Abstract
Recent studies in adult male rats have shown that gonadal hormones influence performance on certain working memory and other types of cognitive tasks that are sensitive to lesions of the medial and/or orbital prefrontal cortices. This study asked whether gonadal hormone modulation of prefrontal cortical function in males also extends to the perirhinal division of the rat prefrontal cortex. Specifically, sham-operated control, gonadectomized, and gonadectomized rats supplemented with testosterone propionate or estradiol were tested on a spontaneous novel object recognition task, a paradigm where performance has been shown to be impaired by perirhinal cortical lesions. Using analyses of variance, regression analyses and post-hoc testing to evaluate group differences, it was found that during both the sample and test trials of the task all four groups spent similar absolute and proportional amounts of time ambulating, rearing, stationary, and exploring the two objects present. All groups also explored each of the two identical objects present during sample trials equally. However, during the test trials, only the control and gonadectomized rats given testosterone showed the expected increase in exploration of the novel objects presented, whereas the gonadectomized and gonadectomized, estradiol-supplemental groups continued to explore the novel and familiar objects equally. That regression analyses also identified significant correlations between low bulbospongiosus muscle weight and impaired novel vs. familiar object discrimination further indicates that gonadectomy in adult male rats adversely affects spontaneous novel object recognition in an androgen-sensitive, estrogen-insensitive manner.
Keywords: Working Memory, Estrogen, Androgen, Prefrontal Cortex, Discrimination Learning, Perirhinal Cortex
INTRODUCTION
Although there may be a longer history of study regarding gonadal hormone influence over the cognitive, mnemonic and executive functions of the prefrontal cortices in females (Berman et al., 1997; Gibbs and Gabor, 2003; Keenan et al., 2001; Korol, 2004), there is growing evidence that gonadal steroids also impact prefrontal cortical operations in males. For example, in both young and aged men, circulating testosterone levels have been positively correlated with performance in prefrontal tasks including mental rotation, verbal recall and divided attention (Cherrier et al., 2002; Cherrier et al., 2001; Christiansen and Knussmann, 1987; Moffat and Hampson, 1996; Yaffe et al., 2002). Studies in animal models also suggest an importance of gonadal steroid stimulation for prefrontal cortical function in males. For example, gonadectomy in adult male rats has been shown to significantly impair performance in maze and operant tests of spatial (Daniel et al., 2003; Kritzer et al., 2007; Kritzer et al., 2001) and non-spatial (Ceccarelli et al., 2001) working memory and behavioral flexibility (Kritzer et al., 2007), which are processes that are dependent on the medial prefrontal cortices (Dias and Aggleton, 2000; Kesner et al., 1996; Lacroix et al., 2002; Schwabe et al., 2004; Taylor et al., 2003), as well as performance on a progressive reward ratio task (Kritzer et al., 2007) which is sensitive to lesions of the orbital prefrontal cortices (Kheramin et al., 2005). In this study, analyses of hormone sensitivity were extended to a task sensitive in part to lesions of the perirhinal prefrontal cortices, a spontaneous novel object recognition (NOR) task, to test the hypothesis that functions mediated by this third major division of the prefrontal cortex may also be sensitive to long-term gonadectomy and hormone replacement in adult male subjects. The novel object recognition task is in part a working memory paradigm that is sensitive to both hippocampal (Gaskin et al., 2003; Gould et al., 2002; Gulinello et al., 2006) and cortical lesions placed in and around the prefrontal areas surrounding the rhinal fissure (Aggleton et al., 1997; Barker et al., 2007; Buffalo et al., 2006; Cowell et al., 2006; Ennaceur et al., 1996; Ennaceur et al., 1997; Moses et al., 2005; Mumby and Pinel, 1994; Winters et al., 2004). It is also a task where hormone sensitivity has been previously established in findings of sex and/or estrous cycle differences in NOR performance (Bisagno et al., 2003; Ghi et al., 1999; Sutcliffe et al., 2007; Walf et al., 2006), and in the attenuation of NOR deficits in ovariectomized female rats (with and without chronic stress) by giving ovariectomized animals estradiol (Luine et al., 2006; Luine et al., 2003; Wallace et al., 2006). Here, NOR performance was assessed for the first time in gonadectomized and hormone-replaced adult male rats. In view of previous evidence for effects of gonadectomy on open field behavior (Adler et al., 1999; Kerr et al., 1996; Slob et al., 1986) quantitative assessments of all major behaviors exhibited by the animals including ambulation and rearing were also made alongside those of object exploration to determine whether and to what extent hormone effects on these ancillary behaviors might affect outcome measures of novel object recognition and/or discrimination in males.
METHODS
A. Animal Subjects
Thirty-one adult male Sprague-Dawley rats (Taconic Farms, Germantown, NY) were used. Rats weighed 200-250g at time of surgery and 275-400g when testing began. Animals were divided into four treatment groups: sham operated controls (n=8, CTRL), rats that were gonadectomized (n=8, GDX), and gonadectomized rats that were supplemented with testosterone propionate (n=7, GDX-TP), or 17-β-estradiol (n=8, GDX-E). Throughout, subjects were housed under a 12/12 light/dark cycle with food and water available ad libitum in home cages that contained 2-3 animals; individuals from each treatment group were housed with a mix of individuals either from other or their own treatment groups. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Stony Brook University and were designed to minimize their stress and discomfort.
