Abstract
Descending supraspinal inputs exert powerful influences on spinal reflex pathways in the legs. Removing these inputs by completely transecting the spinal cord changes the state (i.e. the configuration of the spinal circuitry) of the locomotor network and undoubtedly generates a reorganization of reflex pathways. To study changes in reflex pathways after a complete spinalization, we recorded spinal reflexes during locomotion before and after a complete transection of the spinal cord at the 13th thoracic segment in cats. We chronically implanted electrodes in three cats, to record electromyography (EMG) in several hindlimb muscles and around the left tibial (Tib) nerve at the ankle to elicit reflexes during locomotion before and after spinalization in the same cat. Control values of kinematics, EMGs and reflexes were obtained during intact locomotion for 33–60 days before spinalization. After spinalization, cats were trained 3–5 times a week on a motorized treadmill. Recordings resumed once a stable spinal locomotion was achieved (26–43 days), with consistent plantar foot placement and full hindquarter weight support without perineal stimulation. Changes in Tib nerve reflex responses after spinalization in the same cat during locomotion were found in all muscles studied and were often confined to specific phases of the step cycle. The most remarkable change was the appearance of short-latency excitatory responses in some ipsilateral ankle extensors during stance. Short-latency excitatory responses in the ipsilateral tibialis anterior were increased during stance, whereas in other flexors such as semitendinosus and sartorius, increases were mostly confined to swing. Longer-latency excitatory responses in ipsilateral flexors were absent or reduced. Responses evoked in limb muscles contralateral to stimulation were generally increased throughout the step cycle. These reflex changes after spinalization provide important clues regarding the functional reorganization of reflex pathways during spinal locomotion.
Stimulation of the skin or cutaneous nerves of the foot in anaesthetized preparations at rest and during fictive locomotion produces a mixture of inhibitory and excitatory postsynaptic potentials in several lumbosacral motoneurons, which indicates that parallel excitatory and inhibitory pathways converge on a given motor pool (Burke, 1999; McCrea, 2001). As a result, during normal locomotion stimulating cutaneous nerves evokes a complex pattern of response in multiple hindlimb muscles, which can include inhibitory and excitatory responses within the same muscle (Duysens & Loeb, 1980; Abraham et al. 1985; Loeb, 1993). For example, during swing, short- (P1) and longer- (P2) latency excitatory responses are evoked in ipsilateral flexors at ∼8–10 ms and ∼25–30 ms, respectively. P1 and P2 responses can be modulated independently during the step cycle with large P1 responses being present during early to mid-swing whereas P2 responses are generally more prominent in late stance (Rossignol et al. 2006). In ipsilateral extensors, P1 responses can also be evoked during the swing phase but during stance short-latency inhibitory (N1) and longer-latency excitatory (P2–P3) responses are evoked at ∼8–10 ms and ∼30–45 ms, respectively. In flexors and extensors contralateral to the stimulation, P2 responses are usually observed at ∼20–25 ms (Duysens & Loeb, 1980).
The presence of excitatory and inhibitory responses in ipsilateral extensors suggests that both excitatory and inhibitory last order interneurons converge on motoneurons but that they are dynamically modulated according to the phase of the step cycle. Different structures could be involved in this dynamic modulation. For instance, interneurons interposed in cutaneous reflexes from the foot receive inputs from (1) other peripheral afferents, (2) the spinal central pattern generator (CPG), and (3) supraspinal and propriospinal inputs (Rossignol et al. 2006). Removing any of these inputs creates a new ‘state’, defined as the configuration and excitability of locomotor circuitry, in the spinal CPG and potentially impacts the modulation of inhibitory and excitatory reflex responses during locomotion. Lesions at various levels of the nervous system can modify the state of the system and alter the phase-dependent modulation of spinal reflexes, thereby providing a tool to investigate the role of various structures in the organization and modulation of reflex pathways (Burke, 1999). In a recent study we recorded reflex responses evoked by stimulating the tibial (Tib) nerve at the ankle during locomotion before and after lesioning the mixed nerve supplying the lateral gastrocnemius and soleus (LGS) in otherwise intact cats (Frigon & Rossignol, 2007). The increase in several reflex pathways following the LGS denervation, which could have resulted from a disinhibition of short- and longer-latency excitatory pathways, suggests that afferent inputs from the LGS normally project to interneurons interposed in reflex pathways from the Tib nerve.
Although the pattern of reflex responses has been described during locomotion in intact and spinal cats the functional reorganization of reflexes from an intact to a spinal state in the same cat is unknown. In the present study we recorded reflex responses evoked by stimulating the Tib nerve in the same cat before and after a complete transection of the spinal cord at the 13th thoracic segment (T13). Numerous studies have investigated changes in spinal reflex pathways following spinal cord injury (for a review see Frigon & Rossignol, 2006) but most of these studies have compared reflexes between groups of intact and groups of spinal cord-injured animals. One of the major limitations of such studies is that reflex pathways differ between individuals (Loeb, 1993; Zehr et al. 1997) making the functional reconfiguration of reflex pathways difficult to assess by comparing intact and spinal cord-injured groups. A few studies have used chronic recordings to examine reflex changes after spinal injury in the same animals (Bennett et al. 1999, 2004; Lavrov et al. 2006) but not in a locomotor condition. Descending commands from the brain and brainstem are known to exert powerful influences on spinal reflex pathways (Holmqvist & Lundberg, 1959) and therefore studying the reorganization of reflex pathways during locomotion after complete spinalization could provide important clues as to their intrinsic organization.
Methods
Animals and general procedures
Three adult cats (2 males, 1 female) weighing between 3.0 and 4.5 kg were selected based on their ability to walk for prolonged periods on a treadmill and trained for a few weeks at their preferred speed (0.4–0.5 m s−1). Cats were then chronically implanted with electrodes for electromyographic (EMG) recordings and nerve stimulation, and allowed to recover from the implantation, and then values of EMGs and reflexes were recorded. After stable control data were obtained (33–60 days), a complete spinalization at T13 was performed. Recordings resumed following a few weeks of locomotor training once a stable full weight-bearing spinal locomotion was achieved (26–43 days). Stable locomotion in the intact and spinal states corresponded to periods where stepping was visibly the same from one session to another with similar limb movements, locomotor bursts, and reflexes. It should be emphasized that the modulation of phase-dependent reflexes cannot be rigorously studied unless the animal is capable of achieving long periods of several hundred consecutive steps. Therefore, the gradual evolution of reflexes in the weeks following spinalization before attaining stability of walking could not be studied. Cats were studied for several days after the expression of a stable spinal locomotion (25–37 days). At the termination of experiments, cats were killed with an overdose of sodium pentobarbital.
The experimental protocol was in accordance with the guidelines of the animal Ethics Committee of the Université de Montréal. All surgical procedures were performed under general anaesthesia and aseptic conditions. Prior to surgery, cats were injected with an analgesic (Anafen 2 mg kg−1; subcutaneously) and premedicated (Atravet 0.1 mg kg−1, glycopyrrolate 0.01 mg kg−1, ketamine 0.01 mg kg−1; intramuscularly). Cats were then intubated and maintained under gaseous anaesthesia (isoflurane 2%) while heart rate and respiration were monitored. After surgery, an analgesic (Buprenorphine 0.01 mg kg−1) was administered subcutaneously. An oral antibiotic (cephatab or apo-cephalex, 100 mg day−1) was given for 10 days following surgery.
Implantation of electromyographic electrodes
Chronic EMG electrodes were implanted bilaterally in the following hindlimb muscles: semitendinosus (St: knee flexor/hip extensor), anterior part of sartorius (Srt: hip flexor/knee extensor), vastus lateralis (VL: knee extensor), lateral gastrocnemius (LG: ankle extensor/knee flexor), medial gastrocnemius (MG: ankle extensor/knee flexor), and tibialis anterior (TA: ankle flexor). A pair of Teflon-insulated multistrain fine wires (AS633; Cooner wire, Chatsworth, CA, USA) were directed subcutaneously from head-mounted 15-pin connectors (Cinch Connectors; TTI Inc., Pointe-Claire, Canada) and sown into the belly of each muscle for bipolar EMG recordings. Over the course of the study some EMG recordings were lost in muscles of the left (i.e. ipsilateral to stimulation) and right (contralateral to stimulation) hindlimbs. For instance, the ipsilateral Srt and TA of cat 1, the ipsilateral MG of cat 2 and the contralateral St and MG of cat 3 were lost, as determined by the disappearance of EMG signals. Moreover, some muscles discharged irregularly and the burst could not be delineated although reflex responses could be recorded in these muscles. This is the case for the contralateral Srt of cat 1 and both TAs of cat 3. EMG recordings were bandpass filtered (100–3000 Hz) and amplified (gains of 200–5000) using two Lynx-8 amplifiers (Neuralynx, Tucson, AZ, USA). EMG data were digitized (5000 Hz) using custom-made acquisition software. The duration, mean amplitude and timing of EMG bursts during locomotion relative to ipsilateral St burst onset were calculated using custom software. Onsets and offsets of EMG bursts were determined visually. Mean amplitude was defined as the area under the rectified EMG burst divided by its duration and expressed as a percentage of the averaged intact value to evaluate locomotor EMG burst changes after spinalization.
