Abstract
A novel E41K β-tropomyosin (β-Tm) mutation, associated with congenital myopathy and muscle weakness, was recently identified in a woman and her daughter. In both patients, muscle weakness was coupled with muscle fibre atrophy. It remains unknown, however, whether the E41K β-Tm mutation directly affects regulation of muscle contraction, contributing to the muscle weakness. To address this question, we studied a broad range of contractile characteristics in skinned muscle fibres from the two patients and eight healthy controls. Results showed decreases (i) in speed of contraction at saturated Ca2+ concentration (apparent rate constant of force redevelopment (ktr) and unloaded shortening speed (V0)); and (ii) in contraction sensitivity to Ca2+ concentration, in fibres from patients compared with controls, suggesting that the mutation has a negative effect on contractile function, contributing to the muscle weakness. To investigate whether these negative impacts are reversible, we exposed skinned muscle fibres to the Ca2+ sensitizer EMD 57033. In fibres from patients, 30 μm of EMD 57033 (i) had no effect on speed of contraction (ktr and V0) at saturated Ca2+ concentration but (ii) increased Ca2+ sensitivity of contraction, suggesting a potential therapeutic approach in patients carrying the E41K β-Tm mutation.
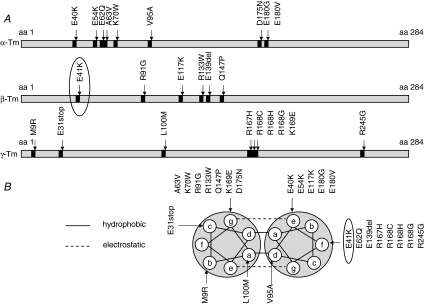
Tropomyosin (Tm) is expressed in all cardiac and skeletal muscle cells. In adult humans there are three major Tm isoforms, α-Tm, β-Tm and γ-Tm, which are encoded by the TPM1, TPM2 and TPM3 genes, respectively (Perry, 2001). Numerous mutations have been reported in these genes (Fig. 1). Mutations in the TPM1 gene, which encodes the α-Tm isoform, are associated with cardiomyopathies (Thierfelder et al. 1994; Nakajima-Taniguchi et al. 1995; Yamauchi-Takihara et al. 1996; Regitz-Zagrosek et al. 2000; Karibe et al. 2001; Olson et al. 2001; Jongbloed et al. 2003). Mutations in the TPM2 gene, which encodes the β-Tm isoform, are associated with skeletal myopathies such as nemaline myopathy (Donner et al. 2002), distal arthrogryposis (Sung et al. 2003; Tajsharghi et al. 2007a) and cap disease (Lehtokari et al. 2007). Mutations in the TPM3 gene, which encodes the γ-Tm isoform, are associated with nemaline myopathy (Laing et al. 1995; Tan et al. 1999; Penisson-Besnier et al. 2007) and congenital fibre type disproportion (Clarke et al. 2008). Mutations in the TPM2 or TPM3 genes are also usually accompanied by muscle weakness. However, multiple mechanisms underlie this phenomenon and appear to be mutation specific (Bottinelli et al. 1998; Michele et al. 1999a,b; Karibe et al. 2001; Heller et al. 2003; Mirza et al. 2005; Ochala et al. 2007; Robinson et al. 2007).
Figure 1. Tm mutations.
A, position of Tm mutations in the primary structures of α-, β- and γ-Tm. B, position of Tm mutations in the heptad repeat of α-, β- and γ-Tm.
A novel β-Tm mutation (E41K) located on the TPM2 gene was recently identified in a woman and her daughter (Tajsharghi et al. 2007b). This mutation was associated with muscle weakness, nemaline myopathy and cap disease (Tajsharghi et al. 2007b). In both patients there were signs of muscle fibre atrophy, but it is not known whether the atrophy is solely responsible for the muscle weakness or whether the E41K β-Tm mutation induces additional alterations in the regulation of contraction. It is well documented that Tm plays an integral role in muscle contraction, as it ‘relays’ information from the Ca2+ sensor, the troponin (Tn) ternary complex, to the actin–myosin interactions, i.e. the cross-bridges. However, Tm is believed to be involved in the regulation of contraction not only by modulating the propagation of Ca2+ signals along the thin filament, i.e. by controlling Ca2+ sensitivity (Brandt et al. 1987; Schachat et al. 1987), but also by modulating cross-bridge cycle kinetics, i.e. by altering the rate of movement of myosin from an attached non-force-generating state to states that generate force (Homsher et al. 2003). We hypothesized that the E41K β-Tm mutation has significant effects on (i) the speed of contraction and (ii) the Ca2+ sensitivity of contraction. To verify these effects, we have used skinned muscle fibre preparations and a single muscle fibre in vitro motility assay to study the regulation of muscle contraction in the two patients carrying the E41K β-Tm mutation and in eight healthy controls. Different contractile parameters have been assessed, including force and stiffness in solutions with varying Ca2+ concentrations (pCa), allowing construction of relative force–pCa and relative stiffness–pCa relationships, maximal Ca2+-activated force normalized to fibre cross-sectional area (CSA), stiffness, the apparent rate constant of force redevelopment (ktr), and maximum unloaded shortening velocity (V0) at saturated Ca2+ concentration. Significant (i) decreases in ktr and V0 and (ii) rightward shifts of the relative force–pCa and relative stiffness–pCa curves were observed in fibres from patients compared with controls. The in vitro motility speed of unregulated actin did not differ between fibres carrying the E41K β-Tm mutation and control fibres, indicating that the altered ktr and V0 were not related to a modified myosin function. Therefore, the E41K β-Tm mutation directly alters regulation of muscle contraction, contributing to the muscle weakness experienced by the patients.
We have also tested the reversibility of the strong negative effects of the mutation on the speed of contraction and Ca2+ sensitivity by using the Ca2+ sensitizer EMD 57033. This has previously been shown (i) to modulate ktr (Lipscomb et al. 2005) and (ii) to increase the sensitivity of force to Ca2+ concentration (Solaro et al. 1993; Kraft & Brenner, 1997; Vannier et al. 1997; Lipscomb et al. 2005). Exposure to EMD 57033 reversed the negative effects of the E41K β-Tm mutation on the relative force–pCa and relative stiffness–pCa relationships. The Ca2+ sensitizer is proposed as a potential therapeutic strategy in patients carrying the E41K β-Tm mutation, but this needs to be tested in vivo in animals carrying this mutation.
