Abstract
Current pancreatic islet transplantation protocols achieve remarkable short-term success, but long-term insulin independence remains elusive. Hypoxic and inflammatory insults cause substantial early post-transplant graft loss while allo/autoimmunity and immunosuppressive toxicity threaten long term graft mass and function. Exendin-4 (Ex4) is a GLP-1 receptor agonist that promotes β-cell proliferation, survival and differentiation. To determine if Ex4 displays potential as a graft-supportive agent, we transplanted 500 murine islets under the kidney capsule of syngeneic or allogeneic streptozotocin-treated recipient mice and immediately initiated daily treatment with vehicle or Ex4. Graft β-cell proliferation, death and vascularity were assessed 1, 3 and 10 days after syngeneic islet transplant. For allogeneic recipients, blood glucose and body weight were assessed until glycemic deterioration. Ex4 did not promote graft β-cell proliferation, reduce β-cell death or enhance graft vascularity over the first 10 days after syngeneic islet transplant. A trend toward prolongation of post-transplant euglycemia was onserved with Ex4 treatment in non-immune suppressed allograft recipients, but its use in this setting was associated with frequent, severe hypoglycemia over the first 2 post-transplant days. Our findings do not support a beneficial effect of Ex-4 on islet grafts during the critical early post-transplant period and demonstrate its significant hypoglycemic potential in the first days after islet transplantation in mice. Optimal application of GLP-1 receptor agonists in the transplant setting may require earlier intervention prior to and/or during islet isolation for peri-transplant cytoprotection and administration beyond the period of engraftment for long term proliferative and survival benefit.
Keywords: GLP-1, insulin, diabetes, hypoglycemia
INTRODUCTION
Despite recent progress, the widespread application of pancreatic islet transplantation to the treatment of type 1 diabetes remains limited by multiple factors that include the scarcity of donor islets, the toxicities of the requisite immunosuppressive agents, and the failure of recipients to maintain long-term insulin independence (19, 39, 41). The Edmonton group recently reported that, despite short-term insulin independence rates exceeding 90%, prolonged insulin independence is rare in their recipients (39). This indicates that islet graft functional capacity declines over time as the graft is subjected to multiple insults.
Islet graft β-cell death is accelerated in the first days after transplantation and the majority of transplanted graft mass may be lost during this time (10, 36, 47). Initial insults include inflammation and hemodynamic instability in donors prior to harvest, hypoxia as islets are devascularized during the isolation procedure, and the acute recipient inflammatory response (7, 8, 14, 34). Islets that survive and successfully engraft must function in an ectopic location while being subjected to allogeneic and autoimmune attack as well as β-cell toxic immunosuppressive agents (9, 21, 22, 29, 30). Novel approaches to supporting islet graft mass and function have demonstrated promise, but until recently none has been translated to the clinical investigational setting (7, 13, 17, 20).
Agonists of the glucagon-like peptide-1 (GLP-1) receptor have multiple β-cell trophic properties, and have been used extensively and safely in humans during their development for the treatment of type 2 diabetes (2, 6, 11, 18, 27). They can promote pancreatic β-cell mass through proliferative and cytoprotective mechanisms, and they potently enhance β-cell function (5, 16, 31, 32, 49). We have also observed a beneficial effect of one of these agents, Exendin-4 (Ex-4), on islet vascularity in intrauterine growth retarded (IUGR) rats (J. N. Ham, B.M. Desai, M. Crutchlow, R. Simmons, and D. Stoffers, unpublished observation). It is not known if these effects will be seen in islet grafts, or whether their application in the islet transplant setting will raise new safety issues. The goal of this study was to employ mouse models to: 1) explore the effects of Ex-4 on islet graft β-cell proliferation, β-cell death and graft revascularization in the early post-transplant period; and 2) determine the impact of Ex-4 on the durability of islet allograft function in non-immune suppressed recipients.
MATERIALS AND METHODS
Animals
Mice were purchased from Charles River Laboratories, housed under standard conditions, and allowed free access to food and water. Recipients were 8–10 week old male C57BL/6 mice, rendered diabetic through the one time intraperitoneal administration of 200mg/kg streptozotocin (Sigma, St. Louis, MO) 5–6 days prior to transplantation, and all had blood glucose concentrations > 350mg/dl on the day of transplantation. Donors were C57BL/6 and BALB/c retired breeder females, aged 6–12 months, in syngeneic and allogeneic transplant experiments, respectively. These studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Exendatide was purchased from Bachem.
Islet transplantation
Anesthesia for both donors and recipients was achieved through intra-peritoneal administration of ketamine (50mg/kg) and xylazine (20mg/kg) Pancreatic islets were isolated through bile duct collagenase perfusion, enzymatic and mechanical pancreas disruption and purification on a Ficoll density gradient (34). After isolation, 500 islets were immediately transplanted into the recipient renal subcapsular space. Pooled islet preparations were used for each set of transplants.
Experimental paradigm
Syngeneic transplant experiment
Three sets of syngeneic islet transplantation experiments were carried out. After transplantation, recipients were randomized to intraperitoneal treatment with Ex-4 or vehicle. Vehicle was composed of 0.9% saline containing 0.1% bovine serum albumin (BSA). Random blood glucose levels were monitored with a Freestyle glucose meter (TheraSense, Alameda, CA) on days 1–3 and 5 of the next 7 days. Intraperitoneal administration of 5-bromo-2-deoxyuridine (BrDU; Sigma, St. Louis, MO; 200mg/kg) was performed prior to graft harvest as described in Results and graft-bearing kidneys were fixed overnight in 4% paraformaldehyde, and embedded in paraffin.