Kinematics
Kinematic data of the left hindlimb were captured before and after spinalization using a Panasonic digital 5100 camera (1/1000 s shutter speed, 30 frames s−1, 60 fields, time resolution of 16.7 ms) and a Sony RDR-GX315 DVD recorder during treadmill locomotion. Small reflective markers were placed over prominent bony landmarks at each joint of the hindlimbs including the iliac crest, greater trochanter, lateral epicondyle, lateral malleolus, metatarso-phalangeal (MTP) joint, and the tip of the fourth toe. Joint angles (hip, knee, ankle, MTP) were reconstructed off-line using custom-made software with a resolution of 60 fields s−1 from ≥ 20 step cycles. Step cycle duration was measured as the time between two successive left foot contacts. The duration of stance was measured as the time between contact and lift of the left foot whereas swing duration was calculated as the difference between step cycle and stance durations.
Spinalization and locomotor training
After establishing stable control (EMG and reflexes) recordings, a laminectomy was performed at T13. The dura was removed and, after local lidocaine application (Xylocaine, 2%), the spinal cord was completely transected with surgical scissors. Hemostatic material (Surgicel) was inserted within the gap and muscles and skin were sown back to close the opening. After a few days, cats were trained 3–5 times a week to walk on the treadmill. In the early days following spinalization, training consisted of having two experimenters move the hindlimbs over the motorized treadmill to simulate locomotion while the forelimbs were positioned on a fixed platform located ∼3 cm above the belt. The skin of the perineal region was stimulated to evoke stepping movements. A Plexiglas separator was placed between the limbs to prevent them from impeding each other because of increased adduction. Initially, the experimenter supported the hindquarters by lifting the tail. No pharmacological agents were used during training. Recording sessions resumed once the animals attained a stable locomotor pattern without perineal stimulation, with full hindquarter weight support and consistent plantar foot placement. The experimenter provided equilibrium by holding the tail (Barbeau & Rossignol, 1987; Bélanger et al. 1996). To effectively compare reflex changes before and after spinalization all bouts of irregular stepping during spinal locomotion were precluded from analysis.
Tibial nerve stimulation and reflexes
A chronic bipolar stimulating electrode composed of wires (AS633; Cooner wire, Chatsworth, CA, USA) embedded in a polymer (Denstply International) cuff (Julien & Rossignol, 1982) was placed around the left Tib nerve at the ankle adjacent to the Achilles' tendon. The Tib nerve was stimulated with a Grass S88 stimulator connected in series with a constant current isolation unit (Grass PSIU6) and a custom-made current measurement unit that monitors the actual current delivered. Initially, stimulation was delivered at different intensities during locomotion with single 1 ms pulses 100 ms after ipsilateral St burst onset to determine the reflex threshold for obtaining small yet consistent short-latency (∼10 ms) responses in the ipsilateral TA. Stimulation current was then set at 1.2 times this threshold (1.2T) to evoke reflexes. The cats did not seem to notice the stimulation during locomotion and as a result long sequences (> 10 min) of stimulation could be obtained. This intensity is slightly above the one required to record the threshold of an afferent volley in the sciatic nerve and reflex responses are thought to be mediated by the largest diameter Aβ afferents (Loeb, 1993). However, influences from group I or II afferents cannot be eliminated since the Tib nerve also supplies intrinsic foot muscles. The stimulation did not visibly alter limb trajectory during intact or spinal locomotion, which is important because perturbations of the limb could introduce responses linked to the movement, such as proprioceptive reflexes. The pattern of responses, as opposed to amplitude, is typically invariant unless very high stimulus intensities are used (Abraham et al. 1985; Loeb, 1993). Therefore, our responses were probably mediated by low-threshold cutaneous afferents.
A computer-generated pseudo-random sequence delivered 10 stimuli in the different 10 subphases of the cycle for a total of approximately 120–200 stimuli, each stimulus being given once every three cycles. Once reflexes were qualitatively and quantitatively reproducible from one session to another for a few weeks (33–60 days) the same stimulation current was used for the remainder of the study before and after spinalization. The current required to reach reflex threshold was assessed postspinalization and did not change by more than a few microamps.
Reflexes were measured as detailed previously (Frigon & Rossignol, 2007, 2008). The EMGs were grouped into stimulated or control (non-stimulated) trials. The step cycle was divided into 10 phases by beginning the cycle from the ipsilateral St burst onset. Averaged EMG responses with stimulation were separated into these 10 bins according to the time in the cycle they were evoked. An average of at least 50 control cycles provided a template of baseline locomotor EMG (blEMG) during the step cycle. The latency of responses, denoted as prominent negative or positive deflections away from the blEMG, were determined manually using predefined latencies as guidelines (Duysens & Stein, 1978; Drew & Rossignol, 1985; Abraham et al. 1985; Pratt et al. 1991; Loeb, 1993) such as P1 or N1 (∼8–10 ms) and P2 (≥ 25 ms), where ‘P’ and ‘N’ are positive (excitatory) and negative (inhibitory) responses, respectively. The latencies of responses are given in Table 2. Note that P2 latencies for St and TA are not provided because in most instances there was no clear distinction between P1 and P2 responses.
Table 2.
Onsets and offsets of muscle bursts relative to left foot contact during intact and spinal locomotion expressed as a percentage of step cycle duration
| Cat 1 | Cat 2 | Cat 3 | |||||
|---|---|---|---|---|---|---|---|
| Muscle | ON | OFF | ON | OFF | ON | OFF | |
| LSt | Intact | 56 ± 3 | 74 ± 2 | 53 ± 3 | 77 ± 8 | 52 ± 3 | 73 ± 3 |
| Spinal | 58 ± 2 | 68 ± 2* | 54 ± 3 | 69 ± 3* | 54 ± 9 | 87 ± 7* | |
| LSrt | Intact | 66 ± 2 | 93 ± 2 | 63 ± 4 | 94 ± 1 | 73 ± 2 | 92 ± 1 |
| Spinal | N/A | N/A | 52 ± 6* | 67 ± 3* | 51 ± 9* | 78 ± 7* | |
| LVL | Intact | −10 ± 5 | 55 ± 3 | −9 ± 4 | 52 ± 4 | −7 ± 2 | 51 ± 3 |
| Spinal | −15 ± 3* | 30 ± 4* | −9 ± 3 | 41 ± 4* | 9 ± 5 | 49 ± 6 | |
| LLG | Intact | −12 ± 2 | 50 ± 3 | −7 ± 1 | 54 ± 7 | −10 ± 1 | 45 ± 3 |
| Spinal | −22 ± 7* | 30 ± 7* | −9 ± 5 | 33 ± 5* | −12 ± 4 | 44 ± 5 | |
| LMG | Intact | −11 ± 1 | 52 ± 1 | N/A | N/A | −11 ± 2 | 47 ± 4 |
| Spinal | −17 ± 4* | 33 ± 5* | N/A | N/A | −12 ± 6 | 44 ± 6 | |
| LTA | Intact | N/A | N/A | 67 ± 2 | 83 ± 3 | 54 ± 4 | 79 ± 3 |
| Spinal | N/A | N/A | 60 ± 4* | 71 ± 4* | N/D | N/D | |
| RSt | Intact | 95 ± 2 | 121 ± 3 | 108 ± 5 | 123 ± 6 | N/A | N/A |
| Spinal | 96 ± 4 | 115 ± 4* | 94 ± 7* | 109 ± 8* | N/A | N/A | |
| RSrt | Intact | 114 ± 3 | 143 ± 6 | 115 ± 5 | 144 ± 6 | 124 ± 4 | 141 ± 4 |
| Spinal | N/D | N/D | 101 ± 7* | 115 ± 5* | 107 ± 2* | 125 ± 2* | |
| RVL | Intact | 35 ± 3 | 105 ± 2 | 34 ± 5 | 107 ± 4 | 46 ± 3 | 99 ± 2 |
| Spinal | 33 ± 4 | 95 ± 3* | 34 ± 3 | 100 ± 4* | 44 ± 3 | 97 ± 4 | |
| RLG | Intact | 38 ± 2 | 98 ± 2 | 40 ± 2 | 98 ± 6 | 42 ± 3 | 98 ± 4 |
| Spinal | 22 ± 4* | 76 ± 9* | 28 ± 5* | 82 ± 4* | 41 ± 8 | 93 ± 5* | |
| RMG | Intact | 41 ± 3 | 93 ± 2 | 40 ± 3 | 94 ± 4 | N/A | N/A |
| Spinal | 25 ± 4* | 81 ± 8* | 28 ± 6* | 84 ± 8* | N/A | N/A | |
| RTA | Intact | 104 ± 2 | 131 ± 4 | 119 ± 3 | 141 ± 3 | 110 ± 3 | 135 ± 3 |
| Spinal | 74 ± 6* | 111 ± 5* | 99 ± 6* | 116 ± 5* | N/D | N/D | |
Each value is the average of 15–40 bursts.