Methods
Subjects
The study was conducted on two patients with congenital myopathy, i.e. a woman, aged 66 years, diagnosed with nemaline myopathy and her daughter, aged 35 years, diagnosed with cap disease. An E41K β-Tm mutation on exon 2 was found in both patients. The mutation was not found in healthy family members. Both patients had neonatal onset of muscle symptoms, delayed motor milestones and a slowly progressive muscle weakness. At the time of the investigation, they had weakness in both proximal and distal muscles. The mother was still ambulant but the daughter had difficulties in walking and used a wheelchair. They both received ventilatory support at night due to nocturnal hypoventilation. There were no signs of cardiac involvement and ECG and echocardiography were normal. Clinical history, muscle morphology and genotype analysis of the two patients have been described in detail elsewhere (Tajsharghi et al. 2007b). Eight healthy men and women (26–67 year), with no history of neuromuscular disease, served as controls. Informed consent was obtained from the patients and control subjects enrolled in the present study. The ethics committee at Gothenburg University approved the protocol and the experiments were carried out according to the guidelines of the Declaration of Helsinki.
Muscle biopsies and muscle fibre membrane permeabilization
Open muscle biopsies of the tibialis anterior or deltoid muscle were performed in patients (one biopsy per patient), under local anaesthesia. Fresh percutaneous conchotome muscle biopsy specimens were obtained from controls' tibialis anterior or vastus lateralis muscles, under local anaesthesia. The biopsy specimens were dissected into two parts. One part was frozen in liquid nitrogen–chilled propane and stored at –80°C. The other was placed in relaxing solution at 4°C, and bundles of ∼50 fibres were dissected free and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. The muscle bundles were then treated with skinning solution (relaxing solution containing glycerol, 50% v/v) for 24 h at 4°C, after which they were transferred to –20°C. The muscle bundles were treated with sucrose, a cryo-protectant, within 1–2 weeks for long-term storage (Frontera & Larsson, 1997). Muscle bundles were detached from the capillary tubes and snap frozen in liquid nitrogen–chilled propane and stored at –160°C.
Single muscle fibre experimental procedure
On the day of an experiment, a fibre segment 1–2 mm long was left exposed to the experimental solution between connectors leading to a force transducer (model 400A, Aurora Scientific) and a lever arm system (model 308B, Aurora Scientific) (Moss, 1979). The total compliance of the attachment system was carefully checked and remained similar for all the single muscle fibres tested (5 ± 0.5% of the fibre length). The apparatus was mounted on the stage of an inverted microscope (model IX70; Olympus). While the fibre segments were in relaxing solution, the sarcomere length was set to 2.75–2.85 μm by adjusting the overall segment length (Larsson & Moss, 1993). The diameter of the fibre segment between the connectors was measured through the microscope at a magnification of ×320 with an image analysis system prior to the mechanical experiments. Fibre depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fibre. The focusing control of the microscope was used as a micrometer. CSA was calculated from the diameter and depth, assuming an elliptical circumference, and was corrected for the 20% swelling that is known to occur during skinning (Moss, 1979).
Relaxing and activating solutions contained (mm): 4 Mg-ATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate, and KCl to adjust the ionic strength to 180 mm. The pH was adjusted to 7.0. The concentrations of free Ca2+ were 10−9m (relaxing solution) and 10−6.3, 10−5.9, 10−5.5, 10−5.1, 10−4.9 and 10−4.5m (activating solutions), expressed as pCa values (i.e. –log [Ca2+]). Apparent stability constants for Ca2+-EGTA were corrected for temperature (15°C) and ionic strength (180 mm). The computer program of Fabiato (Fabiato, 1988) was used to calculate the concentrations of each metal, ligand and metal–ligand complex. EMD 57033 was a gift from Dr N. Beier (E. Merck Pharmaceuticals). It was prepared as a 10 mm stock solution in DMSO and was diluted in relaxing solution to obtain a final EMD 57033 concentration of 30 μm, and a DMSO concentration of 0.3% (v/v). Control solutions were prepared with an equivalent volume of DMSO, which had no effect on the fibres.
At 15°C, immediately preceding each activation, the fibre was immersed for 10–20 s in a solution with a reduced Ca2+-EGTA buffering capacity. This solution was identical to the relaxing solution except that the EGTA concentration was reduced to 0.5 mm, which results in more rapid attainment of steady-state force during subsequent activation.
Force
This was calculated as the difference between the steady-state isometric force in activating solutions and the resting force measured in the same segment while in the relaxing solution. Force was adjusted for CSA, i.e. specific force (SF).
Stiffness
Once steady-state isometric force was reached, small-amplitude sinusoidal changes in length (ΔL: ± 0.2% of fibre length) were applied at 500 Hz at one end of the fibre (Martyn et al. 2007). The resultant force response (ΔF) was measured, and the mean of 20 consecutive readings of ΔL and ΔF was used to determine stiffness. The actual elastic modulus (E) was calculated as the difference between E in activating solutions and resting E measured in the same segment in the relaxing solution. E was determined as follows (McDonald & Fitts, 1995):
Relative force–pCa and stiffness–pCa relationships
Each fibre was exposed to different solutions with pCa values varying from 9.0 to 4.5. Force and stiffness were normalized to maximum force and stiffness at pCa 4.5, allowing the construction of relative force–pCa and relative stiffness–pCa curves. To determine the midpoint (termed pCa50) and the Hill coefficient (nH) from the pCa curves, data were fitted (SigmaPlot 5.0 and Origin 6.1 Professional software; Jandel Scientific) using a three-parameter Hill equation in the following form:
where X is the relative force or relative stiffness, −log[Ca50] is the midpoint (pCa50) and nH is the Hill coefficient.
Apparent rate constant of force redevelopment
Once steady-state isometric force was reached, a slack by 20% of the original fibre length was introduced within 1–2 ms at one end of the fibre, resulting in a rapid reduction of force to near zero. This was followed by a brief period of unloaded shortening (20 ms), after which the preparation was quickly restretched to its original length and the force recovered to its original steady-state value. As previously described (Brenner & Eisenberg, 1986), the apparent rate constant of force redevelopment (ktr) was estimated by linear transformation of the half-time of force redevelopment (t1/2) as follows (Regnier et al. 1998):
Unloaded shortening velocity
At pCa 4.5, once steady-state isometric force was reached, nine slacks of various amplitudes were rapidly introduced (within 1–2 ms) at one end of the fibre (Edman, 1979). Slacks were applied at different amplitudes ranging from 7 to 13% of the fibre length. The fibre was re-extended between releases while relaxed in order to minimize changes in sarcomere length. During the slack test, the time required to take up the imposed release was measured from the onset of the length step to the beginning of the tension redevelopment. A straight line including four or more data points was fitted to a plot of release length versus time, using least-squares regression. The slope of the line divided by the fibre segment length was recorded as the maximum unloaded shortening velocity (V0) for that fibre segment.
