Abstract
The functionality of the endoplasmic reticulum (ER) as a Ca2+ storage organelle is supported by families of Ca2+ pumps, buffers and channels that regulate Ca2+ fluxes between the ER lumen and cytosol. Although many studies have identified heterogeneities in Ca2+ fluxes throughout the ER, the question of how differential functionality of Ca2+ channels is regulated within proximal regions of the same organelle is unresolved. Here, we studied the in vivo dynamics of an ER subdomain known as annulate lamellae (AL), a cytoplasmic nucleoporin-containing organelle widely used in vitro to study the mechanics of nuclear envelope breakdown. We show that nuclear pore complexes (NPCs) within AL suppress local Ca2+ signalling activity, an inhibitory influence relieved by heterogeneous dissociation of nucleoporins to yield NPC-denuded ER domains competent at Ca2+ signalling. Consequently, we propose a novel generalized role for AL – reversible attenuation of resident protein activity – such that regulated AL (dis)assembly via a kinase/phosphatase cycle allows cells to support rapid gain/loss-of-function transitions in cellular physiology.
The endoplasmic reticulum (ER) is an intracellular Ca2+ storage organelle that integrates mechanisms for Ca2+ uptake and release in response to cellular stimulation (Meldolesi & Pozzan, 1998; Baumann & Walz, 2001; Blaustein & Golovina, 2001; Papp et al. 2003). Functioning as a synthetic factory, scrapyard and synchronized distribution network, the ER tailors regional infrastructure and asset localization to support diverse subcellular functions using its sizeable membrane surface area (∼50% total membrane surface) and luminal volume (∼10% of cellular volume; Voeltz et al. 2002) to anchor reactions that support, as well as terminate, cellular viability.
To support such specialization, the ER is highly plastic in morphology and function (Meldolesi & Pozzan, 1998; Baumann & Walz, 2001; Blaustein & Golovina, 2001; Voeltz et al. 2002; Papp et al. 2003; Borgese et al. 2006). Globally, the ER acts as a pervasive network through which ions, proteins and exogenous labels rapidly equilibrate (Terasaki & Jaffe, 1991; Mogami et al. 1997). Such global interconnectivity ensures that ER proteins are able to interact with ER-resident partners whose trans-domains lie within the nucleoplasm or cytoplasm, as well as signalling complexes that sense mitochondrial, extracellular and transcellular environments, underscoring the role of the ER as a key information-relaying superhighway. Despite such interconnectivity, many discrete ER subdomains exist beyond the classic divisions of rough ER, smooth ER and the two nuclear envelope (NE) membranes. These encompass transitional, peroxisomal, junctional and nucleoplasmic ER, the diads, triads and peripheral couplings of sarcoplasmic reticulum, as well as a variety of ER specializations in cells as diverse as oocytes and plant growth tips. In addition, there exist functional heterogeneities in ER Ca2+ uptake, release and buffering that have, as yet, no clear structural basis (Lievremont et al. 1994; Rooney & Meldolesi, 1996; Golovina & Blaustein, 1997). A final complexity is that compartmentalization of ER Ca2+ signals results not only from static heterogeneities in the distribution of Ca2+-handling proteins but also from more ephemeral assemblies of Ca2+-release channels, Ca2+ pumps and Ca2+-binding proteins that alter their localization in response to cell stimulation (Wilson et al. 1998; Liou et al. 2005; Roos et al. 2005; Tateishi et al. 2005; Chalmers et al. 2006). Collectively, all these data suggest that the ER is composed of a collection of rapidly differentiable subdomains with discrete Ca2+-handling properties.
Mechanistic insight into the biogenesis of ER subdomains, and how residency of ER proteins within these specializations affects their functionality, are important considerations in understanding first, how cells regulate their overall responsiveness and second, the targeting, amplification and vectorality of cytoplasmic Ca2+ signals. Therefore, in this manuscript we investigated the cell biology of a discrete ER subdomain, known as annulate lamellae (AL). Numbers of these enigmatic ER structures are elevated in virally infected cells and tumours, as well as oocytes and embryos (Kessel, 1992). Previously viewed as a subdomain of the rER that warehouses excess nuclear pore complexes (NPCs), they have been extensively studied in vitro (Meier et al. 1995; Miller & Forbes, 2000) as a biochemical model for reconstituting the events of NPC assembly and nuclear envelope/germinal vesicle breakdown (NE/GV-BD). Less is known about their in vivo role, despite the observation that in human eggs, formation of AL is a vital postfertilization process, with AL misassembly resulting in early developmental failure (Sutovsky et al. 1998).
We have recently shown that during oocyte maturation, AL contribute to the biogenesis of cortical ER patches and thereby Ca2+ signalling competency in the mature, fertilizable egg (Boulware & Marchant, 2005). AL deliver an IP3R complement from the ER subcortex into the peripheral shell of cortical ER that supports propagation of the fertilization Ca2+ wave. This transfer of the AL-derived IP3Rs is associated with increased IP3R overall responsiveness: whereas IP3Rs within AL in the oocyte have a low propensity to be involved in local Ca2+ signalling, AL-derived IP3Rs within cortical ER patches of the egg act as foci for Ca2+ wave initiation. Succinctly, these two types of ER specialization containing IP3Rs (AL and cortical ER patches) display divergent local Ca2+ signalling activities. As a paradigm for how Ca2+ channel activity can be locally regulated between proximal areas of the same organelle, we resolved to investigate potential in vivo mechanisms underpinning changes in IP3R responsiveness relative to AL dynamics throughout the time course of oocyte maturation.
Methods
Materials
Reagents were sourced as follows: Ca2+ indicators, caged IP3, Alexa Fluor 488-WGA (Alexa Fluor 488 conjugated with wheat germ agglutinin), Alexa Fluor 647-WGA, tetramethylrhodamine- and Alexa Fluor-conjugated dextrans (10 kDa, 70 kDa), and jasplakinolide from Invitrogen (Carlsbad, CA, USA); d-myo-inositol 1,4,5-trisphosphate (IP3) and nocodazole from EMD Biosciences (La Jolla, CA, USA); DsRed2-ER from BD Biosciences (Palo Alto, CA, USA); anti-IP3R (sc-28613) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); mab-414 (Covance MMS-120R) and anti-phospho-vimentin (KAM-CC249) from Stressgen (Victoria, BC, Canada); cytochalasin B, cytochalasin D, progesterone, human chorionic gonadotropin (hCG) and okadaic acid from Sigma-Aldrich (St Louis, MO, USA); Complete protease inhibitors from Roche Diagnostics (Indianapolis, IN, USA). Nup153 conjugated with green fluorescent protein (Nup153-GFP) and GFP-vimentin were generous gifts from Drs Jan Ellenberg (European Molecular Biology Laboratory (EMBL)) and Ronald Liem (Columbia University), respectively.
Handling of oocytes and eggs
Adult Xenopus laevis frogs (NASCO, Fort Atkinson, WI, USA) were anaesthetized by immersion in tricaine methane sulphonate (MS 222, 0.1 g l−1; buffered with 0.7 g l−1 sodium bicarbonate) and monitored during induction and recovery from anaesthetic following protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota. For fractionation experiments, oocytes were obtained following exposure of surgically removed ovarian tissue to collagenase (Sigma Type I, 0.5 mg ml−1) to remove follicular cells. Eggs, obtained by shedding after hCG injection, were de-jellied in 2% cysteine and rinsed in modified Barth's solution (mm: NaCl 88, KCl 1, NaHCO3 2.4, MgSO4 0.83, Ca(NO3)2 0.33, CaCl2 0.41, Hepes 5; and 0.05 mg ml−1 gentamycin, pH 7.4, 18°C) before homogenization. Frogs were killed humanely at the end of experiments. For microinjection experiments, individual stage VI oocytes were isolated and defolliculated manually with watchmakers' forceps. Oocytes were maintained in modified Barth's solution with solution changes every 12 h. cDNA vectors were subsequently injected into the germinal vesicle to ensure appropriate cellular targeting. Cells that expressed DsRed2-ER in the vegetal hemisphere (after ∼48 h) were injected with caged IP3 and fluo4-based Ca2+ indicators (Kd[Ca2+]= 225 nm for imaging local Ca2+ signals or Kd[Ca2+]= 3 μm for imaging global Ca2+ signals). Curve fitting (χ2 as criterion) was performed using Origin (OriginLab Corporation) and mono- and multiexponential fits compared using the partial (sequential) F test. In some experiments cells were injected with fluorescent WGA (final concentration of 5–10 μg ml−1), a concentration lower than that which inhibits pore transport (Finlay et al. 1987). Oocytes were matured in vitro with progesterone (1 μg ml−1).
