INTRODUCTION
Sarcomas are a rare disease, with 9,220 new soft tissue sarcomas diagnoses and 3,560 deaths expected in the United States in 2007.1 Management of soft tissue sarcomas is complicated by the relative rarity of these tumors, the lethality of the disease, and the fact that they can occur as a number of histologies in a variety of sites in the body, each of which has different functional and anatomic considerations regarding optimal management.2 In this section we will concentrate on the radiotherapeutic management of sarcomas occurring in the most common locations: the extremities, the trunk, and the retroperitoneum. An overview of the current radiotherapeutic management of soft-tissue sarcoma is presented as well as a discussion of how surgical management may affect radiotherapeutic management. Finally, we will describe current controversies surrounding the appropriate management of sarcomas with radiotherapy and describe ongoing studies and future areas of research.
Extremity sarcoma: Role of radiotherapy
Prior to the introduction of radiotherapy for extremity soft tissue sarcomas, amputation was the standard therapeutic procedure, often resulting in significant physical and psychological morbidity to the patient. With this radical procedure, local failure is uncommon but as many as 40% of patients continued to die of metastatic disease.3 Studies completed in the 1970’s and 1980’s provided the first evidence that limb salvage was possible and could provide adequate local control without significantly increasing distant failures. Since that time, refinements have been made to further determine how radiotherapy can be best integrated with surgical management to maximize disease control and functional outcomes. A variety of techniques have been employed to improve both disease control and functional outcomes. The timing of radiotherapy for patients with resectable extremity sarcomas, the optimal management and sequencing of therapies for large extremity sarcomas, and the need for radiotherapy in resected low grade lesions are areas which have been, and in some cases still are, controversial.
The first evidence that radiotherapy could be used as a method to improve functional outcomes by avoiding amputation in patients with sarcomas was provided by a prospective randomized trial completed at the NCI.4 In this series, 43 adult patients with high grade soft tissue sarcoma of the extremity were randomized to amputation or limb-sparing resection followed by adjuvant radiation therapy to a total dose of 50 Gy. Patients randomized to the limb sparing group underwent a wide local excision designed to remove gross disease and several centimeters of surrounding normal tissue. In cases in which the pseudocapsule abutted major neurovascular structures, these structures were dissected free even if positive margins were the result. Both groups received chemotherapy (doxorubicin, cyclophosphamide, and methotrexate) post-operatively. Although there were four local recurrences in the limb-sparing group, there was no significant difference in disease-free survival or overall survival between the study arms. Importantly, an association of margin status and local recurrence is not reported in this series. This study confirmed the utility of post-operative radiotherapy; however, radiotherapy did not compensate for an inadequate surgical resection, as patients with a positive margin of resection still had a high risk of local recurrence, despite the addition of radiotherapy.
A subsequent prospective randomized trial also completed at the NCI further defined the role of postoperative external beam radiotherapy after a limb-sparing surgical resection. In this study, patients were randomized to receive or not to receive post-operative adjuvant radiotherapy, while all patients received chemotherapy.5 In patients receiving radiotherapy, there were no local recurrences in patients compared to a local recurrence rate of 22% in patients not receiving radiotherapy. Of additional importance, in this same study, twenty-six patients with a low-grade sarcoma were randomized to receive adjuvant radiotherapy. Excluding desmoids tumors and other neoplasms that did not fit the strict definition of malignant soft tissue sarcoma, 6 of the 19 patients not receiving radiotherapy have recurred and 1 of the 22 patients receiving radiotherapy. This data suggested that a local control benefit is seen with the addition of adjuvant radiotherapy for both low and high grade sarcomas. Additional analysis of both the high grade and low grade groups of patients showed no difference in overall survival with the addition of radiation therapy. Similar results have been confirmed in a number of series leading to the general consensus that radiotherapy delivered after a wide local excision or function preserving surgical procedure provides a significant benefit in local disease control while providing no benefit with respect to overall survival or the incidence of distant metastases.
While these data support a local control benefit for the radiotherapy administered after wide local excision, additional attempts have been made to define a subgroup of patients in whom the risk of local recurrence is sufficiently low to justify avoiding radiotherapy in order to minimize toxicity. However, this remains a significant area of controversy. The NCCN guidelines currently recommends radiation therapy for extremity sarcomas for high grade lesions and for low grade lesions which are larger than 5 cm or have close or positive margins. This is based on the lower risk of local recurrence for small, low grade lesions.6, 7
In some series, additional factors such as age appear to affect rates of local recurrence. For example, in one retrospective series analysis from Memorial Sloan Kettering Cancer Center, age greater than 50 years was identified as an independent predictor of local recurrence, following conservative surgery and radiotherapy.7 In a Princess Margaret Hospital series, younger age, less than 50 years, was predictive of better local control.8 These were retrospective studies and the authors commented that these results may have been confounded by the fact that certain aggressive histologies may be more common in older age groups. Nevertheless, for those patients for whom local excision alone is being contemplated, age should be also be taken into consideration as a possible factor for local recurrence.
Extremity sarcoma: pre-operative versus post-operative radiation therapy
Although the delivery of radiotherapy in large and/or high grade extremity sarcomas is considered standard, significant controversy has existed regarding the appropriate timing of delivery in relation to surgery. Preoperative therapy allows the radiation oncologist to deliver a lower radiation dose (typically 50 Gy versus 66–70 Gy delivered in the adjuvant setting) to a smaller target volume (preoperative extent with margin versus entire operative bed with margin), potentially minimizing toxicity. In addition, marginally resectable tumors may respond to the preoperative radiotherapy in a sufficient manner to allow a negative margin functional resection.