For contractile measurements, strict acceptance criteria were applied. First, the sarcomere length was checked during the experiments, using a high-speed video analysis system (model 901 A HVSL, Aurora Scientific). A muscle fibre was accepted and included in the analyses: (i) if the sarcomere length of a single muscle fibre changed by < 0.10 μm between relaxation and maximum activation, (ii) if maximal force changed by < 10% from first to final activation, and (iii) if r of the slope (plot of release length versus time) for V0 calculation was > 0.96.
After the mechanical measurements, each fibre was placed in urea buffer (120 g urea, 38 g thiourea, 70 ml H2O, 25 g mixed bed resin, 2.89 g dithiothreitol, 1.51 g Trizma base, 7.5 g SDS, 0.004% bromophenol blue) in a plastic microcentrifuge tube and stored at –80°C.
In vitro motility assay
The unregulated actin used was purified from rabbit skeletal muscle essentially and was fluorescently labelled with rhodamine–phalloidin (Molecular Probes). The single fibre in vitro motility system has been described in detail elsewhere (Hook et al. 1999; Hook & Larsson, 2000). In brief, a short muscle fibre segment was placed on a glass slide between two strips of grease, and a nitrocellulose-coated coverslip was placed on top, creating a flow cell with a volume of ∼5 μl volume. Myosin was extracted from the fibre segment through addition of a high-salt buffer (0.5 m KCl, 25 mm Hepes (pH 7.6), 4 mm MgCl2, 4 mm EGTA, 2 mm ATP and 1% 2-mercaptoethanol). After 30 min incubation on ice, a low-salt buffer (25 mm KCl, 25 mm Hepes (pH 7.6), 4 mm MgCl2, 1 mm EGTA and 1% 2-mercaptoethanol) was applied, followed by BSA (1 mg ml−1). Non-functional myosin molecules were blocked with fragmentized F-actin, and rhodamine–phalloidin-labelled actin filaments were subsequently infused into the flow cell, followed by motility buffer (2 mm ATP, 0.1 mg ml−1 glucose oxidase, 23 μg ml−1 catalase, 2.5 mg ml−1 glucose and 0.4% methyl cellulose in low-salt buffer) to initiate movement. The pH of the buffers was adjusted with KOH, and the final ionic strength of the motility buffer was 71 mm. The flow cell was placed on the stage of an inverted epifluorescence microscope (model IX 70; Olympus) and thermostatically controlled at 25°C. Actin movements were filmed with an image-intensified SIT camera (SIT 66; DAGE-MIT) and recorded on tape with a video-cassette recorder. From each single fibre preparation, 20 actin filaments moving at constant speed in an orientated motion were selected for speed analysis. Recordings and analysis were only performed from preparations in which > 90% of the filaments moved bi-directionally. A filament was tracked from the centre of mass, and the speed was calculated from 20 frames at an acquisition rate of five or one frame(s) per second, depending on the fibre type, using an image-analysis package (Image-pro Plus Version 6.0, Media Cybernetics). The mean speed of the 20 filaments was calculated. Since the standard deviation in this group of filaments was small (between 10 and 15% of the mean), the average speed was taken as representative for the muscle fibre (Vf).
Protein isoform expression and quantification
The MyHC isoform composition of single fibres was determined by 6% SDS-PAGE. The acrylamide concentration was 4% (wt/vol) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Sample loads were kept small (equivalent to ∼0.05 mm of fibre segment) to improve the resolution of the MyHC bands (types I, IIa and IIx). Electrophoresis was performed at 120 V for 24 h with a Tris–glycine electrode buffer (pH 8.3) at 15°C (SE 600 vertical slab gel unit, Hoefer Scientific Instruments). The gels were silver-stained and subsequently scanned in a soft laser densitometer (Molecular Dynamics) with a high spatial resolution (50 μm pixel spacing) and 4096 optical density levels.
Tm isoform expression was determined and quantified in single muscle fibres by 12% SDS-PAGE. The acrylamide concentration was 4% (wt/vol) in the stacking gel and 12% in the running gel, and the gel matrix included 10% glycerol. The gels were stained with Coomassie blue (0.5 g Brilliant Blue, 225 ml MeOH, 225 ml distilled H2O and 50 ml acetic acid). The relative contents of α- and β-Tm isoforms in each single muscle fibre were then calculated from the densitometric scanning (see above).
Statistical analysis
Data are presented as means ± standard error of the means (s.e.m.). Sigma Stat software (Jandel Scientific) was used to generate descriptive statistics. Given the small numbers of hybrid (type I/IIa and IIa/IIx) and pure type IIa and IIx fibres observed in these experiments, comparisons were restricted to fibres expressing the β/slow (type I) MyHC isoform. To evaluate the effects of the E41K β-Tm mutation on contractile characteristics, an unpaired Student's t test was applied, and in cases where the data did not meet the criteria of normality (Kolmogorov–Smirnov test, P < 0.05), a non-parametric Mann–Whitney rank-sum test was performed. To assess the impacts of EMD 57033 on contractile properties of fibres from both patients and controls, one-way repeated measures ANOVA was applied, and in cases where the data did not meet the criteria of normality, one-way repeated measures ANOVA on ranks was performed. For ANOVA analysis, when differences were significant, post hoc analysis was carried out by Tukey's test. Otherwise, regressions were performed and relationships were considered significantly different from zero at P < 0.05.
Results
A total of 83 fibres expressing the type I MyHC isoform from the two patients carrying the E41K β-Tm mutation and 98 type I fibres from the eight controls were included in the analyses (33 and 34 type I fibres, respectively, were rejected because they did not meet the criteria for acceptance). The characteristics did not differ between fibres from the two patients and fibres have therefore been pooled.