B. Surgical Procedures and Hormone Replacement
Sham and GDX surgeries were performed 21 days before behavioral testing began under aseptic conditions and using intraperitoneal injections of ketamine (2 mg/100g) and xylazine (1 mg/100g) for anesthesia. For all surgeries, the sac of the scrotum and the underlying layers of tunica were incised. For GDX, the vas deferens was then ligated bilaterally and the testes removed. For the hormone-replaced groups, slow-release pellets that release approximately 3–4 ng of TP per milliliter of blood per day or 25 pg of E per milliliter of blood per day (Innovative Research of America, Sarasota, FL) were then inserted within the tunica. These pellets have been used in previous investigations in this laboratory and have been shown to produce circulating levels of gonadal hormones in GDX rats that fall within physiological ranges (Adler et al., 1999; Kritzer et al., 2007). Incisions were closed using surgical staples.
C. Euthanasiaz
Animals were euthanized two days after completion of behavioral testing by rapid decapitation and their brains were removed and frozen for use in unrelated receptor binding studies. The medial, ventral, and lateral bulbospongiosus muscles (BSM) were also dissected out and weighed at this time.
D. Behavioral Testing: Apparatus and General Procedures
1) Apparatus
Testing was conducted in an open-field arena (80×30×50 cm) that had walls and a floor made of opaque plastic. A grid of 24 10×10 cm squares was marked on the arena floor, and a digital video camera was suspended overhead to record behavior during all testing sessions. A 70% ethanol solution was used to clean the testing arena and all objects (below) before and between all individual trials on all testing days. All tests were conducted in a dimly-lit room with a low level of background white noise (50 dB).
2) Objects
Common, duplicate objects of varying shapes and sizes that included coffee mugs, small and large drinking glasses, teacups, soda cans, and bottles were used for testing object bias and object recognition. All objects weighed at least 450g to discourage their being moved by the animals during testing. The smallest object was 5cm2, and the largest had dimensions of roughly 24cm × 12cm. For object recognition, all objects were approximately cylindrical in shape and ranged from 20-24cm in height and 8-12cm in width.
3) Timeline
Testing took place over a six day period during the animals’ subjective night, between 0800-1500 hours, when the lights in their home environment were on. Prior to testing, the animals’ home cages were placed in the testing room for a period of one hour. The object bias test was conducted first, followed on the next day by an open field test, and on the day after that with an object recognition task habituation trial. After a two day rest interval, the object recognition trials began, with tests using a 1.5 and 4h delay performed sequentially separated by one additional, intervening day of rest. The two delay periods were chosen to model previous studies of hormone manipulations in adult female animals (Luine et al., 2006; Luine et al., 2003; Walf et al., 2006).
E. Behavioral Testing: Paradigm-specific Procedures
1) Object Bias Testing
Animals were placed in the open-field arena with four objects belonging to four categories that were differentiated by size and shape/geometric complexity. Criteria for the categories were: large (more than to 18cm tall), small (less than 12cm tall), smooth (having a regular, cylindrical shape), and complex (having sharp angles, curves, or extending features), and the object categories were: small/smooth objects (e.g., small bowl); large/smooth objects (e.g., soda can); small/complex objects (e.g., teacup) and large/complex objects (e.g., coffee mug). All were located equidistantly from each other and from the arena corners during testing. Animals were placed at the center of the open field to start, with the starting direction and corner positions of the objects falling into the four categories counterbalanced within and across groups.
2) Open Field Test
Animals were placed in the center of the empty arena and allowed a six-minute period to explore. Observation revealed that the principal behaviors engaged in could be categorized as ambulating, rearing or remaining stationary. Times spent on these three behaviors were measured separately for the first and second halves of the six minute testing period.
3) Object Recognition Task Habituation
Identical pairs of objects were placed at one end of the arena 10 cm away from all adjacent walls. Animals were started at the opposite end of the arena facing away from the objects and given three minutes of exploration time. The times spent ambulating, stationary, rearing, and actively exploring objects was scored separately for the first and second halves of the testing period.
4) Object Recognition
The object recognition task consisted of an initial 3-minute sample trial and a subsequent 3-minute test trial. During the sample trial, an identical pair of novel objects (not used in any previous testing) was placed at one end of the arena and in the test trial, one of the pair of sample objects was replaced by a novel object. For all trials, animals were started in the opposite end of the arena facing away from the objects, and the objects used and the positions of the novel and familiar objects during the testing phase were counterbalanced within and across animal groups. All animals were tested first at a 1.5h trial delay and two days later at a 4 hour delay. All behaviors including object exploration and the discrimination index (DI, below) were scored separately for the first and second half of the sample and test trials.
F. Analysis
1) Behavioral Definitions
Behaviors were quantified from digital recordings by a single, blind observer (T.A.). “Ambulation” was defined as the crossing of at least 1 floor grid line within a 3 second period, “stationary” corresponded to the animal remaining unmoving for at least a 3 second duration, and “rearing” was defined as a lifting of the forelimbs and sitting back upon the haunches. Exploration of objects was defined as the animal directing its nose to an item and actively sniffing or whisking; facing or climbing on the object alone did not meet this criteria.
2) Discrimination Index
The differential exploration of two items was quantified by calculating a discrimination index (DI), in which the difference in the amount of time spent with one object vs. the other is expressed as a proportion of total exploration time. When both objects were identical, this was computed according to the following formula, where Obj 1 signified the object on the left, and Obj2 signified the object on the right of the apparatus:
During novel object testing, the DI was calculated as:
With this analysis, a positive score indicated more time spent with the novel object, and a negative score indicated more time spent with the familiar object. Data were collected in separate epochs for the first and second halves of the testing sessions.