P≤ 0.05.
To quantify reflex responses windows were set before and after spinalization in muscles of the ipsilateral and contralateral hindlimbs. For ipsilateral flexors (see Fig. 5), time windows were the same before and after spinalization: St: P1 = 10–25 ms, P2 = 25–55 ms; TA: P1 = 10–30 ms, P2 = 30–55 ms. A time window of 15–50 ms was used for the ipsilateral Srt before and after spinalization because this muscle often shows a prolonged reflex response during that time period and not two distinct responses. For contralateral muscles (Srt, TA, VL) a time window of 15–40 ms was used before and after spinalization. For ipsilateral extensors (see Fig. 3), onsets and offsets of responses were manually determined pre- and postspinalization if an inhibition was present because P2 latencies change somewhat throughout the step cycle as the duration of N1 varies from one phase to another. However, if P1 responses appeared during stance in ankle extensors after spinalization, time windows of 10–25 ms and 25–55 ms were, respectively, used for P1 and P2.
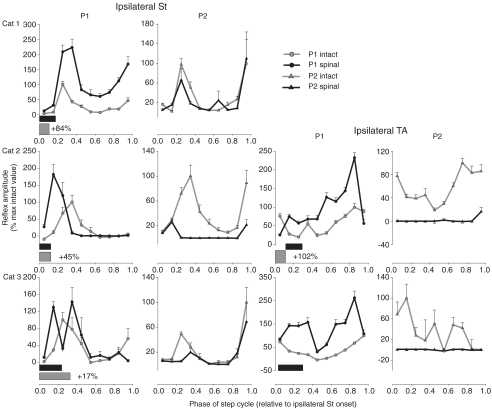
Figure 5. Phase plots summarizing changes in Tib nerve reflexes at 1.2T in ipsilateral flexors (St and TA, respectively) for cat 1 (top panels), cat 2 (middle panels) and cat 3 (bottom panels).
Horizontal bars represent the phase of activity of each muscle during intact (grey) and spinal (black) locomotion normalized to ipsilateral St burst onset. The percentage difference of the mean amplitude of the locomotor EMG burst in the spinal state, expressed as a function of the intact state, is indicated next to the horizontal bar for each muscle. Each data point is the mean ±s.e.m. of approximately 30 responses.
Figure 3. Averaged reflex responses of the ipsilateral St before (grey lines) and after (black lines) spinalization for cat 1 (top panel) and cat 2 (bottom panel).
Values in a single graph are at the same scale in μV. Windows for integration in the ipsilateral St of cats 1 and 2 were 10–25 ms and 25–55 ms for P1 and P2 responses, respectively. The first horizontal trace for a given graph is phase 0.05 of the step cycle synchronized to the ipsilateral St burst onset. Each trace is the average of approximately 10 stimuli. The blEMG was removed for clarity. The rectified, averaged, single burst EMG traces of the ipsilateral St during intact and spinal locomotion are shown on the far right at the same scale.
To measure reflexes, the stimulated and non-stimulated EMG within the determined window were integrated. The non-stimulated integrated EMG within the same window was then subtracted from the integrated stimulated value. Because reflex amplitude is known to scale (i.e. automatic gain control) with the level of EMG activity (Matthews, 1986), the subtracted value was then divided by a fixed 15 ms block of blEMG in the same phase (see Fig. 1 of Frigon & Rossignol, 2008 for an example), thus giving a reflex amplitude normalized to the level of baseline locomotor activity. Inhibitory responses, in our context, can only be quantified when there is a baseline level of EMG. Often the inhibitory response scales with the level of blEMG but the percentage inhibition relative to blEMG remains the same. In order to use a single method to compare inhibitory and excitatory responses in two conditions with different levels of blEMG, we divided the subtracted responses by the level of blEMG. Therefore, reflex responses are expressed as a percentage of the level of baseline locomotor activity in the intact and spinal states. If normalized reflex values in the spinal state were different from those in the intact state, then reflex responses did not simply scale with changes in blEMG that occurred as a result of spinalization, which indicates that there was a change in the strength of the reflex pathway.
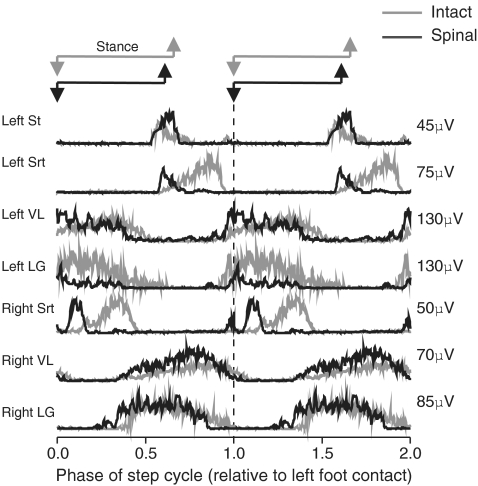
Figure 1. Rectified, averaged, single burst EMG traces for selected muscles during intact (grey) and spinal (black) locomotion in cat 2.
In the intact and spinal states the step cycle begins and ends on left foot contact. Each EMG trace is the average of approximately 60 bursts.
Statistics
For statistical analyses data from three different days in the intact state were pooled and compared with pooled data from three different days after spinalization. Planned comparisons were used to determine statistical differences between intact and spinal locomotion for locomotor EMG bursts (mean amplitude, onsets and offsets), durations of the step cycle, swing and stance, and reflex latencies. A Bonferroni correction for multiple comparisons was made (P is significant when ≤ 0.0071 (0.05/7)). For reflex responses, a repeated measures two-factor ANOVA (2 states × 6–10 phases) was performed to determine significant main effects and interactions between factors. When a significant interaction between state and phase was found, Student's t test for paired data was performed comparing reflex responses evoked in one phase of the step cycle in the intact state with the same phase in the spinal state. For example, P1 responses evoked in the first bin of the step cycle in the ipsilateral St in the intact state were compared with P1 responses evoked in the first bin of the step cycle in the ipsilateral St after spinalization. Significant interactions between state and phase were found for all reflex responses except P2 responses of the ipsilateral MG. All values, unless otherwise stated, are means ± standard error of the mean (s.e.m.).
Results
In the present study EMG bursts and Tib nerve reflexes were quantified during locomotion in the same cat before and after spinalization. All of the studied animals expressed a stable hindlimb locomotion following spinalization at the same treadmill speed as in the intact state, which could be maintained uninterrupted for prolonged periods (≥ 10 min). Cats 1, 2 and 3, respectively, expressed a stable spinal locomotion at 41, 26 and 43 days post-spinalization without perineal stimulation. At this point EMG bursts and Tib nerve reflexes were consistent from one day to another, a prerequisite for quantitative analysis. During spinal locomotion, EMG bursts and the normalized amplitude of Tib nerve reflexes were altered in multiple muscles compared to the intact state.
Changes in the timing and magnitude of locomotor EMG bursts
The EMG activity of several muscles was recorded bilaterally before and after spinalization to quantify changes in the mean amplitude and timing of locomotor bursts after the complete spinal transection. Figure 1 shows locomotor EMG activity for selected muscles in cat 2 during intact and spinal locomotion. In both instances, the step cycle starts at left foot contact and EMGs are represented on the same ordinate scale for comparison. Some changes in the magnitude and timing of several bursts were observed following spinalization. For instance, the burst amplitude was increased in the ipsilateral St and in both VLs whereas that of LG decreased bilaterally. Srt and LG bilaterally started earlier within the normalized step cycle during spinal locomotion.
Table 1 details changes in the mean amplitude of locomotor EMG bursts after spinalization expressed as a percentage difference from the intact state. The EMG burst increased in TA bilaterally in cat 2 and in the right TA of cat 1. Post-spinalization, mean amplitude was increased in the left St of all cats and in the right St of cat 2. Thus, in flexors (St and TA) of the left hindlimb there was an increase in 7/8 recorded muscles. The mean amplitude of Srt, a hip flexor/knee extensor, was unchanged bilaterally in cat 2 and decreased bilaterally in cat 3. In cat 1, activity of the right Srt became clonic after spinalization and the burst could not be quantified (see Methods). In extensors (VL, LG and MG) there was a significant decrease of the mean amplitude in 11/16 muscles. In cat 1, all extensors (VL, LG and MG) were decreased bilaterally. In cat 2, VL and LG activity was, respectively, increased and decreased bilaterally whereas in cat 3 the opposite occurred with decreased and increased activity in VL and LG, respectively. Therefore the mean amplitude of locomotor bursts in flexors was increased in the majority of cases while the mean amplitude of extensors was primarily decreased. Generally, if amplitude increased or decreased in a given muscle it changed in the same direction, albeit not the same magnitude, in the homologous muscle of the contralateral limb.