Arguments also exist supporting the use of post-operative therapy. A significant concern with the delivery of preoperative therapy is the concern for an increase in wound complications with the preoperative approach due to impaired healing in the radiotherapy field, especially when the wound repair is under tension. Although infrequent, complications that occur during radiotherapy may postpone the definitive surgical procedure. Finally, evaluation of the pathologic findings in the setting of preoperative therapy may be complicated by radiation effect.
The decision of whether to utilize pre-operative versus post-operative radiotherapy was examined in a large randomized trial from the NCI Canada Clinical Trial Group.9 This trial evaluated disease control and toxicity in patients with soft tissue sarcoma of the extremity and randomized to treatment by pre-(50 Gy) or post-operative (66 Gy) external radiation in combination with surgery. Local recurrence, locoregional, and progression-free survival were the same between the groups. Of note, 64 of 94 patients in the postoperative group had acute toxic skin effects that were grade 2 or greater, compared with 32 of 88 in the preoperative group. Grade 1 and 2 bowel toxicity did not differ between the two groups and no patient experienced grade 3 or 4 bowel toxicity. Two year toxicity data and functional outcomes data from this series have also been reported.10, 11 In regards to late toxicity, Grade 2 or greater fibrosis occurred in 48.2% of patients in the post-operative arm compared to 31.5% in the pre-operative arm at 2 years. Edema and joint stiffness were more common in the post-operative arm, although this difference was not statistically significant. Patients with fibrosis, joint stiffness, and edema had significantly lower functional scores. Taken together, these data support that preoperative radiotherapy provides equivalent disease control with better long term functional outcomes compared to postoperative radiotherapy.
When deciding between pre-operative versus post-operative radiation therapy, a consideration regarding the type of surgical procedure to be performed, the planned type of wound closure, the amount of expected wound tension, the probable extent of the operative bed, the likelihood of obtaining a margin negative resection, and the histologic grade of the sarcoma should all be considered. Also, a recovery period of three to six weeks is necessary after the completion of pre-operative radiotherapy to allow resolution of acute radiation effects, allow improved wound healing when surgery is performed, and to allow for the desired histologic response. Following eventual surgical resection, a thorough examination of the pathologic specimen is critical to determine the appropriateness of an additional boost of radiation with brachytherapy or external beam radiation therapy if margins are close or positive.
Radiotherapy may be delivered post-operatively instead of pre-operatively for a variety of reasons, including the incidental diagnosis of sarcoma at surgery, upgrading after evaluation at surgery, inability to obtain a negative margin with acceptable functional outcomes, and more extensive lesion than expected based on pre-operative imaging and pathologic data. When radiotherapy is delivered post-operatively, at least 5 cm of margin is used, and a dose of at least 60 Gy or higher is utilized as the dose of radiotherapy delivered in the post-operative setting has been shown to correlate with local control.12 In conclusion, post-operative and pre-operative radiation treatment has both benefits and detriments and should be discussed on an individual basis with each patient in a multi-disciplinary setting.
Brachytherapy in the treatment of soft-tissue sarcomas
In addition to external beam radiation therapy, brachytherapy has been employed for the treatment of sarcomas in an effort to improve the therapeutic ratio by minimizing the extent of normal tissue treated and to simultaneously permit local dose escalation to areas at highest risk. In a prospective randomized trial conducted by Memorial Sloan Kettering Cancer Center, 164 patients were randomized intra-operatively to receive adjuvant brachytherapy or no further treatment after resection of STS of the extremity or trunk.13 The entire radiotherapy treatment in this series was delivered with a brachytherapy implant without external beam irradiation. The tumor bed and surrounding tissue received an iridum-192 implant which delivered 42–45 Gy over 4–6 days. With a median follow-up of 76 months, 5-year local control rates were significantly improved with brachytherapy (82% versus 69%), but this benefit appeared to be limited to patients with high grade lesions. Overall local control was 91% which is comparable to local control rates in post-operative external beam treatments.
Another observation was that patients treated in this trial that had radioactive sources loaded before post-operative day 6 had an increased incidence of wound complications. Once this observation was made, efforts were made to load the catheters after post-operative day 6, so as to not interfere with the granulation phase of wound healing. After this change was made, no difference was noted between the 2 groups with respect to failed tissue healing and this policy was adopted as the preferred standard. As is typical for radiotherapy series for sarcoma, there was no effect on the incidence of distant metastasis or disease-specific survival in any group of patients in this series. There were more major and moderate wound complications in patients in the brachytherapy group (11/23 patients) compared to the unirradiated group (5/21 patients).
A comprehensive review of the technique of placing brachytherapy catheters, treatment planning, and treatment delivery is outside the scope of this brief review. A comprehensive guideline for the performance of brachytherapy for extremity sarcomas has been published by the American Brachytherapy Society.14 In certain instances, brachytherapy should be strictly avoided either due to potential under-treatment or foreseeable toxicity or overdosing of neurovascular structures. Brachytherapy, although an extremely effective treatment, is technically challenging for both the radiation oncologist and surgeon, and should be employed by multidisciplinary teams with significant experience, expertise, and medical physicist support.
The radiotherapy dose delivered with brachytherapy depends on the clinical scenario and indication, although doses of 45 to 50 Gy over 4 to 6 days are often prescribed for low dose rate radiotherapy when delivered as the only form of radiotherapy. In situations in which brachytherapy is used as a boost following a course of external beam radiotherapy, doses of 15 to 25 Gy are typically selected. As mentioned previously, treatment typically commences no sooner than 5 days after wound closure. An example of a brachytherapy treatment is provided in figure 1.