3) Statistics
Times spent on given behaviors were expressed as percents of total (object bias testing) or half (all other testing) trial times for statistical analysis and comparison. Assessments of descriptive statistics (mean, variance) were followed by allowed comparisons using single or repeated-measures ANOVAs that included separate measures of all behaviors (StatView 5.0). The DI and bulbospongiosus muscle weights were also compared using regression analyses. For all statistical assessments, post-hoc testing used the Student-Neuman-Keuls (SNK) test, a p<0.05 level was accepted as significant, and a p<0.09 level was identified as the cut-off for group differences that approached significance.
RESULTS
Effectiveness of Hormone Treatment
Bulbospongiosus muscle (BSM) weights showed expected group differences. Thus, the CTRL and GDX-TP animals had average BSM weights of 1.22 and 1.17g, respectively, whereas GDX and GDX-E animals had average BSM weights of 0.30 and 0.27g, respectively. An ANOVA that compared weights across treatment groups revealed significant main effects of hormone treatment [F(3,27)=59.01, p<0.0001], and post hoc tests confirmed that the androgen-sensitive BSM weights of the control and GDX-TP groups did not differ from one another, that the weights in GDX and GDX-E animals were not significantly different from each other, but that the BSM weights of both the GDX and GDX-E groups were significantly lower than those of both the control and GDX-TP groups (Fig 1).
Figure 1.
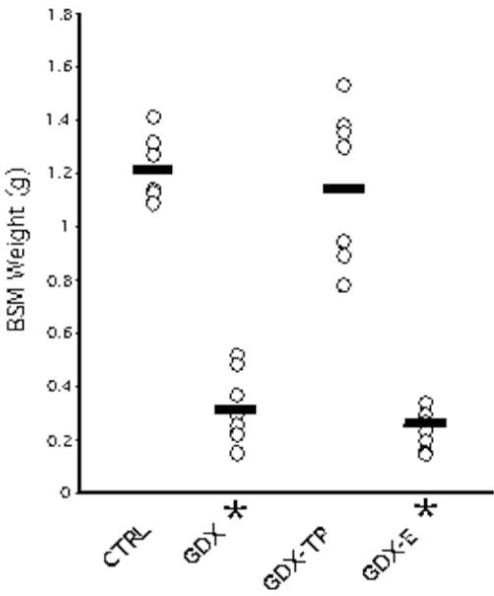
Scatterplots showing bulbospongiosus muscle (BSM) weights in grams (g) of individual animals (dots). Horizontal lines show the average weight for each group. As expected, BSM weights in gonadectomized (GDX) rats and in GDX rats given estradiol (GDX-E) were significantly lower (*) than weights in sham-operated (CTRL) and GDX rats given testosterone propionate (GDX-TP).
Object Bias Test
Testing for object bias revealed consistent differences in the amounts of time that the animals spent exploring four concurrently presented objects belonging to four different size/shape categories, but no group differences in these biases. Thus, the sham-operated, GDX, and GDX-TP and GDX-E cohorts alike all spent roughly 35-38% of total exploration time focusing on large, complex objects, 22-35% of time investigating smaller contoured objects, 20-24% of time on large, smooth objects, and the least amount of time (∼14-19%) exploring the smallest, smoothest objects present (Fig 2). A one-way ANOVA that compared proportionate exploration times confirmed that there were significant main effects of object type/category [F(3, 31)=14.51, p< .0001] but no significant main effects of hormone treatment [F(3, 31)=.440, p=.7263] or hormone treatment by object category interactions [F(3, 31)=1.474, p= .1715] on exploration times. Post-hoc testing also confirmed that most time was spent exploring large, complex objects (p=.0222-.0365), and significantly to near-significantly less time was spent interacting with small, smooth ones (p=.0301-.0593) (Fig 2 A, D). Based on these results, all subsequent testing that required objects used those falling under the classification of large/smooth; objects in this category elicited robust exploration from all animal groups and their relatively simple geometries allowed for a high degree of form continuity among the like and unlike objects used in testing.
Figure 2.
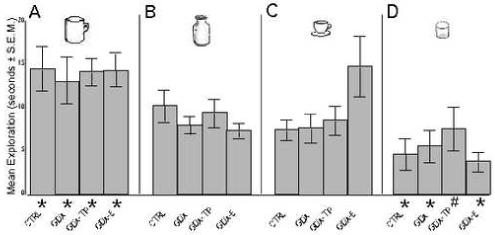
Bar graph showing the mean amount of time (s) animals explored each of four sample objects present in the testing field. Control (CTRL), gonadectomized (GDX), and GDX rats given testosterone propionate (GDX-TP) or estradiol (GDX-E) all spent significantly (*) more time with large/complex objects (A), significantly to near-significantly (#, p<0.07) less time with small/smooth objects (D), and intermediate amounts of time with large/smooth (B) and small/complex (C) objects.
Open Field Test
During Open Field testing, all animal groups engaged in the same three principal behaviors (ambulation, rearing and adopting stationary postures), and all groups apportioned their time among these activities similarly. Thus, during the first half of the trial, animals spent 57-70% ambulating, 20-33% of time stationary and about 6-10% of time rearing. For all groups as well, the percentage of time devoted to ambulating decreased by about 30%, the time spent stationary increased by a similar degree, and the time spent rearing did not appreciably change from the first to the second half of the testing period (Fig. 3). An ANOVA with repeated-measures design that included separate values for time spent on the three principal activities during the first and second halves of the testing period confirmed that there were significant main effects of behavior type [F(2,3)=25.461, p<.0001], and significant main effects of testing half [F(2,3)=71.874, p<.0001], but that there were there were no significant main effects of hormone treatment [F(3,25)=.774, p=.5192] or significant interactions between hormone treatment and behavior type [F(3,6)=.361, p=.9003] or testing halves [F(3,6)=.789, p=.5830, see Figure 3] on open-field activities.
