Abstract
Objective
Platinum/paclitaxel-based chemotherapy is a current treatment for advanced epithelial ovarian cancer. We sought to explore the association between weight change during treatment and survival, as well as the association between pre-chemotherapy body mass index (BMI) and survival.
Methods
A retrospective data review was conducted of 792 advanced ovarian cancer patients who participated in a phase III randomized trial of cisplatin/paclitaxel versus carboplatin/paclitaxel. Pre-chemotherapy BMI was calculated following surgery. Weight change was defined as the ratio of body weight at completion of protocol therapy to pre-chemotherapy body weight. Progression-free survival (PFS) and overall survival (OS), classified by BMI or relative weight change, were estimated by Kaplan-Meier, and associations were assessed using a Cox model controlled for known prognostic variables (age, race, performance status, histology, tumor grade, tumor residual and treatment group).
Results
There was no association between pre-chemotherapy BMI and survival. There was a significant relationship between median OS and weight change as follows: >5% decrease=48.0 months; 0-5% decrease=49.3 months; 0-5% increase=61.1 months; and >5% increase=68.2 months. Adjusted for covariates, the relative risk of death increased by 7% for each 5% decrease in body weight (HR=0.93, 95% CI=0.88-0.99; p=0.013)
Conclusions
Change of body weight during primary chemotherapy was a strong prognostic factor for overall survival. Loss of body weight during primary therapy is an indicator for poor OS; weight gain is an indicator for improved survival. This study supports the development of strategies to minimize weight loss that can be assessed in a prospective, randomized study to improve patient outcomes.
Keywords: body weight, BMI, chemotherapy, ovarian cancer, survival
Introduction
Obesity is implicated as a risk factor in the incidence and mortality due to cancer, and is implicated as being responsible for 14% and 20% of all cancer deaths for men and women, respectively.1 This may in part be due to higher levels of circulating estrogens among those with a higher proportion of adipose tissue that contributes to an increased risk of hormone-dependent cancers.2 Population-based studies have shown increasing risk for cancer mortality with higher body mass index (BMI), which assesses body weight in relation to height, for stomach and prostate cancers in men and breast, endometrial, cervical and ovarian cancers in women.1 Large cohorts of ovarian cancer patients have demonstrated that the risk of ovarian cancer mortality is increased among those with higher BMI.3,4,5 However, there remains conflicting evidence about these findings, primarily because of the lack of control for potential confounding variables, such as surgical or treatment-related factors.
Women who are diagnosed and treated for ovarian cancer are known to experience a variety of chemotherapy-related side effects that can affect quality of life. Ovarian cancer patients may experience cachexia as a result of advanced disease (e.g., bowel obstruction, metabolic changes) and therefore suffer loss of body weight.6 Cachexia has been associated with reduced survival and quality of life in patients with advanced cancers.7 There is also some evidence of weight gain following diagnosis of breast cancer that is largely due to the significant decrease in physical activity following cancer diagnosis.8,9 Furthermore, paclitaxel-based chemotherapy has been associated with patient weight gain in studies of both breast and lung cancer.10,11 Although there has not been a systematic analysis to date, there is some evidence that carboplatin combined with paclitaxel can cause weight gain in ovarian cancer patients.12,13 Therefore, there may be potential risk-factors related to survival related to either high BMI or weight loss.
It is unclear, however, if either an increase or decrease in body weight during chemotherapy could potentially impact survival. Understanding the nature of the problem will enable us to develop future cancer control research that can positively impact the quality of life, and potentially the survival, of ovarian cancer patients during treatment. Nutritional support, dietary intervention, or other interventions to reduce symptoms that may negatively impact appetite or physical activity could decrease any risks associated with weight change if applied appropriately to the problem.