Allogeneic transplant experiments
Recipients were randomized to Ex-4 or vehicle treatment immediately after transplantation and then daily until the recurrence of hyperglycemia, defined as blood glucose>200mg/dl on 2 consecutive days. Random blood glucose measurements were performed on 5 of the first 7 post-transplant days and daily thereafter; body weight was measured on days 1, 5, 9 and 14. Primary graft function was defined in this experiment as average blood glucose concentration<200mg/dl on post-transplant days 7–10. Blood glucose and body weight were monitored as described in Results. Blood was collected from the tail vein (Microvette CB 300 tubes; Sarstedt, Numbrecht), and plasma was separated by centrifugation and submitted for multiplex immunoassay measurement of insulin (detection limit 56pM) and glucagon (Linco Diagnostics; St. Charles, MO.).
Streptozotocin diabetic C57BL6 recipients were transplanted in 3 groups, with all recipients receiving 500 BALB/c islets beneath the kidney capsule. After transplant, recipients were randomized to treatment with Ex-4 or vehicle, immediately and once daily thereafter until the recurrence of hyperglycemia. Serial blood glucose measurements were used to monitor graft function. An initial dose of 20 nmol/kg/day Ex-4 was chosen; however, 2 of 3 recipients randomized to Ex-4 in the first group died within 5 days of transplant, associated in one case with hypoglycemia (blood glucose <35 mg/dl on post-transplant days 1 and 2). The Ex-4 dose was lowered to 10 nmol/kg/day for the second group, but 2 of 4 Ex-4 treated recipients were hypoglycemic (random blood glucose<50mg/dl) on the first post-transplant day, although death did not occur. Therefore, for the third group the Ex-4 dose was escalated from 5 nmol/kg on days 0 and 1, to 10 nmol/kg on days 2–5 and 20 nmol/kg/day thereafter. Neither hypoglycemia nor death was seen in these recipients. In total, 9 Ex-4 and 9 vehicle treated mice survived to be evaluated with 7/9 in each group achieving primary graft function. In a separate experiment to evaluate the mechanism of Ex-4 associated hypoglycemia, recipients received Ex-4 (20nmol/kg) or vehicle immediately after transplantation and on post-transplant days 1 and 2.
Morphologic graft analysis
Sections (6 μm) were rehydrated on a standard alcohol gradient. Primary antisera were: guinea pig anti-insulin (DakoCytomation, Carpinteria, CA), sheep anti-BrDU (US Biologicals, Swampscott, MA), Cy-3 conjugated mouse anti-digoxigenin (Jackson ImmunoResearch, WestGrove, PA), and rabbit anti-Ki67 (Abcam, Cambridge, MA). Secondary antisera (Jackson ImmunoResearch) were labeled with Cy-2 or Cy-3. Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) was performed with the ApopTag Peroxidase In Situ Death Detection Kit (Chemicon International, Temecula, CA) followed by immunofluorescent detection of insulin and digoxigenin. Nuclear labeling was performed with 4, 6-diamidino-2-phenyl-indole, dihydrochloride (DAPI, Molecular Probes, Eugene, OR) and autoflourescence quenched with Sudan Black (BDH Laboratory Supplies, Poole, England). To assess graft vascularity, 100μg Flourescein-Lycopersicon Esculentum Lectin (Vector Laboratories, Burglingame, CA) was infused via the inferior vena cava 5 minutes prior to harvest. These sections were mounted with flourescent mounting medium (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Image capture/analysis
For the determination of β-cell proliferation and death, the entire islet graft on each section was captured in multiple images at 20 X magnification using a Cool Snap digital camera (Photometrics, Huntington Beach, CA) connected to an Eclipse E600 microscope (Nikon, Japan). An average of 1130 β-cell nuclei was counted for each graft and β-cell proliferation rate (rate = BrDU positive β-cell nuclei/all β-cell nuclei x 100) was quantified. β-cell death rates (rate = TUNEL+ β-cell nuclei/total β-cell nuclei) were quantified with the same approach with an average of 1466 β-cell nuclei being counted for each graft. To assess graft vascularity, a QICAM Fast 1394 camera (Q Imaging, Burnaby, BC) captured three graft regions at 20x magnification and image analysis was performed using IP Lab software (Scanalytics, Inc., Fairfax, VA). Four images were captured from each region, with the plane of focus adjusted by 1 micron successively (LEP MAC 5000 Z-Focus Attachment, Ludl Electronic Products Ltd., Hawthorne, NY) and compressed into a 2-dimensional image, which was used for final analysis. Vascularity was calculated as the percent of graft area (β-cell component) occupied by endothelium (endothelial area (uM2)/total graft area (uM2) x 100).
Data analysis
β-cell proliferation and death rates, graft vascularity, random blood glucose concentration, duration of post-allogeneic transplant euglycemia, plasma insulin and glucagon concentrations, and weight change from baseline are expressed as mean +/− SE, and compared between treatment groups with a t test. The incidence of hypoglycemia on post-transplant days 1 and/or 2 was compared between groups with a Fisher’s exact test.