Figure 3.
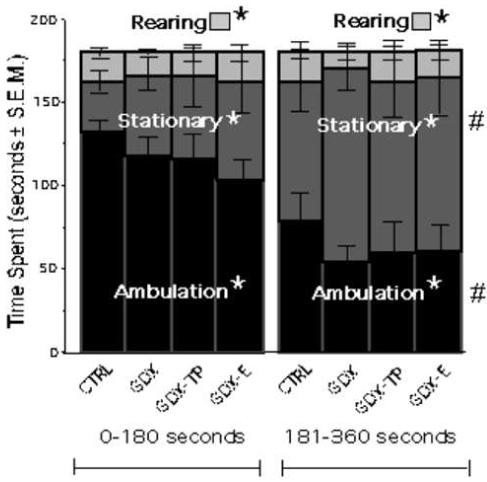
Stacked bar graphs showing the mean amounts of time spent ambulating (black), stationary (dark grey) and rearing (light grey) during the first and final 180 seconds of a six-minute open field session for each group. For control (CTRL), gonadectomized (GDX), and GDX rats given testosterone propionate (GDX-TP) or estradiol (GDX-E), significantly different amounts of time were devoted to each principal behavior (*). In addition, during the second half of the session all groups spent significantly (#) more time stationary and less time ambulating than initially. However, there were no significant main effects of hormone treatment or significant interactions between hormone treatment and behavior.
Object Recognition Task Habituation
Statistical analyses (ANOVA with a repeated-measures design) of a habituation trial for the spontaneous novel object recognition task that included measures of time spent ambulating, rearing, stationary and actively exploring the pair of identical objects that were present in the testing arena revealed significant differences in the amounts of time spent on specific behaviors [F(3, 25)=55.672, p<.0001], and in the proportions of time allotted to them during the first and second halves of testing [F(3, 1)=53.402, p<.0001, see Figure 4A], but no significant main effects of hormone treatment [F(3, 25)=1.504, p=.3863] or significant interactions between hormone treatment and specific behaviors [F(3, 25)=.291, p=.9752] or activities. There were also no significant effects of hormone treatment [F(3, 25)=1.5, p=.3661] or significant effects of hormone treatment on the computed discrimination index (DI) of object exploration [F(3, 25)=.281, p=.8252], whose consistently near zero values indicated that all animals and animal groups tended to interact with each of the two identical objects present for similar amounts of time (Figure 4B).
Figure 4.
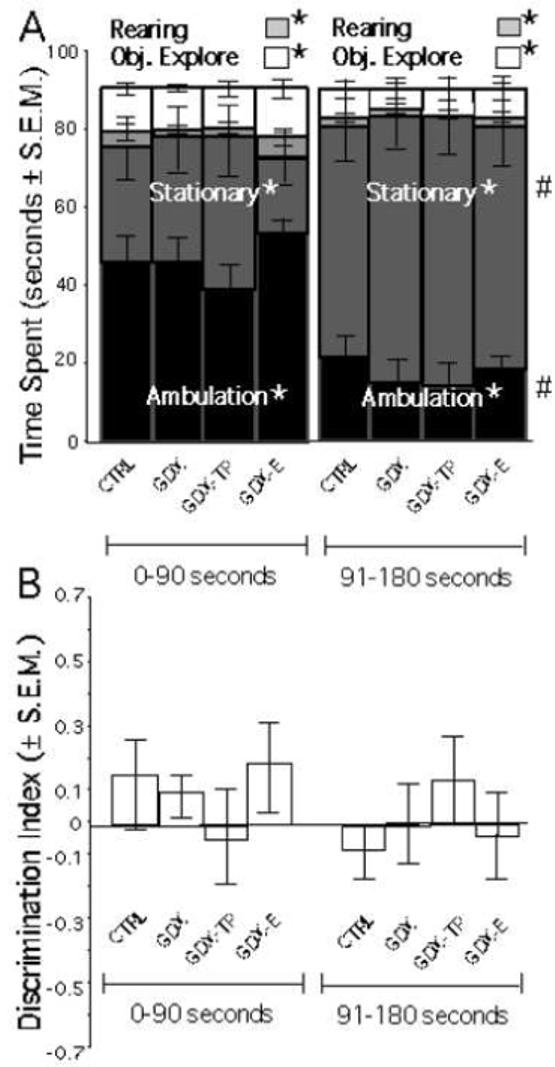
A) Stacked bar graphs showing the mean amounts of time spent ambulating (black), stationary (dark grey), rearing (light grey) and exploring objects (white) during the first and final 90 seconds of a three-minute object recognition task habitation session for each group. For control (CTRL), gonadectomized (GDX), and GDX rats given testosterone propionate (GDX-TP) or estradiol (GDX-E) significantly different amounts of time were devoted to each principal behavior (*). During the second half of the session all groups also spent significantly (#) more time stationary and less time ambulating than initially. No significant main effects of hormone treatment or significant interactions between hormone treatment and object exploration were found. B) Bar graphs showing the Discrimination Index (DI) of object exploration during the first and second halves of the object recognition task habitation trial. For all groups DI values were consistently near zero, indicating that all groups interacted with the two identical objects present for similar amounts of time.
