Abstract
Hyaluronan is an important structural component of extracellular matrices but also interacts instructively with cells during embryonic development, healing processes, inflammation, and cancer. It binds to several different types of cell surface receptors, including CD44, thus leading to co-regulation of important signaling pathways, notably those induced by activation of receptor tyrosine kinases. Consequently, interactions of both stromal and tumor cell-derived hyaluronan with tumor cells play important cooperative roles in several aspects of malignancy. This review focuses on cell autonomous hyaluronan-tumor cell interactions that lead to activation of receptor tyrosine kinases and enhanced drug resistance. Particular emphasis is placed on the role of hyaluronan-CD44 interactions in drug transporter expression and activity, especially in cancer stem-like cells that are highly malignant and resistant to chemotherapy. Antagonists of hyaluronan-CD44 interaction, especially small hyaluronan oligomers, may be useful in therapeutic strategies aimed at preventing tumor recurrence from these therapy-resistant sub-populations within malignant cancers.
Introduction: The relationships between drug resistance, malignancy and cancer stem-like cells
Invasion and metastases of cancer cells and the development of resistance to anticancer therapies are the main causes of morbidity and mortality from cancer. Recently, sub-populations of stem-like cells have been characterized within a variety of cancers. These cells are highly malignant in that they can rapidly regenerate a fully grown tumor when implanted in small numbers in an animal host (1-3) and they may be responsible for tumor metastasis (4, 5). In addition, these cells usually demonstrate resistance to chemotherapy (multi-drug resistance) (6). Expression of the hyaluronan receptor, CD44, is frequently associated with these stem-like cells (1). Both hyaluronan and CD44 have been shown to play a role in drug resistance (7-9), as well as in malignant cell behavior and cell survival (10). Treatment with hyaluronidase enhances the action of various chemotherapeutic agents (11, 12). Moreover, hyaluronan may be extruded from cells by ABC-family drug transporters (13). These and other observations point towards a role for hyaluronan-CD44 interactions in the malignant and drug-resistant properties of cancer cells, and possibly cancer stem-like cells.
Hyaluronan in tumor progression
Hyaluronan is a large, linear glycosaminoglycan composed of 2,000-25,000 disaccharides of glucuronic acid and N-acetylglucosamine: [β1,4-GlcUA-β1,3-GlcNAc-]n, with molecular weights usually ranging from 105 to 107 Da. Hyaluronan is distributed ubiquitously in vertebrate tissues. In adult tissues such as the vitreous, synovial fluid and dermis, it clearly plays an extracellular, structural role that depends on its unique hydrodynamic properties as well as its interactions with other extracellular matrix components. However, hyaluronan is also concentrated in regions of high cell division and invasion, e.g. during embryonic morphogenesis, inflammation, wound repair, and cancer. Thus, in similar fashion to numerous matrix constituents, hyaluronan has an instructive role in terms of cell signaling via hyaluronan receptors on the cell surface, as well as an important structural role (10, 14-17). Although underlying regulatory mechanisms are not well understood, it is clear that hyaluronan-induced signaling is “activated” during dynamic cell processes such as occur in cancer but not under conditions of adult tissue homeostasis.
Considerable experimental evidence implicating hyaluronan in tumor progression has now been obtained in animal models. Several approaches have been used, including manipulation of levels of hyaluronan and perturbation of endogenous hyaluronan-protein interactions. For example, experimental over-expression of the hyaluronan synthase, HAS2, in human fibrosarcoma cells gives rise to elevated hyaluronan production and causes increased tumor cell growth in xenografts in vivo (18). Similar results were obtained in vivo on HAS2 over-expression in a transgenic mouse breast cancer model (19) or on over-expression of HAS3 in human prostate tumor cells (20). On the other hand, transfection of prostate carcinoma cells with antisense Has2 and Has3 reduced subcutaneous tumor growth in nude mice xenografts (21). Tumor growth and metastasis are also inhibited in animal xenograft models by perturbing endogenous hyaluronan-cell receptor interactions in various ways. For example, soluble hyaluronan binding proteins such as the ectodomain of CD44 competitively displace hyaluronan from its endogenous cell surface receptors. Thus over-expression of CD44 ectodomain in mouse mammary carcinoma cells or in human malignant melanoma cells has been shown to inhibit growth, local invasion, and metastasis in vivo (22-25). These effects most likely arise due to induction of apoptosis (23) or cell cycle arrest (24) in vivo. No significant effects were obtained in these studies if the CD44 ectodomain was mutated such that hyaluronan binding was reduced. A soluble form of Rhamm, another hyaluronan receptor, also induces cell cycle arrest and inhibits metastasis (26) and a soluble hyaluronan-binding complex derived from cartilage inhibits both tumor growth and metastasis (27). Likewise, administration of antibodies that block hyaluronan binding to CD44 inhibits tumor growth and invasion (28, 29). In addition, we have found that treatment with small hyaluronan oligosaccharides (oligomers) retards growth of several tumor types in vivo (30-32). These oligomers most likely compete for endogenous polymeric hyaluronan (see Figure 1), thus replacing high affinity, multivalent and cooperative interactions with low affinity, monovalent receptor interactions (33, 34); oligomers containing 6-18 sugar residues are monovalent in their interaction with CD44, whereas larger polymers are multivalent (34).
