Abstract
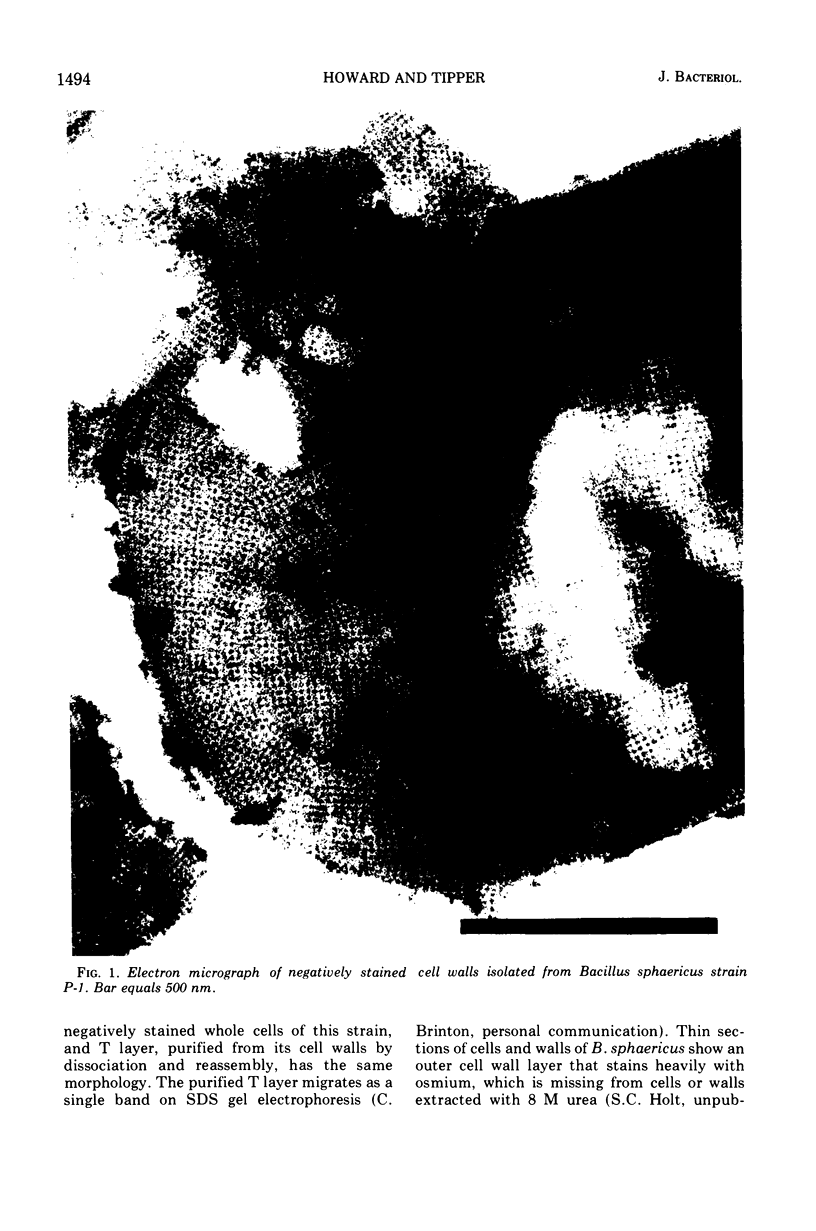
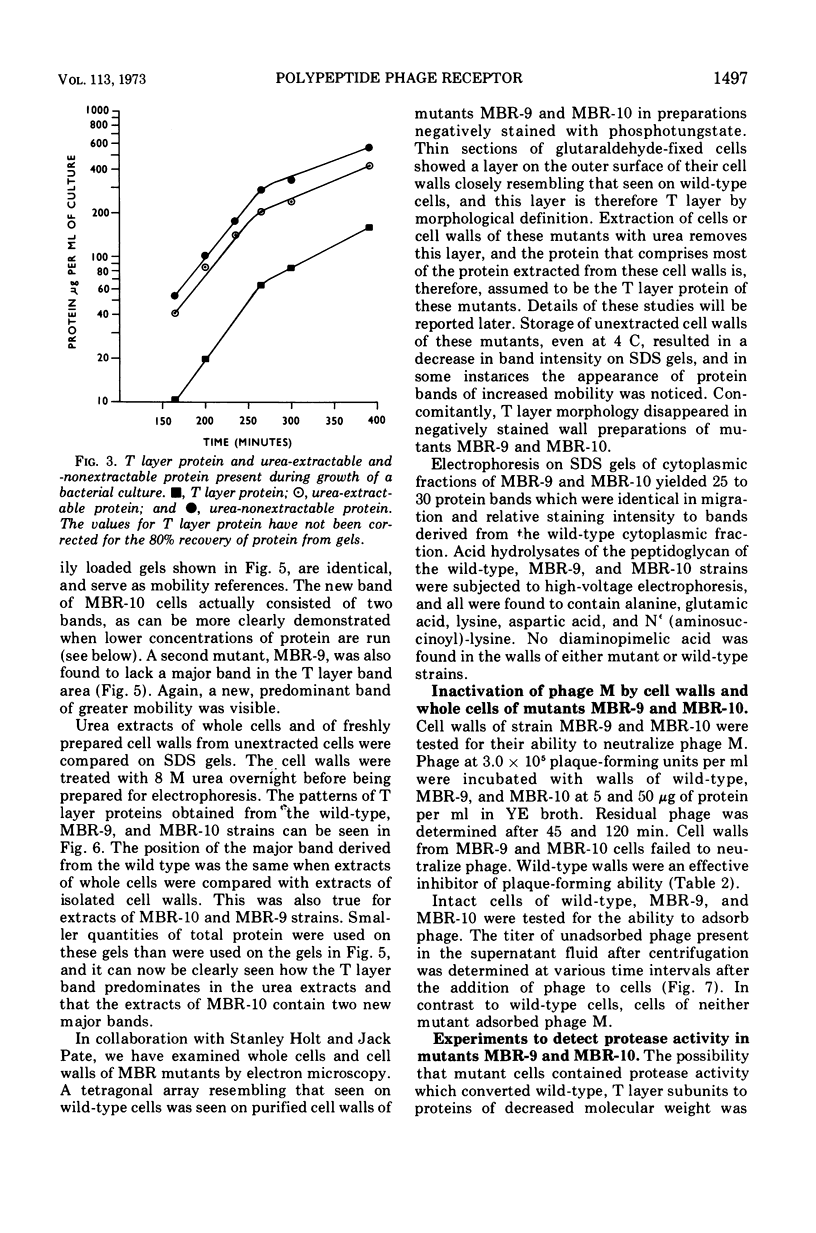
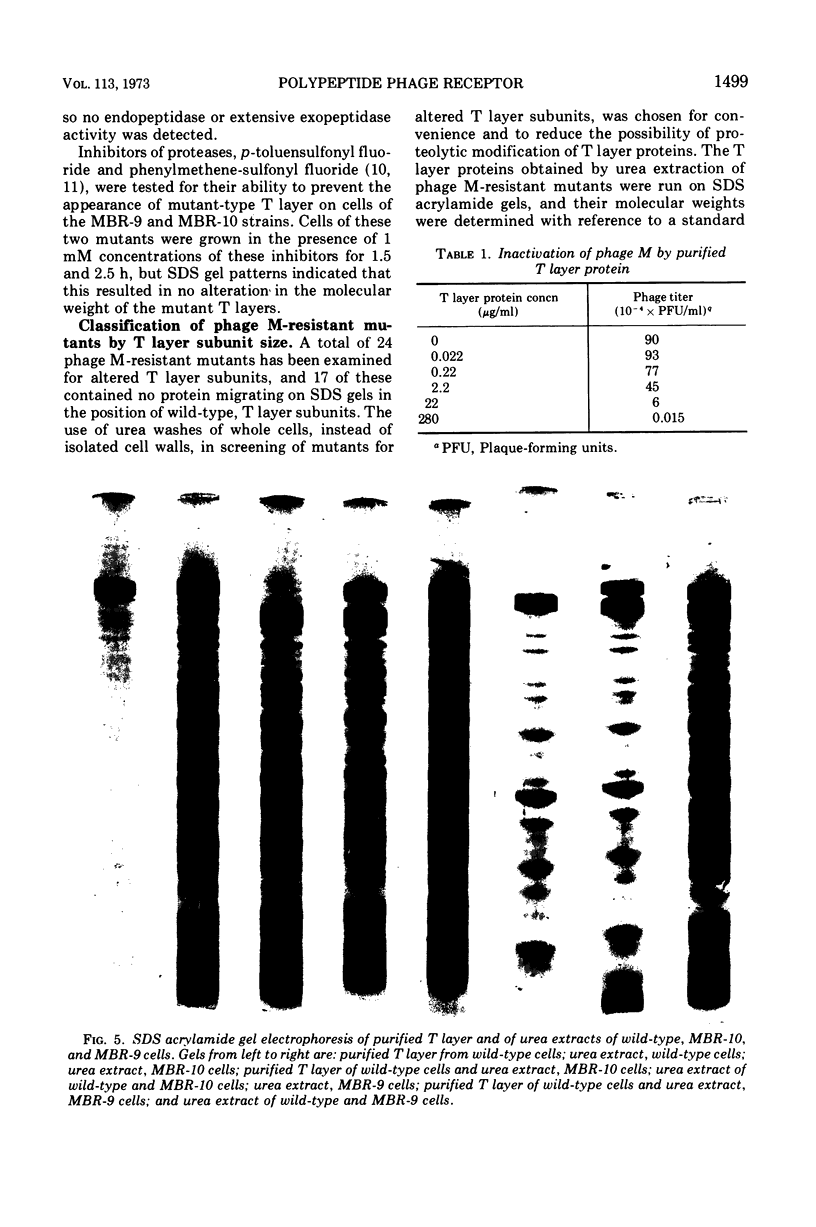
Bacillus sphaericus strain P-1 has previously been shown to have a tetragonally arrayed (T layer) protein which forms the outer layer of the cell wall. The T layer was quantitatively extracted from whole cells by 6 M urea, and the T layer subunits were purified by electrophoresis of the extracts on acrylamide gels containing 0.1% sodium dodecyl sulfate or 6 M urea. Using ethylene diacrylate cross-linked gels, the T layer was found to make up 16% of the total cellular protein. A virulent bacteriophage which is inactivated by purified T layer was isolated from soil. Twenty-four phage-resistant mutants were isolated, of which 17 had T layer subunits of increased mobility on sodium dodecyl sulfate acrylamide gels. No mutants devoid of T layer were found. Mutants were grouped into six classes according to the molecular weight of their T layer subunits. These ranged from that of the wild type, 150,000 down to 86,000. Two mutants from different classes