Novel Object Recognition Tasks (1.5 h and 4 h delay)
During spontaneous novel object recognition testing two CTRL, one GDX and one GDX-TP animal moved from the start position immediately to a corner of the testing box and remained there for the entire sample trial; data from these four animals were thus excluded from the analyses below.
As observed in the Open Field and Object Recognition Task Habituation tests, significant differences were identified in the percentages of time that the animals spent ambulating, rearing and stationary during the first halves of testing and in the redistributions of time spent on these activities during second halves of the three-minute periods for both the 1.5 (all p<.0001, see Figure 5 A, B) and the 4 hour delay tasks (p<.0004-.0001 Figure 6, A, B). For the most part, there were no significant group differences in these measures. However, three subjects from the GDX-TP group did show unusually pronounced decreases in ambulation and rearing during the second half of testing for the 1.5 hour delay sample trial, and the repeated-measures ANOVA that included these data uniquely revealed interactions between hormone treatment and behavior [F(3,9)=3.817, p<.0005] and between hormone treatment and behavior over testing halves [F(3,9)=2.640, p<.0129] that were significant. Post-hoc testing, however, confirmed that these effects were driven largely by the ambulatory and stationary activities of the outliers from the GDX-TP group (Figure 5 B).
Figure 5.
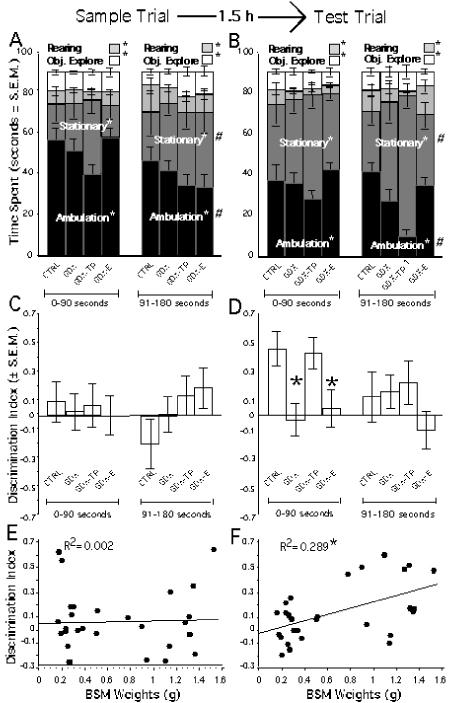
Behavioral data collected during the sample (A, C, E) and test (B, D, F) trials of the spontaneous novel object recognition task with the 1.5 hour delay. Stacked bar graphs showing the mean amounts of time spent ambulating (black), stationary (dark grey), rearing (light grey) and exploring objects (white) during the first and final 90 seconds of a three-minute sample trial (A) and test trial (B) show that the control (CTRL), gonadectomized (GDX), and GDX rats given testosterone propionate (GDX-TP) or estradiol (GDX-E) spent significantly (*) different amounts of time on each principal behavior and that during the second half of the session all groups spent significantly (#) more time stationary and less time ambulating than initially. During the test phase only (B) significant main effects of hormone treatment were observed that were driven by the ambulatory and stationary behaviors of the GDX-TP group (1). Bar graphs showing the Discrimination Index (DI) of object exploration during the first and second halves of the sample (C) and test (D) trials show that all groups explored both sample objects equally (C) and that the CTRL and GDX-TP groups spent significantly (*) more time with the novel object during the first half of the test trial (D) while the GDX and GDX-E groups did not (D). Regression plots graphing individual DI values as a function of bulbospongiosus muscle (BSM) weight in grams (g) show no correlation between the two for the sample trials (E), but a significant (*) correlation between low BSM weight and low DI values/poor object discrimination during the test trial (F).
Figure 6.
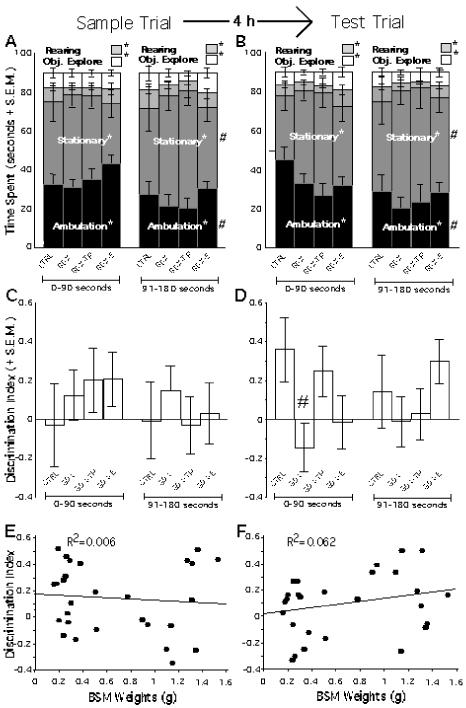
Behavioral data collected during the sample (A, C, E) and test (B, D, F) trials of the spontaneous novel object recognition task with the 4 hour delay. Stacked bar graphs showing the mean amounts of time spent ambulating (black), stationary (dark grey), rearing (light grey) and exploring objects (white) during the first and final 90 seconds of a three-minute sample trial (A) and test trial (B) show that the control (CTRL), gonadectomized (GDX), and GDX rats given testosterone propionate (GDX-TP) or estradiol (GDX-E) spent significantly (*) different amounts of time on each principal behavior and that during the second half of the session all animal groups spent significantly (#) more time stationary and less time ambulating than initially. Bar graphs showing the Discrimination Index (DI) of object exploration during the first and second halves of the sample (C) and test (D) object recognition trials show that all animal groups explored both sample objects equally (C) and that the CTRL and GDX-TP groups spent more time with the novel object during the test trial (D) while the GDX and GDX-E groups spent similar amounts of time with the two objects (D; #, GDX compared to CTRL p=.072). Regression plots graphing individual DI values as a function of bulbospongiosus muscle (BSM) weight in grams (g) show no correlation between the two for the sample trial (E) and a weak relationship between low BSM weight and impaired object discrimination in the test trial (F).