Fig. 1.
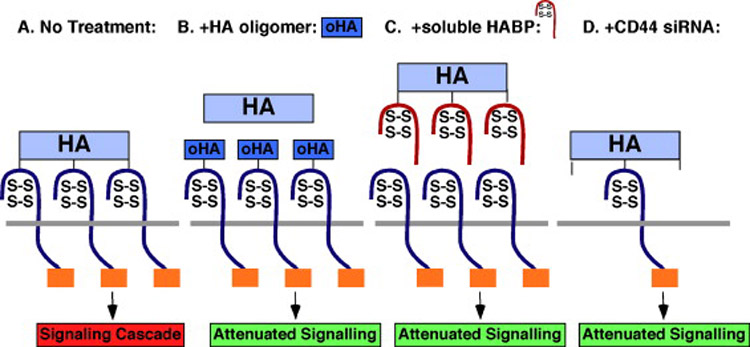
Antagonists of hyaluronan-CD44 signaling (adapted from refs 10 and 51). HA, hyaluronon; HABP, HA-binding protein.
Although these and many other studies have strongly implicated hyaluronan in tumorigenesis, numerous observations have been made that indicate the role of hyaluronan in cancer is complex, especially with respect to hyaluronan processing. First, lower molecular weight hyaluronan, e.g. 10-100 kDa, stimulates angiogenesis but high molecular weight hyaluronan (>1,000 kDa) is inhibitory (35-37). Second, even though elevated hyaluronan production usually promotes tumor progression, extremely high levels of hyaluronan production can be inhibitory (38). Third, glioma progression is promoted by increased hyaluronan production only when hyaluronidase is expressed concomitantly with hyaluronan (39). Similarly, maximum growth of prostate tumors in xenografts was observed on coexpression of both the hyaluronan synthase, HAS2, and the hyaluronidase, HYAL1 (40). Fourth, tumor progression often correlates with both hyaluronan and hyaluronidase levels in human cancers (41). These observations have led several investigators to propose that hyaluronan turnover is essential to the promotion of tumor progression by hyaluronan (23, 39-43). This idea is compounded by the apparent importance of partial degradation of hyaluronan in signaling pathways implicated in inflammation (17), an important factor in the progression of many tumor types (44, 45).
Another complex aspect of the relationship between hyaluronan and tumor progression is the relative contribution of stromal versus cancer cell-produced hyaluronan. In human patients, correlations have been made between increased levels of either stromal or parenchymal hyaluronan and malignant outcomes (reviewed in Tammi et al, Ch. ??). The importance of stromal hyaluronan has been highlighted in a Neu-induced, spontaneous, mouse breast cancer model in which hyaluronan levels were increased by up-regulation of HAS2 (19)(see Kimata and Itano, Ch. ??). Using this model, it was shown that induction of hyaluronan production caused recruitment of stromal cells, deposition of a hyaluronan-enriched stromal matrix and increased angiogenesis, as well as enhanced cell survival signals in the tumor cells themselves (19). However, it was not clear in this study the extent to which stromal cells were the product of epithelial-mesenchymal transition of the carcinoma cells since it is known that hyaluronan can regulate this process (46, 47). Many mechanistic studies have addressed the effects of exogenously added hyaluronan, possibly mimicking the influence of stromal hyaluronan (reviewed in Bourguignon, Ch. ??), but the focus of our work has been on manipulating constitutive hyaluronan interactions in cancer cells themselves. Although both approaches have yielded interesting insights, there is an urgent need for further animal studies that distinguish the effects of stromal and cancer cell-produced hyaluronan.
Cell autonomous regulation of receptor tyrosine kinase activation and anti-apoptotic signaling pathways by endogenously produced hyaluronan
Receptor tyrosine kinases are a class of plasma membrane receptors that bind various regulatory factors, such as EGF, IGF, HGF and PDGF, and activate several intracellular signaling pathways, such as the MAP kinase and phosphoinositide 3-kinase/AKT pathways. Aberrant activities of these receptors, especially members of the ERBB family, have been implicated in the progression of numerous types of human cancers. Increased activity of receptor tyrosine kinases can arise from gene amplification, activating mutations or altered regulation, e.g. by cross-talk between these receptors and integrins or other receptors, or by altered autocrine and paracrine stimulation by various regulatory factors. These changes lead in turn to enhanced tumor cell growth, motility, survival, and resistance to therapies (48-50).