Little is known about the incidence of weight change among ovarian cancer patients. In 2006, Gil and colleagues demonstrated that weight loss occurs after surgery, and weight is regained gradually over the year following surgery, and that the weight regained tended to be body fat, not muscle mass.14 Due to the fact that excess adipose tissue can impact circulating estrogens, weight change may impact disease progression. However, the impact of weight change on survival in this population is unknown. To our knowledge, there have been no studies of ovarian cancer patients that have evaluated weight change during primary chemotherapy as related to survival outcomes among ovarian cancer patients. Few studies have yet assessed BMI and ovarian cancer survival in a randomized trial setting, where potentially confounding therapeutic and patient-related variables may be controlled. Therefore, this study was designed to perform a retrospective examination of the association of pre-chemotherapy BMI and body weight change during chemotherapy with survival from ovarian cancer.
Methods
From April, 1995 through January, 1998, the Gynecologic Oncology Group (GOG) enrolled 792 ovarian cancer patients to GOG-158, “a phase III randomized study of cisplatin and paclitaxel (24-hour infusion) versus carboplatin and paclitaxel (3-hour infusion) in optimal stage III epithelial ovarian carcinoma.” Briefly, GOG-158 was designed to compare the efficacy and toxicity of carboplatin plus paclitaxel with that of cisplatin plus paclitaxel among women with optimal stage III ovarian cancer (tumor residual ≤1cm). Eligible patients were randomly assigned to one of two treatment groups: Arm I: cisplatin 75 mg/m2 plus a paclitaxel 135 mg/m2 (24-hr infusion) (n=400); or Arm II: carboplatin (AUC=7.5) plus paclitaxel 175 mg/m2 (3-hr infusion) (n=392). The final results of the trial were published by Ozols and colleagues in 2003.15 There were no differences in either PFS (hazard ratio for death [HR]=0.88; 95% confidence interval [CI]=0.75 to 1.03) or OS (HR=0.84; 95% CI=0.70 to 1.02) between treatment groups; however, cisplatin/paclitaxel treatment was associated with a statistically significant greater incidence of grade 3-4 leukopenia, gastrointestinal, renal and metabolic toxicity. Throughout the study treatment period, information was collected at each treatment visit (every 21 days for 6 cycles) regarding body weight, side effects and other treatment-related factors. All patients provided written informed consent consistent with all federal, state and local institution requirements prior to receiving protocol therapy.
BMI was calculated using body weight (in kilograms) divided by height squared (in meters). Patients were classified into three groups based on BMI: less than 25.0; 25.0 to 29.9; and greater than or equal to 30.0. These categories correspond with the World Health Organization classification for normal weight, overweight and obese individuals. Mean BMI by patient characteristics (age group, race, performance status, histology, and tumor grade) were assessed by ANOVA. The Tukey-Kramer method was used for pair-wise comparisons. Change in body weight during the treatment period and comparison between treatment groups were assessed using a Mixed model procedure, treating body weight as repeated measures. Only patients who completed the prescribed six cycles of chemotherapy were included in assessment of changes in weight over time. This strategy was taken due to a concern that those patients with missing time point data were likely those with poorer outcomes (e.g., unable to complete therapy), and a random pattern of missing data cannot be assumed. While maintaining homogeneity in the study population, this strategy does limit the inference of study results to patients completing six cycles of primary therapy (86% of all patients in GOG-158 completed six cycles of chemotherapy).
The extent of weight change during therapy was determined by comparing body weight measured pre-treatment (W1) with body weight reassured at the time of the sixth cycle of chemotherapy (W6), and a relative change in body weight was calculated as: [(W6/W1)-1] × 100%. For categorical analyses, patients were classified into four groups based on relative change in body weight: decrease < 5%, decrease 0-5% (0% not included), increase 0-5% (0% included), and increase > 5%. PFS and OS were defined as reported in the parent clinical trial.15 PFS and OS were calculated by the Kaplan-Meier method and the log-rank test was used to compare the survival distribution. Multivariate analysis was conducted with a Cox model adjusted for covariates (age, race, performance status, histology, tumor grade, tumor residual and treatment). The linearity between weight change and risk of death was assessed using a graphical method as suggested by Homer and Lemeshow.16 All reported p values were two sided and the statistical analyses performed on SAS (Statistical Analysis System) version 9.1 (SAS Institute, Cary, NC).