were examined in detail. Cells of the mutant strains did not adsorb phage nor did cell walls isolated from these mutants inactivate phage. The amino acid composition of the T layers from mutants differed little from that of the wild-type T layer.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Glickman R. Method for determination of specific activity of proteins in polyacrylamide gels. Anal Biochem. 1970 Jun;35(2):314–320. doi: 10.1016/0003-2697(70)90190-9. [DOI] [PubMed] [Google Scholar]
- BADDILEY J. TEICHOIC ACIDS AND THE BACTERIAL CELL WALL. Endeavour. 1964 Jan;23:33–37. [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Douglas L. J., Wolin M. J. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry. 1971 Apr 27;10(9):1551–1555. doi: 10.1021/bi00785a007. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Orded substructure in the cell wall of Bacillus cereus. J Bacteriol. 1967 Nov;94(5):1778–1780. doi: 10.1128/jb.94.5.1778-1780.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Effects of protease inhibitors on protein breakdown and enzyme induction in starving Escherichia coli. Nat New Biol. 1971 Nov 10;234(45):51–52. doi: 10.1038/newbio234051a0. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Fleck J., Tipper D. J. Structure of the cell wall peptidoglycan of Lactobacillus casei RO94. Biochemistry. 1969 Sep;8(9):3567–3577. doi: 10.1021/bi00837a012. [DOI] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- Shaw D. R., Mirelman D., Chatterjee A. N., Park J. T. Ribitol teichoic acid synthesis in bacteriophage-resistant mutants of Staphylococcus aureus H. J Biol Chem. 1970 Oct 10;245(19):5101–5106. [PubMed] [Google Scholar]
- Shoer R., Rappaport H. P. Analysis of a Bacillus subtilis proteinase mutant. J Bacteriol. 1972 Feb;109(2):575–583. doi: 10.1128/jb.109.2.575-583.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weppelman R. M., Brinton C. C., Jr The infection of Pseudomonas aeruginosa by RNA pilus phage PP7: the adsorption organelle and the relationship between phage sensitivity and the division cycle. Virology. 1971 Apr;44(1):1–17. doi: 10.1016/0042-6822(71)90147-4. [DOI] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]