Several reports have documented augmentation of receptor tyrosine kinase and downstream signaling pathway activities after treatment of cancer cells with exogenous hyaluronan (reviewed in Bourguignon, Ch. ??). We have shown that manipulations of constitutive hyaluronan production and interactions in cancer cell themselves also have profound effects on these pathways. We found that constitutively high levels of active, i.e. autophosphorylated, ERBB2 in carcinoma cells are dependent on endogenous hyaluronan-CD44 interaction and that experimentally increased hyaluronan production causes elevated ERBB2 phosphorylation in cells that normally exhibit low levels of ERBB2 activity (51). Furthermore, stimulation of hyaluronan production induces assembly of a constitutive, lipid raft-associated, signaling complex containing phosphorylated ERBB2, CD44, ezrin, phosphoinositide 3-kinase, and the chaperone molecules, HSP90 and CDC37; whereas inhibition of endogenous hyaluronan-CD44 interaction causes disassembly of this complex. Antagonists of hyaluronan interactions used in these studies include hyaluronan oligomers, soluble hyaluronan-binding proteins and siRNA against CD44 (Figure 1), all of which caused disassembly of this complex and inactivation of ERBB2 (51). Recent work in our lab shows that hyaluronan antagonists cause rapid internalization of ERBB2 and CD44 accompanied by their disassociation from one another (M. Slomiany & B. Toole, unpublished). Based on the previous work of other groups (see Bourguignon, Ch. ??), it is likely that variants of CD44, rather than standard CD44, are involved in these events; this issue is currently under investigation in our lab. Similar influences of constitutive hyaluronan-CD44 interaction occur with other receptor tyrosine kinases, i.e. EGFR, IGF-1R, PDGFR and c-MET (52), and corresponding effects have been shown for downstream anti-apoptotic and proliferation pathways known to be regulated by these receptor kinases. For example, increased hyaluronan production stimulates the phosphoinositide 3-kinase and MAP kinase pathways whereas antagonists of hyaluronan interactions suppress these pathways (31, 53).
The phosphoinositide 3-kinase/AKT signaling pathway, up-regulated in most malignant cancer cells, is an anti-apoptotic pathway regulated by several receptor tyrosine kinases, e.g. EGFR, ERBB2 and IGF-1R. These receptor kinases are known to be important in malignant cell properties such as deregulated proliferation, anchorage independent colony formation and invasiveness (48-50). In addition to these pro-malignant and anti-apoptotic activities, this pathway leads to increased expression of broadly distributed ABC family multidrug transporters, e.g. P-glycoprotein (MDR1/ABCB1), multidrug resistance-associated protein-1 (MRP-1/ABCC1) and breast cancer resistance protein (BCRP/ABCG2) (8, 54, 55). Not surprisingly, then, recent publications have demonstrated a close relationship between malignant cell properties and resistance to therapy (6, 7, 9, 56-58).
Regulation of multidrug resistance by hyaluronan
Drug resistance can arise in numerous ways, e.g. decreased uptake of drugs due to cell and tissue barriers, activation of repair and detoxification mechanisms, increased activities of anti-apoptotic signaling pathways, or enhanced drug efflux via cell membrane transporters (59-62). Drug efflux from cancer cells is commonly mediated by ATP-dependent efflux pumps such as members of the MDR, MRP and other ABC transporter subgroups, and expression of these transporters is frequently elevated in malignant cancer cells (6, 59).
The possibility that hyaluronan might influence drug resistance was suggested in the finding that hyaluronidase treatment enhances the action of various chemotherapeutic agents, especially when used locally (12). The relation between hyaluronan and multidrug resistance was also studied in multicellular mammary tumor cell spheroids, known to be enriched in therapy-resistant stem-like cells; dispersion of these spheroids with hyaluronidase reverses drug resistance (63, 64). The mechanistic action of hyaluronidase on drug resistance was not understood at the time of these studies but was usually explained in terms of possible effects on cell adhesion barriers (63), drug penetration (12, 65), or cytokine diffusion (66) rather than hyaluronan-specific effects on signaling pathways. However, early studies by our laboratory showed that calcium-independent aggregation of transformed cells, such as occurs in multicellular spheroids, can be due to hyaluronan-mediated, multivalent cross-bridging of receptors on adjacent cells (67). This observation and the finding that hyaluronan constitutively regulates cell survival signaling pathways (31) led us to further investigate the possible role of hyaluronan in drug resistance. As such, increased hyaluronan production was found to stimulate drug resistance in drug-sensitive cancer cells, whereas disruption of endogenous hyaluronan-induced signaling suppresses cell resistance to several drugs, including doxorubicin, taxol, vincristine, and methotrexate (53). This and other studies show that hyaluronan and CD44 promote drug resistance in a variety of cancer cell types, including breast, lung and head and neck carcinomas, and lymphoma (8, 68-71). Although the anti-apoptotic effect of hyaluronan is likely to contribute to these phenomena, hyaluronan-CD44 interactions also regulate expression of drug transporters, including P-glycoprotein (8), MRP2 (8, 71) and BCRP (32). Recent work from our lab suggests that the effect of hyaluronan on transporter expression may be mediated by stabilization of these transporters in the plasma membrane rather than on synthesis. This conclusion is based on experiments in which we show that treatment of cells with hyaluronan oligomers induces rapid internalization of the drug transporters, BCRP and P-glycoprotein (see Figure 2 for details) (M. Slomiany & B. Toole, unpublished).