Muscle fibre size and Tm isoform expression
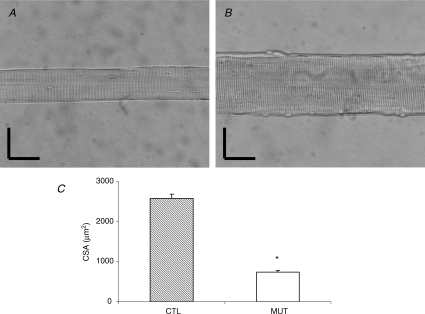
Severe atrophy was confirmed in fibres from patients compared with controls (P < 0.001). The mean CSA of muscle fibres was 730 ± 50 μm2 in patients and 2570 ± 110 μm2 in controls (Fig. 2). It should be noted that (i) the mean CSA of fibres from a patient's tibialis anterior was similar to that of fibres from a patient's deltoid muscle (740 ± 60 μm2 and 710 ± 30 μm2, respectively) and (ii) in the controls, the mean CSA of fibres from tibialis anterior was not significantly different from that of fibres from the vastus lateralis (2330 ± 100 μm2 and 2790 ± 130 μm2, respectively).
Figure 2. Fibre cross-sectional area.
A, image of a fibre from a patient. B, image of a fibre from a control. C, CSA of fibres from patients carrying the E41K β-Tm mutation (open bar, n = 62) and control subjects (hatched bar, n = 84). Values are means ±s.e.m.s. Asterisk indicates significantly different from controls. Vertical and horizontal bars denote 50 μm.
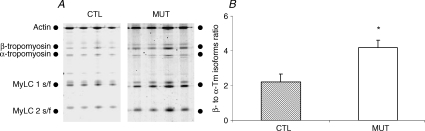
A shift from α- to β-Tm isoform was observed in fibres from patients compared with controls, as demonstrated by the significant increase (P < 0.01) in the β- to α-Tm isoform ratio in patients (4.18 ± 0.43; n = 24) compared with controls (2.21 ± 0.47; n = 22) (Fig. 3).
Figure 3. α- and β-Tm isoform expression.
A, electrophoretic separation of actin, and α- and β-Tm isoforms in fibres from patients (MUT) and controls (CTL). B, β- to α-Tm isoforms ratio in fibres from patients carrying the E41K β-Tm mutation (open bar, n = 24) and control subjects (hatched bar, n = 22). Values are means ±s.e.m. Asterisk indicates significantly different from controls.
Specific force, stiffness, rate constant of force redevelopment, maximum velocity of unloaded shortening and in vitro motility speed
At pCa 4.5, SF and stiffness did not differ significantly between patients' and controls' fibres. Mean SF was 18.10 ± 1.30 N cm−2 in fibres from patients (n = 62) and 21.30 ± 0.60 N cm−2 in control fibres (n = 84). Mean stiffness was 2.20 ± 0.40 kN cm−2 in fibres from patients (n = 20) and 3.10 ± 0.50 kN cm−2 in those from controls (n = 18).
The E41K β-Tm mutation was associated with a significant negative effect on shortening speed. Both ktr and V0 values at pCa 4.5 were lower in fibres from patients compared with controls. The mean ktr values were 53% lower (P < 0.001) in patients (9.80 ± 1.20 s−1; n = 31) than in controls (21.00 ± 1.40 s−1; n = 26). The mean V0 was 47% lower (P < 0.01) in patients (0.46 ± 0.06 Muscle lengths s−1 (ML s−1); n = 24) than in controls (0.87 ± 0.06 ML s−1; n = 30).
In an attempt to test whether these changes were due to the mutation itself or to a secondary modification of the motor protein myosin, the single muscle fibre in vitro motility assay was used in which the motor protein is extracted from muscle fibre segments 1–2 mm long and the interactions with unregulated thin filaments are measured (Hook et al. 1999). The in vitro motility speed (Vf), i.e. the molecular analog of V0 at the cellular level, of actin filaments propelled by myosin did not differ between fibres containing the E41K β-Tm mutation (0.54 ± 0.01 μm s−1; n = 21) and control fibres (0.53 ± 0.03 μm s−1; n = 14).
Ca2+ sensitivity and Hill coefficient
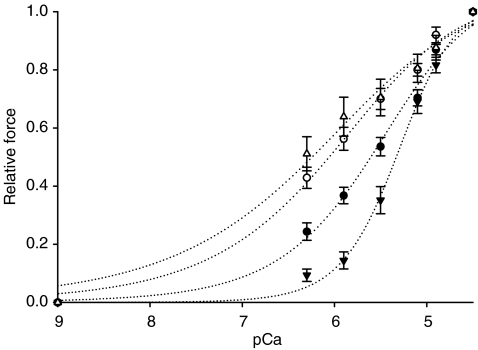
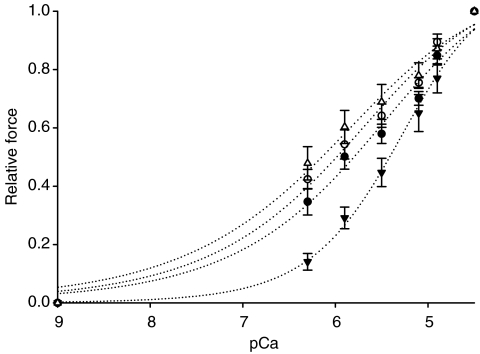
A decreased Ca2+ sensitivity of force was observed in fibres carrying the E41K β-Tm mutation, i.e. pCa50 (5.31 ± 0.04; n = 50) was lower (P < 0.01) in fibres from patients compared with controls (5.66 ± 0.05; n = 54; Fig. 4). However, the Hill coefficient did not differ significantly between patients' and controls' fibres, i.e. mean nH was 4.28 ± 0.77 in fibres from patients (n = 50) and 3.70 ± 0.49 in controls (n = 54).
Figure 4. Relative force–pCa relationships.
Relative force–pCa curves of fibres from patients (▾, n = 50), fibres from patients exposed to the Ca2+ sensitizer (▵, n = 20), fibres from controls (•, n = 54) and fibres from controls exposed to EMD 57033 (○, n = 11). All values are means ±s.e.m.
The Ca2+ sensitivity of stiffness was also found to be lower (P < 0.01) in fibres from patients (pCa50: 5.27 ± 0.08; n = 20) than in those from controls (pCa50: 5.77 ± 0.13; n = 18; Fig. 5). Nevertheless, nH was similar between fibres from patients (4.66 ± 1.34; n = 20) and control fibres (3.43 ± 0.79; n = 13).
Figure 5. Relative stiffness–pCa relationships.
