Abstract
Chronic kidney disease (CKD) is a risk factor for cardiovascular disease (CVD), although shared risk factors may mediate much of the association. We related CKD and CVD in the setting of specific CVD risk factors and determined whether more advanced CKD was a CVD risk equivalent. The Framingham Heart Study original cohort (n=2471, mean age 68 years, 58.9% women) was studied. Glomerular filtration rate (eGFR) was estimated using the simplified Modification of Diet in Renal Disease Study equation. CKD was defined as eGFR < 59 mL/min per 1.73 m2 (women) and < 64 (men) and Stage 3b CKD defined as eGFR 30-44 (women) and 30-50 (men). Cox Proportional Hazard models adjusting for CVD risk factors were used to relate CKD to CVD. We tested for effect modification by CVD risk factors. Overall, 23.2% of the study sample had CKD (n=574; mean eGFR 50 mL/min per 1.73 m2) and 5.3% had Stage 3b CKD (n=131; mean eGFR 42 mL/min per 1.73 m2). In multivariable models (mean follow-up time 16 years), Stage 3 CKD was marginally associated with CVD (HR=1.17, 95% CI 0.99-1.38, p=0.06), whereas Stage 3b CKD was associated with CVD [HR=1.41, 95% CI 1.05-1.91, p=0.02]. Upon testing CVD risk equivalency, the risk of CVD for Stage 3b CKD among participants with prior CVD was significantly lower as compared to participants with prior CVD and no Stage 3b CKD (age- and sex-adjusted HR for CVD = 0.66 [95% CI 0.47 to 0.91], p=0.01). Low HDL modified the association between CKD and CVD (p-value=0.004 for interaction). Stage 3b CKD is associated with CVD but is not a CVD risk equivalent. In conclusion, CVD risk in the setting of CKD is higher in the setting of low HDL cholesterol.
Introduction
We sought to study the association between CKD and CVD in an unselected, community based cohort in order to quantify the CVD risk associated with more advanced CKD within the Stage 3 CKD category. We also sought to determine if this more advanced subset of Stage 3 CKD may in fact constitute a CVD risk equivalent. Finally, we tested the association of CKD with CVD in the setting of different CVD risk factor profiles.
Materials and Methods
The Framingham Heart Study is a community-based prospective cohort study of cardiovascular disease and its risk factors, which began in 1948, consisting of 5209 men and women in the original cohort.1 Participants were invited to attend examinations every two years. The study sample for the present investigation consists of original cohort participants attending examination cycle 15 (1977 to 1979). Of 2632 participants attending the 15th examination cycle, 67 had missing covariate data, and 94 had missing creatinine values, leaving a final sample size of 2471. All analyses with the end points of CVD and CHD as end-points excluded individuals with prevalent CVD (n=396) and had a total of 2075 participants. All analyses with the endpoints of all-cause mortality and CVD-mortality included those with prevalent CVD and therefore had a total of 2471 participants.
We used the simplified Modification of Diet in Renal Disease (MDRD) Study equation2,3 to estimate kidney function by glomerular filtration rate (eGFR). We defined CKD based on the National Kidney Foundation Disease Outcome Quality Initiative working group definition of kidney disease, which defines CKD as GFR < 60 mL/min per 1.73 m2.2 We have found that the use of this cut point classifies approximately 50% more women as having kidney disease than men; therefore we modified the definition of CKD and reclassified kidney disease as a GFR at or below the sex-specific fifth percentile (59 mL/ min per 1.73 m2 in women and 64 mL/min per 1.73 m2 in men).4 We also examined stage 3a CKD, defined as GFR 45 to <59 mL/min per 1.73 m2 in women and 51 to <64 mL/min per 1.73 m2 in men, and stage 3b CKD, defined as GFR 30-44 mL/min per 1.73 m2 in women and GFR 30-50 mL/min per 1.73 m2 in men.