Fig. 2.
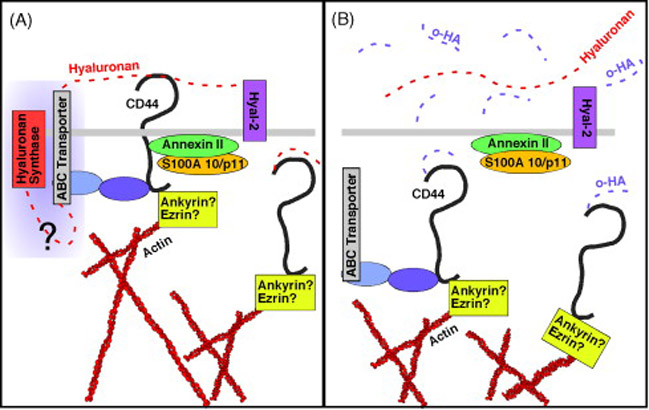
A. Hyaluronan is tethered by CD44 at the plasma membrane whereby it stabilizes actin-linked CD44-transporter complexes in lipid microdomains. Hyaluronan is cleaved by Hyal-2 and internalized via CD44 in an orderly manner. B. Oligomers of hyaluronan (o-HA) stimulate CD44 internalization en masse, destabilizing transporter complexes.
Activation of the MDR1 upstream promoter, associated with P-glycoprotein overexpression, correlates with metastases to the lymph nodes in breast carcinoma cells (57). In accordance with the role of CD44 in malignant cell behavior and metastases, it was shown by confocal microscopic co-localization and fluorescence resonance energy transfer (FRET) studies in NIH3T3 cells that P-glycoprotein is closely associated with CD44 and other components of plasma membrane lipid microdomains, commonly known as lipid rafts (72). Interestingly, P-glycoprotein was found to be anchored to the cytoskeleton. CD44 is known to bind to the actin cytokeleton through ERM-family proteins (73) or ankyrin (74). Thus, these results suggest that CD44 resides in close molecular vicinity (<10 nm) to P-glycoprotein and may be one of the proteins responsible for the cytoskeletal association of this transporter. Furthermore, raft localization of P-glycoprotein seems to be of functional importance since cholesterol depletion results in strong inhibition of transporter activity (72). In addition, a study comparing multidrug resistant cell lines of breast, oral, and ovarian origin that overexpress P-glycoprotein with their respective P-glycoprotein-negative, drug-sensitive, parental cell lines demonstrated a positive correlation in the expression of CD44 and P-glycoprotein. The two were found to co-immunoprecipitate, and drugs that interfere with the function of P-glycoprotein also interfere with cell motility and invasion, both hallmarks of CD44 receptor activity (7). We also have observed that CD44 co-localizes in the plasma membrane of cancer cells with the transporters, P-glycoprotein and BCRP, and that treatment of the cells with hyaluronan oligomers rapidly induces internalization of the transporters and CD44 into the cell. Interestingly, it has been noted that drugs that interfere with P-glycoprotein can also affect localization of CD44 on the cell membrane and promote CD44 capping, and therefore might act via inhibition of actin polymerization (7). Similarly, we have seen that the CD44 internalization process is inhibited if the cells are co-treated with an inhibitor of actin polymerization, latrunculin, thus suggesting that the transporters and CD44 are anchored to actin filaments (M. Slomiany & B. Toole, unpublished). Our interpretation of these results is shown in Figure 2.
Relatedly, it has been demonstrated that expression of CD44 and P-glycoprotein are co-regulated, whereby modulation of CD44 expression correspondingly affected P-glycoprotein expression (7). P-glycoprotein-targeted siRNA also decreased the rate of cell migration (7), which agrees with the observation that both P-glycoprotein and CD44 need to be active in T lymphocytes for their proper migration to lymph nodes (75). Most likely these effects are due to alterations in CD44 expression, and therefore its migration-inducing activity (76, 77), by inhibition of P-glycoprotein expression.