Confocal imaging
For live cell imaging experiments, we employed two distinct confocal systems built around the same inverted microscope (Olympus IX81 ×40–60 objective lenses, NA 1.35–1.42). This system (shown in Fig. 1 of the online Supplemental material), permitted the interchangeable use of video-rate confocal and flash photolysis (for Ca2+ imaging), as well as multicolour confocal timelapse imaging (for morphology) in the same cell. The need for this dual approach derived from the discrete benefits of either confocal approach. The video-rate raster-scanning laser spot system (Callamaras & Parker, 1999) well suited for localizing Ca2+ puffs (duration of tens of milliseconds) over short periods (seconds) within restricted regions of the ER was deleterious to the observation of ER morphology over the long time course of oocyte maturation (≤ 14 h). A swept-field confocal approach (using an arc lamp-based spinning disc system to facilitate multiwavelength imaging) was preferable for timelapse confocal imaging where low light intensities and long exposure intervals sufficed, but this method was reciprocally futile for resolving local Ca2+ signals at acceptable illumination intensities.
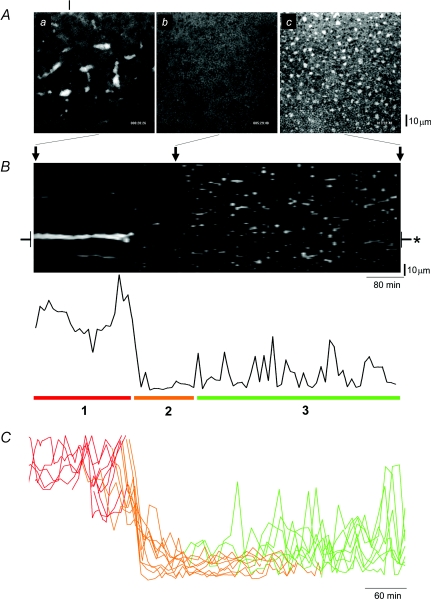
Figure 1. ER subdomain morphology during Xenopus oocyte maturation.
A, lateral (‘xy’) confocal images of DsRed2-ER fluorescence in the vegetal hemisphere of the same Xenopus oocyte, taken ∼20 min (a), 5.5 h (b) and 14 h (c) from a timelapse movie (Supplemental Movie 2) started on addition of progesterone (1 μg ml−1). The vertical line indicates the linescan position used for the projection in B. B, upper panel, projection (‘xt’) of a timelapse stack into a single ‘lamellogram’ image, showing fluorescence across a single spatial dimension (vertical axis) over time (horizontal axis). Positions in this projection corresponding to the images shown in A are indicated by arrows; lower panel, fluorescence intensity profile measured from region (asterisked) on lamellogram. C, overlay of fluorescence profiles from multiple lamellograms (n = 10) revealing three phases of ER remodelling (1, red; 2, orange; 3, green) are conserved in different cells. Given the variable timeframe of maturation in single cells, profiles are aligned to the end of phase ‘1’ in each timelapse record.
For short-term Ca2+ imaging (seconds to minutes), we used the ‘home-brew’ resonant-galvanometer, laser(488 nm)-point scanning system built around the video-port of the microscope, which provided sufficient temporal resolution (15–60 Hz) and sensitivity to resolve individual Ca2+ puffs (Callamaras & Parker, 1999). Ca2+ signals were represented as pseudo-ratios (F/F0) of fluorescence at each pixel during a response (F) relative to resting pixel fluorescence (F0). For timelapse imaging, we preferred an arc-lamp-based spinning-disc confocal module (Olympus DSU) with electron-multiplication CCD camera (Hamamatsu, C9100-12) attached to the epifluorescence port. The arc-lamp-based approach enabled imaging of fluorophores with discrete excitation spectra (e.g. ER (red, DsRed2-ER), NPCs (far-red, Alexa Fluor 647-WGA)), while the sensitivity of the EM-CCD and wide-field imaging approach minimized exposure times that we believe minimized phototoxicity over the lengthy timeframe of these experiments (≤ 14 h per cell). Consequently, ER remodelling could be followed in multiple AL simultaneously using timelapse confocal imaging, and then individual AL from the same field selected at appropriate time points for local Ca2+ imaging using video-rate confocal microscopy.
Immunoblotting
The protein content of samples was quantified using a Qubit fluorimeter and Quant-it protein assay kit (Invitrogen). Equal amounts of protein from specific fractions were subjected to electrophoresis and Western blot analysis using the Invitrogen NuPage large protein analysis system. Nitrocellulose membranes were blocked with 12% (w/v) non-fat dried milk (Flavorite) and 1% (w/v) bovine serum albumin (BSA) and probed with antibodies against IP3R and nup62 (mab-414, see Materials). Signals were visualized using appropriate secondary antibodies coupled to horseradish peroxidase using an enhanced chemiluminescence system (Pierce, Rockford, IL, USA).
Immunocytochemistry
Cells were fixed by immersion and incubation (> 2 h) in 1% formaldehyde in methanol at −20°C. Cells were warmed to room temperature and rehydrated in PBS in a stepwise fashion (5 min rinses in a 75%–50%–25% MeOH series into 100% PBS). Cells were permeabilized (0.1% Triton X-100) and blocked (4% BSA, 1 h) and then incubated (2 h) with primary antibody in BSA (2%). Cells were rinsed in PBS (1 h) and incubated in secondary antibody in BSA (2%) overnight at 4°C. Finally, cells were rinsed (1 h, PBS) and mounted on glass coverslips using mounting media (Ted Pella Inc.).
Maturation-promoting factor (MPF) protein kinase assay
Cells were snap frozen in a minimal volume of kinase assay buffer (mm: Tris 50, NaCl 500, EDTA 4, EGTA 2, β-glycerophosphate 25, sodium orthovanadate 1, 2-mecaptoethanol 50 and a Complete protease inhibitor tablet, pH 7.5). Cells were thawed, supplemented with 50 μl kinase assay buffer per oocyte and homogenized by vigorous pipetting before being spun at 10 000 g for 15 min. Kinase assays (absorbance at λ= 490 nm) were performed using the Mesacup Cdc2 Kinase Assay Kit (MBL International Corp., Woburn, MA, USA) according to the manufacturer's instructions using 2.5 μl of supernatant collected as described above.
Cell fractionation and 45Ca2+ flux assay
Xenopus oocytes or eggs were homogenized in buffer (pH 7.25) containing 50 mm Tris-HCl, 250 mm sucrose and a Complete protease inhibitor tablet. The resulting homogenate was centrifuged at 1500 g for 15 min to yield a supernatant which was then centrifuged at 142 000 g for 35 min to yield a microsomal pellet. The pellet was resuspended in a minimal volume of the same buffer and re-homogenized using a tight-fitting ground glass homogenizer. The sample was then layered on top of a discontinuous sucrose gradient composed of three layers at 56%, 46% and 43% sucrose in 50 mm Tris-HCl pH 7.25 and spun at 237,000 g in a SW55 swinging bucket rotor for 2.5 h. Visible material was isolated at each interface, diluted into homogenization buffer and spun at 142 000 g for 40 min to yield the final pellets which were solubilized in 50 mm Tris-HCl, 300 mm sucrose and a Complete protease inhibitor tablet before being snap frozen in liquid nitrogen.