RESULTS
Ex-4 administered after allogeneic murine islet transplantation under the kidney capsule
The first 2 weeks after islet transplantation has been identified as a critical time period during which β-cell death is initially accelerated, β-cell mass declines, and the critical process of graft revascularization occurs (10, 35). Therefore, we assessed the impact of Ex-4 on graft β-cell proliferation and death, and graft vascularity over the first 10 post-transplant days employing a syngeneic model to avoid the superimposition of allo-immune phenomena. Guided by published rodent studies demonstrating the β-cell trophic actions of Ex-4 at doses ranging from 0.1–24nmol/kg administered once daily, a dose of 10nmol/kg administered by once-daily intraperitoneal injection was chosen for this model (32, 42, 43, 45, 46, 49). Streptozotocin induced diabetic recipients received 500 islets under the kidney capsule and were then randomized to treatment with Ex-4 or vehicle (n=14/group), immediately and once daily thereafter. Blood glucose levels tended to be lower in Ex-4 treated recipients throughout the post-transplant period, and were significantly lower on days 2 and 3 (Figure 1).
Figure 1. Glycemic response to syngeneic islet transplantation.
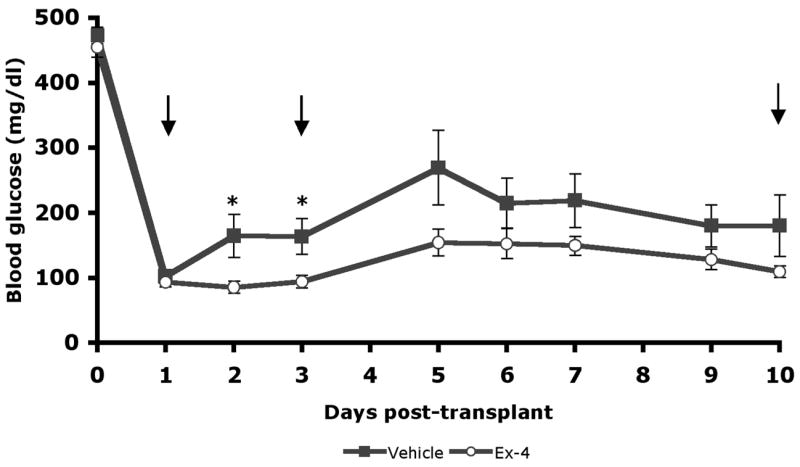
Streptozotocin diabetic recipients received kidney capsule transplants of 500 donor islets (curative dose) and were treated with 10nmol/kg/d Ex-4(n=13) or vehicle(n=14). Random blood glucose (mg/dl) measurements of graft bearing mice prior to harvest depicted as mean +/− SE (*P<0.05). Grafts were harvested for morphologic analysis at 1, 3, and 10 days as indicated by arrows; the number of mice represented decreases correspondingly.
No effect of Ex-4 on graft β-cell proliferation in the early post-transplant period
Graft-bearing kidneys were harvested from 14 vehicle treated recipients (5, 4, and 5 at 1, 3, and 10 days, respectively), and 13 Ex-4 treated recipients (5, 4, and 4 at 1, 3, and 10 days, respectively; 1 death). BrDU was administered 6 hours prior to graft harvest at 1 and 3 days. Although BrDU incorporation was detected throughout the renal cortex of graft bearing kidneys, no graft BrDU+/insulin+ cells were observed. Lack of graft β-cell proliferation at these time points was confirmed by the absence of nuclear Ki67 expression. Therefore, BrDU was administered 24 and 6 hours prior to graft harvest at 10 days post-transplantation to increase sensitivity for low proliferation rates. β-cell proliferation was easily detected (Figure 2A). Rates of β-cell proliferation were similar between vehicle (n=5) and Ex-4 (n=3) treated recipients (Figure 2B: vehicle vs. Ex-4: 3.5 +/− 0.14% vs. 2.4 +/− 0.74%; P=0.25). Notably, the early difference in glycemia between groups did not appear to influence proliferation rates, although a non-significant trend toward lower proliferation was observed in Ex-4 treated recipients. Nuclear Ki67 labeling was examined and demonstrated in all grafts, confirming increased proliferation compared to the earlier time points (data not shown).
Figure 2. Ex-4 does not impact graft β-cell proliferation, graft β-cell death, or graft vascularity.