In addition, there may be a direct relationship between hyaluronan and drug transport since manipulation of hyaluronan in a cell-free system inhibits drug transport (S. Misra, S. Ghatak & B. Toole, unpublished). Moreover, based on studies documenting association of polysaccharide export with ABC-transporters in bacteria (78), recent work indicates that hyaluronan might be secreted through multidrug transporters in vertebrate cells (13, 79). Studies employing a battery of inhibitors as well as siRNA to sort out possible transporters involved in hyaluronan export led to the conclusion that MRP5 is the most likely hyaluronan transporter in human fibroblasts (13). The MRP5 gene is ubiquitously expressed, with the highest levels of expression found in skeletal muscle and the brain. However, whereas MRP5 knockout mice are viable (80), hyaluronan deficiency is most likely incompatible with life in vertebrates (46). Thus hyaluronan export probably requires alternative or backup transport systems that can compensate for the lack of MRP5. Also, it would not be surprising to find other members of multidrug resistant transporters as hyaluronan exporters since these transporters are often expressed in a tissue specific pattern. These may include MRP1 (75) or ABCC11 or ABCC12 due to their close phylogenetic relationship (13).
Although this evidence supports a role for drug transporters in hyaluronan secretion, other studies strongly suggest that constitutive export of hyaluronan requires only the hyaluronan synthases themselves (81). Moreover, definitive evidence for hyaluronan export through ABC transporters, rather than regulation by transporter activity, is lacking. Nevertheless it is likely that such export does occur at least under certain circumstances. Indeed, our findings demonstrate that hyaluronan production or export is inhibited by treatment of cells with hyaluronan oligomers (52) and that treatment of cells with hyaluronan oligomers induces rapid internalization of the drug transporters, BCRP and P-glycoprotein (M. Slomiany & B. Toole, unpublished). Thus, the finding that drugs used in the inhibition of drug transporters may also inhibit hyaluronan synthesis may open novel ways for treatment of diseases characterized by hyaluronan overproduction such as edema formation after injuries and inflammation, or in metastasis.
Hyaluronan-CD44 interactions, cancer stem cells and resistance to chemotherapy
Of particular relevance to the relationship of malignant cell properties to chemoresistance are the properties of a small sub-population of stem-like cells that has now been characterized within many cancers. These cells have been variously named: “cancer stem cells”, “cancer progenitor cells” and “tumor-initiating cells”. These cells are highly malignant in that a very small number can rapidly regenerate a fully grown tumor when implanted in an animal host (1-3) and they may include the metastatic sub-population of tumors (4, 5). These cells are also resistant to chemotherapeutic agents (6) and to radiation (82, 83).
CD44 is one of the most common markers used for isolation of cancer stem-like cells (84-87), and recent studies of leukemia stem cells indicate that CD44 may be functionally important as well (88, 89). Other studies point to a possible role for another hyaluronan-binding protein, Rhamm, in myeloma progenitors (90, 91). However, virtually nothing is known about the potential role of hyaluronan, the major ligand for CD44 and Rhamm, although hyaluronan appears to have a role in normal stem cell behavior (92-95) and hyaluronan synthases are altered in myeloma progenitors (96, 97).
The exact nature of these tumor sub-populations is controversial, especially with respect to their precise relationship to stem cells (2, 98-100) but the presence of highly malignant, therapy-resistant sub-populations within human tumors is reasonably well-established. Consequently, recent work has focused on the nature of their resistance to therapy and in particular, the elevated expression of ABC-family drug transporters by these cells that may be central to tumor recurrence or persistence after chemotherapy (6, 58). Recently we have begun to examine the effects of perturbing hyaluronan interactions with hyaluronan oligomers on the malignant and therapy-resistant properties of stem-like cells isolated from cancer cell lines and from patient-derived tumors. As discussed above, these oligomers most likely displace constitutively bound hyaluronan polymer from its receptors, resulting in attenuation of hyaluronan-induced signaling (31, 51, 52). We find that the oligomers inhibit growth of a very aggressive stem-like sub-population isolated from C6 glioma cells in a novel spinal cord engraftment model that replicates invasive behaviors of human gliomas in the central nervous system (32). The oligomers cause increased apoptosis and decreased proliferation in these tumors. The stem-like cells show elevated activation of EGFR and AKT, expression of the BCRP drug transporter and resistance to treatment with methotrexate, when compared with the parental cells. All of these parameters were reduced by treatment with the oligomers (32), indicating the potential importance of hyaluronan in the properties of these cells.