For 45Ca2+ uptake, equal amounts of protein from each fraction were thawed and diluted into a cytoplasmic-like buffer (mm: Pipes 20, KCl 140, NaCl 20, MgCl2 2, EGTA 1 and CaCl2 0.3, supplemented with 7 mm ATP, 10 μm FCCP and 30 μCi ml−1 of 45Ca2+ at pH 7.0) for 20 min at room temperature (21°C). After loading, microsomes were diluted into non-supplemented cytoplasmic-like buffer containing the indicated concentration of IP3 or 0.1% Triton X-100 for 3 min before filtering and rinsing on a GF/B filter. Residual radioactivity was estimated by liquid scintillation counting (LS6500, Beckman).
Results
Changes in vegetal hemisphere ER morphology were followed during progesterone-evoked maturation by timelapse confocal imaging (images captured every 10 min over ∼10–14 h) in cells expressing a luminal ER marker (DsRed2-ER). Before the addition of progesterone, annulate lamellae (AL) were resolved as subcortical ER densities (‘cigar’-shaped structures, 10–25 μm long, ∼5 μm wide; Boulware & Marchant, 2005) that remained relatively immobile in the arrested oocyte (Fig. 1Aa; see also Movie 1 in online Supplemental material). However, AL disappear within ∼4–5 h after progesterone addition (Fig. 1Ab), appearing to sink deeper into the cytoplasm, an event which precedes the emergence of patch-like densities in the ER cortex of the maturing cell (Fig. 1Ac, Supplemental Movie 2).
A simple way of visualizing the process of ER remodelling throughout maturation in a single cell, that facilitates comparison of experimental manipulations is shown in Fig. 1B. This figure (a ‘lamellogram’) is a thresholded ‘xt’ (‘linescan’) projection of a ∼14 h timelapse in a single cell that tracks ER morphology (vertical) over time (horizontal). Although considerable spatial information is lost, the simple plot condenses all pertinent morphological and kinetic information into a single, comprehensible plot. The lamellogram and associated fluorescence profile (Fig. 1B) reveal three phases of AL remodelling: (1) ‘AL persistence’ after progesterone addition, (2) the short, abrupt phase of ‘AL disappearance’, and finally (3) the phase of ‘ER patch emergence’, in which smaller, motile ER patches develop that frequently transect the scanline (see later). Each of these phases was observed in all maturing oocytes, although the duration of specific phases showed appreciable variability between cells (Fig. 1C).
To image NPC dynamics within AL during maturation, we expressed a GFP-tagged nucleoporin (Nup153-GFP, a basket nucleoporin; Stoffler et al. 1999) or labelled endogenous nucleoporins (including nup62, nup98 and nup214; Miller et al. 2000) with fluorescent wheat germ agglutinin (WGA) conjugates. Timelapse imaging of NPC dynamics using either approach (Fig. 2A) showed that both NPC labels were rapidly lost from AL around the time that this ER subdomain disappeared (‘phase 2’, Fig. 1B) and neither NPC label reappeared in cortical ER densities during the late phase of maturation (Supplemental Movie 3). Simultaneous dual-emission imaging of DsRed2-ER and Alexa Fluor 488-WGA (Fig. 2A) confirmed the differential behaviour of NPC and ER markers in the same cell over the entire maturation time course. Further, the fluorescence profiles associated with these dual wavelength projections indicated a small delay (range of 20–90 min; mean of 41 ± 28 min, n = 5) between the loss of NPCs and the disappearance of AL (asterisk, Fig. 2A). To substantiate this observation, lateral (‘xy’) confocal images, captured at shorter time intervals during this period, were analysed. These images surprisingly revealed a heterogeneous, ‘patchy’ dissociation of NPC label from AL, leaving a variegated ER subdomain of adjacent NPC-replete and NPC-denuded regions (Fig. 2Ba, Supplemental Movie 4). In higher-magnification images of individual AL, vesicle-like structures labelled with NPC markers could be seen in the vicinity of AL during this period of NPC dissociation (Fig. 2Bb, Supplemental Movie 5).
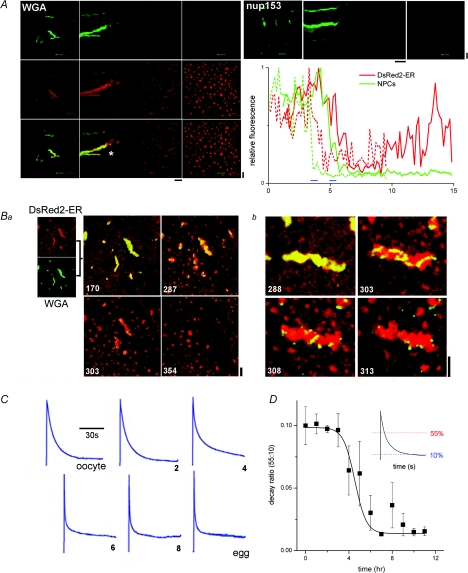
Figure 2. Nucleoporin and macroscopic Ca2+ dynamics during oocyte maturation.
A, time course of nucleoporin dissociation from AL. Left, composite lamellogram of a maturing cell expressing DsRed2-ER (red), injected with Alexa Fluor 488-WGA (green), with the dual-channel fluorescence overlay shown at bottom. Confocal (‘xy’) images are shown at the start (t= 1 h, left) and the end (t= 15 h, right) of a timelapse record (Supplemental Movie 3). Right, time course of nup153-GFP dissociation during maturation. Representative fluorescence profiles of ER remodelling (red) and NPC dissociation (green) in cells expressing DsRed2-ER and nup153 (dotted lines) or DsRed2-ER alone and injected with Alexa Fluor 488-WGA (green). Time delays between NPC and AL disappearance were estimated at half-maximal relative fluorescence, and indicated by blue bars. Horizontal time bars = 1 h. Ba, confocal images of a maturing cell expressing DsRed2-ER (red) and injected with Alexa Fluor 488-WGA (green). Individual fluorescence images are merged as an overlay shown at various times (numbered in minutes) after progesterone addition (Supplemental Movie 4). Image stills show NPC dissociation from AL over ∼1 h interval, captured ∼5 h after progesterone exposure. Bb, higher magnification images of AL morphology during NPC dissociation. C, cellular Ca2+ signals evoked by photolytic release of saturating concentrations of IP3 at indicated time points (hours) during maturation. There was no difference in the magnitude of Ca2+ signals, IP3R sensitivity or maturation competence between cells expressing DsRed2-ER and non-expressing controls. D, analysis of time course of changes in the macroscopic Ca2+ transient during maturation indexed by following changes in the rate of decay of the transient (ratio of time to decay to 55% and 10% of peak, inset). Vertical scale bars = 10 μm.
Do macroscopic Ca2+ dynamics change during this phase of ER remodelling? Previous comparison of cellular Ca2+ dynamics only at the extremes of the maturation process (i.e. immature oocyte versus fertilizable egg; Boulware & Marchant, 2005) demonstrated an increase in magnitude, initial rate of cytosolic Ca2+ clearance and Ca2+ signal duration (owing to regenerative propagation of the fertilization Ca2+ wave) after maturation in the vegetal hemisphere. In essence, the initial component of the decline in the oocyte Ca2+ transient is accelerated, while the terminal phase(s) are lengthened – adaptations that can easily be tracked via a single ratio (t55: t10; ratio of times taken for fluorescence to decay to 55% and 10% of peak value). Using a low-affinity Ca2+ indicator to track Ca2+ dynamics throughout maturation, it was observed that the period of ER remodelling correlated temporally with these transitions in the cellular Ca2+ transient (Fig. 2C and D). That ER remodelling occurs contemporaneously with changes in Ca2+ signalling should not imply a sole causative relationship – it is probably one of many factors to impact the whole-cell Ca2+ signature (El-Jouni et al. 2005) – however, we do infer from these data that the extensive AL remodelling occurs while the Ca2+ signal of the maturing cell is being customized to support fertilization.