Figure 1.

Brachytherapy for extremity sarcoma. A twenty-two year old male presented with 4 × 1.8 × 1.8 cm mass on the dorsum of his left foot in a field previously irradiated for Ewing’s Sarcoma. Biopsy was consistent with synovial sarcoma. After excision of the mass, the tumor bed was irradiated to 20 Gy intraoperatively with electrons via a specialized cone (A). Brachytherapy catheters were then placed across the tumor bed and secured (B) prior to flap placement and wound closure (C). After documentation of catheter placement with treatment planning images (D), an additional dose of 25 Gy was delivered with an Ir-192 brachytherapy implant.
Pre-operative regimens for extensive or locally advanced sarcomas
A variety of regimens have been evaluated as pre-operative therapy for extremity and truncal sarcomas to improve resectability while maximizing functional outcomes including chemotherapy alone, radiotherapy alone, or chemoradiation. The use of chemotherapy as an adjuvant therapy in sarcomas is controversial based on the variable results achieved in regards to disease control and response rates in prospective studies and metaanalyses.15–17 Despite the controversy in the use of adjuvant chemotherapy for sarcomas, the use of neoadjuvant chemotherapy has also been explored as a possible strategy for improving treatment of a soft-tissue sarcoma. It has been proposed that the use of neoadjuvant therapy may allow a reduction in radiation volumes or allow selection of patients who respond to chemotherapy for additional adjuvant treatment.
In an effort to evaluate whether neoadjuvant therapy improves outcomes for patients with locally advanced or high grade tumors, investigators at MD Anderson Cancer Center evaluated 3 cycles of pre-operative doxorubicin and dacarbazine, cyclophosphamide and ADIC patients with Stage IIIB extremity sarcoma. The DFS and OS in patients who received 3 cycles of preoperative doxorubicin and dacarbazine, cyclophosphamide and ADIC was similar to that achieved in historical studies where patients were randomized post-operative chemotherapy.18 Unfortunately, even the subset of patients classified as responders to the neoadjuvant regimen did not achieve any significant benefit in local recurrence free survival, distant metastasis free survival, or overall survival. A randomized trial of pre-operative chemotherapy consisting of doxorubicin and ifosfamide versus local treatment alone through the EORTC has been completed and final results are pending (www.clinicaltrials.gov).
An alternative regimen to improve outcomes of soft tissue sarcoma is a combined radiation and chemotherapy approach. Perhaps the best example of this approach is a single institution series reported from Massachusetts General Hospital of a pre-operative chemotherapy regimen consisting of mesna, adriamycin, ifosfamide, and dacarbazine (MAID) alternating with radiotherapy followed by resection and post-operative chemotherapy with or without radiotherapy.19 Patients with high grade extremity soft-tissue sarcoma greater than 8 cm were treated with 3 cycles of pre-operative chemotherapy combined with 44 Gy of radiotherapy followed by surgery. For patients with positive surgical margins, 16 Gy was delivered post-operatively. Five year local control, freedom from distant metastases, disease free survival and overall survival was were all improved compared to historical controls. Overall survival increased from 58% to 87% which represented a significant gain in disease free survival compared with the historical data.
Based on these promising results, this regimen was subsequently tested in a Phase II RTOG trial.20 A total of 66 patients with a high grade soft tissue sarcoma greater than 8 cm in diameter received 3 cycles of neoadjuvant MAID chemotherapy, alternating with radiotherapy and 3 cycles of post-operative MAID chemotherapy. Estimated 3 year rates of disease-free, distant-disease-free and overall survival were 56.6%, 64.5%, and 75.1% in this series. Toxicity was significant with his approach, with 84% experiencing a grade 4 toxicity (78% experienced grade 4 hematologic toxicity and 19% experienced grade 4 non-hematologic toxicity). Of the five amputations in the study, 2 were considered to be treatment-related. The conclusion of the authors is that such an aggressive neoadjuvant regimen can be used, however it needs to be performed in the setting of a clinical trial given the significant toxicity.
Unresectable sarcomas
Sarcomas may be deemed unresectable at presentation for a variety of reasons. For tumors invading major neurovascular or bony structures, such as those involving the pelvic girdle or structures of the shoulder, resection may lead to unacceptable morbidity and poor functional outcomes. Additionally, complex involvement of major neurovascular structures may preclude surgical extirpation. In these situations, neoadjuvant therapy as described above may be employed with the hopes of downsizing the tumor to facilitate resection. If the extent of disease is such that downsizing would be unlikely to result in a viable surgical option, definitive radiotherapy may be employed with the hopes of offering durable local control 21.
Alternatively, patients may present with medical comorbidities that preclude aggressive surgical management. For example, the option of definitive radiotherapy may provide local control after a core biopsy or in the case of a marginal excision when further surgery is contraindicated or in the case of a positive margin without reexcision 22 23. In some instances, the burden of metastatic disease in addition to the extent of local disease may result in the decision to forego an aggressive local surgical procedure.