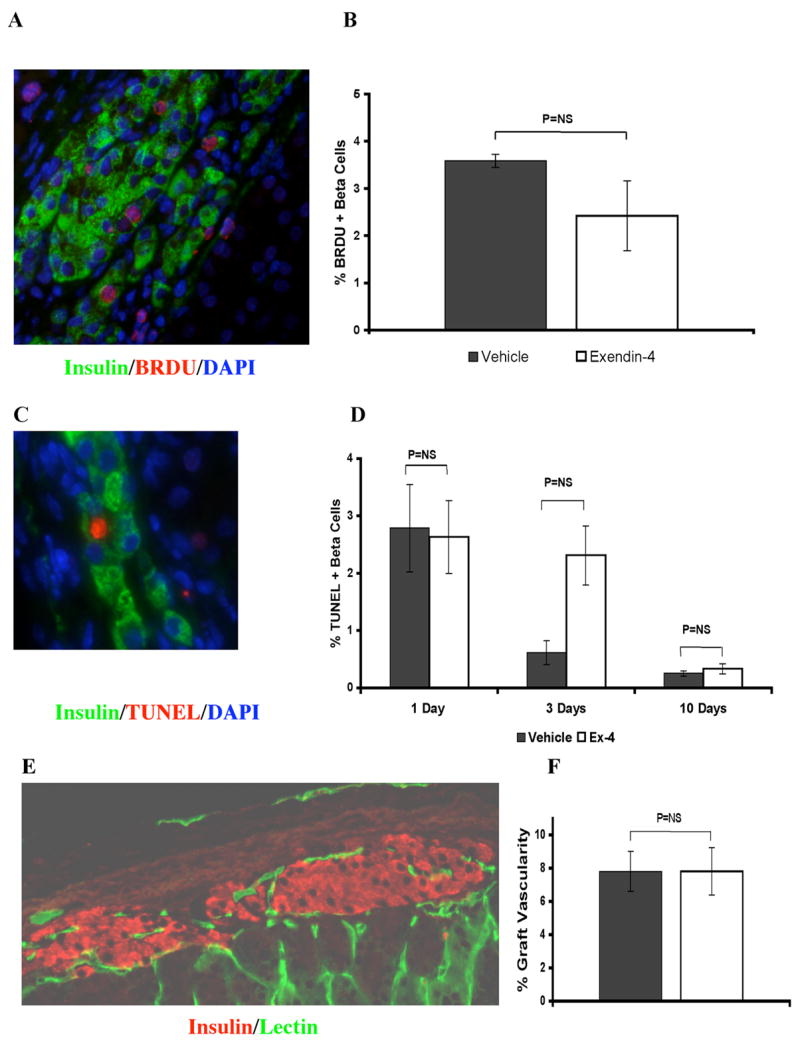
Recipients were treated with 10nmol/kg/d Ex-4 or vehicle and grafts harvested at 1, 3, and 10 days post-transplant. A, B: Graft β-cell (green-insulin+) proliferation, assessed by BRDU incorporation (red), was absent in all grafts at 1, and 3 days and similar with Ex-4 (n=3) or vehicle (n=5) at 10 days. C, D: Graft β-cell death rates, assessed by TUNEL labeling (red) were similar with Ex-4 (n=5, 3, and 4 at 1, 3, and 10 days, respectively) or vehicle (n=4 at all time points) at each time point. E, F: Density of functional graft capillary network within the β-cell component (red-insulin+) of islet grafts was assessed at 10 days after transplant by 5-minute pre-harvest intravenous infusion of FITC-conjugated Lycopersicon Esculentum Lectin (green). By computer based image analysis no difference between Ex-4 (n=4) or vehicle (n=3) was found. All values are mean +/− SE.
Ex-4 does not improve β-cell survival during the early post-transplant period
Graft β-cell death rates, assessed by TUNEL labeling, were highest 1 day after transplant and declined to very low levels by 10 days (Figure 2C). Rates of TUNEL labeling were similar between treatment groups at 1 and 10 days after transplantation, while a trend was seen towards increased death in Ex-4 treated recipients at 3 days (Figure 2D: vehicle vs. Ex-4: 1 day: 2.8 +/− 0.8 vs. 2.6 +/− 0.6%, P=0.88; 3 days: 0.6 +/− 0.2 vs 2.3 +/− 0.5%, P=0.064; 10 days: 0.25 +/− 0.04 vs. 0.33 +/− 0.08%, P=0.46). β-cell death rates were not significantly affected by early hypoglycemia in the Ex-4 treated group.
No impact of Ex-4 on islet graft vascularity
Graft vascularity was assessed at 10 days after transplant, around the time of maximal graft revascularization (35, 47). Endothelium was labeled through the pre-harvest intravenous infusion of a FITC-conjugated endothelial-avid lectin (Figure 2E), and image analysis determined the percentage of graft β-cell area, delineated by insulin staining, occupied by endothelium. No difference was seen between treatment groups (Figure 2F; vehicle (n=3) vs. Ex-4 (n=4): 7.8 +/− 1.2 % vs. 7.8 +/− 1.4 %, P=0.99), suggesting that Ex-4 does not improve islet graft revascularization under these experimental conditions.
Impact of Ex-4 on duration of post-allogeneic transplant euglycemia
Due to significant dose-dependent hypoglycemia in Ex-4 treated transplant recipients (described in Methods), an escalating dosing regimen was developed (5 nmol/kg on days 0 and 1, to 10 nmol/kg on days 2–5 and 20 nmol/kg/day thereafter). Neither hypoglycemia nor death was seen in these recipients. There was a trend toward increased duration of euglycemia in all allogeneic transplant recipients (Ex-4 vs. vehicle: 16.7 +/− 1.7 vs. 12.4 +/−0.8 days, P=0.05; Figures 3A-C) with 3 of 7 Ex-4 treated recipients maintaining euglycemia for 20 or more days. The precipitous recurrence of severe hyperglycemia in all recipients suggested that euglycemia was dependent on graft function and not on the recovery of endogenous β-cell function.
Figure 3. Ex-4 prolongs euglycemia after allogeneic transplant.