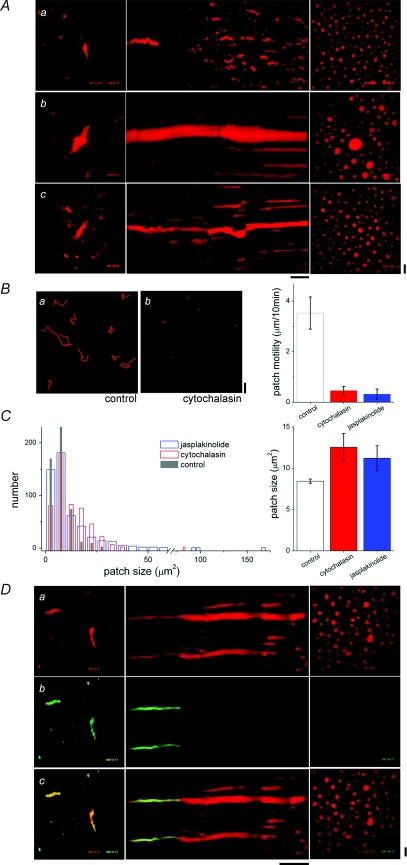
Next, we attempted to image localized IP3R activity in AL throughout maturation to resolve at what point IP3R activity increased in these ER subdomains. In particular, the simultaneous occurrence of NPC-replete and NPC-depleted areas within AL (Fig. 2B), allowed testing of a hypothesis that NPC dissociation relieved attenuation of IP3R activity. However, the fact that AL sank beyond resolvable imaging depth during normal maturation precluded imaging analysis of IP3R functionality during this period of particular interest. Therefore, we investigated the effects of cytoskeletal inhibition on ER remodelling, with the goal of impeding the AL movements that precluded continuous imaging throughout maturation. Incubation of oocytes in nocodazole (70 μm), a microtubule-disrupting agent, had little effect on AL in resting oocytes and cortical ER patch dynamics during oocyte maturation (Fig. 3Aa). In contrast, manipulation of actin microfilaments, with cytochalasin or jasplakinolide, markedly altered the dynamics of ER remodelling. Both agents inhibited the sinking and normal fragmentation of AL into smaller patches (phase 2), such that individual AL could now be tracked throughout maturation as they condensed into single, often abnormally large, cortical ER patches (Fig. 3Ab and c, Supplemental Movie 6). As with nocodazole, neither compound disrupted AL organization in the resting oocyte. Quantitative analyses of cortical ER patch properties revealed that microfilament disruption inhibited ER patch movement (Fig. 3B) and increased average patch size (Fig. 3C). Notably, the population histogram of patch size displayed rightward shifts in the presence of the actin-modifying agents, with the increased population mean (cytochalasin 12.5 ± 1.7 μm2, jasplakinolide 11.2 ± 1.5 μm2versus control 8.4 ± 0.3 μm2) reflecting the population of ‘mega’-patches condensed from individual AL (Fig. 3C). Since this manipulation permitted resolution of AL throughout the maturation process, we investigated whether cytoskeletal disruption affected NPC dissociation. Treatment with either cytochalasin (Fig. 3D) or jasplakinolide (data not shown) did not impact NPC dissociation judged by the time course or the ‘mosaic’ appearance of AL during NPC disassembly. Therefore, we exploited this method to permit imaging of AL Ca2+ dynamics during NPC dissociation within the live cell.
Figure 3. Effect of cytoskeletal disruption on ER remodelling during maturation.
A, lamellograms of maturing oocytes subjected to treatment with cytoskeletal-disrupting agents. Sequences show cells treated with 70 μm nocodazole (a), 2.5 μm jasplakinolide (b, Supplemental Movie 6), and 50 μm cytochalasin B (c). Horizontal time bar represents 1 h (a and b) and 30 min (c). B, patch motility measured from timelapse records of mature control cells and mature cells treated with cytochalasin or jasplakinolide. Left, tracking patch motility in control (a) and cytochalasin-treated cells (b) at 10 min intervals from hour-long timelapse records. Right, average patch motility for indicated treatments. C, left, histogram showing distribution of patch sizes (cross-sectional area, μm2) in mature control cells (grey), or cells treated with cytochalasin (red) or jasplakinolide (blue). Right, average patch size for different treatments. D, composite lamellogram of a cell expressing DsRed2-ER (a) and injected with Alexa Fluor 488-WGA (b) matured in the presence of cytochalasin (middle) with the overlay (c) shown at bottom. Horizontal time bar represents 1 h. All vertical scale bars = 10 μm.
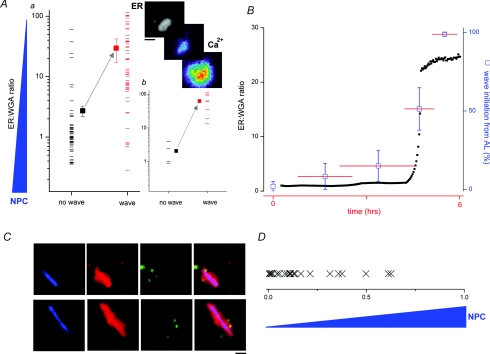
First, we looked at numerous AL (at different points during maturation), and correlated the capacity of IP3Rs within AL to trigger Ca2+ waves (Fig. 4A, inset) relative to the density of NPCs on individual AL at that time point. As an index of NPC presence, we used the ratio of DsRed2-ER fluorescence intensity (ER label) to WGA fluorescence intensity (NPC label). The ratio (ER: WGA) increased as NPCs dissociated from AL. Considering the population of AL as a whole, this dataset revealed that the higher the intensity of WGA staining, the lower the likelihood of AL IP3Rs initiating Ca2+ waves in response to IP3 (Fig. 4Aa, Supplemental Movie 7). Even in a single cell, at the same time point, where AL in different parts of the vegetal hemisphere could be resolved at different stages of NPC disassembly, individual NPC-depleted AL were more active than AL decorated with NPCs (Fig. 4Ab).
Figure 4. NPC disassembly relieves inhibition of IP3Rs within AL.
Aa, AL population measurement showing correlation between NPC presence on AL (ER: WGA ratio, i.e. value increases with NPC dissociation) and probability of Ca2+ wave initiation (black = no wave; red = wave for individual measurements). Filled squares depict population average. Ab, single time point data from a single cell, correlating NPC density on different AL (ER: WGA ratio) throughout the ooplasm with Ca2+ signalling phenotype. Top, and Supplemental Movie 7, example of WGA-depleted AL (DsRed2-ER, greyscale) initiating a Ca2+ wave (pseudocolour) seen at time points ∼500 ms apart. B, temporal correlation between ER: WGA ratio (black squares, left ordinate) and Ca2+ wave initiation from AL pooled over different time bins after progesterone addition. Horizontal lines (red) represent overlapping time windows (x-axis), vertical lines (blue) represent error bars for Ca2+ measurements (right ordinate). C, representative image stills showing distribution of WGA (blue), DsRed2-ER (red) and Ca2+ puffs (green) from two different AL captured during NPC dissociation. Composite three-channel overlay is shown at right. Scale bar = 5 μm D, population measurements of Ca2+ puff localization on AL during NPC dissociation revealed that the majority of Ca2+ puffs localized (‘X’) to regions of AL showing lower staining with WGA. Horizontal axis represents normalized WGA fluorescence within the AL encompassing the gradient of WGA fluorescence intensities from ‘0’ (lowest WGA) to ‘1’ (maximal WGA intensity).