Outcomes with this approach are clearly inferior to surgically-based approaches with 5 year local control rates approximating 29%–45% in single institution reviews.21, 23–25 Factors negatively influencing the ability to obtain local control with definitive radiotherapy in these series include increasing tumor size (<5cm vs 5–10 cm vs >10 cm) and lower radiation dose (<63 Gy vs >63 Gy). The use of chemotherapy does not correlate with improved outcomes in this population.21
In any situation in which definitive radiotherapy is used, care must be taken to appropriately design fields to exclude sensitive structures. The likelihood of toxicity following definitive radiotherapy depends at least partially on the size and location of the tumor, factors which affect the amount and type of normal tissue exposed to higher doses. In some series, doses in excess of 68 Gy or 70 Gy have been associated with an increased risk of major radiotherapy complications.21, 24 The incidence of major complications that may result from radiotherapy in the definitive setting vary depending on the site treated, but may include soft tissue necrosis, fibrosis, neuropathy, bone fracture, and bowel injury.21, 24
Newer technologies such as intensity modulated radiotherapy (IMRT), proton therapy, and helical tomotherapy may allow delivery of effective definitive radiotherapy while reducing the risk of complications for patients with sarcomas.26–28 These technologies each may allow more conformal radiation delivery, allowing for effective tumor coverage with the target radiation dose while minimizing the amount of uninvolved normal tissue that is exposed to higher doses. These technologies are actively being investigated in this setting (www.clinicaltrials.gov).
In addition to newer radiotherapy techniques, chemotherapy delivered concurrently with radiotherapy has been investigated as a mechanism to improve resectability. While some series have found up to 25% objective responses with chemotherapy in advanced sarcomas,29 evidence that induction chemotherapy can result in downsizing sufficient to allow resection or prolonged local control is lacking. For this reason, induction chemotherapy remains investigational.
Other multimodality approaches including techniques such as isolated limb perfusion strategies with or without radiotherapy have been evaluated as a mechanism for increasing resectability. Limb perfusion with agents such as doxorubicin, melphalan, and tumor necrosis factor with or without with radiation have been reported with response rates of 53–85% and significant improvements in resectability.30–32 Long-term follow-up has revealed high risks of necrosis and eventual requirement for amputation due to significant vascular morbidity in some series of multimodality management including radiation with limb perfusion.33, 34 Due to the complexity of the procedure and the potential for significant morbidity, limb perfusion with or without radiotherapy is not in widespread clinical use at this time.
Retroperitoneal sarcoma
Retroperitoneal sarcomas present a unique therapeutic challenge. Because of their location, these tumors often become quite large before presenting signs and symptoms develop. In addition, these tumors are often adjacent to a number of critical structures. These factors complicate both surgical and radiotherapeutic management. Finally, the relative rarity of these tumors has resulted in mainly retrospective evaluations of radiotherapy in addition to surgery precluding the ability to make definitive treatment recommendations.
Because of the relatively high likelihood of positive surgical margins and eventual local recurrence, radiotherapy has been applied in the pre-operative, intra-operative, and post-operative settings. The use of radiotherapy in addition to surgical resection has achieved 5 year local control rates of 51% to 71% in several retrospective series. 35–38 However, toxicity with this approach is a major concern due to the proximity of these tumors to many organs and structures with relatively low radiation tolerances. Organs such as the spinal cord, kidneys, liver, and small bowel limit radiotherapy doses below what is typically considered appropriate for soft tissue sarcomas in the adjuvant setting (60 to 70 Gy). Based on these considerations, a radiotherapy dose of 45 to 50 Gy is often considered the maximal safe dose for this region of the body with conventional approaches, a dose that is considered reasonable as a preoperative dose for sarcomas.
Similar to extremity sarcomas, the timing of radiotherapy has significant implications in regards to targeting and potential toxicity. Although use of post-operative radiotherapy allows the selection of patients at highest risk of recurrence based on margin status, there are numerous reasons why preoperative therapy is preferred. In the post-operative setting, the tumor has been resected or debulked, allowing normal tissues to move into and become adhered to the tumor bed. This potentially results in a higher radiotherapy dose to some sensitive normal tissues, such as bowel, that would typically move in and out of the field of radiation if delivered in the setting of preoperative therapy. In addition, preoperative treatment volumes are often smaller because they are based on treating the tumor volume with a margin for microscopic extension while post-operative therapy often includes the entire tumor bed, clips, with additional margin. An additional theoretical benefit to preoperative therapy is the improved oxygenation of the tumor bed in the pre-operative setting, a factor known to enhance sensitivity of tumors to damage caused by ionizing radiation.
Typically, pre-operative radiotherapy is considered for intermediate or high grade retroperitoneal sarcomas that are likely to be resected with positive margins. If preoperative therapy is considered, a biopsy is required to verify histology prior to initiating radiotherapy. Prospective trials evaluating the role of preoperative radiotherapy with intraoperative therapy or as part of multimodality regimens have found promising disease control rates.39–42 A recent report of long term data from patients treated on two of these prospective series found a 5 year disease free survival rate of 46% and a 5 year survival rate of 50%. For patients in this series who completed radiotherapy and underwent a macroscopically complete resection the 5 year local recurrence free survival rates were 60%.
Toxicities of external bean radiotherapy (EBRT) for retroperitoneal sarcomas may include mild to moderate acute toxicities of enteritis, nausea, and vomiting. 35, 36, 43 and late toxicities of bleeding gastric ulcers, small bowel obstruction, strictures, and impaired wound healing.36, 43 Rates of toxicity range widely in reported series and with chosen radiation delivery method, but in general appear to be less with preoperative approaches44.