During both the sample and test trials for the two NOR tasks evaluated (1.5 and 4 h delay) all animal groups engaged in similar absolute and proportional amounts of object exploration time (Figures 5 A, B, 6 A, B). The DIs of object exploration measured during the sample trials also showed that all groups explored the two identical objects present equally (Figures 5C, 6C). Statistical analyses of the sample trial (ANOVAs) confirmed that there were no significant main effects of hormone treatment [1.5 hour delay: F(3, 25)=.327, p=.8061; 4 hour delay: F(3, 25)=.319, p=.8118] or testing halves on the DI [1.5 hour delay: F(3, 1)=.012, p=.9143; 4 hour delay: F(3, 1)=.559, p=.4618], nor significant interactions between hormone treatment and DI [1.5 hour delay: F(3, 1)=.607, p=.6172; 4 hour delay; F(3, 1)=.325, p<.8073].
In the test trials, the CTRL and GDX-TP groups spent proportionately more time exploring the novel compared to the familiar object present, whereas GDX and GDX-E groups continued to divide exploration time equally between the old and new objects (Figures 5D, 6D). This group difference was especially pronounced during the first half of the test trials and less so during the second half, during which time the GDX and GDX-E animals persisted in splitting their time evenly between objects and the CTRL and GDX-TP groups attended to the novel and familiar objects more and more similarly. Analyses of variance with repeated-measures designs confirmed these observations and revealed that for the 1.5 hour delay there was a significant main effect of hormone treatment on DI during the first half [F(3,24)=6.034, p< .0033, Figure 5D] but not the second half of the test trial. Allowed post-hoc testing further showed that while the DI’s of GDX and GDX-E groups on the one hand and of the CTRL and GDX-TP groups on the other did not differ from each other, the DI’s of the GDX and GDX-E cohorts were both significantly different from those of the CTRL and GDX-TP groups for the first but not the second half of testing. Corresponding analyses for the 4 hour delay study revealed similar trends in the data that approached but did not reach significance (Figure 6D). Finally, regression analyses that compared individual animal DI values with measures of their BSM weights (Figures 5 E, F, 6 E, F) likewise also showed that there was a significant correlation between high BSM weight and a high DI value (i.e., novel object preference) for the test (R2=.289, p< .0032, Figure 5F) but not the sample trial (R2=.002, p<.8211, Figure 5E) of the 1.5 hour study, and correlations between BSM and DI value that were stronger for the test than the sample trial but did not reach significance for the four hour study (Figure 6 E, F).
DISCUSSION
Recent studies in adult male rats have identified modulatory roles for gonadal steroids on working memory and other types of cognitive tasks that are known to be sensitive to lesions of the medial (Ceccarelli et al., 2001; Daniel et al., 2003; Kritzer et al., 2007; Turvin et al., 2007) and orbital (Kheramin et al., 2003; Kritzer et al., 2007) divisions of the prefrontal cortices in rats. The studies presented here now demonstrate gonadal hormone sensitivity for a spontaneous NOR task that is sensitive in part to lesions of the third major subdivision of the rat PFC – the perirhinal prefrontal cortices. Specifically, long-term gonadectomy in adult male rats was found to significantly and selectively decrease the initial discrimination index in a spontaneous object recognition task at a 1.5 hour delay. Like previously observed effects of gonadectomy on other prefrontal cortical functions (Kritzer et al., 2007), the especially apparent deficits that marked the first half of testing of the 1.5 hour NOR task were attenuated by supplementing GDX animals with testosterone propionate but not estradiol and were significantly correlated with the somatic measure of circulating androgens, the weights of the bulbospongiosus muscle group (measured post-mortem within 2 days of the completion of behavioral testing). That hormone effects were greatest during the first half of test trials also aligns with previous data showing that in intact animals it is primarily during the initial part of the recognition phase of the test trials that a novel object is preferentially explored (Dix and Aggleton, 1999; Moses et al., 2005); after this initial exploration, novel objects rapidly become familiar, and control DI values decline, leaving in essence no room for deficits on novelty recognition to be resolved. Such basement effects may also explain why hormone effects seen in this study at the 4 hour delay were small and failed to reach significance. More specifically, given the relatively low DI of the CTRL group and the consequent reduction in the magnitude of between-group differences, the power needed to resolve group these differences statistically may have been raised beyond that possible with the experimental n’s of this study.