Second, we collated the activity of IP3Rs within AL subdomains over different time periods during maturation and compared the time course of relief of IP3R inhibition with the time course of NPC disassembly. A strong temporal correlation was observed between the abrupt change in NPC integrity (ER: WGA ratio) and the capacity of AL to nucleate Ca2+ waves (Fig. 4B). In summary, both spatial (Fig. 4A) and temporal (Fig. 4B) macroscopic datasets indicate that the presence of intact NPCs on AL correlates with decreased local IP3R activity.
Using video-rate confocal microscopy, Ca2+ puffs were mapped at low levels of stimulation and their localization correlated with AL morphology during the period of NPC disassembly. To analyse these triple colour imaging experiments, composite overlays of NPC distribution throughout AL were generated to assess the location of active IP3R clusters relative to NPC distribution (Fig. 4C). Visual inspection of these records demonstrated that Ca2+ puffs within AL first appeared in regions free from NPCs (Fig. 4C), with NPC-replete regions of the same ER structure remaining devoid of Ca2+ puff activity. Such observations were quantified by collating the WGA fluorescence intensity at Ca2+ puff sites relative to the range of WGA fluorescence values observed throughout the AL at the same time point (Fig. 4D). These data show that sites of Ca2+ puff activity occurred within regions of a single AL with low NPC occupancy (Fig. 4D), again suggesting that NPC dissociation indirectly/directly facilitated a gain-of-functionality transition in Ca2+ signalling competency of IP3Rs.
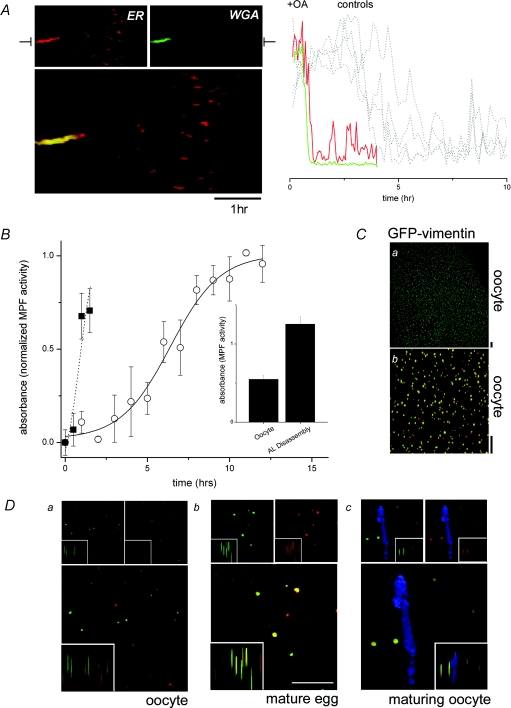
What triggers NPC dissociation from AL? Injection of okadaic acid in the absence of progesterone, a protein phosphatase 1 and 2A inhibitor, caused rapid AL disassembly and decreased the duration of phase 1 (‘AL persistence’) from 4.3 ± 1.5 h to ≤ 1 h (Fig. 5A). Consistent with models for regulation of NPC (dis)assembly in the nuclear envelope, these data implicate a kinase/phosphatase cycle controlling AL remodelling (Onischenko et al. 2005). A likely candidate kinase is the maturation promoting factor (MPF) kinase (a cyclin B and cdc2/cdk1 heterodimer), given its role in maturation and as an in vitro effector of NPC disassembly at nuclear envelope breakdown (NEBD) (Favreau et al. 1996; Onischenko et al. 2005; Muhlhausser & Kutay, 2007). Indeed, whole-cell kinase assays demonstrated that the time course of MPF activation correlated well with AL remodelling (Fig. 5B). Moreover, measurements of MPF activity in single cells performed when NPCs were observed to be dissociating from AL (by live cell imaging) were higher than in cells where AL were static and NPCs were intact (Fig. 5B, inset). Finally, okadaic acid caused a rapid increase in MPF activity (Fig. 5B), mimicking the accelerated time course of AL disassembly (Fig. 5A).
Figure 5. MPF is a candidate kinase for AL disassembly.
A, okadaic acid (OA) triggers rapid remodelling of AL. Left, lamellogram showing rapid AL disappearance in an OA-treated cell. Right, associated fluorescence profiles taken from the indicated lamellogram section showing the time course of ER (DsRed2-ER, red) and NPC (WGA, green) disappearance compared with DsRed2-ER profiles from four maturing, parallel controls not exposed to OA (multiple dashed lines). B, MPF kinase assay (A490, normalized to levels observed in mature eggs) in maturing oocytes (○) and oocytes treated with OA alone (▪). Inset, relative levels of MPF activity (A490) in oocytes and maturing oocytes in which AL were visibly remodelling, assessed by live cell imaging of NPC dissociation. Ca, confocal (xy) image of an oocyte expressing GFP-vimentin (Supplemental Movie 8). Cb, fluorescence overlay of confocal images taken at t= 0 (red) and t= 2 s (green) highlight considerable motility of some of the GFP-vimentin puncta. Scale bars = 10 μm. D, immunofluorescence staining of phospho-vimentin (red, top right), compared with GFP-vimentin (green, top left) by dual colour overlay (bottom) in both lateral (‘xy’) and 3-D projection (insets). Representative images are shown from an oocyte (a), an egg (b) and from the vicinity of an AL (WGA, blue) in a maturing cell (c). Scale bar = 10 μm.
These data imply that MPF activation parallels the timeframe of AL disappearance, and that the kinase is ‘globally’ active in cells where AL remodelling is occurring, but do not demonstrate that MPF is active in the local vicinity of AL prior to NPC dissociation, as expected if this kinase was a key trigger for NPC dissociation in vivo. Therefore, to monitor endogenous MPF activity, we expressed a GFP-tagged vimentin, a substrate for MPF that targets the oocyte cortex (Dent et al. 1992). Vimentin is phosphorylated by MPF at a site in the NH2-terminus (Ser-55), the phosphorylation status of which can be resolved using phospho-specific antibodies. Therefore, by monitoring the co-localization of punctate GFP-vimentin fluorescence with phosphorylation-dependent immunoreactivity, it was possible to identify when, and where, endogenous MPF became active. Figure 5C shows the punctate profile and dynamics of vimentin-GFP in the periphery of a live oocyte (Supplemental Movie 8). After immunostaining, little co-localization of GFP fluorescence with phospho-antibody staining was observed in oocytes (Fig. 5Da), in contrast to a strong fluorescence overlap observed in eggs (Fig. 5Db), as expected if the assay correctly reports MPF activity. Analysis of fluorescence staining in oocytes (fixed at ∼15 min intervals during maturation), at the time point immediately preceding observation of NPC dissociation in live cells, revealed local phosphorylation of vimentin epitope within the vicinity of AL (Fig. 5D). Such images suggest that MPF is active locally in the maturing oocyte cortex, immediately prior to AL remodelling.
How does the presence of NPCs regulate local IP3R activity in AL? Both direct and indirect mechanisms are plausible: direct mechanisms would rely on structural or functional interactions between NPCs and IP3Rs; indirect regulation would depend upon the architectural effects of ER membranes themselves (e.g. membrane curvature; Botelho et al. 2006), effects of IP3R clustering on IP3R function, or (in)accessibility of sensitizing/inhibitory regulators. Distinguishing these possibilities in the context of AL organization in the intact cell is not straightforward. Two pieces of experimental evidence, however, lend weight to indirect architectural regulation of IP3Rs within AL.