Retroperitoneal sarcomas remain a challenging problem, with local control rates clearly inferior to those obtained in extremity sarcomas. Attempts to improve the local control benefit obtained with radiotherapy while minimizing toxicity have led to alternative targeting methods, the use of highly conformal therapies, and alternative delivery methods. The use of local radiation dose escalation at the anticipated site of a positive margin has been explored with promising results. A single institution series in which the entire tumor with margin was treated preoperatively to a dose of 45 Gy with selective escalation with a boost to 57.5 Gy to areas expected to be at high risk of a positive margin was well tolerated with 88% of patients undergoing a gross total resection and a resultant two year local control rate of 80%.45 Studies evaluating treatment planning with highly conformal therapies such as IMRT and helical tomotherapy have shown the potential to reduce toxicity with these newer planning technologies by allowing avoidance of high dose regions in organs with low radiation tolerance46, 47.
Intra-operative radiotherapy (IORT) was developed as a potential mechanism of increasing the deliverable dose while minimizing toxicity by delivering radiotherapy at the time of surgery when many sensitive normal tissues could be retracted and removed from the direct path of the radiation beam. IORT requires a specialized operating room equipped with the proper equipment to deliver the radiotherapy. This specialized equipment is currently available at a few institutions. IORT can be delivered alone or in combination with additional external radiotherapy delivered preoperatively or postoperatively.
Several series of IORT for retroperitoneal sarcomas have shown favorable disease control, however at a cost of toxicity. These toxicities are different than those typically reported for external beam radiation alone and are likely due to the tissues exposed to the large fraction sizes typically employed and include neuropathy, ureteral fistula, hydronephrosis, and bowel obstruction.42, 48, 49 Patient selection for IORT both preoperatively and intraoperatively is critical for selecting patients that may have a lower risk of toxicity. Assessment of the total field size required, normal tissues to be included in the field, and the anticipated toxicity of the therapy may lead to intraoperative determination that the therapy is inappropriate in a significant number of patients.40
Brachytherapy is an alternative technique for delivering local tumor bed boosts. The use of brachytherapy in this setting has typically been combined with standard doses of preoperative external beam radiotherapy. Toxicity in these series is significant with reoperation rates of up to 21.5%.39, 50 There is some indication that brachytherapy to the upper abdomen may be poorly tolerated, with higher rates of late toxicity and long-term complications.39 Early local control rates in these series are promising but longer follow up in larger prospective series is needed.39, 50
Planning and Delivery of Radiation Therapy
Once the decision to utilize external beam radiation therapy has been reached, technical aspects such as dosage, timing, and margins need to be considered. Radiation therapy for sarcomas can usually be delivered in the outpatient setting, with the exception of interstitial brachytherapy. The use of brachytherapy requires inpatient admission for reasons of radiation protection.
For external beam radiation treatment planning, the ability to properly target the areas at highest risk is of critical importance. Radiation oncologists typically rely on a variety of data to determine target volumes for treatment planning, including physical examination, pre-operative imaging, operative reports, radio opaque clips, scars, and drain sites. CT imaging and MR imaging provide complimentary information for radiotherapeutic treatment planning and should be obtained whenever possible. It is critical that the imaging obtained preoperatively encompass the entire area of radiographic abnormality with margin to allow for adequate tumor targeting with radiotherapy. In addition, obtaining these images with the patient in the radiation treatment planning position (ie. a treatment planning MR or CT) can be facilitate treatment planning by allowing an acceptable fusion of these image series to the treatment planning images, significantly improving target delineation.
For radiotherapy delivered in the pre-operative setting, a discussion with the surgeon who will operate can allow avoidance of high dose regions in areas destined to be incision or drain sites, potentially reducing wound complications. In general, radiation oncologists attempt to exclude sensitive structures from the radiation field to minimize toxicity, including uninvolved compartments, joint spaces, genitalia, and visceral organs. In addition, attempts are made to avoid treating the entire circumference of a limb due to the risk of lymphedema. Placement of surgical incisions, drain sites, and biopsy sites overlying or adjacent to these sensitive structures may result in the need to include them in the radiation field, hence increasing the risk of toxicity. A thorough discussion of the multimodality treatment plan with members of the oncology team may allow therapy to be delivered in a fashion that maximizes disease control and minimizes toxicity.
In the post-operative setting, radio opaque surgical clips placed at the time of resection can assist in delineating the tumor bed and the area at high risk. For patients with microscopically positive surgical margins in whom brachytherapy was not delivered, a post-operative external beam boost of 16 Gy may be delivered at 2.5–3 weeks after surgery or once wound healing is adequate. In either the pre-operative or post-operative setting, it is crucial to spare an uninvolved compartment of the limb from receiving full dose to spare lymphatics. Typically, the radiation plan is also optimized to reduce dose to poorly vascularized areas such as the pre-tibial, pre-patellar and pre-olecranon skin. A multidisciplinary approach towards management of sarcomas can significantly improve the ability of the radiation oncologist to meet these complex dose planning goals while minimizing toxicity. Clearly, involvement of the surgeon and radiation oncologist for planning of both the surgical and radiation procedures can significantly affect the treatment plan. Representative plans from patients with extremity and retroperitoneal sarcoma are presented in figure 2 and figure 3.
Figure 2.

External beam radiation for extremity sarcoma. A fifty-five year old man presented with popliteal mass with core biopsy consistent with malignant fibrous histiocytoma. Neurovascular function was intact. Preoperative radiotherapy was delivered to a total dose of 50 Gy in 2 Gy daily fractions to the popliteal tumor with 5 cm margins longitudinally. Care was taken to provide adequate circumferential margin while limiting the exposure of the knee joint and anterior tissues of the leg by employing opposed lateral fields directed at the popliteal fossa. The tumor volume is outlined in pale yellow, other colored lines represent isodose curves (orange =52.5 Gy, red = 50 Gy, green = 47.5 Gy).