Relative stiffness–pCa curves of fibres from patients (▾, n = 20), fibres from patients exposed to the Ca2+ sensitizer (▵, n = 11), fibres from controls (•, n = 18) and fibres from controls exposed to EMD 57033 (○, n = 6). All values are means ±s.e.m.
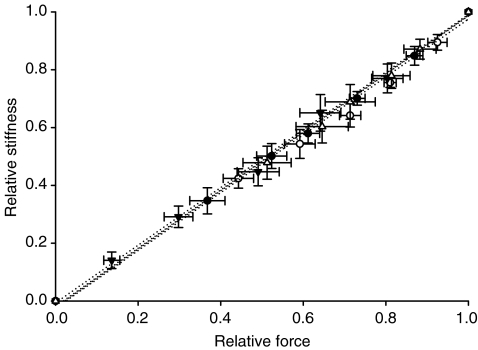
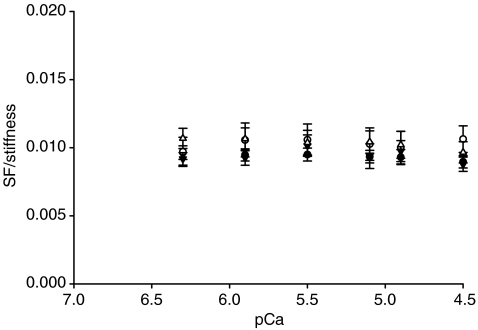
Stiffness is dependent on the number of attached cross-bridges, the force/compliance of each cross-bridge, and the compliance of the structures in series with the cross-bridges (the thick filament, proteins along the thin filament and probably also Z-discs) (Higuchi et al. 1995; Seow et al. 1997; Galler & Hilber, 1998). The strong linear relationship between the relative stiffness and the relative force in fibres from both patients and controls (Fig. 6) demonstrates that stiffness is primarily dependent on the number of attached cross-bridges at all levels of Ca2+ activation (Regnier et al. 2004). The SF/stiffness ratio may therefore be closely related to the force/compliance of each cross-bridge (McDonald & Fitts, 1995). The maintained SF/stiffness–pCa relationships in fibres from patients (Fig. 7), suggest that the force/compliance of each cross-bridge is preserved at all Ca2+ concentrations.
Figure 6. Relative stiffness–relative force relationships.
Relative stiffness–relative force curves of fibres from patients (▾, n = 20), fibres from patients exposed to the Ca2+ sensitizer (▵, n = 11), fibres from controls (•, n = 18) and fibres from controls exposed to EMD 57033 (○, n = 6). All values are means ±s.e.m.
Figure 7. SF/stiffness–pCa relationships.
SF/stiffness–pCa curves of fibres from patients (▾, n = 20), fibres from patients exposed to the Ca2+ sensitizer (▵, n = 11), fibres from controls (•, n = 18) and fibres from controls exposed to EMD 57033 (○, n = 6). All values are means ±s.e.m.
Effects of the Ca2+ sensitizer
Out of the 83 analysed type I fibres from patients, 27 fibres were further exposed to the Ca2+ sensitizer EMD 57033. Of the 98 type I fibres from controls, 16 were exposed to EMD 57033. At pCa 4.5, SF, stiffness and shortening speeds (ktr and V0) did not differ when patients' fibres were exposed to 30 μm EMD 57033. When treated with 30 μm EMD 57033, mean SF was 22.20 ± 2.10 N cm−2 (n = 27), mean stiffness was 2.40 ± 0.30 kN cm−2 (n = 11), mean ktr was 13.00 ± 1.10 s−1 (n = 9), and mean V0 was 0.54 ± 0.08 MLs−1 (n = 7). When control fibres were exposed to the same concentration of EMD 57033, ktr and V0 were maintained; however, SF and stiffness increased (P < 0.01, P < 0.05, respectively). When treated with 30 μm EMD 57033, mean SF was 26.50 ± 1.20 N cm−2 (n = 16), mean stiffness was 3.70 ± 0.60 kN cm−2 (n = 6), mean ktr was 21.60 ± 3.40 s−1 (n = 5), and mean V0 was 0.80 ± 0.08 ML s−1 (n = 5).
The relative force–pCa relationship was affected by 30 μm EMD 57033 exposure, and increased Ca2+ sensitivity was observed both in fibres containing the E41K β-Tm mutation (P < 0.001) and in control fibres (P < 0.01; Fig. 4). When treated with 30 μm EMD 57033, mean pCa50 was 6.18 ± 0.19 in fibres from patients (n = 20) and 6.00 ± 0.06 in fibres from controls (n = 11). The Hill coefficient did not differ significantly, i.e. mean nH was 2.82 ± 0.91 in fibres from patients (n = 20) and 3.18 ± 0.97 in controls (n = 11), when exposed to 30 μm EMD 57033.
The relative stiffness–pCa relationship was also affected by the sensitizer and an increase (P < 0.01) in pCa50 was observed in the patients' fibres (Fig. 5). The same trend was observed in controls' fibres, but did not reach the level of statistical significance. When exposed to 30 μm EMD 57033, mean pCa50 was 6.04 ± 0.17 in fibres from patients (n = 11) and 5.89 ± 0.09 in fibres from controls (n = 6). nH was similar in fibres from patients (3.32 ± 0.93; n = 11) and from controls (3.34 ± 1.29; n = 6).
The linear relative stiffness versus relative force relationship was not affected by exposure to 30 μm EMD 57033 in either patient or control fibres (Fig. 6), demonstrating that strong dependence of stiffness on the number of attached cross-bridges was maintained at all levels of Ca2+ activation under all conditions. The SF/stiffness–pCa relationships were also unchanged (Fig. 7), suggesting that the force/compliance of each cross-bridge was preserved at all Ca2+ concentration under all conditions.
Discussion
In this study we explored a broad range of contractile parameters in muscle fibres from patients carrying the E41K β-Tm mutation. This mutation directly reduces (i) the shortening speeds and (ii) the Ca2+ sensitivity of contraction, suggesting that it has negative effects on contractile function contributing to the muscle weakness in the patients. Some of these negative effects of the mutation are reversible. Muscle fibres from patients exposed to the Ca2+ sensitizer EMD 57033 exhibited an increased Ca2+ sensitivity of contraction, indicating a potential therapeutic role of this drug in patients carrying the E41K β-Tm mutation.