Table 1.
Changes in mean amplitude of EMG bursts for all recorded muscles after spinalization expressed as the percentage difference from the intact state
| Cat 1 | Cat 2 | Cat 3 | |
|---|---|---|---|
| LSt | +84% | +45% | +17% |
| LSrt | N/A | = | −33% |
| LVL | −36% | +60% | −49% |
| LLG | −67% | −42% | +21% |
| LMG | −73% | N/A | −21% |
| LTA | N/A | +102% | N/D |
| RSt | = | +33% | N/A |
| RSrt | N/D | = | −58% |
| RVL | −24% | +101% | −49% |
| RLG | −54% | −27% | +69% |
| RMG | −82% | +10% | N/A |
| RTA | +53% | +117% | N/D |
Values are difference in mean amplitude as a percentage of intact. Some muscles were lost (N/A) over the course of the study and others could not be delineated (N/D). Each value is the average of 40–60 bursts.
Table 2 provides onsets and offsets of EMG bursts during intact and spinal locomotion, expressed as a percentage of the step cycle. In intact and spinal locomotion, left foot contact indicated the beginning of the step cycle. In St bilaterally, burst onset occurred earlier in 1/5 muscles. Offset was significantly different in 5/5 St muscles with an earlier offset in 4/5. In TA bilaterally, onset and offset occurred earlier in 3/3 muscles. In Srt bilaterally, burst onset and offset occurred earlier in 4/4 muscles. In extensors bilaterally (VL, LG, MG) burst onset occurred earlier in 7/16 muscles with 6/7 of these being gastrocnemii. In these same extensors burst offset occurred earlier in 12/16 muscles. Therefore, in St and VL burst onset was primarily unchanged whereas that of Srt, TA and gastrocnemii occurred earlier. In most muscles the bursts terminated earlier within the normalized step cycle.
Table 3 gives the durations (in ms) of the step cycle and of swing and stance phases in the intact and spinal states for each cat at the same treadmill speed. In all cats there was a significant decrease (P≤ 0.05) in the duration of the step cycle and of swing and stance phases after spinalization. To maintain the same treadmill speed as before spinalization cats walked at a faster rate (steps per unit of time). Expressing swing and stance durations as a function of step cycle duration in the intact and spinal states revealed that stance and swing occupied an identical percentage of the step cycle after spinalization in cat 1 whereas in cats 2 and 3 the percentage of swing increased slightly while that of stance decreased. Therefore, the structure of the step cycle is relatively unchanged after spinalization.
Table 3.
Absolute values of durations of the step cycle, swing, and stance phases before and after spinalization in cats 1–3
| Cat 1 | Cat 2 | Cat 3 | ||||
|---|---|---|---|---|---|---|
| ms | % | ms | % | ms | % | |
| Step cycle duration | ||||||
| Intact | 1082 ± 9 | — | 1021 ± 15 | — | 983 ± 12 | — |
| Spinal | 751 ± 6* | — | 697 ± 6* | — | 697 ± 14* | — |
| Swing duration | ||||||
| Intact | 358 ± 4 | 33 | 345 ± 8 | 34 | 341 ± 8 | 35 |
| Spinal | 250 ± 4* | 33 | 273 ± 5* | 39 | 267 ± 12* | 38 |
| Stance duration | ||||||
| Intact | 725 ± 8 | 67 | 676 ± 12 | 66 | 641 ± 10 | 65 |
| Spinal | 501 ± 5* | 67 | 424 ± 8* | 61 | 430 ± 10* | 62 |
P≤ 0.05.
Changes in Tib nerve reflexes
The left Tib nerve was stimulated before and after spinalization in the same cats during locomotion to evaluate reflex changes taking place following a complete spinal transection. For comparison, reflex responses for a given muscle of the ipsilateral hindlimb in the 10 bins of the step cycle are separated into four events (shown in Figs 3 and 4): swing (first 3 phases), the swing-to-stance transition (phase 4), stance (phases 5–9), and the stance-to-swing transition (phase 10).
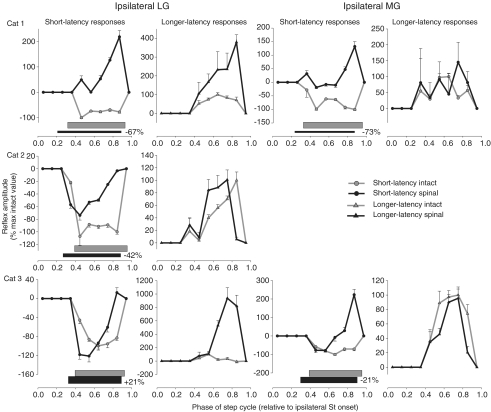
Figure 4. Phase plots summarizing changes in Tib nerve reflexes at 1.2T in ipsilateral ankle extensors (LG and MG, respectively) for cat 1 (top panels), cat 2 (middle panels) and cat 3 (bottom panels).
Horizontal bars represent the phase of activity of each muscle during intact (grey) and spinal (black) locomotion. The percentage difference of the mean amplitude of the locomotor EMG burst in the spinal state, expressed as a function of the intact state, is indicated next to the horizontal bar for each muscle. Each data point is the mean ±s.e.m. of approximately 30 responses.
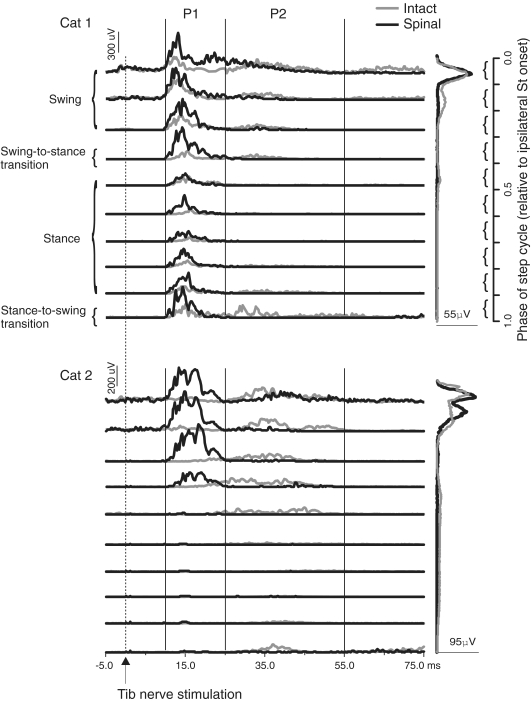
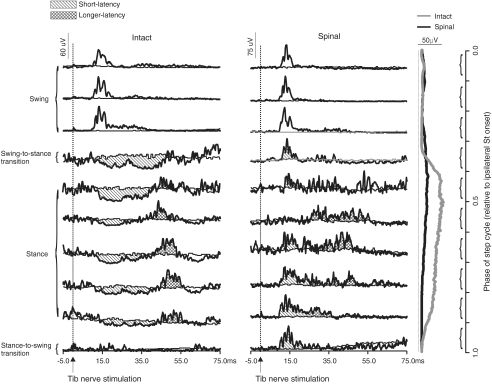
The most remarkable change in reflexes after spinalization was the appearance of a short-latency excitation in some ankle extensors during the stance phase instead of the more common short latency inhibition. Figure 2 shows reflex responses evoked by stimulating the left Tib nerve during different phases of the step cycle in cat 1 before (left panel) and after (right panel) spinalization in the ipsilateral LG. Short-latency inhibitory (N1) responses during stance (i.e. phases 5–9 in the intact state) disappeared and excitatory (P1) responses appeared in the spinal state. The P1 responses evoked during swing (i.e. the first 3 phases) were largely unaffected after spinalization. Thus, excitatory responses in ipsilateral extensors can appear in some phases of the step cycle even though bursting activity was similar, albeit decreased, during spinal locomotion (see scale and EMGs on the right side). When present, the appearance of short-latency excitatory responses during stance persisted for the duration of the study in several testing sessions.
Figure 2. Averaged reflex responses of the ipsilateral LG in the intact and spinal states for cat 1.
The thin lines in each phase indicate the blEMG during intact and spinal locomotion. Values in a single graph are at the same scale in microvolts (μV) but scales differ before and after spinalization. Windows for integrating N1 responses were manually determined in the intact state but after spinalization in this cat fixed windows were used, similar to flexors, because there was a change from inhibition to excitation in most phases of the step cycle, making the delineation between responses difficult. The first horizontal trace for a given graph is phase 0.05 of the step cycle synchronized to the ipsilateral St burst onset. Each trace is the average of approximately 10 stimuli. The rectified, averaged, single burst EMG traces of the ipsilateral LG during intact and spinal locomotion are shown on the far right at the same scale.