Studies in several types of cancer cells have demonstrated that it is possible to define and isolate an enriched tumor-initiating population using the so-called “side population” phenotype (101); this phenotype depends on efflux of the Hoechst 33342 dye by drug transporters, especially BCRP. In a study of lung cancer cells (102), the side population cells were found to be significantly enriched for tumorigenicity and invasiveness, possessed stem cell properties such as multidrug resistance, high telomerase activity, and tumor-repopulating capacity, and consistently expressed ABC transporters, including BCRP, ABCA2, P-glycoprotein, and MRP1 (and related subfamily members MRP2 to MRP9), that consequently increased their resistance to multiple chemotherapeutic drugs. However, expression of CD44, and other commonly used cancer stem cell markers, varied amongst side population preparations and was present in side population and non-side population cells, pointing to remaining heterogeneity amongst these subpopulations and lack of exclusivity of CD44 to a particular subpopulation (102). This is in agreement with recent studies that confirm the difficulty of employing Hoechst dye exclusion alone to isolate homogenous side populations of stem-like cells, as drug transporter type and expression may vary widely, even amongst populations with similar tumorigenicities (98). Thus, the immediate challenge to understanding the relationship between hyaluronan, CD44, and multidrug resistance in cancer stem-like cells will come from the isolation of homogenous populations to better characterize the transporters involved and their relationship to CD44 expression.
Despite these caveats, it is reasonable to expect that antagonists of hyaluronan-CD44 interaction, e.g. small hyaluronan oligomers, may be useful in therapeutic strategies aimed at preventing tumor recurrence from therapy-resistant sub-opulations within malignant cancers.
Acknowledgments
Recent work from our lab that is described herein was supported by grants to B.P.T. from the National Institutes of Health (CA073839 and CA082867), the Department of Defense (OC050368) and The Charlotte Geyer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 2.Hill RP, Perris R. “Destemming” cancer stem cells. J Natl Cancer Inst. 2007;99:1435–1440. doi: 10.1093/jnci/djm136. [DOI] [PubMed] [Google Scholar]
- 3.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 4.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 7.Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN, Rodriguez-Rodriguez L. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–6667. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 8.Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–20315. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- 9.Colone M, Calcabrini A, Toccacieli L, Bozzuto G, Stringaro A, Gentile M, Cianfriglia M, Ciervo A, Caraglia M, Budillon A, Meo G, Arancia G, Molinari A. The Multidrug Transporter P-Glycoprotein: A Mediator of Melanoma Invasion? J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701082. in press, published on web. [DOI] [PubMed] [Google Scholar]
- 10.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 11.Kerbel RS, Rak J, Kobayashi H, Man MS, St Croix B, Graham CH. Multicellular resistance: a new paradigm to explain aspects of acquired drug resistance of solid tumors. Cold Spring Harb Symp Quant Biol. 1994;59:661–672. doi: 10.1101/sqb.1994.059.01.076. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner G, Gomar-Hoss C, Sakr L, Ulsperger E, Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors--experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:85–99. [PubMed] [Google Scholar]
- 13.Prehm P, Schumacher U. Inhibition of hyaluronan export from human fibroblasts by inhibitors of multidrug resistance transporters. Biochem Pharmacol. 2004;68:1401–1410. doi: 10.1016/j.bcp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 15.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 16.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 18.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 19.Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N. Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol. 2007;170:1086–1099. doi: 10.2353/ajpath.2007.060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001;61:5207–5214. [PubMed] [Google Scholar]
- 21.Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol. 2002;161:849–857. doi: 10.1016/S0002-9440(10)64245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson RM, Yu Q, Stamenkovic I, Toole BP. Perturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascites. Am J Pathol. 2000;156:2159–2167. doi: 10.1016/S0002-9440(10)65086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, Herrlich P, Simon JC. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399–3408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- 26.Mohapatra S, Yang X, Wright JA, Turley EA, Greenberg AH. Soluble hyaluronan receptor RHAMM induces mitotic arrest by suppressing Cdc2 and cyclin B1 expression. J Exp Med. 1996;183:1663–1668. doi: 10.1084/jem.183.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu N, Lapcevich RK, Underhill CB, Han Z, Gao F, Swartz G, Plum SM, Zhang L, Gree SJ. Metastatin: a hyaluronan-binding complex from cartilage that inhibits tumor growth. Cancer Res. 2001;61:1022–1028. [PubMed] [Google Scholar]