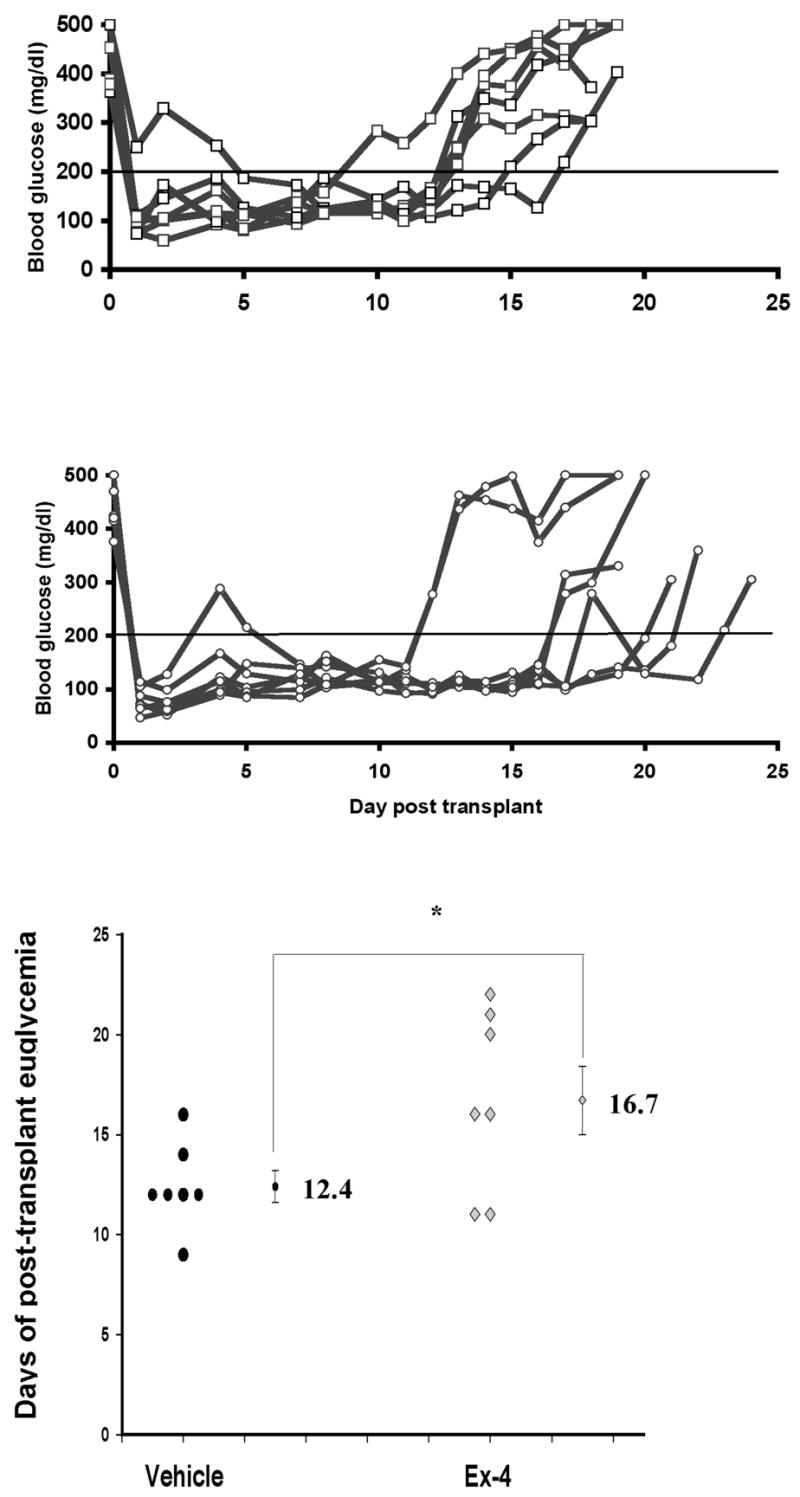
A–B. Random blood glucose (mg/dl) measurements of streptozotocin diabetic recipients treated with vehicle (A) or Ex-4 (B) after allogeneic transplant. C. Data points indicate duration of euglycemia (days) for individual recipients; mean duration +/− SE also depicted (*P=0.05).
Ex-4 promotes hypoglycemia and weight loss in the early post-allogeneic transplant period
A second experiment was performed to investigate Ex-4 associated hypoglycemia. Donor, recipient and transplant characteristics were unchanged, and recipients were randomized to treatment with 20 nmol/kg Ex-4 (n=5) or vehicle (n=4) immediately and once daily on post-transplant days 1 and 2. Blood glucose and body weight were measured frequently over the first 48 hours after transplantation (Figures 4A, B), and plasma was collected for measurement of insulin and glucagon at baseline, 6 and 16 hours (Table 1).
Figure 4. Ex-4 promotes early post-transplant hypoglycemia and weight loss in islet graft recipients.
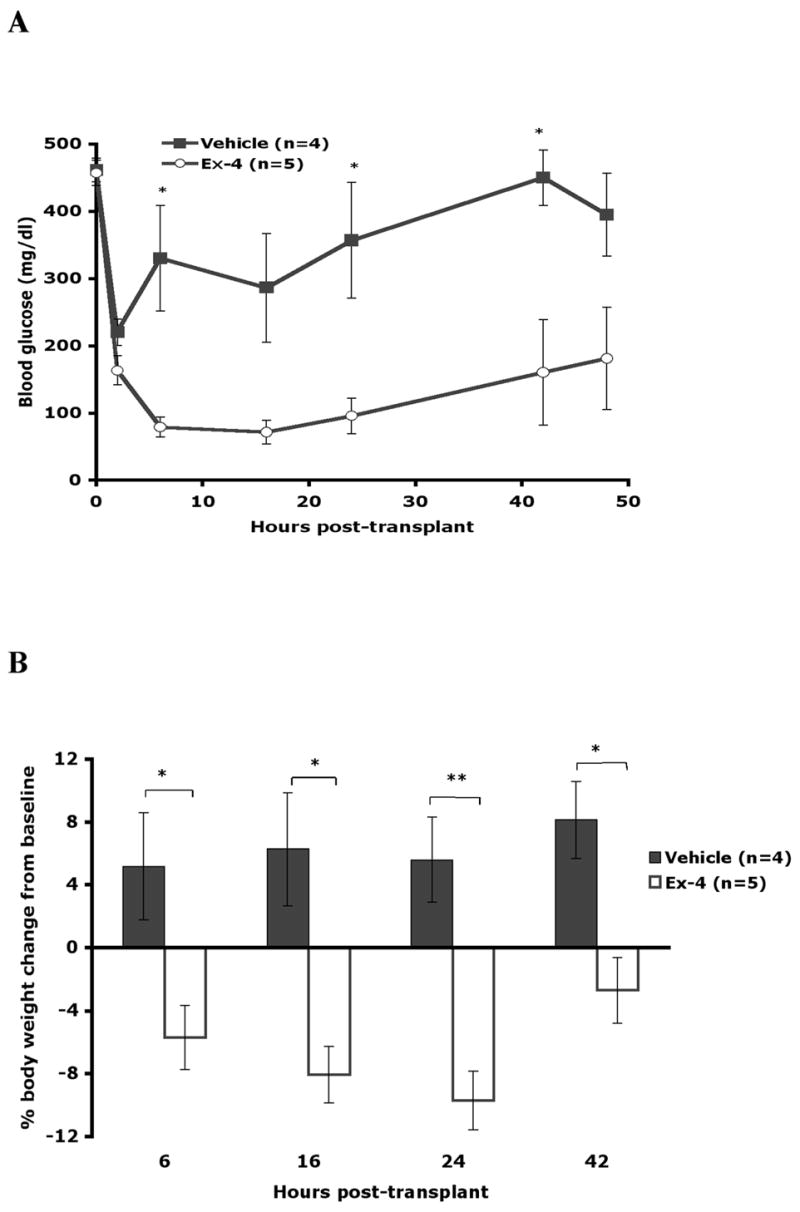
A. Mean blood glucose (mg/dl) measurements for vehicle (n=4) and Ex-4 (n=5) recipients over the 48-hour post-transplant follow-up period. B. Percent weight change from baseline-6 to 42 hours post-transplant. All data are mean +/−SE (*P<0.05; **P<0.01).