Figure 3 illustrates responses evoked by Tib nerve stimulation in the ipsilateral knee flexor St for stimuli applied in different phases of the step cycle in cat 1 (top panel) and cat 2 (bottom panel) before (grey line) and after (black line) spinalization. After spinalization, P1 responses in cats 1 and 2 were increased while P2 responses were decreased throughout the step cycle even though locomotor burst activity was similar between intact and spinal locomotion. Reflex changes in the ipsilateral St of cat 3 were similar to cat 2 and are not illustrated.
Table 4 provides the latency of responses before and after spinalization in each cat for selected muscles. The latency of P1 responses was significantly reduced in 4/7 ipsilateral flexors (St, Srt, TA) but never by more than 1–2 ms. In ipsilateral extensors (VL, LG, MG) the latency of N1 or P1 responses was unchanged in 8/8 muscles. After spinalization, the latency of P2 responses was significantly reduced in 5/8 ipsilateral extensors (i.e. VL, LG and MG). The latency of responses in muscles of the contralateral leg (Srt, VL, TA), contralateral to the stimulation, was reduced in 6/6 muscles.
Table 4.
Latency of reflex responses for selected muscles in each before and after spinalization
| Cat 1 | Cat 2 | Cat 3 | |||||
|---|---|---|---|---|---|---|---|
| Muscle | N1 or P1 | P2 | N1 or P1 | P2 | N1 or P1 | P2 | |
| LSt | Intact | 11 ± 1 | — | 11 ± 1 | — | 10 ± 2 | — |
| Spinal | 10 ± 1* | — | 10 ± 1* | — | 9 ± 1 | — | |
| LSrt | Intact | — | — | 18 ± 3 | — | 16 ± 4 | — |
| Spinal | — | — | 16 ± 4* | — | 17 ± 3 | — | |
| LVL | Intact | 10 ± 1 | 45 ± 4 | 13 ± 1 | 48 ± 3 | 13 ± 2 | 45 ± 3 |
| Spinal | 10 ± 2 | 26 ± 8* | 12 ± 2 | 47 ± 7 | 11 ± 2 | 32 ± 4* | |
| LLG | Intact | 11 ± 1 | 42 ± 4 | 11 ± 1 | 35 ± 4 | 11 ± 1 | 29 ± 6 |
| Spinal | 10 ± 1 | 24 ± 5* | 11 ± 1 | 40 ± 5* | 10 ± 1 | 29 ± 4 | |
| LMG | Intact | 10 ± 1 | 40 ± 5 | — | — | 11 ± 2 | 33 ± 7 |
| Spinal | 10 ± 1 | 26 ± 3* | — | — | 12 ± 1 | 31 ± 5 | |
| LTA | Intact | — | — | 12 ± 1 | — | 10 ± 1 | — |
| Spinal | — | — | 9 ± 1* | — | 10 ± 1 | — | |
| RSrt | Intact | 21 ± 5 | — | 23 ± 5 | — | 18 ± 1 | — |
| Spinal | 14 ± 4* | — | 17 ± 6* | — | 16 ± 2* | — | |
| RVL | Intact | 17 ± 3 | — | 18 ± 3 | — | 21 ± 3 | — |
| Spinal | 13 ± 2* | — | 15 ± 3* | — | 16 ± 3* | — | |
| RTA | Intact | 18 ± 4 | — | 22 ± 4 | — | 22 ± 5 | — |
| Spinal | 14 ± 2* | — | 18 ± 3* | — | 20 ± 4* | — | |
Each value is the mean ± the standard deviation of ∼20 responses.
P≤ 0.05.
Summary of reflex changes
Figures 4–7 provide a more detailed account of modifications of the normalized amplitude of reflex pathways from the left Tib nerve in muscles of the ipsilateral (Figs 4–6) and contralateral (Fig. 7) legs. Responses are grouped into 10 phases relative to ipsilateral St onset and expressed as a function of the maximal intact value. Responses in each phase are divided by the blEMG occurring in the same phase to provide a measure of reflex responses. Therefore, by measuring the normalized amplitude of reflexes before and after spinalization, changes in reflexes are independent of changes in the background level of muscle activity, which can influence reflex amplitude (Matthews, 1986). As can be seen from these figures, reflexes in most muscles were significantly altered in each cat during spinal locomotion.
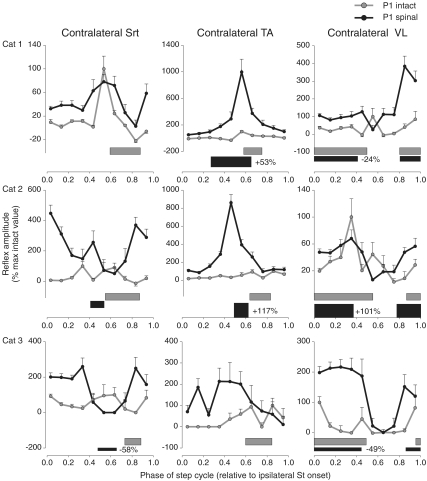
Figure 7. Phase plots summarizing changes in Tib nerve reflexes at 1.2T in contralateral muscles (Srt, TA and VL, respectively) for cat 1 (top panels), cat 2 (middle panels) and cat 3 (bottom panels).
Horizontal bars represent the phase of activity of each muscle during intact (grey) and spinal (black) locomotion normalized to ipsilateral St burst onset. The percentage difference of the mean amplitude of the locomotor EMG burst in the spinal state, expressed as a function of the intact state, is indicated next to the horizontal bar for each muscle. Each data point is the mean ±s.e.m. of approximately 30 responses.
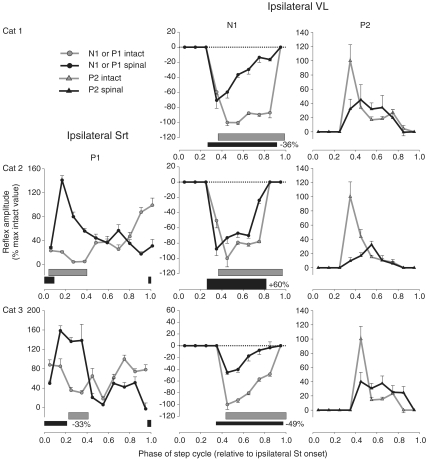
Figure 6. Phase plots summarizing changes in Tib nerve reflexes at 1.2T in the hip flexor/knee extensor Srt and the knee extensor VL for cat 1 (top panels), cat 2 (middle panels) and cat 3 (bottom panels).
Horizontal bars represent the phase of activity of each muscle during intact (grey) and spinal (black) locomotion normalized to ipsilateral St burst onset. The percentage difference of the mean amplitude of the locomotor EMG burst in the spinal state, expressed as a function of the intact state, is indicated next to the horizontal bar for each muscle. Each data point is the mean ±s.e.m. of approximately 30 responses.
Ipsilateral LG and MG
Figure 4 quantifies short-latency reflex responses in the ipsilateral LG and MG of cats 1–3 before and after spinalization. For the group, during stance (i.e. data points at 0.45–0.85), short-latency responses were significantly modified in 10/10 cases in the ipsilateral LG and MG. After spinalization, short-latency excitatory responses appeared during stance in some cases while in other cases the inhibition was primarily decreased, except for LG of cat 3 where it was increased in 2/5 bins. Therefore, in ipsilateral gastrocnemii muscles the short-latency inhibition during stance is either reduced or short-latency excitation becomes apparent. During stance, longer-latency responses of the ipsilateral LG were significantly modified in 3/5 bins (data points at 0.65–0.85) for the group whereas those of the ipsilateral MG were not significantly altered after spinalization. P2 responses in the ipsilateral LG were mostly increased during stance after spinalization. Increased longer-latency responses could be observed in muscles with decreased (e.g. ipsilateral LG of cat 1) or increased (e.g. ipsilateral LG of cat 3) mean amplitude of the locomotor burst.
Ipsilateral St and TA
In the ipsilateral St (Fig. 5, two left-most panels), P1 responses were significantly modified in 10/10 bins after spinalization for the group. During swing (i.e. responses at 0.05–0.25) P1 responses were primarily increased postspinalization. In the early part of swing (data points at 0.05 and 0.15), during which the ipsilateral St is active, P1 responses increased in all cats after spinalization. P1 responses were increased during stance in cat 1 but unchanged in cats 2 and 3. At the transition from swing to stance (i.e. response at 0.35), P1 responses were increased in cat 1 but decreased in cat 2 after spinalization. At the transition from stance to swing (i.e. response at 0.95), P1 responses were increased in cat 1 and decreased in cat 3. Therefore, the most consistent change between cats was an increase in P1 responses during the swing phase. P2 responses of the ipsilateral St during swing were significantly modified in 7/10 bins for the group. In cat 2, P2 responses were decreased in most bins of the step cycle whereas in cats 1 and 3 P2 responses were mostly unchanged or decreased after spinalization. Decreases in P1 and/or P2 responses could be found even though ipsilateral St burst increased in all cats.