Figure 3.
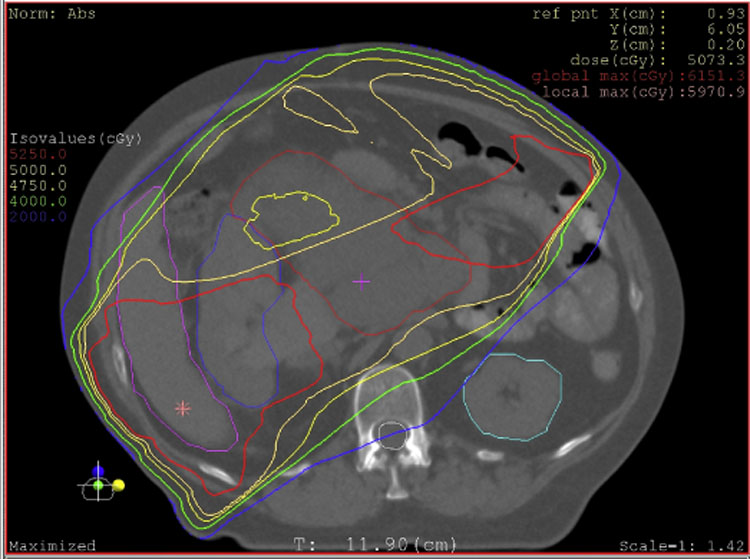
External beam radiation for retroperitoneal sarcoma. A 62 year old woman presented with increasing abdominal girth and was noted to have a large retroperitoneal mass on CT. Biopsy was consistent with high grade liposarcoma. Preoperative radiotherapy to a total dose of 50.4 Gy in 1.8 Gy fractions was delivered followed by margin negative resection. The tumor is outlined in dark red, other colored lines represent isodose curves (red = 52.5 Gy, yellow = 50 Gy, pale yellow = 47.5 Gy). A complex beam arrangement was used to minimize dose to the left kidney while providing adequate margin on tumor.
Future directions
While significant improvements have been made in the local management of extremity sarcomas, a significant number of patients experience local failures and toxicity of therapy. The relatively few cases diagnosed in the United States further complicates matters and makes conducting large trials and studies to improve management not feasible. Although management of soft tissue sarcoma of the extremity has improved greatly and there are generally accepted methods used to improve general outcomes for patients with retroperitoneal and unresectable sarcomas, the overall prognosis remains poor for this particular subset of sarcomas. Several areas of ongoing research hope to address issues that may continue to improve outcomes in patients with these diseases.
From a radiotherapeutic setting, the main focus of ongoing research includes improved targeting of therapy to minimize toxicity and maximize disease control. From a systemic therapy point of view, current chemotherapy regimens can be quite toxic and effects can be multiplied when used in a sandwich and concomitant setting. This toxicity has lead to the manipulation of molecular targets within tumors to enhance local effects of radiation. Additional efforts have focused on identification of individual factors such as single nucleotide polymorphisms or gene expression patterns that may predict for toxicity from radiotherapy.
Because unresected sarcoma requires high doses of radiation therapy in order to achieve the best chance of local control, doses of this magnitude come with the attendant possibility of significant normal tissue toxicity. In order to mitigate this possibility, various other radiation therapy modalities have been investigated such as IMRT and other forms of highly conformal therapies. The goal with these approaches is to spare as much normal tissue within the affected limb or region of the body from high dose irradiation as possible to minimize both acute and late toxicities of therapy. As discussed previously, these techniques are currently under investigation and may provide an additional method to improve the therapeutic ratio.
Utilizing different types of particulate radiation therapy has also been of some interest due to the ability to deliver highly conformal plans with these technologies. Proton beam therapy has been increasingly utilized because it takes advantage of the Bragg peak and deposits little energy in tissue until near the end of the proton range where the residual energy is lost over a short distance, ideally the span of the tumor thickness. Heavy charged particles have also been thought to be advantageous as there is an increase in linear energy transfer (LET) and increased energy deposition in the tissue. There is some data that shows that higher LET radiation is less affected by tissue oxygenation and less sensitive to variations in the cell cycle which may be important in sarcomas.51 However, few studies in other tumor sites have demonstrated a statistically significant benefit of neutrons over photons, and this approach remains investigational.
Summary
Sarcomas represent a heterogeneous, challenging and rare group of tumors that present many management challenges. Significant progress has already been made in determining the benefit of post-operative radiotherapy, the role of pre-operative versus post-operative radiotherapy, and the indications for conformal technologies such as brachytherapy and intra-operative therapy. Refinement of surgical and radiation techniques have improved functional and disease outcomes. A number of management issues, including the sequencing of therapies, the benefit of chemotherapy, defining the subset of patients who will benefit from radiotherapy, and the use of radiation sensitizers to improve local control are all an active area of research. The use of highly conformal therapies such as IMRT and the use of charged particles such as protons is being refined as well. There are clear indications for the use of radiation therapy to improve local control. The evaluation and management of patients with sarcoma in a multi-disciplinary setting is critical so that treatment is optimized and individualized for each patient.
Acknowledgements
“This research was supported in part by the Intramural Research Program of the NIH.” Special thanks to Jason Duelge and Scot Fisher with their assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aradhana Kaushal, Radiation Oncology Branch, National Cancer Institute, Building 10, Hatfield CRC, B2-3500, 10 Center Drive, Bethesda, MD 20892, (301) 496-5457 (phone), (301) 480-5439 (fax), kaushala@mail.nih.gov.