We used the modified Jaffe method to measure serum creatinine. We calibrated our creatinine values using a 2-step process in order to account for variation of creatinine across laboratories. First, National Health and Nutritional Examination Survey III creatinine values were calibrated to the Cleveland Clinic Laboratory, requiring a correction factor of 0.23 mg/dL (20.3 μmol/L).5 Age-and-sex specific (20-39, 40-59, 60-69, ≥70 yrs) creatinine values were then aligned with the corresponding corrected National Health and Nutrition Examination Survey III age-and-sex specific means.4
At the 15th examination cycle, all the participants underwent a routine physical examination, anthropometry, and laboratory assessment of vascular risk factors as has been previously described. Plasma glucose, total and HDL cholesterol were measured on non-fasting morning samples. Participants with a systolic blood pressure ≥140 mm Hg, or a diastolic blood pressure ≥ 90 mm Hg (mean reading of 2 readings taken by an examining physician) or receiving medication for treatment of hypertension were defined as having hypertension. Diabetes was defined as a casual glucose ≥ 200 mg/dL or treatment with either insulin or an oral hypoglycemic agent. Participants were considered to be current smokers if they smoked at least one cigarette per day for the last year. Medication use was ascertained by a physician- administered questionnaire. Body mass index (BMI) was defined as weight in kilograms divided by the square of height in meters.
Cardiovascular disease and mortality endpoints were determined by continuous events surveillance and adjudicated by a three-physician panel as has been previously described.6 We defined a cardiovascular (CVD) event as the occurrence of coronary death, myocardial infarction, coronary insufficiency, angina pectoris, atherothrombotic stroke, intermittent claudication, or cardiovascular death. Coronary heart disease (CHD) events were defined as coronary death, myocardial infarction, or hospitalized coronary insufficiency. The follow-up period was from the 15th examination to December 31, 2004.
Descriptive statistics of the exposure and covariates were performed. Cox proportional hazard models were used to relate CKD with CVD, CHD, CVD mortality and all-cause mortality. The first set of analyses sought to quantify the risk of CVD, CHD, all-cause mortality and CVD-mortality among individuals with overall and Stage 3b CKD. Multivariable analyses models for the outcomes of CVD and CHD adjusted for age, sex, body mass index, total/HDL cholesterol, smoking, diabetes, hypertension treatment and systolic blood pressure among individuals free of CVD at baseline. Analyses of all-cause mortality or CVD mortality included individuals with prevalent CVD at baseline and additionally adjusted for prevalent CVD.
In order to determine whether Stage 3b CKD constituted a CVD risk equivalent, we determined the hazard ratios for incident CVD and CHD comparing the subgroup with Stage 3b CKD, no prevalent CVD to the referent subgroup with no Stage 3b CKD/prevalent CVD. Additionally, we compared the risks of incident CVD and CHD for the following three subgroups each compared with the referent group (no Stage 3b CKD, no prevalent CVD): 1) + Stage 3b CKD, no prevalent CVD; 2) no Stage 3b CKD, + prevalent CVD; 3) + Stage 3b CKD, +prevalent CVD. We constructed Kaplan-Meir curves for CVD-mortality by CKD status.
To quantify CVD risk among individuals with different risk factor profiles, 4 categories were created. For example, to quantify the CVD risk among individuals with both CKD and hypertension, the age- and sex-adjusted hazard ratios for incident CVD were analyzed for the following three groups each compared to the referent group (no CKD/no HTN): 1) +CKD/no HTN; 2) no CKD/+HTN; and 3) +CKD/+HTN. Similar analyses were performed for diabetes mellitus, low HDL (< 40 mg/dL in men and < 50 mg/dL in women) and obesity (BMI ≥ 30 kg/m2). Finally, we tested for effect modification (on a multiplicative scale) of associations between CKD and CVD by diabetes, hypertension and low HDL cholesterol, and smoking.
We tested the adequacy of proportional hazard assumption by evaluating the Schoenfeld residual plots for each of the predictors in the model and the slope of generalized linear regression of the scaled Schoenfeld residuals on functions of time. The assumption of proportionality of hazards was met for the CVD and CHD outcomes but was violated for mortality outcomes using the entire duration of follow-up. Therefore we truncated follow-up for mortality at 10 years of follow-up. With the follow-up period truncated at ten years the proportionality hazards assumption was met. Therefore models considering the outcome of mortality were based on ten-years of maximum follow-up time.