In the ipsilateral TA (Fig. 5, two rightmost panels), for the group (cats 2 and 3), P1 responses were significantly modified in 9/10 bins. During swing and stance, P1 responses were primarily increased. Thus, as with the ipsilateral St, a knee flexor, short-latency excitatory responses in the ipsilateral TA, an ankle flexor, are also increased during swing in the majority of cases. For the group, P2 responses in the ipsilateral TA were modified in 10/10 bins and were almost always reduced after spinalization. Thus, in ipsilateral flexors, such as St and TA, short-latency excitatory responses are consistently increased during swing and longer-latency excitatory responses are generally reduced after spinalization.
Ipsilateral Srt and VL
In the ipsilateral Srt (Fig. 6, left-most panels), for the group (cats 2 and 3), P1 responses were significantly modified in 6/10 bins. Responses of the hip flexor Srt were generally increased during swing post-spinalization similar to other ipsilateral flexors such as St and TA. In both cats, responses in the ipsilateral Srt were increased at the swing-to-stance transition and decreased at the stance-to-swing transition. Thus responses could be increased in some phases, particularly during swing and the transition to stance, even though left Srt burst activity was unchanged (cat 2) or decreased (cat 3) during spinal locomotion.
In the ipsilateral VL of cats 1–3 (Fig. 6, two right-most panels), short-latency inhibitory responses (N1) during stance were significantly modified in 5/5 bins for the group. Similar to other ipsilateral extensors, such as gastrocnemii muscles (Fig. 4), short-latency inhibition was reduced throughout the stance phase in the ipsilateral VL. However, contrary to LG and MG there were no short-latency excitatory responses during stance that appeared after spinalization. For the group, P2 responses of the ipsilateral VL were significantly modified in 3/6 bins. The large decrease in P2 responses in phases 0.35 or 0.45 occurred irrespective of locomotor burst activity because ipsilateral VL activity was increased in cat 2.
Contralateral muscles
For the group, excitatory responses in muscles of the limb contralateral to the stimulation were increased after spinalization in most phases of the step cycle (Fig. 7).
For example, responses in the contralateral Srt (left-most panels) were increased in 9/10 bins. In the contralateral TA (middle panels) responses were increased in 4/10 bins. In the contralateral VL (right-most panels), responses were increased in 7/10 bins. In cats 1 and 3 the burst of the contralateral VL decreased after spinalization even though reflex responses in this muscle were mostly increased. Thus, irrespective of whether a muscle contralateral to the stimulation is a flexor (TA), an extensor (VL), or bifunctional (Srt), reflex responses are generally increased post-spinalization.
In summary, reflex responses were altered in all muscles studied post-spinalization and changes were often confined to specific phases of the step cycle. For instance, in ipsilateral flexors, such as St, TA and Srt, short-latency excitatory responses were consistently increased during the swing phase from one cat to another. During stance, in ipsilateral extensors, such as LG, MG and VL, there was less inhibition and in some ankle extensors there was the appearance of short-latency excitatory responses. In muscles of the limb contralateral to the simulation reflex responses were generally increased.
Discussion
The present study showed for the first time how, in the same cat, reflexes evoked by stimulating the Tib nerve through a stable chronically implanted nerve cuff were modified following a complete spinal transection during locomotion. Changes in reflexes were observed in flexors and extensors in both limbs as well as in bifunctional muscles. The clues provided by recording reflexes in the same animal before and after spinalization as to the organization of reflex pathways during locomotion and the reorganization from the intact to the spinal states will be discussed.
Methodological considerations
Changes in the properties of the stimulating cuff and recording electrodes over time could have accounted for altered reflexes. However, stable responses were recorded for prolonged periods before spinalization during which time substantial scar tissue formed around electrodes and stabilized them. In several cases, changes in reflex responses were confined to specific phases of the step cycle. For instance, P1 responses in the ipsilateral Srt were mostly increased during swing (Fig. 6) and could be decreased in later phases. A change in the effective stimulation intensity would have affected all phases similarly. Moreover, the appearance of short-latency excitatory responses during the stance phase in some ankle extensors (Figs 2 and 4) cannot be due to changes in the effective stimulus because such changes would only have affected the size of reflex responses and not the phasic modulation of responses. Therefore, we conclude that reflex changes were not due to changes in the physical conditions at stimulating or recording electrodes.
Unlike previous studies (Forssberg et al. 1975, 1977, 1979) that investigated responses to perturbations in spinal cats, we evaluated reflexes before and after spinalization in the same cat during locomotion to determine changes in reflex pathways from the Tib nerve. Variability in spinal reflexes from one animal or individual to another makes comparisons between intact and lesioned groups problematic and probably reflect differences in neural circuitry that are genetically predetermined and shaped during development and by experience or training (Loeb, 1993). Our approach of recording reflexes in the same animal before and after a lesion allows us to highlight similarities and differences in reflex changes from one cat to another.
Locomotor activity
Modifications in EMG bursts during locomotion were evaluated to determine if changes in reflexes paralleled those in the magnitude and timing of locomotor bursts. As in previous studies, changes in muscle activity could vary somewhat from one animal to another after spinalization, although in general, the locomotor pattern was similar to intact stepping (Smith et al. 1982; Lovely et al. 1986; Barbeau & Rossignol, 1987; Lovely et al. 1990; Bélanger et al. 1996). As previously described (Smith et al. 1982; Lovely et al. 1990; Bélanger et al. 1996) step cycle, swing and stance durations were reduced after spinalization (Table 3).
Since reflex amplitude is influenced by the level of pre-existing motoneuron activity, or automatic gain control (Matthews, 1986; Duysens et al. 1993; Gritsenko et al. 2001) changes in the level of activity of motoneurons after spinalization could simply have accounted for changes in reflexes. Indeed, the loss and recovery of persistent inward currents and plateau potentials after spinal cord injury influences motoneuron and interneuron excitability and hence spinal reflexes (Bennett et al. 2001a,b; Heckman et al. 2003; Brownstone, 2006). However, reflex modifications presented here could not be due solely to altered motoneuron activity because we measured reflexes by dividing reflex responses by the baseline locomotor EMG occurring in the same phase. Moreover, increased or decreased responses could occur in muscles where mean amplitude of locomotor bursts were, respectively, decreased or increased, showing a clear dissociation between changes in reflexes and locomotor EMG bursts. Therefore, premotoneuronal mechanisms must be involved in modifying reflex responses.
Shifts in onsets and offsets of EMG bursts were observed for some muscles (Table 2) and could have influenced reflex responses. However, these shifts would only result in the appearance (or disappearance) of responses in one or two phases of the step cycle, which was not consistently observed. Again, dividing reflex responses by the blEMG in the same phase eliminates the underlying muscle activity as a factor in reflex changes.
If changes in burst amplitude of a given muscle occurred after spinalization, then the homologous muscle of the contralateral limb changed in the same direction (Table 1) suggesting that after complete spinalization the spinal CPG increases or decreases the activity of homologous muscles in parallel, which could be important for bilateral adjustments during locomotion. Parallel changes in homologous muscles could also be due to reciprocal connections. For instance, changes in muscle strength in one muscle through resistance training will increase the strength of the contralateral homologous non-trained muscle (Carroll et al. 2006).
Reflex changes after spinalization
Our results show not only how reflex responses from the Tib nerve are modified following spinalization but also provide clues as to the organization and modulation of these reflex pathways in the intact state. For instance, in ipsilateral extensors, during intact locomotion, short-latency (8–10 ms) excitatory responses can be evoked in the swing phase whereas during stance, short-latency (8–10 ms) inhibitory responses are followed by longer-latency (30–50 ms) excitatory responses (see Fig. 2). Short-latency excitatory inputs to ankle extensors evoked by stimulating cutaneous nerves of the foot have been demonstrated in reduced preparations (Wilson, 1963; LaBella & McCrea, 1990; Rybak et al. 2006) and in spinal cats (Forssberg et al. 1975) but a clear demonstration in the same cat before and after spinalization had never been shown. After spinalization short-latency excitatory responses during swing are unchanged, but during stance inhibitory responses are reduced or short-latency excitatory responses appear. At the same time longer-latency responses begin at an earlier latency. The most likely explanation for these changes after spinalization is that there was a shift in the excitatory/inhibitory balance within spinal sensorimotor circuits that impacted the ‘normal’ phase-dependent modulation of reflex pathways during locomotion. Firstly, in the intact state the short-latency excitatory pathway to ipsilateral extensors is probably actively suppressed during stance by the short-latency inhibitory reflex pathways, by the extensor half of the spinal CPG and/or by supraspinal inputs. Spinalization altered this modulation and as a result short-latency excitatory responses appeared during stance or the inhibition was reduced. Secondly, the short-latency inhibitory pathway probably also gates or competes with the longer-latency excitatory pathway. Consequently, because inhibition is reduced following spinalization longer-latency excitatory responses begin earlier (Table 4). In addition, reflex changes could be due to altered transmission in group I and II pathways because the Tib nerve also innervates intrinsic foot muscles. The contribution of group I and II pathways in the Tib nerve during locomotion in the cat are unknown and require further investigation in the intact and spinal states.