Results
Body Mass Index (BMI) and Clinical Outcomes
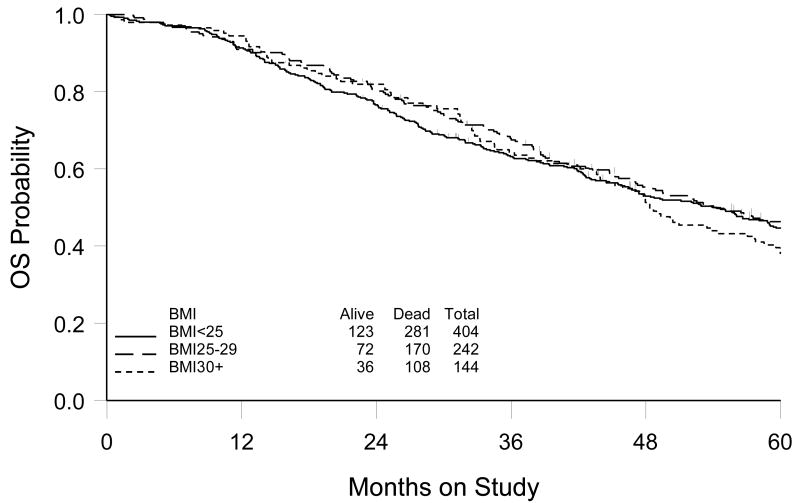
Of the 792 patients enrolled to GOG-158, 790 were included in this analysis (two patients had missing height or weight records at study enrollment). Median BMI at study enrollment (pre-chemotherapy) was 24.9 (range 13.7 – 52.9). Of the 790 patients, 404 (51.1%) were normal weight (BMI <25.0), 242 (30.6%) were overweight (BMI 25.0-29.9), and 144 (18.2%) were obese (BMI ≥30.0) before chemotherapy. Average BMI was statistically different by race and tumor grade. African-American patients and patients with well-differentiated tumors (grade 1) had statistically significantly higher BMI (Table 1). Within a median follow-up period of 48 months, 629 disease recurrences and 559 deaths were identified. Median PFS was estimated to be 19.5, 21.4 and 18.7 months and median OS was 54.3 months, 54.1 months and 48.4 months for the BMI<25.0, 25.0-29.9 and ≥30.0 groups, respectively. There was no evidence that BMI was associated with either PFS (p=0.56) or OS (p=0.62). Figure 1 shows the estimate of OS by BMI group. These results remained consistent with multivariate analysis after controlling for covariates. The causes of death by BMI group were also examined. Of 559 deaths, 87% were ovarian cancer-related, 1% caused by treatment and 12% from other causes. This distribution was consistent across the three groups. Furthermore, 685 (86%) patients completed all six prescribed chemotherapy cycles. There were no differences in cycle completion rates by BMI group.
Table 1. Body Mass Index (BMI) by Patient Characteristics (N=790).