- 28.Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- 29.Zahalka MA, Okon E, Gosslar U, Holzmann B, Naor D. Lymph node (but not spleen) invasion by murine lymphoma is both CD44- and hyaluronate-dependent. J Immunol. 1995;154:5345–5355. [PubMed] [Google Scholar]
- 30.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer. 1998;77:396–401. doi: 10.1002/(sici)1097-0215(19980729)77:3<396::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 32.Gilg AG, Tye SL, Tolliver LB, Wheeler WG, Visconti RP, Duncan JD, Kostova FV, Bolds LN, Toole BP, Maria BL. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-1228. in press. [DOI] [PubMed] [Google Scholar]
- 33.Underhill CB, Chi-Rosso G, Toole BP. Effects of detergent solubilization on the hyaluronate-binding protein from membranes of simian virus 40-transformed 3T3 cells. J Biol Chem. 1983;258:8086–8091. [PubMed] [Google Scholar]
- 34.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 35.West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp. 1989;143:187–201. doi: 10.1002/9780470513774.ch12. [DOI] [PubMed] [Google Scholar]
- 36.Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- 37.Rahmanian M, Pertoft H, Kanda S, Christofferson R, Claesson-Welsh L, Heldin P. Hyaluronan oligosaccharides induce tube formation of a brain endothelial cell line in vitro. Exp Cell Res. 1997;237:223–230. doi: 10.1006/excr.1997.3792. [DOI] [PubMed] [Google Scholar]
- 38.Itano N, Sawai T, Atsumi F, Miyaishi O, Taniguchi S, Kannagi R, Hamaguchi M, Kimata K. Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem. 2004;279:18679–18687. doi: 10.1074/jbc.M313178200. [DOI] [PubMed] [Google Scholar]
- 39.Enegd B, King JA, Stylli S, Paradiso L, Kaye AH, Novak U. Overexpression of hyaluronan synthase-2 reduces the tumorigenic potential of glioma cells lacking hyaluronidase activity. Neurosurgery. 2002;50:1311–1318. doi: 10.1097/00006123-200206000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Simpson MA. Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am J Pathol. 2006;169:247–257. doi: 10.2353/ajpath.2006.060032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lokeshwar VB, Schroeder GL, Selzer MG, Hautmann SH, Posey JT, Duncan RC, Watson R, Rose L, Markowitz S, Soloway MS. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer. 2002;95:61–72. doi: 10.1002/cncr.10652. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delpech B, Laquerriere A, Maingonnat C, Bertrand P, Freger P. Hyaluronidase is more elevated in human brain metastases than in primary brain tumours. Anticancer Res. 2002;22:2423–2427. [PubMed] [Google Scholar]
- 44.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 45.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278:45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 48.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 49.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 50.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 51.Ghatak S, Misra S, Toole BP. Hyaluronan regulates constitutive ErbB2 phosphorylation and signal complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 52.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 53.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multi-drug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 54.Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, Summer R, Fine A, Quesenberry PJ, Walsh K. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 55.Lee JT, Jr, Steelman LS, McCubrey JA. Phosphatidylinositol 3’-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 56.Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res. 2003;1:420–427. [PubMed] [Google Scholar]
- 57.Raguz S, Tamburo De Bella M, Tripuraneni G, Slade MJ, Higgins CF, Coombes RC, Yague E. Activation of the MDR1 upstream promoter in breast carcinoma as a surrogate for metastatic invasion. Clin Cancer Res. 2004;10:2776–2783. doi: 10.1158/1078-0432.ccr-03-0517. [DOI] [PubMed] [Google Scholar]
- 58.Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Bophys Res Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- 59.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 60.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 61.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 63.Kerbel RS, St Croix B, Florenes VA, Rak J. Induction and reversal of cell adhesion-dependent multicellular drug resistance in solid breast tumors. Hum Cell. 1996;9:257–264. [PubMed] [Google Scholar]
- 64.St Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett. 1998;131:35–44. doi: 10.1016/s0304-3835(98)00199-2. [DOI] [PubMed] [Google Scholar]
- 65.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 66.Vincent T, Molina L, Espert L, Mechti N. Hyaluronan, a major non-protein glycosaminoglycan component of the extracellular matrix in human bone marrow, mediates dexamethasone resistance in multiple myeloma. Br J Haematol. 2003;121:259–269. doi: 10.1046/j.1365-2141.2003.04282.x. [DOI] [PubMed] [Google Scholar]
- 67.Underhill CB, Toole BP. Receptors for hyaluronate on the surface of parent and virus-transformed cell lines: binding and aggregation studies. Exp Cell Res. 1981;131:419–423. doi: 10.1016/0014-4827(81)90248-2. [DOI] [PubMed] [Google Scholar]