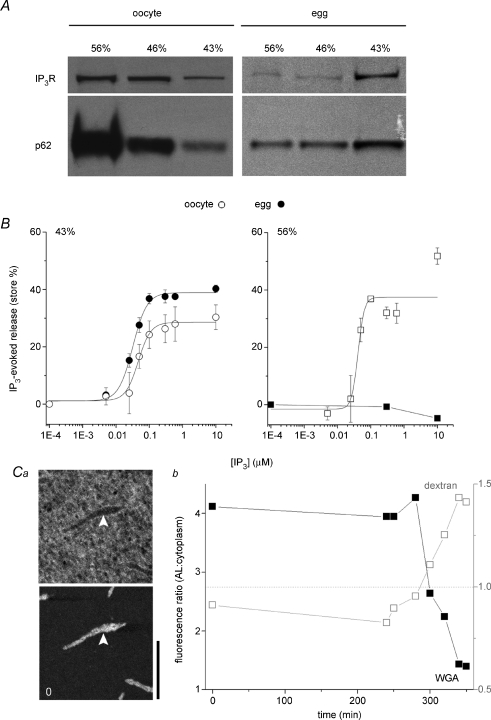
First, there is no fundamental difference in IP3R sensitivity between ER- and AL-resident IP3Rs when intact cell architecture is disrupted by homogenization. To test IP3R functionality within AL, we optimized a homogenization and fractionation protocol to isolate AL-derived membrane vesicles (marked by enrichment of the nucleoporin p62) using discontinuous sucrose gradient fractionation (Meier et al. 1995). In oocytes, an AL-derived vesicle population, shown by immunoblotting to be enriched in p62 and IP3Rs was isolated in the ‘heavy’ (56%) fraction (Fig. 6A). Notably, the immunoreactivity of this fraction decreased in eggs (Fig. 6A), consistent with the observed disassembly of AL during meiotic maturation (Boulware & Marchant, 2005). In parallel, IP3R immunoreactivity was transferred into the lighter (43%), cortical ER fraction recapitulating the IP3R redistribution from AL into cortical ER patches observed in live cells (Fig. 3). IP3 responsiveness of the relevant vesicle populations was determined in 45Ca2+ efflux assays (Fig. 6B). As expected, the light (43%) cortical ER fractions derived from whole eggs and oocytes both released Ca2+ in response to IP3 (EC50 32 ± 2 nm and 47 ± 13 nm, respectively, n = 3) with a larger observed store size in eggs than oocytes (38.9 ± 3.4% and 28.5 ± 8.0%, n = 6). Noticeably, the AL fraction from oocytes (56%) also released Ca2+ in response to IP3 over a similar concentration range (EC50 of 41 ± 6 nm) to that seen in cortical ER fractions. These results suggest that there is no fundamental difference in IP3 sensitivity between these ER fractions that is bridged, for example, by a gain-of-function phosphorylation or loss of an inhibitory factor during maturation. AL in oocytes do contain IP3Rs capable of activation and their activity is more likely attenuated by architectural considerations within intact AL that are disrupted by homogenization, or physiologically reorganized during maturation by MPF-triggered disassembly of NPCs (Figs 2, 4, 5 and 6). No Ca2+ release was observed from the AL (56%) fraction in eggs (n = 1) indicating that the Ca2+ release in the oocyte fraction was not an artifact of contaminating ER (Fig. 6B).
Figure 6. Structural inhibition of IP3Rs in AL.
A, immature oocytes were homogenized and fractionated through a discontinuous sucrose gradient to yield ‘heavy’ vesicles derived from AL (56%), a population of cortical ER vesicles (43%) and an intermediate mixed vesicular population (46%). The same protocol was utilized on naturally shed eggs. Equal amounts of protein from each fraction were subjected to immunoblotting with anti-IP3R or anti-p62 antibodies. B, left, IP3 evoked 45Ca2+ release from the light membrane fraction (43%) extracted from oocytes (○) or eggs (•). Right, IP3 evoked 45Ca2+ release from the heaviest membrane fraction (AL) extracted from oocytes (○) or eggs (•). C, altered membrane architecture of AL following NPC dissociation: a, image stills showing distribution of a 70 kDa fluorescent dextran (top) and a fluorescently tagged WGA conjugate (bottom) with respect to the same AL (white arrows) before maturation (t= 0 min, scale bar = 20 μm); b, ratio of fluorescence of WGA (▪, left ordinate) and dextran fluorescence (□, right ordinate) labelling of AL relative to randomly chosen surrounding cytoplasmic area at the indicated time points during maturation (minutes). Values below dashed grey line (ratio = 1, right ordinate) indicates exclusion from AL.
Second, a significant architectural reorganization of ER membranes ensues after NPC dissociation, as evidenced by a permeability transition to high molecular weight dextrans previously excluded from AL. AL membranes in oocytes did not pose a barrier to ∼10 kDa dextran conjugates. However, several types of 70 kDa fluorescently tagged dextran molecules were initially excluded from AL when injected alone into oocytes. During maturation, the permeability of AL to 70 kDa dextrans increased (Fig. 6C, Supplemental Fig. 2) consistent with a morphological reorganization associated with NPC dissociation. Therefore, both the 45Ca2+ flux and morphological data support the general concept of indirect attenuation of IP3R activity, relieved by nucleoporin disassembly.
Discussion
Understanding ER heterogeneity is important: the overall functionality of an organelle integrates the properties and prevalence of the subdomains existing within a cell at any given time. In Xenopus oocytes, the vast majority of NPCs (> 80%) are not found as would be expected within the nuclear envelope, but in understudied specializations of the rough ER known as annulate lamellae (Cordes et al. 1995). Here, we discuss three issues relevant to AL: What is their cellular role? How do they assemble and disassemble? How is IP3R activity locally attenuated within AL?
A new role for annulate lamellae
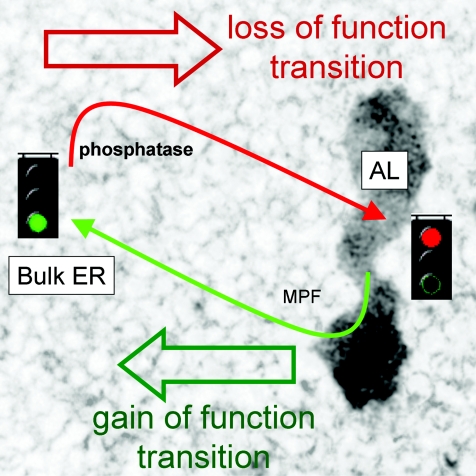
Over the years, a variety of roles have been ascribed to AL (Kessel, 1992) with the prevalent consensus being that AL are stockpiles of excess nucleoporins to support subsequent, rapid cell divisions. However, careful quantification of AL number and nucleoporin localization during proliferation in early Drosophila embryos challenged this idea (Onischenko et al. 2004). Our data also suggest a wider role for AL beyond solely nucleoporin ‘warehousing’ that stresses the functional consequences of ER protein residency within these subdomains. We propose that targeting of ER proteins into AL may attenuate their functionality, suppressing their involvement in cellular events up to the point when AL are remodelled and AL-resident proteins rejoin the bulk ER/cytosol (Fig. 7). Therefore, the genesis of AL provides a cell with a chronic mechanism (≥ hours) for regulating ER protein activity, by localizing proteins within these discrete ER subdomains where their activity is suppressed. Scenarios of AL biogenesis would then encompass preparative, physiological adaptations to support rapid, ‘gain-of-function’ transitions in cellular behaviour on AL remodelling, but also as a way of minimizing the impact of proteins that may impair ER functionality. In this context, it is noteworthy that several studies report increased numbers of AL (≤ 3-fold) in virally infected cells, consistent with targeting of viral glycoproteins into these ER subdomains (Kessel, 1992; Cardinali et al. 1998).
Figure 7. Proposed role for AL in regulating ER protein activity.
Hypothesized role for AL assembly/disassembly in regulating the activity of ER resident proteins. ER proteins resident within AL (right) display attenuated function compared to their properties when resident within the bulk ER (left). Therefore regulated assembly/disassembly of AL, effected via a kinase (probably MPF)/phosphatase cycle, is associated with a loss (red)/gain (green) of function transition in protein activity. Regulated AL disassembly provides a mechanism for physiological ‘gain-of-function’ transitions, and pathologically AL assembly is probably an adaptation to sequester proteins when exogenous protein overload (e.g. viral infection) may impair ER functionality.