Deborah Citrin, Section of Imaging and Molecular Therapeutics, Radiation Oncology Branch, National Cancer Institute, Building 10, Hatfield CRC, B2-3500, 10 Center Drive, Bethesda, MD 20892, citrind@mail.nih.gov.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan–Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004 Mar–Apr;54(2):94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 3.Hoekstra HJ, Thijssens K, van Ginkel RJ. Role of surgery as primary treatment and as intervention in the multidisciplinary treatment of soft tissue sarcoma. Ann Oncol. 2004;15 Suppl 4:iv181–iv186. doi: 10.1093/annonc/mdh924. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982 Sep;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998 Jan;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Karakousis CP, Emrich LJ, Rao U, Khalil M. Limb salvage in soft tissue sarcomas with selective combination of modalities. Eur J Surg Oncol. 1991 Feb;17(1):71–80. [PubMed] [Google Scholar]
- 7.Geer RJ, Woodruff J, Casper ES, Brennan MF. Management of small soft-tissue sarcoma of the extremity in adults. Arch Surg. 1992 Nov;127(11):1285–1289. doi: 10.1001/archsurg.1992.01420110027007. [DOI] [PubMed] [Google Scholar]
- 8.LeVay J, O'Sullivan B, Catton C, et al. Outcome and prognostic factors in soft tissue sarcoma in the adult. Int J Radiat Oncol Biol Phys. 1993 Dec 1;27(5):1091–1099. doi: 10.1016/0360-3016(93)90529-5. [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. The Lancet. 2002 Jun 29;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 10.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005 Apr;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Davis AM, O'Sullivan B, Bell RS, et al. Function and Health Status Outcomes in a Randomized Trial Comparing Preoperative and Postoperative Radiotherapy in Extremity Soft Tissue Sarcoma. J Clin Oncol. 2002 November 15;20(22):4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 12.Fein DA, Lee WR, Lanciano RM, et al. Management of extremity soft tissue sarcomas with limb-sparing surgery and postoperative irradiation: do total dose, overall treatment time, and the surgery-radiotherapy interval impact on local control? Int J Radiat Oncol Biol Phys. 1995 Jul 15;32(4):969–976. doi: 10.1016/0360-3016(95)00105-8. [DOI] [PubMed] [Google Scholar]
- 13.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996 Mar;14(3):859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 14.Nag S, Shasha D, Janjan N, Petersen I, Zaider M. The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2001 Mar 15;49(4):1033–1043. doi: 10.1016/s0360-3016(00)01534-0. [DOI] [PubMed] [Google Scholar]
- 15.Tierney JF, Mosseri V, Stewart LA, Souhami RL, Parmar MK. Adjuvant chemotherapy for soft-tissue sarcoma: review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995 Aug;72(2):469–475. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramwell V, Rouesse J, Steward W, et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1994 Jun;12(6):1137–1149. doi: 10.1200/JCO.1994.12.6.1137. [DOI] [PubMed] [Google Scholar]
- 17.Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001 Jan Mar;19(5):1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 18.Pisters PW, Patel SR, Varma DG, et al. Preoperative chemotherapy for stage IIIB extremity soft tissue sarcoma: long-term results from a single institution. J Clin Oncol. 1997 Dec;15(12):3481–3487. doi: 10.1200/JCO.1997.15.12.3481. [DOI] [PubMed] [Google Scholar]
- 19.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. International Journal of Radiation Oncology*Biology*Physics. 2003 Jul 15;56(4):1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 20.Kraybill WG, Harris J, Spiro IJ, et al. Phase II Study of Neoadjuvant Chemotherapy and Radiation Therapy in the Management of High-Risk, High-Grade, Soft Tissue Sarcomas of the Extremities and Body Wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006 February 1;24(4):619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 21.Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. International Journal of Radiation Oncology*Biology*Physics. 2005 Nov 1;63(3):852–859. doi: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Kepka L, Suit HD, Goldberg SI, et al. Results of radiation therapy performed after unplanned surgery (without re-excision) for soft tissue sarcomas. J Surg Oncol. 2005 Oct 1;92(1):39–45. doi: 10.1002/jso.20351. [DOI] [PubMed] [Google Scholar]
- 23.DeLaney TF, Kepka L, Goldberg SI, et al. Radiation Therapy for Control of Soft-Tissue Sarcomas Resected With Positive Margins. International Journal of Radiation Oncology*Biology*Physics. 2007 Apr 1;67(5):1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Slater JD, McNeese MD, Peters LJ. Radiation therapy for unresectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1986 Oct;12(10):1729–1734. doi: 10.1016/0360-3016(86)90312-3. [DOI] [PubMed] [Google Scholar]
- 25.Tepper JE, Suit HD. Radiation therapy alone for sarcoma of soft tissue. Cancer. 1985 Aug 1;56(3):475–479. doi: 10.1002/1097-0142(19850801)56:3<475::aid-cncr2820560311>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.DeLaney TF, Trofimov AV, Engelsman M, Suit HD Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control. 2005 Jan–Feb;12(1):27–35. doi: 10.1177/107327480501200104. [DOI] [PubMed] [Google Scholar]