Results
The mean age of our study sample was 68 years and 58.9% were women. The mean overall follow-up time was 16 years. Among study participants, 574 (23.2%) had CKD. Of these, 131 (22.8%) had Stage3b CKD. Those with CKD were older, tended to have lower mean HDL-cholesterol levels, and had a higher prevalence of hypertension and diabetes (Table 1). Relative to those with Stage 3a CKD, individuals with Stage 3b CKD were older, more likely to be hypertensive, have diabetes, and had high rates of prevalent CVD. The eGFR among those with stage 3b CKD was 42 mL/min per 1.73 m2 compared to 54 mL/min per 1.73 m2 among those with Stage 3a CKD.
Table 1. Baseline characteristics for sample including those with prevalent cardiovascular disease at baseline examination (n=2471), n's represent mean ± standard deviation, or % where indicated.
| Variable | No CKD
(n=1897) |
CKD* (n=574) |
Stage 3a** CKD
(n=430) |
Stage 3b† CKD
(n=131) |
|---|---|---|---|---|
| Age (years) | 68±7 | 71±8 | 70±7 | 74±7 |
| Women | 1123 (59.2%) | 333 (58.0%) | 242 (56.3%) | 83 (63.4%) |
| Estimated Glomerular Filtration Rate (mL/min per 1.73 m2) | 93 ± 31 | 50±8 | 54 ±5 | 42 ± 5 |
| Body Mass Index (kg/m2) | 26.5±4.3 | 26.2±4.31 | 26.2±4.2 | 26.3±4.8 |
| Total cholesterol (mg/dL) | 231±41 | 228±44 | 228±42.6 | 228±46 |
| Systolic Blood Pressure (mmHg) | 136±19 | 138±20 | 137±19.7 | 139±20 |
| Diastolic Blood Pressure (mmHg) | 76±10 | 75±11 | 76±10.6 | 74±12 |
| High density lipoprotein (HDL) cholesterol (mg/dL) | 50±15 | 48±16 | 49±15.9 | 46±16 |
| Total/HDL cholesterol ratio | 5.0±1.6 | 5.1±1.8 | 5.0 ±1.7 | 5.4 ±1.7 |
| Hypertension | 689 (36.4%) | 277 (48.4%) | 194 (45.3%) | 74 (56.5%) |
| Diabetes Mellitus | 146 (7.7%) | 75 (13.1%) | 51 (11.9%) | 22 (16.8%) |
| Smoker | 559 (29.5%) | 120 (20.9%) | 96 (22.3%) | 23 (17.6%) |
| Prevalent cardiovascular disease ‡ | 260 (13.7%) | 136 (23.7%) | 98 (22.8%) | 35 (26.7%) |
| Prevalent coronary heart disease § | 109 (5.8%) | 49 (8.5%) | 37 (8.6%) | 10 (7.6%) |
CKD=chronic kidney disease
HDL=high density lipoprotein
GFR < 59 mL/min per 1.73 m2 in women and < 64 mL/min per 1.73 m2 in men (this category includes Stage 3 and Stage 4 CKD)
GFR 45 to <59 mL/min per 1.73 m2 in women and 51 to <64 mL/min per 1.73 m2 in men
GFR 30-44 mL/min per 1.73 m2 in women and 30-50 mL/min per 1.73 m2 in men
Cardiovascular disease events were defined as coronary death, myocardial infarction, coronary insufficiency, angina pectoris, atherothrombotic stroke, intermittent claudication, or cardiovascular death.
Coronary heart disease events were defined as coronary death, myocardial infarction, or hospitalized coronary insufficiency.
Age-and sex-adjusted CVD event rates are shown in Table 2. Overall, there were 874 CVD events, 199 of which were among participants with CKD. In age-and sex-adjusted models, participants with both overall CKD and stage 3b CKD had elevated risks of CVD (Table 3). Overall CKD was marginally associated with CVD upon multivariable adjustment (p=0.06), whereas stage 3b CKD was significantly associated with CVD upon multivariable adjustment. Stage 3a CKD was not associated with CVD in either age-and sex- or multivariable-adjusted models.