Behavioral Impact of GDX on the NOR Task
Although there are several behavioral paradigms in rats that tap perirhinal prefrontal cortical functions, the focus here on gonadal hormones gave the selection of a NOR task several significant advantages. For example, given evidence for effects of gonadectomy and/or hormone replacement in adult male rats on measures of motivation including break point in a water-but not food-rewarded progressive reward ratio task (Kritzer et al., 2007; van Hest et al., 1988), acquisition of conditioned place preference, and in intracerebroventricular hormone self-administration (Wood, 2004), it is important that the NOR paradigm unlike other perirhinal tasks relies on spontaneous rather than rewarded activity. Further, because some of the effects of GDX on working memory paradigms include task acquisition (Kritzer et al., 2007), that the NOR task requires no training allowed hormone effects on mnemonic components of the task to be demonstrated in relative isolation from learning. Nonetheless, there were also balancing concerns about using the NOR paradigm. Most notably, as has been described in other studies like the present that used fixed trial times (Dix and Aggleton, 1999; Ennaceur and Delacour, 1988; Luine et al., 2003), outcome measures related to object exploration can be influenced by the amounts of time that subjects spend on other, essentially open field behaviors during testing (Moses et al., 2005). This was of particular concern here since significant differences in open field activity have been identified across the sexes (Blizard et al., 1975; Slob et al., 1981; Slob et al., 1986; Swanson, 1966), between ovariectomized and hormonally replaced female rats (Frye and Walf, 2004; Heinsbroek et al., 1988; Palermo-Neto and Dorce, 1990), and among gonadectomized and hormone-replaced adult male rats (Adler et al. 1999; Swanson et al., 1966; but see Slob et al. 1981). The effects of GDX and hormone replacement in adult male rats on various measures of anxiety have also been identified in elevated plus maze (Frye and Walf, 2004; (Walf and Frye, 2005a); Walf and Frye, 2005b), avoidance (Edinger and Frye, 2007; Frye et al., 2004) and acoustic startle response paradigms (Turvin et al., 2007), thus introducing the further possibility that anxiety in the GDX and/or hormone replaced subjects could affect NOR testing and outcome. For these reasons, detailed analyses of videotaped trials were included in the present studies. Importantly, these additional assessments failed to find any evidence for potentially confounding group differences along behavioral dimensions other than the DI of object exploration. Thus, none of the animals exhibited any obvious thigmotaxis or freezing behavior indicative of elevated anxiety, and quantitative analyses of the major behaviors that were observed in addition to object exploration—ambulation, rearing and remaining stationary—revealed no group differences in either the amounts of time devoted to these activities, or in the systematic changes in the proportions of time allotted to them that occurred as the trials progressed. Critically, there were also no significant group differences in the amounts of time that animals or animal groups spent interacting with objects, which compared to the other behaviors evaluated was also surprisingly stable from the first to the second halves of testing for all animal groups. Taken together, these analyses indicate that it is unlikely that hormone effects on potentially interfering behaviors (e.g. ambulation) contributed to the group differences in the DI. Rather, whereas the central nervous system effects of gonadectomy and hormone replacement are widespread, behavioral effects of the manipulations on the spontaneous NOR task seem focused on the principal construct of this paradigm—novel object recognition.
Hormone Sensitivity of the NOR Task: Comparison to Previous Studies
Previous studies have examined spontaneous novel object recognition across the sexes or in hormone-manipulated female rats or mice. In these, although there are discrepancies regarding effects of the estrous cycle on NOR performance (Sutcliffe et al., 2007; Walf et al., 2006), evidence of superior NOR in gonadally intact female compared to male rats (Ghi et al., 1999; Sutcliffe et al., 2007) and in gonadally intact and ovariectomized female rats supplemented with estradiol compared to ovariectomized controls (Luine et al., 2003; Wallace et al., 2006) suggest that circulating estrogens positively influence the exploration and discrimination of novel objects in females. Other studies using delays of 24 hours or more also found facilitating effects of administering estradiol, progesterone and their major metabolites, and even dihydrotestosterone and other non-aromatizable testosterone metabolites immediately after the sample trial on subsequent novel object recognition in female subjects (Frye and Lacey, 2001; Gresack and Frick, 2006; Walf et al., 2006). Our findings provide evidence that gonadal hormones are also important in the NOR task in males and that androgens may play especially critical roles in its modulation in this sex. Further, our findings of abnormally low values of the DI with GDX that are attenuated by replacement with TP but not E are an intriguing fit with clinical evidence of diminished recognition memory in cases of hypogonadism (Salminen et al., 2004; Salminen et al., 2005) and NOR deficits of schizophrenia in male patients (Heckers et al., 2000), which are a part of the cluster of negative, prefrontal cortically mediated symptoms in this disease for which severity has also been directly linked to decreased circulating testosterone levels (Akhondzadeh et al., 2006; Goyal et al., 2004; Huber et al., 2005; Ko et al., 2007; Shirayama et al., 2002; Taherianfard and Shariaty, 2004).
Summary, Conclusions and Future Directions
The findings from this study suggest that in adult male rats, gonadal hormones influence mnemonic functions associated not only with the medial and orbital, but also with the perirhinal division of the prefrontal cortex. Like effects on the spatial working memory processes of the medial prefrontal cortex demonstrated previously (Daniel et al., 2003; Kritzer et al., 2007; Kritzer et al., 2001), the adverse effects of GDX on the NOR task observed here were attenuated by supplementing GDX animals with TP but not E, and were significantly correlated with a bioassay of circulating androgens-- BSM weight. Although not examined in this study, there are several reasons to suspect that correlations previously found between the effects of GDX on medial prefrontal working memory function and on the density of its functionally critical dopamine (DA) innervation (Kritzer et al., 2007) may also extend to the perirhinal-dependent processes of the NOR task, since the effects of GDX on DA innervation in perirhinal prefrontal fields are qualitatively and quantitatively similar to those observed in medial prefrontal areas and are likewise androgen dependent (Kritzer et al., 2003), and because performance on NOR tasks, like that of the medial prefrontal cortical-mediated spatial working memory (Stam et al., 1989; Zahrt et al., 1997) is also impaired by selective perturbations of the prefrontal dopamine system (Morrow et al., 2000). Accordingly, as the study of gonadal hormone influence in males over the executive and mnemonic functions of the prefrontal cortex in rats continues to expand, it may be increasingly important to explore the DAergic endpoints in parallel with behavior.