If this idea is correct, what is the physiological rationale for compartmentalization of proteins shown to localize to AL (IP3Rs, NPCs, cyclin-B2 and viral glycoproteins)? For IP3Rs, the transient, gain-of-function of IP3Rs released from AL in the vegetal hemisphere during oocyte maturation probably facilitates propagation of the fertilization Ca2+ wave (Boulware & Marchant, 2005). For NPCs, although presence in AL is futile in terms of transport (mediating cytoplasmic-to-cytoplasmic transfer), NPCs are the keystones that coordinate AL formation. Further, NPC compartmentalization provides a rapidly mobilizable pool of NPCs to impact nucleocytoplasmic transport and gene expression when integrated back into the nuclear envelope. Future studies of NPC density, a parameter that varies with cellular metabolic activity (Hetzer et al. 2005), to address whether increases in NPC density can facilitate gene transcription and mRNA export during transcriptional activation of the zygotic genome are warranted. Similarly, correlation between changes in nucleocytoplasmic transport observed in transformed, cancerous cells (Poon & Jans, 2005) and increases in NPC compartmentalization within AL merits investigation. Finally, the targeting of cyclin-B2 (as a pre-MPF component) to AL (Beckhelling et al. 2003) probably facilitates localized activation and release of a bolus of MPF when cued (Beckhelling et al. 2003), but may also prevent inappropriate activation of cdc2 by segregation of pre-MPF into an ER domain where Ca2+ signalling is attenuated.
Mechanisms of AL (dis)assembly
The NPC is a large, complex structure. More than 100 proteins must be recruited from the cytoplasm (multiple copies of individual nucleoporins) to assemble a single NPC. At 125 MDa, it occupies a considerably larger volume than AL-contained ribosomes or IP3Rs (∼35-fold and ∼110-fold smaller volumes, respectively). There are few consistently reported differences in NPC composition between NE and AL (Cordes et al. 1997; Miller & Forbes, 2000), although AL lack nuclear lamina, matrix and chromatin-associated proteins. Structurally, AL comprise an interconnected stack of ER membranes, with individual cisternae separated by ∼100 nm (Cordes et al. 1995). Ultrastructural analyses resolve that the intercisternal gap is bridged by NPCs, implying that AL formation is mediated by NPC interactions (Kessel, 1992; Meier et al. 1995). Our in vivo imaging data showing that NPC presence is essential for AL integrity supports this model (Fig. 2). Consequently, the structural organization of AL by NPCs imparts a regulable cycle for AL (dis)assembly (Fig. 7), contrasting with the variety of organized smooth ER structures formed by low-affinity interactions between abundant, or overexpressed, ER proteins (Snapp et al. 2003), which lack a unifying mechanism to rapidly reverse their formation.
While reversible phosphorylation of a subset of NPC components has long been implicated in the mitotic disassembly of nuclear envelope NPCs, the identity of the relevant in vivo kinases (cdk1, casein kinase, PKA and GSK3α all phosphorylate specific nucleoporins in vitro), as well as the sites phosphorylated by particular kinases, is surprisingly sparse. Strong in vivo evidence for a cdk1/phosphatase cycle in regulating AL NPC (dis)assembly derives from an elegant study showing that NPC assembly/localization depends on cdk1 activity, altered experimentally by temperature shifts using a conditional cdk1tsDrosophila mutant (Onischenko et al. 2005). These data are consistent with our demonstration of localized MPF activity in the subcortex of maturing cells when and where AL are remodelling (Fig. 5), the inhibitory action of local elevations of Ca2+ on the initiation of AL remodelling (data not shown), together with the rapid inhibition of AL perdurance after okadaic acid injection (Fig. 5).
Finally, in addition to NPC phosphorylation, cytoskeletal-dependent ‘shearing’ events facilitate NEBD. In mammalian somatic cells, evidence supports a role for microtubules and microtubule-associated motors (Beaudouin et al. 2002; Salina et al. 2002). In contrast, our data implicate microfilament-critical steps in AL remodelling (Fig. 3; Muhlhausser & Kutay, 2007). Microfilament disruption inhibited the morphological rearrangements that accompany both AL sinking and AL disaggregation into smaller, cortical ER densities. Whether this difference reflects specialized roles for microtubules and microfilaments in NEBD and AL remodelling, or simply differences in the role of cytoskeletal filaments between mammalian and non-mammalian systems remains to be established.
IP3R functionality within AL
We believe decreased IP3R activity within AL is most likely an indirect consequence of the architectural role of NPCs in forming this ER subdomain, rather than any direct effects of NPCs on IP3R. A speculative mechanism may relate to changes in optimal IP3R architecture enforced by spatial constraints on IP3R clustering resulting from the high density of NPCs and IP3Rs within intact AL (40–70 NPCs μm−2 in AL; Cordes et al. 1995; Hoppener et al. 2005). The elementary, functional unit of IP3-dependent local Ca2+ signalling in intact cells is the ‘Ca2+ puff’ (Parker et al. 1996; Bootman et al. 1997), an activity-dependent measure of Ca2+ channel recruitment within an IP3R ‘cluster’. In the absence of structural data, the underlying IP3R architecture is inferred from confocal linescan images of Ca2+ release profiles, interpreted as being consistent with coordinated opening of ∼30 (25–35) IP3Rs within a region ∼550 nm (300–800 nm) wide (Shuai et al. 2006). Therefore, the optimal IP3R ‘cluster’ producing Ca2+ puffs in the bulk ER appears (at least from functional measurements) to be a loosely corralled unit (mean separation between active IP3Rs of ∼80 nm, a distance ∼4-fold greater than the lateral dimension of a tetrameric IP3R; Taylor et al. 2004). Modelling predictions that support the ‘diffuse’ organization of active IP3Rs within Ca2+ puffs (Shuai et al. 2006) are consistent with microscopic Ca2+ release patterns within individual Ca2+ puff sites (Bootman et al. 1997; Demuro & Parker, 2008). If such an organization of IP3Rs is a prerequisite for observation of local IP3R activity, then attenuated responsiveness of IP3Rs in AL may simply result from an inability to adopt this favoured distribution. Higher IP3R density, and increased IP3R proximity enforced by NPC packing (free space between NPCs of only ∼50 nm in AL) may preclude organization of IP3Rs into architectures optimal for local Ca2+ signalling – perhaps via modulation of local Ca2+ feedback effects (Means et al. 2006) or direct conformational uncoupling – until NPC disassembly has occurred (Fig. 4). Consistent with this theme, others have speculated that the high density packing of IP3Rs observed in cerebellar Purkinje cells inhibits IP3R function (Satoh et al. 1990; Katayama et al. 1996). Smooth ER cisternal stacks/vesicles (containing IP3Rs at ∼10-fold the density of adjacent rER, Satoh et al. 1990; or as arrays of IP3Rs where individual channels physically contact each other, Katayama et al. 1996) increase rapidly in hypoxic scenarios, possibly as an adaptation to decrease ER Ca2+ efflux (Satoh et al. 1990; Takei et al. 1994; Katayama et al. 1996). Such a pool of high density, attenuated IP3Rs is consistent with the low IP3 sensitivity of intact Purkinje neurons (tens of micromolar; Khodakhah & Ogden, 1993). Similarly, higher-order IP3R ‘aggregations’ (those visible by fluorescence microscopy) seen in the ER after maintained IP3R stimulation, are associated with suppression of whole-cell Ca2+ signals (Wilson et al. 1998; Tateishi et al. 2005; Chalmers et al. 2006; Means et al. 2006). None-the-less, further studies of AL organization and composition would be needed to better understand the regulatory environment of IP3Rs in AL. Although the morphological continuity between the ER membranes often leads to the assumption of equivalency, the composition of different ER regions is divergent, a finding underscored by large-scale, subtractive proteomics (Schirmer et al. 2003).
We conclude that relief of IP3R inhibition is correlated with the physiological dismantling of NPCs from the same, local regions of ER. Physiological disassembly of NPCs during oocyte maturation, effected via targeted phosphorylation of nucleoporins by maturation kinases, notably MPF, yields NPC-denuded ER domains that we show via multicolour imaging experiments correlate spatially and temporally with increased IP3R activity.