Table 1.
Glucose, insulin and glucagon levels in vehicle and Ex-4 treated allogeneic transplant recipientsa
| Glucose (mg/dl)
|
Insulin (pM)
|
Glucagon (pM)
|
||||
|---|---|---|---|---|---|---|
| Hours | Vehicle | Ex-4 | Vehicle | Ex-4 | Vehicle | Ex-4 |
|
|
|
|||||
| 0 | 462 ± 18 | 457 ± 19 | ND | ND | 76 ± 28 | 56 ± 18 |
| 6 | 331 ± 78 | 79 ± 15* | 782 ± 249 | 366 ± 19 | 49 ± 6 | 47 ± 12 |
| 16 | 287 ± 81 | 72 ± 17 | 510 ± 161 | 370 ± 105 | 42 ± 7 | 30 ± 8 |
Values are mean +/−SE.
P<0.05 vs. vehicle
vehicle (n=4) and Ex-4 (20nmol/kg, n=5)
ND, not detected
Blood glucose was significantly lower in Ex-4 treated recipients at all time points. In accordance with the first transplant experiment using this dose, hypoglycemia (<50mg/dl) occurred in 2/5 Ex-4 treated recipients at both 6 and 16 hours, but was not observed in vehicle treated recipients. Plasma insulin concentrations were similar between treatment groups, although trending lower in Ex-4 treated recipients (Table 1), while the ratio of insulin(pM):glucose(mg/dl) from 6 to 16 hours trended higher in Ex-4 treated recipients (Ex-4 vs. vehicle: 5.3+/−0.8 vs 3.0+/−1.3) and was highest in the hypoglycemic Ex-4 recipients (6.2, 8.1) suggesting inappropriate hyperinsulinemia in the setting of hypoglycemia. Glucagon levels trended downward in both groups after transplant (Table 1) and failed to rise in the hypoglycemic Ex-4 treated mice (baseline: 88+/−24 pM, 6 hours: 69+/−11 pM, 16 hours: 42+/−13 pM), suggesting impaired transplant and native □ cell function. Significant weight loss occurred in Ex-4 recipients and was greatest, nearly 10% of initial body weight, at 24 hours, while vehicle treated recipients had a small weight gain (Figure 4B). Weight loss was similar between the hypoglycemic and euglycemic Ex-4 treated recipients.
Considering all allogeneic transplant recipients in this study, 5/12 treated with 10 or 20 nmol/kg of Ex-4 on post-transplant days 0 and 1 became hypoglycemic on day 1 and/or day 2 after transplant, compared with 0/13 vehicle treated recipients (42% vs. 0%, P=0.015) and 0/4 recipients treated with 5 nmol/kg Ex-4. Mice treated with any dose of Ex-4 (5–20 nmol/kg) experienced a significant weight loss over the first day after transplant (Δ weight Ex-4 vs. vehicle: −8.6 +/− 0.97% vs. +0.3 +/− 1.8%, P<0.001). Ex-4 treated recipients that achieved primary graft function recovered weight completely by 5 days (Δ weight Ex-4 vs. vehicle 5 days: +0.48+/−2.58% vs. +2.27+/−2.4%, P=0.62; 9 days: +7.2+/−2.4% vs. +7.2+/−2.4%, P=0.99) indicating that this effect was limited to the early post-transplant period.
DISCUSSION
We employed murine models to explore the effects of Ex-4 on islet graft β-cell proliferation, β-cell death and graft revascularization in the early post-transplant period and to determine the impact of Ex-4 on the durability of islet allograft function in non-immune suppressed recipients. We hypothesized that Ex-4 would limit graft β-cell death through its demonstrated cytoprotective actions against inflammatory cytokines and oxidative stress, both of which likely contribute to β-cell death in this setting. However, we did not observe any benefit of Ex-4 on graft morphology in a syngeneic transplant model and observed only a trend towards prolonged graft function in the setting of allogeneic islet transplantation.