ACKNOWLEDGEMENTS
The authors thank Dr. Victoria Luine for her encouragement in using the NOR and her assistance in the initial design of the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before in its published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Adler A. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–54. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull. 1997;43:279–87. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:405–10. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Barker GR. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–41. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V. Functional aspects of estrogen neuroprotection. Endocrine. 2003;21:33–41. doi: 10.1385/endo:21:1:33. [DOI] [PubMed] [Google Scholar]
- Blizard DA. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–8. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Buffalo EA. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learn Mem. 2006;13:638–43. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123:65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Cherrier MM. Cognitive effects of short-term manipulation of serum sex steroids in healthy young men. J Clin Endocrinol Metab. 2002;87:3090–6. doi: 10.1210/jcem.87.7.8570. [DOI] [PubMed] [Google Scholar]
- Cherrier MM. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–8. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Cowell RA. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. J Neurosci. 2006;26:12186–97. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching-and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–66. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–19. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Frye CA. 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004;29:1019–27. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci. 2001;1:172–82. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–13. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Gaskin S. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–9. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Ghi P. Sex differences in memory performance in the object recognition test. Possible role of histamine receptors. Pharmacol Biochem Behav. 1999;64:761–6. doi: 10.1016/s0091-3057(99)00143-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–43. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Effects of hippocampal lesions on patterned motor learning in the rat. Brain Res Bull. 2002;58:581–6. doi: 10.1016/s0361-9230(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Goyal RO. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci. 2004;1032:291–4. doi: 10.1196/annals.1314.042. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gulinello M. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–60. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biol Psychiatry. 2000;48:651–7. doi: 10.1016/s0006-3223(00)00919-7. [DOI] [PubMed] [Google Scholar]
- Heinsbroek RP. Effects of progesterone on open field behavior of food deprived ovariectomized female rats. Physiol Behav. 1988;43:779–82. doi: 10.1016/0031-9384(88)90376-9. [DOI] [PubMed] [Google Scholar]
- Huber TJ. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30:111–4. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Keenan PA. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–90. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kerr JE. Androgens selectively modulate C-fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience. 1996;74:757–66. doi: 10.1016/0306-4522(96)00219-9. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–8. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kheramin S. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–52. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kheramin S. Role of the orbital prefrontal cortex in choice between delayed and uncertain reinforcers: a quantitative analysis. Behav Processes. 2003;64:239–250. doi: 10.1016/s0376-6357(03)00142-6. [DOI] [PubMed] [Google Scholar]
- Ko YH. Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology. 2007;32:385–91. doi: 10.1016/j.psyneuen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–23. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience. 2003;122:757–72. doi: 10.1016/s0306-4522(03)00548-7. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–94. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–74. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lacroix L. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- Luine V. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–7. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine VN. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–37. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Morrow BA. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000;52:519–23. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Moses SN. Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Res Bull. 2005;67:62–76. doi: 10.1016/j.brainresbull.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behav Neurosci. 1994;108:11–8. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Dorce VA. Influences of estrogen and/or progesterone on some dopamine related behavior in rats. Gen Pharmacol. 1990;21:83–7. doi: 10.1016/0306-3623(90)90600-q. [DOI] [PubMed] [Google Scholar]
- Salminen EK. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–82. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- Salminen EK. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer. 2005;103:1381–7. doi: 10.1002/cncr.20962. [DOI] [PubMed] [Google Scholar]
- Schwabe K. Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behav Brain Res. 2004;153:21–34. doi: 10.1016/j.bbr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Shirayama Y. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Slob AK. Effects of gonadectomy and exogenous gonadal steroids on sex differences in open field behaviour of adult rats. Behav Brain Res. 1981;2:347–62. doi: 10.1016/0166-4328(81)90017-6. [DOI] [PubMed] [Google Scholar]
- Slob AK. Ontogeny of sex differences in open-field ambulation in the rat. Physiol Behav. 1986;37:313–5. doi: 10.1016/0031-9384(86)90239-8. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–25. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Swanson HH. Sex differences in behaviour of hamsters in open field and emergence tests: effects of pre- and post-pubertal gonadectomy. Anim Behav. 1966;14:522–9. doi: 10.1016/s0003-3472(66)80054-4. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J Med Sci. 2004;58:3–9. [PubMed] [Google Scholar]
- Taylor CL. Impaired delayed spatial win-shift behaviour on the eight arm radial maze following excitotoxic lesions of the medial prefrontal cortex in the rat. Behav Brain Res. 2003;147:107–14. doi: 10.1016/s0166-4328(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Turvin JC. On again, off again effects of gonadectomy on the acoustic startle reflex in adult male rats. Physiol Behav. 2007;90:473–82. doi: 10.1016/j.physbeh.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hest A. The behavior of male and female Wistar rats pressing a lever for food is not affected by sex differences in food motivation. Behav Brain Res. 1988;27:215–21. doi: 10.1016/0166-4328(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Winters BD. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83:279–89. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–12. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- Zahrt J. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