Effects of the E41K β-Tm mutation on contractile function
Maximal Ca2+-activated force normalized to fibre cross-sectional area (specific force, SF), stiffness and SF/stiffness ratio at pCa 4.5 were preserved in fibres from patients carrying the E41K β-Tm mutation. Hence, this mutation does not appear to have a direct impact on the total number of cross-bridges recruited or on the force/compliance per cross-bridge at a saturated Ca2+ concentration. These findings are in line with those in other α-Tm and γ-Tm mutations (Bottinelli et al. 1998; Michele et al. 1999a,b; Mirza et al. 2005). However, fibres from the patients carrying the mutation showed reductions in contractile speed, i.e. both the apparent rate constant of force redevelopment (ktr) and maximum velocity of unloaded shortening (V0) at saturated Ca2+ concentration, indicating that Tm directly modulates contractile speed by regulating kinetic steps of the cross-bridge cycle without affecting the total number of cross-bridges recruited or the force/compliance per cross-bridge. Similar observations have been reported for other α-Tm mutations associated with dilated cardiomyopathy (E40K) (Mirza et al. 2005) or hypertrophic cardiomyopathy (V95A) (Karibe et al. 2001). At pCa 4.5 (i) ktr reflects fapp+gapp defined as the cross-bridge attachment and detachment rates, respectively (Brenner & Eisenberg, 1986); (ii) V0 is mainly limited by gapp (Edman, 1979); and (iii) force is proportional to fapp/(fapp+gapp) (Brenner & Eisenberg, 1986). Hence, changes in ktr and V0 are expected to be related to changes in force and vice versa. However, changes in fapp and gapp are more sensitively detected by force development kinetics than by the force itself (Kruger et al. 2005) and we suggest this to be the most likely reason why we did not observe any significant changes in SF between fibres from patients and controls.
A desensitization of force to Ca2+ concentration was found in fibres from patients carrying the E41K β-Tm mutation compared with controls (Fig. 4). This is in accordance with other α-Tm and γ-Tm mutations (Michele et al. 1999b; Mirza et al. 2005) and underlines the predominant role of Tm in regulating the propagation of Ca2+ signals along the thin filament from the Tn complex to the cross-bridges (Brandt et al. 1987; Schachat et al. 1987). It remains unclear how the E41K β-Tm mutation leads to a Ca2+ desensitization of force. To investigate whether this effect was related to a decrease in the number of cross-bridges recruited or in the strong binding of each cross-bridge, i.e. force/compliance per cross-bridge, at submaximal Ca2+ concentrations, the relative stiffness–pCa and SF/stiffness–pCa relationships were constructed, respectively. The relative stiffness–pCa curve showed desensitization to the Ca2+ concentration in fibres from the patients compared with the controls (Fig. 5), whereas the SF/stiffness–pCa relationship was maintained (Fig. 7). These observations indicate that the present mutation decreases the relative number of cross-bridges recruited at submaximal Ca2+ concentrations without altering the force/compliance per cross-bridge. The E41K β-Tm mutation is located in the outer domain of the strand in the ‘f’ position of the heptad repeat. It therefore appears unlikely that the mutation lowers the Ca2+ sensitivity by altering the chain–chain interaction between the two Tm strands that wind around each other, and a direct Tm–actin interaction appears more likely. According to the steric blocking model (Poole et al. 1995; Vibert et al. 1997), the mutation-induced switching from a negative to a positive charge may produce a local inflexibility, which is expected to change Tm–actin affinity (Brown et al. 2001; Singh & Hitchcock-DeGregori, 2003, 2006), destabilizing the On states, i.e. increasing the probability of being in the Off state (Cammarato et al. 2005), and lowering the On–Off equilibrium as shown with the neighbour E40K α-Tm mutation associated with dilated cardiomyopathy (Mirza et al. 2007). Higher Ca2+ concentrations may be needed to switch Tm to the On state, leading to a decrease in the relative number of cross-bridges recruited and in the relative force produced at submaximal Ca2+ concentrations. In addition to a possible change in Tm–actin interaction, the mutation may affect the Tn–Tm interface. The E41K β-Tm mutation is located in the N-terminal region of the Tm segment, close to one TnT-anchoring region. TnT contributes to the inhibition of muscle contraction via Tm in the absence of Ca2+ (Tobacman et al. 2002). If the mutation enhances TnT ability to promote stabilization of the Off state, then cross-bridge attachment may be partially prevented (Chang et al. 2005). Future experiments are needed to improve our understanding of how the E41K β-Tm mutation alters actin and TnT interactions.
Surprisingly, the β- to α-Tm isoform ratio was increased in fibres carrying the E41K β-Tm mutation (Fig. 3). This may potentially result in an increased effect of the β-Tm isoform on function in muscle cells carrying the present mutation. Over-expression of β-Tm is known to induce an increase in Ca2+ sensitivity of force (Palmiter et al. 1996; Wolska et al. 1999) and a decrease in specific force (Wolska et al. 1999). It is therefore unlikely that the altered Ca2+ sensitivity in fibres carrying the E41K β-Tm mutation is related to the higher β- to α-Tm isoform ratio.
Effects of the Ca2+ sensitizer on contractile function
SF, stiffness, SF/stiffness ratio, ktr and V0 at pCa 4.5 were all preserved when fibres from patients carrying the E41K β-Tm mutation were exposed to 30 μm EMD 57033. These observations differ from those from controls, where only SF and stiffness increased in response to 30 μm EMD 57033. The effects of EMD 57033 may be mitigated by the present mutation at saturated Ca2+ concentration, resulting in maintenance of the total number of cross-bridges recruited, the force/compliance per cross-bridge and the kinetic steps of cross-bridge cycle. Further experiments are needed to clarify the mechanisms underlying the differences between fibres carrying the E41K β-Tm mutation and controls in response to EMD treatment.
Hypersensitization of force and stiffness to Ca2+ concentration was found in fibres both from patients carrying the E41K β-Tm mutation and from controls, exposed to the Ca2+ sensitizer (Figs 4 and 5). EMD 57033 is effective in fibres carrying the E41K β-Tm mutation; it completely counterbalances the negative action of the mutation at submaximal Ca2+ concentrations. It increases the relative number of cross-bridges recruited at various submaximal pCa values without modifying the force/compliance per cross-bridge, as attested by the SF/stiffness–pCa curves (Fig. 7). The causes of such phenomenon remain unclear. Nevertheless, EMD 57033 is known to bind directly to TnC and thereby to increase the affinity of TnC for the inhibitory region of TnI (Wang et al. 2001). This displaces the inhibitory region of TnI from actin towards TnC, thereby inducing Tm movement towards the inner domain of actin, i.e. from the Off state to the On states, facilitating cross-bridge recruitment (Lipscomb et al. 2005).
