8674(91)90577-l. [DOI] [PubMed] [Google Scholar]
- Hiller MM, Finger A, Schweiger M, Wolf DH. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci USA. 1994a;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 1994b;13:2686–2698. doi: 10.1002/j.1460-2075.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. Split ubiquitin: a sensor of protein interactions in vivo. In: Bartel PL, Fields S, editors. The Yeast Two Hybrid System. Oxford, United Kingdom: Oxford University Press; 1997. pp. 316–332. [Google Scholar]
- Jungnickel B, Rapoport TA. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995;82:261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Krieg UC, Walter P, Johnson AE. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci USA. 1986;83:8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Wiedmann M, Girshovich AS, Bochkareva ES, Bielka H, Rapoport TA. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986;320:634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell. 1997;88:85–96. doi: 10.1016/s0092-8674(00)81861-9. [DOI] [PubMed] [Google Scholar]
- Maarse AC, Blom J, Grivell LA, Meijer M. MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J. 1992;11:3619–3628. doi: 10.1002/j.1460-2075.1992.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack KE, Plath K, Misselwitz B, Rapoport TA. Protein transport by purified yeast Sec complex and Kar2p without membranes. Science. 1997;277:938–941. doi: 10.1126/science.277.5328.938. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A, Wiedmann M, Rapoport TA. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992;69:343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R. A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol. 1988;107:851–863. doi: 10.1083/jcb.107.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, Brown JD, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg SC, Poritz MA, Walter P. Signal recognition particle receptor is important for cell growth and protein secretion in Saccharomyces cerevisiae. Mol Biol Cell. 1992;3:895–911. doi: 10.1091/mbc.3.8.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P, Kuranda MJ, Albright CF. Analysis of glycoproteins from Saccharomyces cerevisiae. Methods Enzymol. 1991;194:682–697. doi: 10.1016/0076-6879(91)94050-m. [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Rapoport TA, Jungnickel B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- Rossi F, Charlton CA, Blau HM. Monitoring protein-protein interactions in intact eukaryotic cells by β-galactosidase complementation. Proc Natl Acad Sci USA. 1997;94:8405–8410. doi: 10.1073/pnas.94.16.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt JA, Deshaies RJ, Sanders SL, Daum G, Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman RW. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Sapperstein S, Berkower C, Michaelis S. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol Cell Biol. 1994;14:1438–1449. doi: 10.1128/mcb.14.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Newman AP, Ferro-Novick S. The BOS1 gene encodes an essential 27-kDa putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J Cell Biol. 1991;113:55–64. doi: 10.1083/jcb.113.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SM, Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991;65:371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Stagljar I, Korostensky C, Johnsson N, te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Walter P, Ibrahimi I, Blobel G. Translocation of proteins across the endoplasmic reticulum I. Signal recognition protein (SRP) binds to in vitro assembled polysomes synthesizing secretory protein. J Cell Biol. 1981;91:545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson BM, Critchley AJ, Stirling CJ. Determination of the transmembrane topology of yeast Sec61p, an essential component of the endoplasmic reticulum translocation complex. J Biol Chem. 1996;271:25590–25597. doi: 10.1074/jbc.271.41.25590. [DOI] [PubMed] [Google Scholar]