Table 2. Cardiovascular disease, coronary heart disease, all-cause mortality* and cardiovascular disease-mortality event rates *.
| Variables | Number at Risk | Number of Events | Age- and Sex- Adjusted Event Rate
(per 1000 person year) |
|---|---|---|---|
| Cardiovascular Disease | |||
| No CKD | 1637 | 675 | 3.15 |
| CKD** | 438 | 199 | 3.97 |
| Stage 3a CKD† | 332 | 143 | 3.28 |
| Stage 3b CKD‡ | 96 | 51 | 5.12 |
| Coronary Heart Disease | |||
| No CKD | 1637 | 484 | 2.12 |
| CKD | 438 | 149 | 2.75 |
| Stage 3a CKD† | 332 | 112 | 2.59 |
| Stage 3b CKD‡ | 96 | 35 | 4.13 |
| Mortality | |||
| No CKD | 1897 | 514 | 2.27 |
| CKD | 574 | 228 | 3.06 |
| Stage 3a CKD† | 430 | 154 | 2.65 |
| Stage 3b CKD‡ | 131 | 63 | 4.16 |
| Cardiovascular Disease Mortality | |||
| No CKD | 1897 | 188 | 0.82 |
| CKD | 574 | 92 | 1.22 |
| Stage 3a CKD† | 430 | 65 | 1.14 |
| Stage 3b CKD‡ | 131 | 24 | 1.91 |
CKD=chronic kidney disease
Mortality and CVD mortality follow-up truncated at ten-years
Cardiovascular disease events were defined as coronary death, myocardial infarction, coronary insufficiency, angina pectoris, atherothrombotic stroke, intermittent claudication, or cardiovascular death. Coronary heart disease events were defined as coronary death, myocardial infarction, or hospitalized coronary insufficiency.
Analysis done in persons without prevalent CVD
GFR < 59 mL/min per 1.73 m2 in women and < 64 mL/min per 1.73 m2 in men
GFR 45 to <59 mL/min per 1.73 m2 in women and 51 to <64 mL/min per 1.73 m2 in men
GFR 30-44 mL/min per 1.73 m2 in women and 30-50 mL/min per 1.73 m2 in men
Table 3. Chronic kidney disease and the risk of incident cardiovascular disease, coronary heart disease and mortality among Framingham Heart Study Offspring Participants.
| CVD | p-value | CHD | p-value | All-cause mortality | p-value | CVD-mortality | p-value | |
|---|---|---|---|---|---|---|---|---|
| Chronic Kidney Disease* | ||||||||
|
| ||||||||
| Age and sex | 1.19(1.02-1.40) | 0.03 | 1.23(1.02-1.48) | 0.03 | 1.24(1.06-1.45) | 0.01 | 1.39(1.08-1.79) | 0.01 |
| Multivariable | 1.17(0.99-1.38) | 0.06 | 1.21(1.00-1.50) | 0.05 | 1.14(0.96-1.34) | 0.13 | 1.27(0.98-1.64) | 0.08 |
| Stage3a Chronic Kidney Disease** | ||||||||
| Age and Sex | 1.10(0.92-1.32) | 0.31 | 1.17(0.95-1.44) | 0.13 | 1.14(0.95-1.37) | 0.15 | 1.32(0.99-1.75) | 0.06 |
| Multivariable | 1.08(0.90-1.30) | 0.42 | 1.17(0.94-1.44) | 0.16 | 1.06(0.88-1.28) | 0.53 | 1.24(0.92-1.66) | 0.16 |
| Stage 3b Chronic Kidney Disease† | ||||||||
| Age and Sex | 1.50(1.12-2.01) | 0.007 | 1.39(0.98-1.97) | 0.07 | 1.37(1.05-1.80) | 0.02 | 1.50(0.96-2.32) | 0.07 |
| Multivariable | 1.41(1.05-1.91) | 0.02 | 1.31(0.91-1.86) | 0.14 | 1.20(0.90-1.59) | 0.21 | 1.29(0.82-2.01) | 0.27 |
CVD=cardiovascular
Multivariable models adjusted for age, sex, body mass index, total/HDL cholesterol, smoking, diabetes, hypertension treatment and systolic blood pressure
Mortality and CVD mortality follow-up truncated at ten-years
GFR < 59 mL/min per 1.73 m2 in women and < 64 mL/min per 1.73 m2 in men
GFR 45 to <59 mL/min per 1.73 m2 in women and 51 to <64 mL/min per 1.73 m2 in men
GFR 30-44 mL/min per 1.73 m2 in women and 30-50 mL/min per 1.73 m2 in men
Overall, there were 633 CHD events, 149 of which were among participants with CKD. In age-sex and multivariable-adjusted models, CKD was associated with CHD (Table 3). Among those with Stage 3b CKD, the magnitude of the HR for CHD was slightly lower than as compared to CVD (multivariable-adjusted HR 1.31, 95% CI [0.91-1.86], p-value=0.14). No significant associations were observed for Stage 3a CKD and CHD.