| No. patients | (%) | BMI (kg/m2) | P value | ||
|---|---|---|---|---|---|
| Mean | (SD) | ||||
| Age Group (years) | 0.09 | ||||
| < 55 | 350 | (44.3) | 26.2 | (6.3) | |
| 55 - 64 | 225 | (28.5) | 25.9 | (5.0) | |
| ≥65 | 215 | (27.2) | 25.1 | (5.2) | |
| Race | <0.001 | ||||
| White | 679 | (86.0) | 25.7 | (5.6) | |
| Black | 50 | (6.3) | 29.1 | (7.0) | |
| Other | 61 | (7.7) | 24.6 | (3.9) | |
| GOG Performance Status | 0.98 | ||||
| 0 | 348 | (44.1) | 25.8 | (5.3) | |
| 1 | 379 | (48.0) | 25.8 | (5.8) | |
| 2 | 63 | (8.0) | 26.0 | (6.7) | |
| Histology | 0.62 | ||||
| Serous | 569 | (72.0) | 25.7 | (5.5) | |
| Mucinous/Clear cell | 50 | (6.3) | 26.5 | (7.1) | |
| Other | 171 | (21.7) | 25.9 | (5.7) | |
| Tumor Grade | 0.008 | ||||
| 1 | 78 | (9.9) | 27.3 | (7.2) | |
| 2 | 279 | (35.3) | 26.2 | (5.8) | |
| 3 | 433 | (54.8) | 25.3 | (5.2) | |
| Residual disease | 0.13 | ||||
| None/Microscopic | 281 | (35.6) | 25.4 | (5.7) | |
| Gross | 509 | (64.4) | 26.1 | (5.7) | |
| Treatment | 0.77 | ||||
| Cisplatin/paclitaxel | 398 | (50.4) | 25.8 | (5.7) | |
| Carboplatin/paclitaxel | 392 | (49.6) | 25.9 | (5.6) | |
ANOVA performed to test the difference in mean BMI across groups; P value for global test reported; Tukey-Kramer method used for pair-wise comparison (results not shown in table).
Figure 1.
Kaplan-Meier estimate of overall survival (OS) by body mass index (BMI) prior to chemotherapy
Body Weight Change during Chemotherapy and Clinical Outcomes
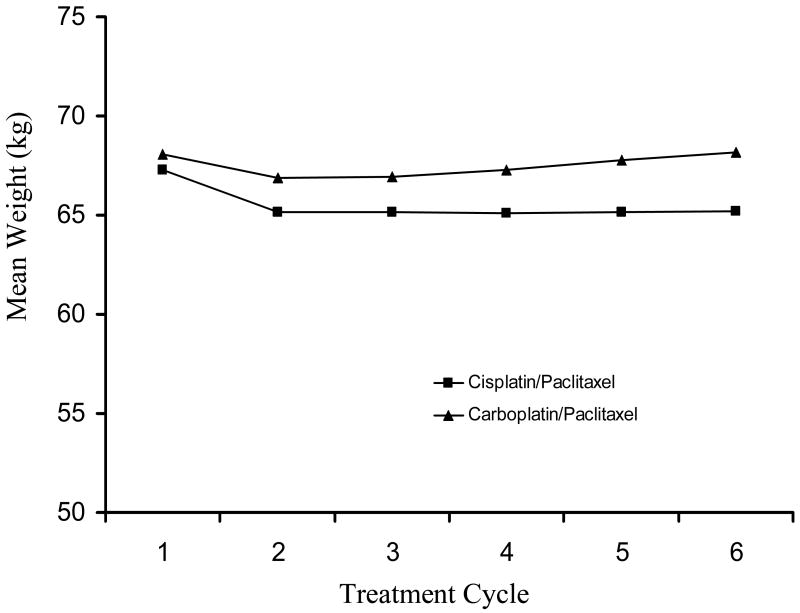
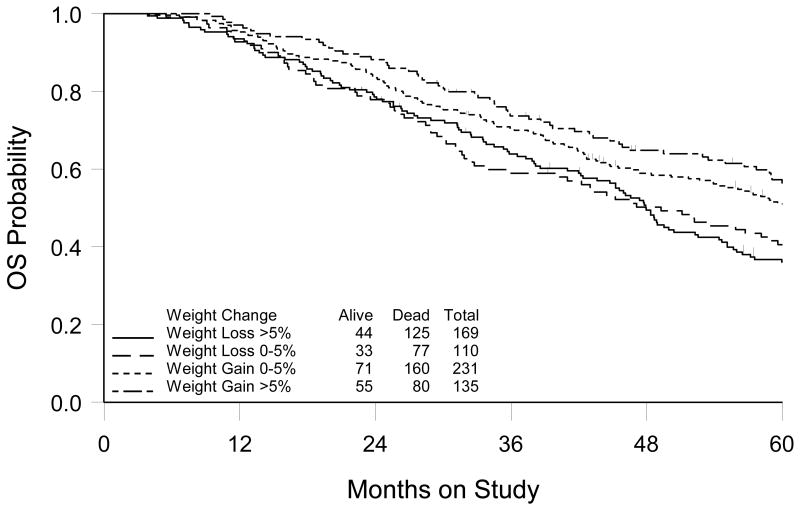
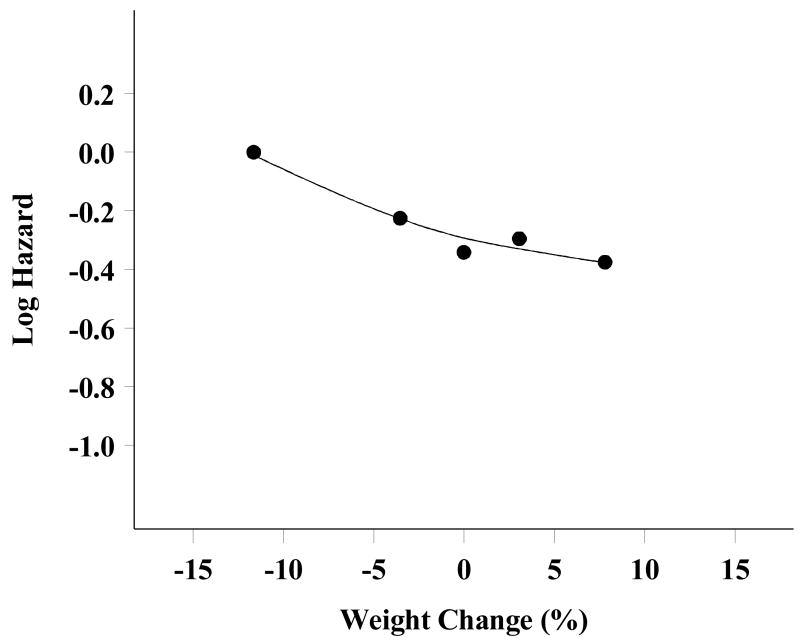
Of the 685 patients who completed all six cycles of chemotherapy, 645 (94%) had complete weight data available for analysis (e.g. body weight recorded at each course of therapy). The pattern of weight change during the chemotherapy period differed by treatment group (Figure 2). Body weight changes were significantly different over the treatment period between the cisplatin/paclitaxel and carboplatin/paclitaxel treatment groups (p<0.001). Patients treated on both treatment arms experienced a decrease in body weight after the first cycle of chemotherapy (-2.2 kg for the cisplatin/paclitaxel arm versus -1.2 for the carboplatin/paclitaxel arm). Patients on the cisplatin/paclitaxel regimen did not tend to regain the initial weight lost, but maintained a lower, stable body weight throughout the treatment period, whereas patients on the carboplatin/paclitaxel regimen tended to increase body weight again after the second cycle until returning to pre-treatment body weight. Of all patients, 51% lost weight on the cisplatin/paclitaxel arm as compared to 35% on the carboplatin/paclitaxel arm. There were no differences in OS and PFS related to pre-treatment body weight or related to end-of-treatment body weight, as examined individually. However, increasing body weight during therapy was associated with improved OS (Figure 3). When patients were classified into four groups: decrease by > 5% (n=169); decrease by >0 - 5% (n=110); increase by 0 - 5% (n=231); and increase by > 5% (n=135), median OS was estimated to be 48.0, 49.3, 61.1 and 68.2 months, respectively. Based on the survival curves, the difference in survival distribution across groups was statistically significant (p=0.006). Multivariate analysis, controlling for age, race, performance status, histology, tumor grade, tumor residual and treatment group, supported a linear relationship between weight change and risk of death (Figure 4), with an estimate that a 5% increase in body weight during chemotherapy was associated with a 7% decrease in risk of death (HR=0.93; 95% CI= 0.88-0.99, p=0.01) (Table 2). This association was consistent for both treatment groups. The causes of death were identical for the four groups, suggesting the increased risk for death for patients who had body weight loss during treatment was unlikely to be caused by non-cancer related co-morbidities. There was no evidence that the change in body weight during chemotherapy was related to disease progression.