- 27.Alektiar KM, Hong L, Brennan MF, Della-Biancia C, Singer S. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: preliminary results. Int J Radiat Oncol Biol Phys. 2007 Jun 1;68(2):458–464. doi: 10.1016/j.ijrobp.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 28.Griffin AM, Euler CI, Sharpe MB, et al. Radiation planning comparison for superficial tissue avoidance in radiotherapy for soft tissue sarcoma of the lower extremity. Int J Radiat Oncol Biol Phys. 2007 Mar 1;67(3):847–856. doi: 10.1016/j.ijrobp.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer. 2007 May 1;109(9):1863–1869. doi: 10.1002/cncr.22609. [DOI] [PubMed] [Google Scholar]
- 30.Hegazy MA, Kotb SZ, Sakr H, et al. Preoperative isolated limb infusion of Doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Ann Surg Oncol. 2007 Feb;14(2):568–576. doi: 10.1245/s10434-006-9138-1. [DOI] [PubMed] [Google Scholar]
- 31.Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol. 2007 Jan;14(1):230–238. doi: 10.1245/s10434-006-9040-x. [DOI] [PubMed] [Google Scholar]
- 32.Grunhagen DJ, de Wilt JH, Graveland WJ, Verhoef C, van Geel AN, Eggermont AM. Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer. 2006 Apr 15;106(8):1776–1784. doi: 10.1002/cncr.21802. [DOI] [PubMed] [Google Scholar]
- 33.Hoven-Gondrie ML, Thijssens KM, Van den Dungen JJ, Loonstra J, van Ginkel RJ, Hoekstra HJ. Long-term locoregional vascular morbidity after isolated limb perfusion and external-beam radiotherapy for soft tissue sarcoma of the extremity. Ann Surg Oncol. 2007 Jul;14(7):2105–2112. doi: 10.1245/s10434-007-9365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Ginkel RJ, Thijssens KM, Pras E, van der Graaf WT, Suurmeijer AJ, Hoekstra HJ. Isolated limb perfusion with tumor necrosis factor alpha and melphalan for locally advanced soft tissue sarcoma: three time periods at risk for amputation. Ann Surg Oncol. 2007 Apr;14(4):1499–1506. doi: 10.1245/s10434-006-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zlotecki RA, Katz TS, Morris CG, Lind DS, Hochwald SN. Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol. 2005 Jun;28(3):310–316. doi: 10.1097/01.coc.0000158441.96455.31. [DOI] [PubMed] [Google Scholar]
- 36.Feng M, Murphy J, Griffith KA, et al. Long-term outcomes after radiotherapy for retroperitoneal and deep truncal sarcoma. Int J Radiat Oncol Biol Phys. 2007 Sep 1;69(1):103–110. doi: 10.1016/j.ijrobp.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Youssef E, Fontanesi J, Mott M, et al. Long-term outcome of combined modality therapy in retroperitoneal and deep-trunk soft-tissue sarcoma: analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 2002 Oct 1;54(2):514–519. doi: 10.1016/s0360-3016(02)02942-5. [DOI] [PubMed] [Google Scholar]
- 38.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001 Jul 15;92(2):359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 39.Jones JJ, Catton CN, O'Sullivan B, et al. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol. 2002 May;9(4):346–354. doi: 10.1007/BF02573869. [DOI] [PubMed] [Google Scholar]
- 40.Pisters PW, Ballo MT, Fenstermacher MJ, et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003 Aug 15;21(16):3092–3097. doi: 10.1200/JCO.2003.01.143. [DOI] [PubMed] [Google Scholar]
- 41.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006 Apr;13(4):508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 42.Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001 May 1;50(1):127–131. doi: 10.1016/s0360-3016(00)01589-3. [DOI] [PubMed] [Google Scholar]
- 43.Gilbeau L, Kantor G, Stoeckle E, et al. Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol. 2002 Dec;65(3):137–143. doi: 10.1016/s0167-8140(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 44.Ballo MT, Zagars GK, Pollock RE, et al. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys. 2007 Jan 1;67(1):158–163. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Tzeng JBF Ching-Wei D, Popple Richard A, PabloArnoletti J, Russo Suzanne M, Urist Marshall M, Bland Kirby I, Heslin Martin J. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107(2):371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 46.Pezner RD, Liu A, Han C, Chen Y-J, Schultheiss TE, Wong JYC. Dosimetric comparison of helical tomotherapy treatment and step-and-shoot intensity-modulated radiotherapy of retroperitoneal sarcoma. Radiotherapy and Oncology. 2006 Oct;81(1):81–87. doi: 10.1016/j.radonc.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Bossi A, De Wever I, Van Limbergen E, Vanstraelen B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. International Journal of Radiation Oncology*Biology*Physics. 2007 Jan 1;67(1):164–170. doi: 10.1016/j.ijrobp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Bobin JY, Al-Lawati T, Granero LE, et al. Surgical management of retroperitoneal sarcomas associated with external and intraoperative electron beam radiotherapy. Eur J Surg Oncol. 2003 Oct;29(8):676–681. doi: 10.1016/s0748-7983(03)00139-2. [DOI] [PubMed] [Google Scholar]
- 49.Alektiar KM, Hu K, Anderson L, Brennan MF, Harrison LB. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2000 Apr 1;47(1):157–163. doi: 10.1016/s0360-3016(99)00546-5. [DOI] [PubMed] [Google Scholar]
- 50.Dziewirski W, Rutkowski P, Nowecki ZI, et al. Surgery combined with intraoperative brachytherapy in the treatment of retroperitoneal sarcomas. Ann Surg Oncol. 2006 Feb;13(2):245–252. doi: 10.1245/ASO.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 51.Habrand JL, Le Pechoux C. Radiation therapy in the management of adult soft tissue sarcomas. Ann Oncol. 2004;15 Suppl 4:iv187–iv191. doi: 10.1093/annonc/mdh925. [DOI] [PubMed] [Google Scholar]