Overall, there were 742 deaths (Table 2). CKD and Stage 3b CKD were related to all-cause mortality in age-and sex-adjusted models but not in multivariable-adjusted models (Table 3). Stage 3a CKD was not associated with all-cause mortality in either age-and sex-adjusted or in multivariable-adjusted models. CKD was associated with CVD-mortality in age and sex adjusted models but only marginally upon multivariable adjustment (p=0.08). Stage 3b CKD was marginally associated with CVD-mortality in age and sex adjusted models (p=0.07); this was attenuated upon multivariable adjustment.
Among individuals with Stage 3b CKD but without CVD (+Stage 3b CKD/no CVD), the age- and sex-adjusted HR for CVD was 1.51 (95% CI 1.13-2.02, p-value=0.005) (Table 4). Among individuals without Stage 3b CKD but with CVD (no Stage 3b CKD/+CVD), the age- and sex-adjusted HR for CVD was 2.39 ([95% CI 2.01-2.83], p-value<0.0001).
Table 4. Stage3b chronic kidney disease as a cardiovascular disease risk equivalent: hazard ratios with 95% confidence intervals for incident cardiovascular disease and coronary heart disease.
| CVD | p-value | CHD | p-value | |
|---|---|---|---|---|
| CKD+/no CVD (n=96) | ||||
| Age and Sex | 1.51(1.13-2.02) | 0.005 | 1.42(1.00-2.01) | 0.05 |
| Multivariable | 1.44(1.07-1.94) | 0.02 | 1.34(0.94-1.91) | 0.10 |
|
| ||||
| No CKD/CVD+ (n=260) | ||||
| Age and Sex | 2.39(2.01-2.83) | <0.0001 | 2.58(2.13-3.12) | <0.0001 |
| Multivariable | 2.05(1.72-2.44) | <0.0001 | 2.21(1.81-2.69) | <0.0001 |
|
| ||||
| CKD+/CVD+ (n=35) | ||||
| Age and Sex | 2.38(1.48-3.83) | 0.0004 | 2.58(2.13-3.12) | <0.0001 |
| Multivariable | 1.99(1.23-3.22) | 0.005 | 2.21(1.81-2.69) | <0.0001 |
CKD=chronic kidney disease
CVD=cardiovascular disease
CHD=coronary heart disease
Reference group is no CKD/ no CVD (n=1637)
Multivariable models adjusted for age, sex, body mass index, total/HDL cholesterol, smoking, diabetes, hypertension treatment and systolic blood pressure
GFR < 59 mL/min per 1.73 m2 in women and < 64 mL/min per 1.73 m2 in men
GFR 30-44 mL/min per 1.73 m2 in women and 30-50 mL/min per 1.73 m2 in men
In order to test the hypothesis of risk equivalency, we compared CVD risks among those with Stage 3b CKD with and without CVD at baseline. In age- and sex-adjusted models, the risks of CVD were significantly lower in participants with Stage 3b CKD but without CVD as compared to participants without Stage 3b CKD but with CVD (HR for CVD = 0.66 [95% CI 0.47 to 0.91], p=0.01). Similar results were observed for CHD (HR 0.60 [95% CI 0.41 to 0.88], p=0.009).