In conclusion, in this study we addressed the effects of a novel β-Tm mutation on the contractile function. The E41K β-Tm mutation mainly causes reductions in (i) the speed of contraction and (ii) the sensitivity of contraction to Ca2+ concentration, thus negatively affecting muscle function in these patients. The Ca2+ sensitizer EMD 57033 partially reversed these effects of the mutation on regulation of muscle contraction and it may represent a potential therapeutic strategy in patients carrying the E41K β-Tm mutation. However, this potential therapeutic strategy needs to be evaluated in vivo in animals carrying the E41K β-Tm mutation.
Acknowledgments
This study was supported by grants from the Swedish Institute and Association Française contre les Myopathies to J.O., from the Association Française contre les Myopathies and Swedish Research Council (07122) to A.O., and from the Swedish Research Council (08651), Association Française contre les Myopathies, Cancer Foundation and National Institutes of Health (AR045627, AR047318) to L.L. EMD 57033 was provided by Merck KGaA, Darmstadt, Germany (Dr Norbert Beier). We are grateful to Yvette Hedström and Ann-Marie Gustafsson for excellent technical assistance. We thank the patients and control subjects for making this work possible.
References
- Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, Watkins H, Reggiani C. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity. Circ Res. 1998;82:106–115. doi: 10.1161/01.res.82.1.106. [DOI] [PubMed] [Google Scholar]
- Brandt PW, Diamond MS, Rutchik JS, Schachat FH. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987;195:885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-Degregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci U S A. 2001;98:8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarato A, Craig R, Sparrow JC, Lehman W. E93K charge reversal on actin perturbs steric regulation of thin filaments. J Mol Biol. 2005;347:889–894. doi: 10.1016/j.jmb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Chang AN, Harada K, Ackerman MJ, Potter JD. Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in α-tropomyosin. J Biol Chem. 2005;280:34343–34349. doi: 10.1074/jbc.M505014200. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Kolski H, Dye DE, Lim E, Smith RL, Patel R, Fahey MC, Bellance R, Romero NB, Johnson ES, Labarre-Vila A, Monnier N, Laing NG, North KN. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol. 2008;63:329–337. doi: 10.1002/ana.21308. [DOI] [PubMed] [Google Scholar]
- Donner K, Ollikainen M, Ridanpaa M, Christen HJ, Goebel HH, De Visser M, Pelin K, Wallgren-Pettersson C. Mutations in the β-tropomyosin (TPM2) gene – a rare cause of nemaline myopathy. Neuromuscul Disord. 2002;12:151–158. doi: 10.1016/s0960-8966(01)00252-8. [DOI] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Meth Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Larsson L. Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve. 1997;20:948–952. doi: 10.1002/(sici)1097-4598(199708)20:8<948::aid-mus3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K. Tension/stiffness ratio of skinned rat skeletal muscle fibre types at various temperatures. Acta Physiol Scand. 1998;162:119–126. doi: 10.1046/j.1365-201X.1998.0272f.x. [DOI] [PubMed] [Google Scholar]
- Heller MJ, Nili M, Homsher E, Tobacman LS. Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility. J Biol Chem. 2003;278:41742–41748. doi: 10.1074/jbc.M303408200. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yanagida T, Goldman YE. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J. 1995;69:1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Nili M, Chen IY, Tobacman LS. Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J. 2003;85:1046–1052. doi: 10.1016/S0006-3495(03)74543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook P, Larsson L. Actomyosin interactions in a novel single muscle fiber in vitro motility assay. J Muscle Res Cell Motil. 2000;21:357–365. doi: 10.1023/a:1005614212575. [DOI] [PubMed] [Google Scholar]
- Hook P, Li X, Sleep J, Hughes S, Larsson L. In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J Physiol. 1999;520:463–471. doi: 10.1111/j.1469-7793.1999.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed RJ, Marcelis CL, Doevendans PA, Schmeitz-Mulkens JM, Van Dockum WG, Geraedts JP, Smeets HJ. Variable clinical manifestation of a novel missense mutation in the α-tropomyosin (TPM1) gene in familial hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:981–986. doi: 10.1016/s0735-1097(02)03005-x. [DOI] [PubMed] [Google Scholar]
- Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, Arai AE, Ortiz A, Roberts R, Homsher E, Fananapazir L. Hypertrophic cardiomyopathy caused by a novel α-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation. 2001;103:65–71. doi: 10.1161/01.cir.103.1.65. [DOI] [PubMed] [Google Scholar]
- Kraft T, Brenner B. Force enhancement without changes in cross-bridge turnover kinetics: the effect of EMD 57033. Biophys J. 1997;72:272–281. doi: 10.1016/S0006-3495(97)78666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Zittrich S, Redwood C, Blaudeck N, James J, Robbins J, Pfitzer G, Stehle R. Effects of the mutation R145G in human cardiac troponin I on the kinetics of the contraction–relaxation cycle in isolated cardiac myofibrils. J Physiol. 2005;564:347–357. doi: 10.1113/jphysiol.2004.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, et al. A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy NEM1. Nat Genet. 1995;10:249. doi: 10.1038/ng0695-249. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtokari VL, Ceuterick-De Groote C, De Jonghe P, Marttila M, Laing NG, Pelin K, Wallgren-Pettersson C. Cap disease caused by heterozygous deletion of the β-tropomyosin gene TPM2. Neuromuscul Disord. 2007;17:433–442. doi: 10.1016/j.nmd.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Lipscomb S, Preston LC, Robinson P, Redwood CS, Mulligan IP, Ashley CC. Effects of troponin C isoform on the action of the cardiotonic agent EMD 57033. Biochem J. 2005;388:905–912. doi: 10.1042/BJ20041841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, Fitts RH. Effect of hindlimb unloading on rat soleus fiber force, stiffness, and calcium sensitivity. J Appl Physiol. 1995;79:1796–1802. doi: 10.1152/jappl.1995.79.5.1796. [DOI] [PubMed] [Google Scholar]
- Martyn DA, Smith L, Kreutziger KL, Xu S, Yu LC, Regnier M. The effects of force inhibition by sodium vanadate on cross-bridge binding, force redevelopment, and Ca2+ activation in cardiac muscle. Biophys J. 2007;92:4379–4390. doi: 10.1529/biophysj.106.096768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Albayya FP, Metzger JM. Direct, convergent hypersensitivity of calcium-activated force generation produced by hypertrophic cardiomyopathy mutant α-tropomyosins in adult cardiac myocytes. Nat Med. 1999a;5:1413–1417. doi: 10.1038/70990. [DOI] [PubMed] [Google Scholar]