The absence of a protective effect in this study suggests that GLP-1 receptor agonists do not confer protection in this setting or that the precise regimen was suboptimal. Pre-treatment prior to the delivery of an insult has been employed in most models demonstrating GLP-1 receptor agonist-mediated β-cell cytoprotection, and is requisite in at least one (25, 31, 32). In support of this concept, a recent report indicates a beneficial effect of Ex-4 treatment on transplantation outcomes using islets maintained in culture for a period of time prior to transplantation (28). In our syngeneic model, islets were devascularized for 2–4 hours and may have been exposed to recipient inflammatory responses prior to Ex-4 treatment. Substantial β-cell injury certainly occurs by this time, and the window of opportunity for early Ex-4 mediated cytoprotection may have passed. It also remains possible that continuous infusion of Ex-4 might be more effective than once-daily administration. However, superiority of continuous administration of Ex-4 has not been demonstrated in other studies and the possibility of GLP-1 receptor desensitization after continued agonist exposure is supported by several studies (1, 3, 12).
Absence of β-cell proliferation at 1 and 3 days post-transplant likely reflects the stress imposed upon islet grafts in the peri-transplant period, which Ex-4 was unable to overcome. Lack of effect at 10 days is more surprising, but not inconsistent with previous rodent studies in which already and perhaps maximally elevated rates of β-cell proliferation were not further increased by Ex-4, as observed in the rat 90% partial pancreatectomy model (36, 49). At later time points, graft β-cell proliferation rates are similar to those observed in native pancreatic islets and it remains possible that chronic GLP-1 receptor agonist treatment will have a proliferative effect on islet grafts (36). Consistent with our findings over the first 10 post-transplant days, King and colleagues found that 2 weeks of Ex-4 treatment after transplant did not improve the long term function of marginal dose syngeneic islet grafts (28).
Our data unexpectedly suggest that Ex-4 treatment may be associated with increased graft β-cell death at 3 days after transplant, although the observed trend did not reach statistical significance (Fig. 3). This may reflect the involution of “excess” β cell mass in the setting of improved β cell function. Reductions of β cell mass that are not needed to meet metabolic demands have been reported in the setting of excessive islet transplantation, transplanted insulinomas and post-pregnancy (4, 36, 40). Ex-4 could also influence the clearance of dying cells by as yet unknown immune mechanisms, thereby enhancing their detection. Regardless of the mechanism, the trend to increased death at 3 days post transplant was not associated with any deterioration in glucose homeostasis compared with vehicle treated transplant recipients.
The trend towards prolongation of euglycemia after allo-transplantation seen with Ex-4 treatment in this study may be a consequence of multiple factors. Ex-4 treatment might have conferred temporary protection against lymphocyte derived mediators of allo-rejection, or simply enhanced the function of graft β-cells without affecting the rate of graft loss (33). Further, islet independent actions including suppression of glucagon secretion, slowing of gastric motility, appetite suppression, and the stimulation of insulin independent glucose uptake by liver and skeletal muscle, could all decrease insulin requirements and thereby prolong euglycemia (23, 26, 48).
Ex-4 associated hypoglycemia was particularly surprising and may have been due to relative hyperinsulinemia and impaired hypoglycemic defense mechanisms coinciding over the first 2 post-transplant days. Unregulated insulin release from dying graft β-cells likely occurred in both treatment groups and contributed to the occurrence of hypoglycemia, although the similar between-group β-cell death rates seen at 1 day in our syngeneic models argues against the possibility that this process was enhanced by Ex-4. Human subjects treated with GLP-1 receptor agonists in pharmacologic doses have experienced brief hypoglycemic episodes associated with delayed suppression of insulin secretion (15, 44), although this response appears distinct from the sustained hypoglycemia observed in mice 6–24 hours after Ex-4 dosing in this study.
An underlying impairment of native and grafted α-cells has been demonstrated in streptozotocin treated diabetic rats and in human pancreatic islet transplant recipients and may have existed in the transplant recipients in this model (24, 37, 50). An alternative explanation is that that Ex-4 mediated appetite suppression was coupled with an underlying impairment of α cell function resulting in insufficient hypoglycemia defense against unregulated insulin secretion from dying β-cells. Finally, Ex-4 may have stimulated insulin independent hepatic and/or skeletal muscle glucose uptake, a possibility consistent with the disparity in blood glucose between Ex-4 and vehicle treated recipients after transplant despite similar insulin concentrations (26, 38, 48).