In ipsilateral flexors (Fig. 5), stimulation of cutaneous nerves from the foot evokes short- and longer-latency excitatory responses during intact and spinal locomotion. After spinalization short-latency excitatory responses are increased during early swing (St) or throughout most of the stance phase (TA) whereas longer-latency excitatory responses are reduced. Increased P1 responses in St during swing could result from a disinhibition of the short-latency excitatory pathway by the flexor half of the spinal CPG whereas increases in P1 responses in the TA during stance could be due to a disinhibition by the extensor half. The presence of P2 responses in ipsilateral flexors and extensors was shown in chronic spinal cats during fictive locomotion (Andersson et al. 1978; LaBella et al. 1992) and confirms that they can be organized within the spinal cord. The reduction of P2 responses in ipsilateral flexors, however, suggests an important supraspinal contribution to the excitability of this pathway. Spino-bulbo-spinal reflex pathways project primarily to flexors (Shimamura et al. 1991), which would explain why P2 responses were considerably reduced in St and TA (Fig. 5) after spinalization while longer-latency excitatory responses in ipsilateral extensors were unchanged or even increased (Fig. 4). Decreased P2 responses could also be due to increased refractoriness of motoneurons activated by the short-latency excitatory pathway. However, this could only partly explain changes in P2 responses because there is no clear relationship between P1 and P2 responses after spinalization (Fig. 5).
In muscles contralateral to the stimulation, excitatory responses were increased (Fig. 7) and occurred at an earlier latency (Table 4). Again it is probable that spinalization caused a disinhibition and/or enhanced facilitation of excitatory crossed pathways. The earlier onset of responses could be due to the fact that larger responses are easier to delineate and/or that an inhibitory influence was removed. For instance, a short-latency inhibitory pathway projects from cutaneous afferents of the foot to contralateral extensors (Edgley & Aggelopoulos, 2006; Frigon & Rossignol, 2008) and may project to several motor pools. In this scenario, spinalization reduced the efficacy of the crossed inhibitory pathways and consequently responses occurred earlier, although this requires further investigation.
Therefore, the present results revealed how spinal reflex pathways are functionally reorganized during locomotion after spinalization. Evidently, locomotor training was responsible for some of the reflex changes because transmission in cutaneous and group I reflex pathways are modified by treadmill training, as recently shown during fictive locomotion (Cote et al. 2003; Cote & Gossard, 2004). In the present study, the effects of spinalization and locomotor training on changes in reflex pathways cannot be dissociated because non-trained cats do not express a sufficiently stable locomotor pattern to allow rigorous quantification, as was done here. Moreover, the quality of spinal locomotion did not differ substantially from one cat to another because cats that could step remarkably well after spinalization were required for the quantitative analyses. Locomotor training facilitates the expression of spinal locomotion, and as suggested by others a ‘normalization’ of the excitability of reflex pathways following spinalization could be one of the underlying mechanisms (Cote et al. 2003; Cote & Gossard, 2004; Frigon & Rossignol, 2006). In humans, locomotor training can also modify reflex pathways after spinal cord injury (for a review see Frigon & Rossignol, 2006). In two case studies, changes in reflexes with locomotor training correlated with reduced symptoms of spasticity (Kiser et al. 2005) and improved walking ability (Trimble et al. 1998). Therefore, in animal models and humans spinal cord injury increases reflex excitability, which can be normalized by locomotor training. Further investigation is required to determine whether changes in reflex pathways are causal factors in the recovery of locomotor and/or motor functions.
Spinalization and locomotor training undoubtedly alter the excitatory/inhibitory balance within the spinal cord, hence modifying the excitability of reflex pathways. Indeed, post-spinalization reflex changes could be due to modifications in inhibitory influences within the spinal cord, such as altered presynaptic and/or reciprocal inhibition (Holmqvist & Lundberg, 1959; Nelson & Mendell, 1979; Nelson et al. 1979; Calancie et al. 1993; Faist et al. 1994; Frigon & Rossignol, 2006). There is also evidence that treadmill training can modify the balance between excitation and inhibition within the spinal cord by reducing glycine- and GABA-mediated inhibition in adult spinal cats (de Leon et al. 1999; Tillakaratne et al. 2000). Cutaneous reflexes, such as those from the Tib nerve, are mediated through polysynaptic pathways and changes could reflect modified gating or transmission at several pre- and postsynaptic loci at interneuronal levels and at the motoneuron. Although we favour a shift in the excitatory/inhibitory balance to explain most of our reflex changes, other mechanisms cannot be excluded, such as activation of latent connections, changes in synaptic strength via long-term potentiation or depression, and formation of new connections through axonal sprouting (Edgerton et al. 2004; Rossignol, 2006; Cai et al. 2006; Maier & Schwab, 2006).
In a given cat, changes in reflexes could differ between close synergists such as LG and MG (see LG and MG of cat 3 in Fig. 6). The different changes in reflex responses between close synergist could be due to the fact that LG and MG can receive different inputs from a given cutaneous nerve (LaBella et al. 1989) and that these inputs can be modulated differently (Degtyarenko et al. 1996). As a result, after spinalization the different input connections to both gastrocnemius muscles can be differently impacted leading to dissimilar reflex changes.
Functional significance
The general control model of locomotion is tripartite comprising a spinal CPG, descending supraspinal and propriospinal inputs, and sensory afferents from the periphery (Rossignol et al. 2006). On an ongoing basis the spinal CPG probably optimizes available sources of inputs to generate the appropriate locomotor pattern. For example, during normal walking, cutaneous feedback from the foot soles can contribute to locomotor activity as the foot contacts the ground, but during swimming proprioceptive feedback from the moving muscles undoubtedly becomes an increasingly more important source of input to the spinal CPG. If the spinal CPG is already configured to optimize available inputs in the intact state it is anticipated that it will maximize remaining sources of inputs after injury to generate the locomotor pattern.
Although the spinal CPG appears relatively unchanged after a complete spinal transection, because the locomotor pattern is similar in the intact and spinal states, its input connections are drastically different. For instance, all descending inputs from supraspinal and propriospinal sources rostral to the lesion have been abolished and consequently the voluntary control of locomotion and that of posture and equilibrium is lost. As shown in this study reflex pathways from the Tib nerve are also altered. Reflex pathways, if not integral components of the CPG, certainly have powerful projections to it (Schomburg, 1990; McCrea, 2001; Rossignol et al. 2006) and we suggest that the spinal CPG maximizes available inputs (i.e. reflex pathways) to compensate for the loss of supraspinal influences. This compensatory principle was shown with other lesions. For example, after a cutaneous denervation of the hindpaws in cats corticospinal efficacy is increased (Bretzner & Drew, 2005b) as are reflexes from an intact cutaneous nerve (Bernard et al. 2007). In a similar vein, after partial denervation of ankle extensors in cats the excitability of cutaneous reflexes is increased (Frigon & Rossignol, 2007). Sensory feedback from cutaneous receptors in the hindpaws and ankle extensors normally provides important signals to the locomotor circuitry to guide phase transitions and/or control the magnitude of locomotor bursts (Rossignol et al. 2006). After removing these inputs the locomotor system can compensate for the loss indicating that the spinal CPG can use other available sources of inputs to generate the locomotor pattern.
As stated by Sherrington (1906), all parts of the nervous system are interconnected and consequently removing any input potentially influences the excitability of all others. Descending supraspinal inputs are known to influence the excitability in cutaneous reflex pathways in anaesthetized cats (Fleshman et al. 1988) and in intact cats during locomotion (Bretzner & Drew, 2005a; Bouyer & Rossignol, 2003). After spinalization the excitability from supraspinal inputs is abolished and cutaneous reflex pathways are altered. It stands to reason that cutaneous feedback from the periphery assumes a greater role after spinalization to generate locomotion. In the present study, several reflex changes were observed in phases of the step cycle where they would be most functionally relevant. For example, in the ipsilateral St and Srt, a knee flexor and hip flexor, respectively, increases in reflex responses were mostly recorded during the swing phase (Figs 5 and 6). Greater sensory feedback leading to the activation of St and Srt could reinforce and/or assist the initiation of swing. Additionally, reduced inhibition and/or increased excitation to ankle extensors (Fig. 4) could reinforce extensor activity during stance, thus explaining the absence or reduction of ankle yield during spinal locomotion (Smith et al. 1982; Bélanger et al. 1996). Large increases in short-latency responses in the ipsilateral TA (Fig. 5), an ankle flexor, during the stance phase could increase the stiffness around the ankle joint by coactivating flexors and extensors of the ankle.