- Michele DE, Albayya FP, Metzger JM. A nemaline myopathy mutation in α-tropomyosin causes defective regulation of striated muscle force production. J Clin Invest. 1999b;104:1575–1581. doi: 10.1172/JCI7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Marston S, Willott R, Ashley C, Mogensen J, Mckenna W, Robinson P, Redwood C, Watkins H. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- Mirza M, Robinson P, Kremneva E, Copeland O, Nikolaeva O, Watkins H, Levitsky D, Redwood C, El-Mezgueldi M, Marston S. The effect of mutations in α-tropomyosin (E40K and E54K) that cause familial dilated cardiomyopathy on the regulatory mechanism of cardiac muscle thin filaments. J Biol Chem. 2007;282:13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length–tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Taniguchi C, Matsui H, Nagata S, Kishimoto T, Yamauchi-Takihara K. Novel missense mutation in α-tropomyosin gene found in Japanese patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol. 1995;27:2053–2058. doi: 10.1016/0022-2828(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Ochala J, Li M, Tajsharghi H, Kimber E, Tulinius M, Oldfors A, Larsson L. Effects of a R133W β-tropomyosin mutation on regulation of muscle contraction in single human muscle fibres. J Physiol. 2007;581:1283–1292. doi: 10.1113/jphysiol.2007.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of α-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol. 2001;33:723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of β- for α-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J Biol Chem. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Penisson-Besnier I, Monnier N, Toutain A, Dubas F, Laing N. A second pedigree with autosomal dominant nemaline myopathy caused by TPM3 mutation: a clinical and pathological study. Neuromuscul Disord. 2007;17:330–337. doi: 10.1016/j.nmd.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- Poole V, Evans G, Rosenbaum G, Lorenz M, Holmes K. The effect of cross-bridges on the calcium sensitivity of the structural change of the regulated thin filament. Biophys J. 1995;68:365. [Google Scholar]
- Regitz-Zagrosek V, Erdmann J, Wellnhofer E, Raible J, Fleck E. Novel mutation in the α-tropomyosin gene and transition from hypertrophic to hypocontractile dilated cardiomyopathy. Circulation. 2000;102:E112–E116. doi: 10.1161/01.cir.102.17.e112. [DOI] [PubMed] [Google Scholar]
- Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004;87:1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M, Martyn DA, Chase PB. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J. 1998;74:2005–2015. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P, Lipscomb S, Preston LC, Altin E, Watkins H, Ashley CC, Redwood CS. Mutations in fast skeletal troponin I, troponin T, and β-tropomyosin that cause distal arthrogryposis all increase contractile function. FASEB J. 2007;21:896–905. doi: 10.1096/fj.06-6899com. [DOI] [PubMed] [Google Scholar]
- Schachat FH, Diamond MS, Brandt PW. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987;198:551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Seow CY, Shroff SG, Ford LE. Detachment of low-force bridges contributes to the rapid tension transients of skinned rabbit skeletal muscle fibres. J Physiol. 1997;501:149–164. doi: 10.1111/j.1469-7793.1997.149bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Hitchcock-Degregori SE. Local destabilization of the tropomyosin coiled coil gives the molecular flexibility required for actin binding. Biochemistry. 2003;42:14114–14121. doi: 10.1021/bi0348462. [DOI] [PubMed] [Google Scholar]
- Singh A, Hitchcock-Degregori SE. Dual requirement for flexibility and specificity for binding of the coiled-coil tropomyosin to its target, actin. Structure. 2006;14:43–50. doi: 10.1016/j.str.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, Lakatta EG. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- Sung SS, Brassington AM, Krakowiak PA, Carey JC, Jorde LB, Bamshad M. Mutations in TNNT3 cause multiple congenital contractures: a second locus for distal arthrogryposis type 2B. Am J Hum Genet. 2003;73:212–214. doi: 10.1086/376418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsharghi H, Kimber E, Holmgren D, Tulinius M, Oldfors A. Distal arthrogryposis and muscle weakness associated with a β-tropomyosin mutation. Neurology. 2007a;68:772–775. doi: 10.1212/01.wnl.0000256339.40667.fb. [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Ohlsson M, Lindberg C, Oldfors A. Congenital myopathy with nemaline rods and cap structures caused by a mutation in the β-tropomyosin gene (TPM2) Arch Neurol. 2007b;64:1334–1338. doi: 10.1001/archneur.64.9.1334. [DOI] [PubMed] [Google Scholar]
- Tan P, Briner J, Boltshauser E, Davis MR, Wilton SD, North K, Wallgren-Pettersson C, Laing NG. Homozygosity for a nonsense mutation in the α-tropomyosin slow gene TPM3 in a patient with severe infantile nemaline myopathy. Neuromuscul Disord. 1999;9:573–579. doi: 10.1016/s0960-8966(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Thierfelder L, Watkins H, Macrae C, Lamas R, Mckenna W, Vosberg HP, Seidman JG, Seidman CE. α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Tobacman LS, Nihli M, Butters C, Heller M, Hatch V, Craig R, Lehman W, Homsher E. The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem. 2002;277:27636–27642. doi: 10.1074/jbc.M201768200. [DOI] [PubMed] [Google Scholar]
- Vannier C, Lakomkine V, Vassort G. Tension response of the cardiotonic agent (+)-EMD-57033 at the single cell level. Am J Physiol Cell Physiol. 1997;272:C1586–C1593. doi: 10.1152/ajpcell.1997.272.5.C1586. [DOI] [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Wang X, Li MX, Spyracopoulos L, Beier N, Chandra M, Solaro RJ, Sykes BD. Structure of the C-domain of human cardiac troponin C in complex with the Ca2+ sensitizing drug EMD 57033. J Biol Chem. 2001;276:25456–25466. doi: 10.1074/jbc.M102418200. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, De Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express β-tropomyosin. Circ Res. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Nakajima-Taniguchi C, Matsui H, Fujio Y, Kunisada K, Nagata S, Kishimoto T. Clinical implications of hypertrophic cardiomyopathy associated with mutations in the α-tropomyosin gene. Heart. 1996;76:63–65. doi: 10.1136/hrt.76.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]