Acknowledgments
This work was supported by NIH (to J.S.M., grant no. NS046783) and a NSF CAREER Fellowship (to J.S.M., grant no. 0237946).
Supplementary material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.153379/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2008.153379
References
- Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- Beckhelling C, Chang P, Chevalier S, Ford C, Houliston E. Pre-M phase-promoting factor associates with annulate lamellae in Xenopus oocytes and egg extracts. Mol Biol Cell. 2003;14:1125–1137. doi: 10.1091/mbc.E02-08-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Niggli E, Berridge MJ, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Francolini M, Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr Opin Cell Biol. 2006;18:358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho AV, Huber T, Sakmar TP, Brown MF. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys J. 2006;91:4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MJ, Marchant JS. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr Biol. 2005;15:765–770. doi: 10.1016/j.cub.2005.02.065. [DOI] [PubMed] [Google Scholar]
- Callamaras N, Parker I. Construction of a confocal microscope for real-time x-y and x-z imaging. Cell Calcium. 1999;26:271–279. doi: 10.1054/ceca.1999.0085. [DOI] [PubMed] [Google Scholar]
- Cardinali G, Gentile M, Cirone M, Zompetta C, Frati L, Faggioni A, Torrisi MR. Viral glycoproteins accumulate in newly formed annulate lamellae following infection of lymphoid cells by human herpes virus 6. J Virol. 1998;72:9738–3976. doi: 10.1128/jvi.72.12.9738-9746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers P, Schell MJ, Thorn P. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem J. 2006;394:57–66. doi: 10.1042/BJ20051130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Franke WW. High content of a nuclear pore complex protein in cytoplasmic annulate lamellae of Xenopus oocytes. Eur J Cell Biol. 1995;68:240–255. [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Rackwitz HR, Franke WW. Identification of protein p270/Tpr as a constitutive component of the nuclear pore complex-attached intranuclear filaments. J Cell Biol. 1997;136:515–529. doi: 10.1083/jcb.136.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Parker I. Multi-dimensional resolution of elementary Ca2+ signals by simultaneous multi-focal imaging. Cell Calcium. 2008;43:367–374. doi: 10.1016/j.ceca.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA, Cary RB, Bachant JB, Domingo A, Klymkowsky MW. Host cell factors controlling vimentin organization in the Xenopus oocyte. J Cell Biol. 1992;119:855–866. doi: 10.1083/jcb.119.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Newmeyer DD, Price TM, Forbes DJ. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function and dynamics of the nuclear periphery. Ann Rev Cell Dev Biol. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- Hoppener C, Siebrasse JP, Peters R, Kubitscheck U, Naber A. High-resolution near-field optical imaging of single nuclear pore complexes under physiological conditions. Biophys J. 2005;88:3681–3688. doi: 10.1529/biophysj.104.051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama E, Funahashi H, Michikawa T, Shiraishi T, Ikemoto T, Iino M, Hirosawa K, Mikoshiba K. Native structure and arrangement of inositol-1,4,5-trisphosphate receptor molecules in bovine cerebellar Purkinje cells as studied by quick-freeze deep-etch electron microscopy. EMBO J. 1996;15:4844–4851. [PMC free article] [PubMed] [Google Scholar]
- Kessel RG. Annulate lamellae: a last frontier in cellular organelles. Int Rev Cytol. 1992;133:43–120. doi: 10.1016/s0074-7696(08)61858-6. [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci U S A. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievremont J-P, Hill A-M, Hilly M, Mauger J-P. The inositol 1,4,5-trisphosphate receptor is localized on specialized sub-regions of the endoplasmic reticulum in rat liver. Biochem J. 1994;300:419–427. doi: 10.1042/bj3000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim LM, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+ store depletion triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means S, Smith AJ, Shepherd J, Shadid J, Fowler J, Wojcikiewicz RJH, Mazel T, Smith GD, Wilson BS. Reaction diffusion modeling of calcium dynamics with realistic ER geometry. Biophys J. 2006;91:537–557. doi: 10.1529/biophysj.105.075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E, Miller BR, Forbes DJ. Nuclear pore complex assembly studied with a biochemical assay for annulate lamellae formation. J Cell Biol. 1995;129:1459–1472. doi: 10.1083/jcb.129.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The heterogeneity of ER Ca2+ stores has a key role in non-muscle cell signaling and function. J Cell Biol. 1998;142:1395–1398. doi: 10.1083/jcb.142.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Forbes DJ. Purification of the vertebrate nuclear pore complex by biochemical criteria. Traffic. 2000;1:941–951. [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Powers M, Park M, Fischer W, Forbes DJ. Identification of a new vertebrate nucleoporin, Nup188, with the use of a novel organelle trap assay. Mol Biol Cell. 2000;11:3381–3396. doi: 10.1091/mbc.11.10.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Nakano K, Tepikin AV, Petersen OH. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell. 1997;88:49–55. doi: 10.1016/s0092-8674(00)81857-7. [DOI] [PubMed] [Google Scholar]
- Muhlhausser P, Kutay U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J Cell Biol. 2007;178:595–610. doi: 10.1083/jcb.200703002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onischenko EA, Gubanova NV, Kieselbach T, Kiseleva EV, Hallberg E. Annulate lamellae play only a minor role in the storage of excess nucleoporins in Drosophila embryos. Traffic. 2004;5:152–164. doi: 10.1111/j.1600-0854.2004.0166.x. [DOI] [PubMed] [Google Scholar]
- Onischenko EA, Gubanova NV, Kiseleva EV, Hallberg E. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol Biol Cell. 2005;11:5152–5162. doi: 10.1091/mbc.E05-07-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp S, Dziak E, Michalak M, Opas M. Is all of the endoplasmic reticulum created equal? The effects of the heterogeneous distribution of endoplasmic reticulum Ca2+-handling proteins. J Cell Biol. 2003;160:475–479. doi: 10.1083/jcb.200207136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- Poon IKH, Jans DA. Regulation of nuclear transport: central role in development and transformation. Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Rooney A, Meldolesi J. The endoplasmic reticulum in PC12 cells. J Biol Chem. 1996;271:29304–29311. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold reveals concentration in an ER subcompartment. J Cell Biol. 1990;111:615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Florens L, Guan T, Yates JR, III, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- Shuai J, Rose HJ, Parker I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4033–4044. doi: 10.1529/biophysj.106.088880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, Borgese N, Lippincott-Schwartz J. Formation of stacked ER cisternae by low affinity protein interactions. J Cell Biol. 2003;163:257–269. doi: 10.1083/jcb.200306020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffler D, Fahrenkrog B, Aebi U. The nuclear pro complex: from molecular architecture to functional dynamics. Curr Opin Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Simerly C, Hewitson L, Schatten G. Assembly of nuclear pore complexes and annulate lamellae promotes normal pronuclear development in fertilized mammalian oocytes. J Cell Sci. 1998;111:2841–2854. doi: 10.1242/jcs.111.19.2841. [DOI] [PubMed] [Google Scholar]
- Takei K, Mignery GA, Mugnaini E, Südhof TC, De Camilli P. Inositol 1,4,5-trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells. Neuron. 1994;12:327–342. doi: 10.1016/0896-6273(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Tateishi Y, Hattori M, Nakayama T, Iwai M, Bannai H, Nakamura T, Michikawa T, Inoue T, Mikoshiba K. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J Biol Chem. 2005;280:6816–6822. doi: 10.1074/jbc.M405469200. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Da Fonseca PCA, Morris EP. IP3 receptors: the search for structure. Trends Biochem Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991;114:929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Smith AJ, Oliver JM, Oberdorf JA, Wojcikiewicz RJH. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol Biol Cell. 1998;9:1465–1478. doi: 10.1091/mbc.9.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.