- 68.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 69.Wang SJ, Bourguignon LY. Hyaluronan-CD44 promotes phospholipase C-mediated Ca2+ signaling and cisplatin resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:19–24. doi: 10.1001/archotol.132.1.19. [DOI] [PubMed] [Google Scholar]
- 70.Cordo Russo RI, Garcia MG, Alaniz L, Blanco G, Alvarez E, Hajos SE. Hyaluronan oligosaccharides sensitize lymphoma resistant cell lines to vincristine by modulating P-glycoprotein activity and PI3K/Akt pathway. Int J Cancer. 2008;122:1012–1018. doi: 10.1002/ijc.23122. [DOI] [PubMed] [Google Scholar]
- 71.Ohashi R, Takahashi F, Cui R, Yoshioka M, Gu T, Sasaki S, Tominaga S, Nishio K, Tanabe KK, Takahashi K. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett. 2007;252:225–234. doi: 10.1016/j.canlet.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 72.Bacso Z, Nagy H, Goda K, Bene L, Fenyvesi F, Matko J, Szabo G. Raft and cytoskeleton associations of an ABC transporter: P-glycoprotein. Cytometry A. 2004;61:105–116. doi: 10.1002/cyto.a.20081. [DOI] [PubMed] [Google Scholar]
- 73.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP(3) receptor in lipid rafts promotes hyaluronan-mediated Ca(2+) signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 75.Honig SM, Fu S, Mao X, Yopp A, Gunn MD, Randolph GJ, Bromberg JS. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tzircotis G, Thorne RF, Isacke CM. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J Cell Sci. 2005;118:5119–5128. doi: 10.1242/jcs.02629. [DOI] [PubMed] [Google Scholar]
- 77.Tzircotis G, Thorne RF, Isacke CM. Directional sensing of a phorbol ester gradient requires CD44 and is regulated by CD44 phosphorylation. Oncogene. 2006;25:7401–7410. doi: 10.1038/sj.onc.1209724. [DOI] [PubMed] [Google Scholar]
- 78.Ouskova G, Spellerberg B, Prehm P. Hyaluronan release from Streptococcus pyogenes: export by an ABC transporter. Glycobiology. 2004;14:931–938. doi: 10.1093/glycob/cwh115. [DOI] [PubMed] [Google Scholar]
- 79.Schulz T, Schumacher U, Prehm P. Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J Biol Chem. 2007;282:20999–21004. doi: 10.1074/jbc.M700915200. [DOI] [PubMed] [Google Scholar]
- 80.de Wolf CJ, Yamaguchi H, van der Heijden I, Wielinga PR, Hundscheid SL, Ono N, Scheffer GL, de Haas M, Schuetz JD, Wijnholds J, Borst P. cGMP transport by vesicles from human and mouse erythrocytes. Febs J. 2007;274:439–450. doi: 10.1111/j.1742-4658.2006.05591.x. [DOI] [PubMed] [Google Scholar]
- 81.Weigel PH, Deangelis PL. Hyaluronan synthases: A decade-plus of novel glycosyltransferases. J Biol Chem. 2007 doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 82.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 83.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 84.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 87.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 89.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 90.Crainie M, Belch AR, Mant MJ, Pilarski LM. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: identification of three distinct RHAMM variants. Blood. 1999;93:1684–1696. [PubMed] [Google Scholar]
- 91.Maxwell CA, Rasmussen E, Zhan F, Keats JJ, Adamia S, Strachan E, Crainie M, Walker R, Belch AR, Pilarski LM, Barlogie B, Shaughnessy J, Jr, Reiman T. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood. 2004;104:1151–1158. doi: 10.1182/blood-2003-11-4079. [DOI] [PubMed] [Google Scholar]
- 92.Pilarski LM, Pruski E, Wizniak J, Paine D, Seeberger K, Mant MJ, Brown CB, Belch AR. Potential role for hyaluronan and the hyaluronan receptor RHAMM in mobilization and trafficking of hematopoietic progenitor cells. Blood. 1999;93:2918–2927. [PubMed] [Google Scholar]
- 93.Nilsson SK, Haylock DN, Johnston HM, Occhiodoro T, Brown TJ, Simmons PJ. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101:856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- 94.Matrosova VY, Orlovskaya IA, Serobyan N, Khaldoyanidi SK. Hyaluronic acid facilitates the recovery of hematopoiesis following 5-fluorouracil administration. Stem Cells. 2004;22:544–555. doi: 10.1634/stemcells.22-4-544. [DOI] [PubMed] [Google Scholar]
- 95.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 96.Calabro A, Oken MM, Hascall VC, Masellis AM. Characterization of hyaluronan synthase expression and hyaluronan synthesis in bone marrow mesenchymal progenitor cells: predominant expression of HAS1 mRNA and up-regulated hyaluronan synthesis in bone marrow cells derived from multiple myeloma patients. Blood. 2002;100:2578–2585. doi: 10.1182/blood-2002-01-0030. [DOI] [PubMed] [Google Scholar]
- 97.Adamia S, Reiman T, Crainie M, Mant MJ, Belch AR, Pilarski LM. Intronic splicing of hyaluronan synthase 1 (HAS1): a biologically relevant indicator of poor outcome in multiple myeloma. Blood. 2005;105:4836–4844. doi: 10.1182/blood-2004-10-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2-cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 99.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2007 doi: 10.1002/ijc.23130. in press, published on web. [DOI] [PubMed] [Google Scholar]
- 101.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 102.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]