These studies demonstrate both limitations and possible side effects of GLP-1 receptor agonist therapy in the early post-islet transplant period, while suggesting potential future approaches to its application. In the clinical setting, β-cell injury may begin prior to isolation from the donor with the hemodynamic instability and inflammatory phenomena associated with brain death. Therefore, maximal protection during isolation and transplantation may be seen if GLP-1 receptor agonists are administered prior to and during pancreas harvest and islet isolation (8). Dose-dependent hypoglycemia was seen in our mouse model, and awareness of this possibility will be important in the future as GLP-1 receptor agonists are employed in the clinical islet transplant setting. Finally, the trend toward a prolongation of post allo-transplant euglycemia seen here provides some evidence that GLP-1 receptor agonists may improve allograft functional durability and be a productive adjuvant to immunosuppressive agents in chronic graft-supportive regimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baggio L, Adatia F, Bock T, Brubaker PL, Drucker DJ. Sustained expression of exendin-4 does not perturb glucose homeostasis, beta-cell mass, or food intake in metallothionein-preproexendin transgenic mice. J Biol Chem. 2000;275:34471–34477. doi: 10.1074/jbc.M005119200. [DOI] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004;18:531–554. doi: 10.1016/j.beem.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes. 2004;53 Suppl 3:S205–214. doi: 10.2337/diabetes.53.suppl_3.s205. [DOI] [PubMed] [Google Scholar]
- 4.Blume N, Skouv J, Larsson LI, Holst JJ, Madsen OD. Potent inhibitory effects of transplantable rat glucagonomas and insulinomas on the respective endogenous islet cells are associated with pancreatic apoptosis. J Clin Invest. 1995;96:2227–2235. doi: 10.1172/JCI118278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt A, Katschinski M, Arnold R, Polonsky KS, Goke B, Byrne MM. GLP-1-induced alterations in the glucose-stimulated insulin secretory dose-response curve. Am J Physiol Endocrinol Metab. 2001;281:E242–247. doi: 10.1152/ajpendo.2001.281.2.E242. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 7.Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernandez JA, Griffin JH, Eckhoff DE. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004;53:2804–2814. doi: 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- 8.Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson JA, Chaudry IH, Eckhoff DE. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52:2935–2942. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico E, Hui H, Khoury N, Di Mario U, Perfetti R. Pancreatic beta-cells expressing GLP-1 are resistant to the toxic effects of immunosuppressive drugs. J Mol Endocrinol. 2005;34:377–390. doi: 10.1677/jme.1.01655. [DOI] [PubMed] [Google Scholar]
- 10.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 12.Delmeire D, Flamez D, Moens K, Hinke SA, Van Schravendijk C, Pipeleers D, Schuit F. Prior in vitro exposure to GLP-1 with or without GIP can influence the subsequent beta cell responsiveness. Biochem Pharmacol. 2004;68:33–39. doi: 10.1016/j.bcp.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Duvivier-Kali VF, Omer A, Parent RJ, O'Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:1698–1705. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 14.Eckhoff DE, Eckstein C, Smyth CA, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Anthony Thompson J, Chaudry IH, Contreras JL. Enhanced isolated pancreatic islet recovery and functionality in rats by 17beta-estradiol treatment of brain death donors. Surgery. 2004;136:336–345. doi: 10.1016/j.surg.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Edwards CM, Todd JF, Ghatei MA, Bloom SR. Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci (Lond) 1998;95:719–724. doi: 10.1042/cs0950719. [DOI] [PubMed] [Google Scholar]
- 16.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes JR, Duvivier-Kali VF, Keegan M, Hollister-Lock J, Omer A, Su S, Bonner-Weir S, Feng S, Lee JS, Mulligan RC, Weir GC. Transplantation of islets transduced with CTLA4-Ig and TGFbeta using adenovirus and lentivirus vectors. Transpl Immunol. 2004;13:191–200. doi: 10.1016/j.trim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 19.Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: progress and challenge. Arch Med Res. 2005;36:273–280. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Ocana A, Vasavada RC, Cebrian A, Reddy V, Takane KK, Lopez-Talavera JC, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 21.Hahn HJ, Dunger A, Laube F, Besch W, Radloff E, Kauert C, Kotzke G. Reversibility of the acute toxic effect of cyclosporin A on pancreatic B cells of Wistar rats. Diabetologia. 1986;29:489–494. doi: 10.1007/BF00453499. [DOI] [PubMed] [Google Scholar]
- 22.Hirano Y, Fujihira S, Ohara K, Katsuki S, Noguchi H. Morphological and functional changes of islets of Langerhans in FK506-treated rats. Transplantation. 1992;53:889–894. doi: 10.1097/00007890-199204000-00033. [DOI] [PubMed] [Google Scholar]
- 23.Holst JJ. Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab Res Rev. 2002;18:430–441. doi: 10.1002/dmrr.328. [DOI] [PubMed] [Google Scholar]
- 24.Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the "switch-off" hypothesis. Diabetes. 2004;53:1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- 25.Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5'-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144:1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- 26.Ionut V, Hucking K, Liberty IF, Bergman RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia. 2005;48:967–975. doi: 10.1007/s00125-005-1709-3. [DOI] [PubMed] [Google Scholar]
- 27.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 28.King A, Lock J, Xu G, Bonner-Weir S, Weir GC. Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia. 2005 doi: 10.1007/s00125-005-1922-0. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002;51:691–698. doi: 10.2337/diabetes.51.3.691. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001;15:1758–1767. doi: 10.1210/mend.15.10.0702. [DOI] [PubMed] [Google Scholar]
- 31.Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48:1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 33.Martinez OM, Rosen HR. Basic concepts in transplant immunology. Liver Transpl. 2005;11:370–381. doi: 10.1002/lt.20406. [DOI] [PubMed] [Google Scholar]
- 34.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–1366. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 35.Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;38 Suppl 1:199–201. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 36.Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozotocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- 38.Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Penacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43–50. doi: 10.1016/s0303-7207(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 40.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 42.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52:734–740. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- 43.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 44.Toft-Nielsen M, Madsbad S, Holst JJ. Exaggerated secretion of glucagon-like peptide-1 (GLP-1) could cause reactive hypoglycaemia. Diabetologia. 1998;41:1180–1186. doi: 10.1007/s001250051049. [DOI] [PubMed] [Google Scholar]
- 45.Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 46.Tourrel C, Bailbe D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50:1562–1570. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 47.Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation. 1995;60:123–127. [PubMed] [Google Scholar]
- 48.Villanueva-Penacarrillo ML, Puente J, Redondo A, Clemente F, Valverde I. Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine. 2001;15:241–248. doi: 10.1385/ENDO:15:2:241. [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 50.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha–cell function by the beta-cell during hypoglycemia in Wistar rats: the "switch-off" hypothesis. Diabetes. 2004;53:1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]