The present paper showed how sensory feedback from the Tib nerve is altered during spinal locomotion by evaluating reflex changes before and after spinalization in the same cats but could not ascertain whether these changes are required from expressing spinal locomotion. From one cat to another, there were many similarities in reflex changes after spinalization, which could indicate that specific modifications take place in reflex pathways as a result of spinalization and locomotor training. Although an open question, interanimal differences in reflex changes could indicate why some cats express a spinal locomotion earlier and why the quality of spinal locomotion differs between cats.
Making use of the complex sensorimotor circuitry within the spinal cord by facilitating reflex changes might be effective in restoring walking ability after spinal cord injury. The methodology presented here can be used to systematically evaluate changes in numerous reflex pathways after spinal lesions of varying extents, which could provide more clues regarding the functional reorganization of spinal sensorimotor pathways after spinal cord injury. The methodology can also be used to test interventions, such as electrical or pharmacological stimulation, that could promote changes in reflex pathways. From an applied perspective, rehabilitative approaches specifically aimed at normalizing or shaping reflex pathways could facilitate the recovery of locomotion following spinal cord injury and deserve further investigation.
Acknowledgments
We thank Janyne Provencher and Dr Hugues Leblond for technical assistance. A special thanks to Drs Jean-Pierre Gossard and Grégory Barrière for their helpful comments, and to Dr Valeriya Gritsenko and Miguel Chagnon for their help with the statistical analysis. This investigation was supported by a doctoral studentship from the Groupe de Recherche sur le Système Nerveux Central (GRSNC) and by the Tomas Reader Prize to A. Frigon and by individual and group grants from the Canadian Institute of Health Research and from a Tier 1 Canada Chair on spinal cord research to S. Rossignol.
References
- Abraham LD, Marks WB, Loeb GE. The distal hindlimb musculature of the cat. Cutaneous reflexes during locomotion. Exp Brain Res. 1985;58:594–603. doi: 10.1007/BF00235875. [DOI] [PubMed] [Google Scholar]
- Andersson O, Forssberg H, Grillner S, Lindquist M. Phasic gain control of the transmission in cutaneous reflex pathways in motoneurones during ‘fictive’ locomotion. Brain Res. 1978;149:503–507. doi: 10.1016/0006-8993(78)90493-6. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol. 2001a;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001b;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Bernard G, Bouyer LJG, Provencher J, Rossignol S. Study of cutaneous reflex compensation during locomotion after nerve section in the cat. J Neurophysiol. 2007;97:4173–4185. doi: 10.1152/jn.00797.2006. [DOI] [PubMed] [Google Scholar]
- Bouyer LJG, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion: 2. Spinal cats. J Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J Neurophysiol. 2005a;94:673–687. doi: 10.1152/jn.01247.2004. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Changes in corticospinal efficacy contribute to the locomotor plasticity observed following unilateral cutaneous denervation of the hindpaw in the cat. J Neurophysiol. 2005b;94:2911–2927. doi: 10.1152/jn.00254.2005. [DOI] [PubMed] [Google Scholar]
- Brownstone RM. Beginning at the end: repetitive firing properties in the final common pathway. Prog Neurobiol. 2006;78:156–172. doi: 10.1016/j.pneurobio.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci. 2006;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101:1514–1522. doi: 10.1152/japplphysiol.00531.2006. [DOI] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Burke RE. Differential modulation of disynaptic cutaneous inhibition and excitation in ankle flexor motoneurons during fictive locomotion. J Neurophysiol. 1996;76:2972–2985. doi: 10.1152/jn.1996.76.5.2972. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Forelimb responses to cutaneous nerve stimulation during locomotion in intact cats. Brain Res. 1985;329:323–328. doi: 10.1016/0006-8993(85)90543-8. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol. 1980;44:1024–1037. doi: 10.1152/jn.1980.44.5.1024. [DOI] [PubMed] [Google Scholar]
- Duysens J, Stein RB. Reflexes induced by nerve stimulation in walking cats with implanted cuff electrodes. Exp Brain Res. 1978;32:213–224. doi: 10.1007/BF00239728. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Trippel M, Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Res. 1993;613:230–238. doi: 10.1016/0006-8993(93)90903-z. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Aggelopoulos NC. Short latency crossed inhibitory reflex actions evoked from cutaneous afferents. Exp Brain Res. 2006;171:541–550. doi: 10.1007/s00221-005-0302-9. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Fleshman JW, Rudomin P, Burke RE. Supraspinal control of a short-latency cutaneous pathway to hindlimb motoneurons. Exp Brain Res. 1988;69:449–459. doi: 10.1007/BF00247299. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Plasticity of reflexes from the foot during locomotion after denervating ankle extensors in intact cats. J Neurophysiol. 2007;98:2122–2132. doi: 10.1152/jn.00490.2007. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Short-latency crossed inhibitory responses in extensor muscles during locomotion in the cat. J Neurophysiol. 2008;99:989–998. doi: 10.1152/jn.01274.2007. [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Mushahwar V, Prochazka A. Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. J Physiol. 2001;533:299–311. doi: 10.1111/j.1469-7793.2001.0299b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. On the organization of the supraspinal inhibitory control of interneurons of various reflex arcs. Arch Ital Biol. 1959;97:340–356. [Google Scholar]
- Julien C, Rossignol S. Electroneurographic recordings with polymer cuff electrodes in paralyzed cats. J Neurosci Methods. 1982;5:267–272. doi: 10.1016/0165-0270(82)90078-4. [DOI] [PubMed] [Google Scholar]
- Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- LaBella LA, Kehler JP, McCrea DA. A differential synaptic input to the motor nuclei of triceps surae from the caudal and lateral cutaneous sural nerves. J Neurophysiol. 1989;61:291–301. doi: 10.1152/jn.1989.61.2.291. [DOI] [PubMed] [Google Scholar]
- LaBella LA, McCrea DA. Evidence for restricted central convergence of cutaneous afferents on an excitatory reflex pathway to medial gastrocnemius motoneurons. J Neurophysiol. 1990;64:403–412. doi: 10.1152/jn.1990.64.2.403. [DOI] [PubMed] [Google Scholar]
- LaBella L, Niechaj A, Rossignol S. Low-threshold, short-latency cutaneous reflexes during fictive locomotion in the ‘semi-chronic’ spinal cat. Exp Brain Res. 1992;91:236–248. doi: 10.1007/BF00231657. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Loeb GE. The distal hindlimb musculature of the cat: interanimal variability of locomotor activity and cutaneous reflexes. Exp Brain Res. 1993;96:125–140. doi: 10.1007/BF00230446. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cat. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SG, Collatos TC, Niechaj A, Mendell LM. Immediate increase in Ia-motoneuron synaptic transmission caudal to spinal cord transection. J Neurophysiol. 1979;42:655–664. doi: 10.1152/jn.1979.42.3.655. [DOI] [PubMed] [Google Scholar]
- Nelson SG, Mendell LM. Enhancement in Ia-motoneuron synaptic transmission caudal to chronic spinal cord transection. J Neurophysiol. 1979;42:642–654. doi: 10.1152/jn.1979.42.3.642. [DOI] [PubMed] [Google Scholar]
- Pratt CA, Chanaud CM, Loeb GE. Functionally complex muscles of the cat hindlimb. IV. Intramuscular distribution of movement command signals and cutaneous reflexes in broad, bifunctional thigh muscles. Exp Brain Res. 1991;85:281–299. doi: 10.1007/BF00229407. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361:1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol. 2006;577:641–658. doi: 10.1113/jphysiol.2006.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT, USA: Yale University Press; 1906. [Google Scholar]
- Shimamura M, Tanaka I, Livingston RB. Longitudinal conduction systems serving spinal and brainstem coordination (spino-bulbo-spinal reflex) In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. pp. 241–255. [Google Scholar]
- Smith JL, Smith LA, Zernicke RF, Hoy M. Locomotion in exercised and non-exercised cats cordotomized at two or twelve weeks of age. Exp Neurol. 1982;76:393–413. doi: 10.1016/0014-4886(82)90217-5. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Kukulka CG, Behrman AL. The effect of treadmill gait training on low-frequency depression of the soleus H-reflex: comparison of a spinal cord injured man to normal subjects. Neurosci Lett. 1998;246:186–188. doi: 10.1016/s0304-3940(98)00259-6. [DOI] [PubMed] [Google Scholar]
- Wilson VJ. Ipsilateral excitation of extensor motoneurones. Nature. 1963;198:290–291. doi: 10.1038/198290b0. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]