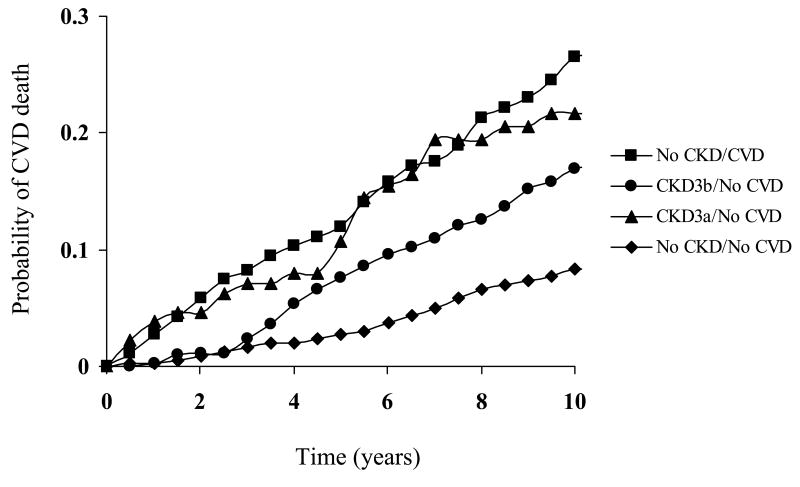
Figure 1 demonstrates the KM curves for CVD mortality; CVD-mortality was highest among those with prevalent CVD and no CKD, followed by those with Stage 3b CKD alone and then by those with Stage 3a CKD alone.
Figure 1.
Kaplan-Meir survival curves for cardiovascular disease mortality by chronic kidney disease status.
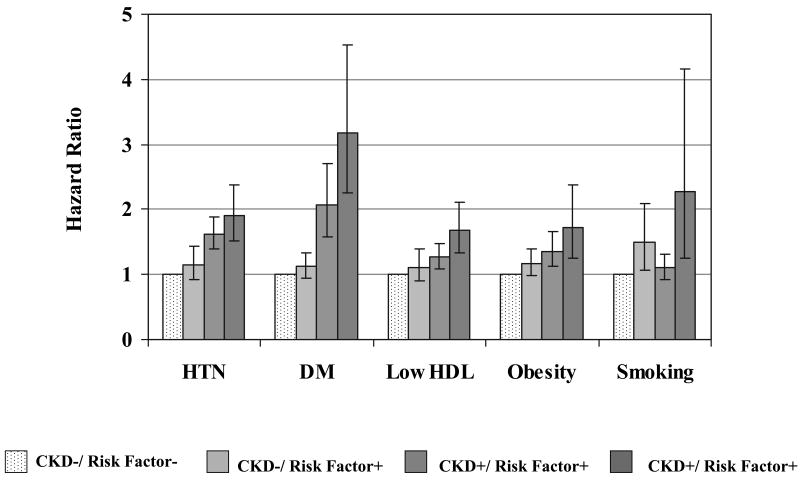
Individuals with CKD and low HDL were more likely to develop CVD on follow-up as compared to those with neither condition (age-and sex-adjusted HR=1.68, 95% CI=1.33-2.12, p<0.0001) (Figure 2). A significant interaction was observed between HDL and CKD (p-value for interaction term=0.004), suggesting that the combination of low HDL and CKD conferred a greater risk of developing CVD than either individual condition alone. Among individuals with both CKD and DM, the HR for CVD was 3.19 (95% CI 2.25-4.52, p <0.001), which was higher than for either individual condition, although the interaction term was not significant (p-value=0.17). Similar patterns were seen for subgroups of CKD and hypertension with regards to the outcome of CVD (p-value interaction 0.93; age-and sex-adjusted HR for those with CKD and hypertension vs. neither condition =1.90, 95% CI=1.52-2.39, p<0.0001), obesity (p-value interaction 0.72; age-and sex-adjusted HR=1.71, 95% CI=1.24-2.38, p<0.0001) and smoking (p-value interaction 0.36; age-and sex-adjusted HR=2.28, 95% CI=1.25-4.17, p=0.007).
Figure 2.
Chronic kidney disease and incident cardiovascular disease: risk factor subgroups. Bars represent 95% confidence intervals. Age- and sex-adjusted p-values for the interaction of CKD with the following risk factors: hypertension p=0.93; diabetes p=0.17; low HDL cholesterol p=0.004; obesity p=0.72 smoking p=0.35.
Sample sizes for figure.
| CKD-/Risk factor- | CKD-/Risk factor+ | CKD+/Risk factor- | CKD+/Risk factor+ | |
|---|---|---|---|---|
| Hypertension | 1042 | 559 | 46 | 44 |
| Diabetes Mellitus | 1501 | 105 | 76 | 14 |
| Low HDL cholesterol | 995 | 611 | 44 | 46 |
| Obesity | 1345 | 261 | 74 | 16 |
| Smoking | 1125 | 74 | 481 | 16 |
CKD=chronic kidney disease; HDL=high density lipoprotein
Discussion
In a community-based cohort of men and women, Stage 3 CKD was associated with a 20% increased risk of CVD and CHD after adjustment for age and sex, whereas Stage 3b CKD was associated with a 50% increased risk of CVD, which remained significant in multivariable-adjusted models. CKD and Stage 3b CKD were associated with all-cause mortality in age-and sex-adjusted models but not after accounting for CVD risk factors and prevalent CVD. Stage 3b CKD did not constitute a CVD risk equivalent. The association between CKD and CVD varied based on different CVD risk factor combinations and was highest among those with DM. Lastly, those with CKD and low HDL had a significantly higher risk of CVD than either condition alone.
Our study demonstrated modest multivariable-adjusted associations between Stage 3 CKD and CVD. This finding is consistent with prior studies of kidney disease and CVD which were conducted in low risk community-based cohorts similar to the Framingham Heart Study.7-9 The hazard ratios for associations between CKD and CVD from the present investigation were somewhat lesser in magnitude as compared to those demonstrated in a previous investigation in the Atherosclerosis Risk in Communities (ARIC) Study (multivariable adjusted HR for CVD=1.58, 95% CI:1.01-2.47).10 Whereas the ARIC study was comprised of one-quarter black individuals, the Framingham Heart Study is largely comprised of nearly 100% white individuals of European ancestry. In a separate study utilizing pooled data from ARIC, the Framingham Heart Study and the Cardiovascular Health Study, CKD risks were higher in black as compared to white individuals for the composite endpoint of all-cause mortality or CVD-mortality.11 Furthermore, prior data from the Third National Health Examination Survey suggests increased CVD prevalence with successively lower GFR among black as compared to white individuals even after accounting for traditional CVD risk factors.12 Therefore racial differences may explain the lower magnitude of effect size in our study as compared to prior investigations.
Our findings that Stage 3b CKD is significantly associated with CVD and CHD are consistent with prior data from patients in a large integrated healthcare database that demonstrated a graded relationship between CKD and CVD which rose most sharply at a GFR < 45 mL/min per 1.73 m2.13 The study sample utilized in this prior analysis likely had higher associated CVD risk given the need to have a medical indication for creatinine assessment in a clinical setting than our community-based cohort. Indeed, the corresponding hazard ratios for CVD for individuals with GFRs corresponding to the Stage 3b range in the previous analysis (or 30-44 mL/min per 1.73 m2) were higher than in the prior study (HR=2.0; 95 % CI=1.9-2.1) as compared to in our study (1.41; 95 % CI=1.05-1.91). Our study provides evidence extending prior findings that among a relatively low risk sample unselected for CKD, Stage 3b CKD is associated with incident CVD.
The “risk equivalent” concept has been highlighted by recent epidemiologic evidence demonstrating that diabetes mellitus may confer an equivalent risk of incident myocardial infarction as compared to a prior diagnosis of MI.14 The risk equivalency status of diabetes mellitus is further reflected in the National Cholesterol Education and Prevention Advanced Treatment Panel III guidelines which recommend the same treatment thresholds for individuals with diabetes or prior CHD.15
Prior data from the ARIC study demonstrate that CKD is not a CVD risk equivalent.16 We extend these findings by demonstrating that Stage 3b CKD does not confer the same risk of CVD as a prior diagnosis of CVD in a community based sample. Whether more advanced forms of Stage 3b CKD indeed confer CVD risk equivalency is an important area of further investigation.17,18
Our findings that low HDL and CKD have a synergistic effect on CVD risk is a novel finding. A previous study in the Cardiovascular Health Study of elderly community dwelling individuals (age ≥ 65 years) did not find a positive interaction between HDL < 40 mg/dL and CKD on risk of CVD death.19 Older aged individuals, lack of sex-specific HDL cut-points and a use of a different endpoint (CVD death versus CVD) are potential explanations for differing results between our study as compared to the previous study.19 If confirmed in subsequent studies, the question of whether HDL levels can provide discrimination in CVD risk stratification among those with CKD should be an area of future research.
The combination of CKD and DM conferred a 3-fold risk increase in CVD, greater than those with CVD alone, suggesting that individuals with both CKD and DM may constitute a CVD risk-equivalent. Given that DM is already considered a CHD risk-equivalent, this finding is less important in terms of clinical guidelines. However, it underscores the high risk of CVD among individuals with both of these conditions.
Our finding that having both CKD as well as hypertension conferred a greater relative risk of CVD compared to CKD or hypertension alone is not entirely surprising. Previous data suggest high relative risks for hypertension and CHD in the setting of CKD.20
Obesity is associated with CKD21 and components of the metabolic syndrome.22 National data suggests a steady rate of CKD over the past twenty years,23 a rise in obesity prevalence over the past several decades,24,25 and an increase in obesity incidence over the past fifty years.26 The joint association between obesity and CKD on CVD risk should be examined in future studies given escalating obesity rates and demonstrated pathophysiologic links between obesity and CKD.
Within a community-based cohort, a subset of individuals with more advanced CKD (Stage 3b CKD) are at increased risk for CVD. The findings of previous studies of CKD and CVD in higher risk, selected cohorts should not necessarily be extrapolated to the community. Our findings are important given that Stage 3 CKD has a much greater prevalence (7.7%) as compared to more advanced CKD (Stage 4 + Stage 5 CKD prevalence=0.35%) and represents the majority of CKD in the United States.27 Our findings do not support the concept that individuals with Stage 3b CKD should receive equivalent risk modification as compared to those with prevalent CVD solely in terms of CVD risk prevention.
The strengths of our study include a community-based sample not selected for CKD, reducing the risk of referral or selection bias. The Framingham Heart Study has detailed assessment of CVD risk factors and rigorously adjudicated CVD outcomes. Several limitations should be acknowledged. Our study sample is limited both geographically and ethnically, as our participants are primarily white individuals of European ancestry. Our definition of CKD is limited to a single measurement of serum creatinine on one occasion, not measured over a period of three months or greater as has been defined by the National Kidney Foundation. Further, we used the simplified MDRD study equation to estimate GFR, instead of measuring it directly. In order to improve the validity and accuracy of the MDRD equation, we indirectly calibrated our creatinine values. Nonetheless, if our GFR estimates were misclassified as a result of estimation from the MDRD equation, this would have biased our estimates towards the null value. We were unable to account for albuminuria, which may have led to an underestimation of CKD in our study. Lastly, we may have had limited statistical power to detect very modest effects between CKD and CVD or CHD outcomes.
Acknowledgments
Dr. Fox had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsor: The funding sources had no role in the study design, analyses, or drafting of the manuscript. The NHLBI reviews all manuscripts submitted for publication but it was not involved in the decision to publish.
The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (N01-HC-25195)
Footnotes
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dawber TR, Kannel WB. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 6.Cupples LA, D'Agostino RB., Sr . Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Heart Study: an Epidemiologic Investigation of Cardiovascular Disease. Washington, DC: NIH Publication; 1987. pp. 87–203. [Google Scholar]
- 7.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 8.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28:557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 11.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen HT, Stack AG. Ethnic disparities in cardiovascular risk factors and coronary disease prevalence among individuals with chronic kidney disease: findings from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1716–1723. doi: 10.1681/ASN.2005010056. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 14.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 15.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol. 2006;48:1183–1189. doi: 10.1016/j.jacc.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 19.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 21.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 26.Parikh NI, Pencina MJ, Wang TJ, Lanier KJ, Fox CS, D'Agostino RB, Vasan RS. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med. 2007;120:242–250. doi: 10.1016/j.amjmed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]