Figure 2.
Mean body weight during chemotherapy period by treatment group
Figure 3.
Kaplan-Meier estimate of overall survival (OS) by body weight change
Figure 4.
Risk of death (Log HR) versus weight change (%), adjusted for covariates (smoothed); negative value in weight change indicating weight loss and positive value indicating weight gain.
Table 2. Association of Body Weight with Progression-free Survival (PFS) and Overall Survival (OS) (N=645).
| HR |
PFS (95% C.I.) |
P value | HR |
OS (95% C.I.) |
P value | |
|---|---|---|---|---|---|---|
| Weight prior to chemotherapy | ||||||
| Increase per 1 kg | 1.00 | (1.00-1.01) | 0.50 | 1.00 | (0.99-1.01) | 0.83 |
| Weight at end of treatment | ||||||
| Increase per 1 kg | 1.00 | (1.00-1.01) | 0.61 | 1.00 | (0.99-1.00) | 0.44 |
| Relative Weight Change | ||||||
| Increase per 5 percent | 1.00 | (0.99-1.01) | 0.71 | 0.93 | (0.88-0.99) | 0.01 |
Hazard ratio (HR) for disease progression or death estimated by Cox model, adjusted for age, race, baseline performance status, histology, tumor volume residual and treatment.
Discussion
In this study, increase in body weight during primary therapy for advanced ovarian cancer was a positive prognostic factor in stage III epithelial ovarian cancer patients (p=0.006). Patients who experienced a loss of body weight during chemotherapy experienced poor overall survival (median 48.0 months); whereas weight gain during the treatment period was associated with improved overall survival (median 68.2 months). The relative risk of death increased by 7% with each 5% decrease in body weight (HR=0.93, 0.88-0.99, p=0.013). There was no evidence that body weight change during therapy was associated with progressive disease, as reasons for death were similar among patients in all weight change categories. When controlling for prognostic factors (age, race, performance status, histology, tumor grade, tumor residual and treatment group), body weight change during chemotherapy was significantly associated with overall survival.
Although some trials have documented shorter OS from ovarian cancer associated with BMI, there was no corresponding association between pre-treatment BMI and survival in this study.17,18,19 However, this finding could be in part due to the low prevalence of obesity among those enrolling to GOG-158 (e.g., more than half of all patients were already in the lowest BMI category at study enrollment). This discrepancy may also be related to the fact that prior work has utilized retrospective hospital chart review or did not standardize patient care, so there may have been important differences in therapeutic regimens and dose between obese and normal or under-weight women that were not accounted for in these analyses. This study may help clarify this inconsistency due to the homogeneous population (e.g., optimal stage III disease with minimal co-morbidity) standardized therapy (e.g., paclitaxel + platinum) and assessment methods used (e.g. patients followed on a scheduled basis for survival endpoints). Other prospective trials with standardized treatment regimens and methodology should be evaluated to confirm this finding.
The findings of this study also contradict what has been reported in some other cancers. For example, excess body weight and BMI are established risk factors for both the initiation and progression of breast cancer. This is largely due to the epidemiologic and clinical data demonstrating that increased or prolonged circulating estrogen promotes the initiation and progression of breast cancer. Nearly 80% of breast cancers are estrogen receptor (ER) positive.20 Excess adipose tissue enhances circulating estrogens, which in turn contributes to the increased risk. Ovarian cancer, on the other hand, has not been shown to be hormone dependent, with less than 40% of these cancers demonstrating ER positivity.21 Therefore, it is very likely this is one potential mechanism of ovarian cancer initiation and progression that differs from the BMI and body weight data in breast cancer that has shown increased risk for both higher BMI and increased body weight.
In this study, there was also no association between body weight change and PFS. This is not surprising due to the fact that the decision to undergo second-look surgery was made by the patient, and was not required as part of the study protocol. Less than half (41%, n=325) of patients underwent surgical restaging or had progressive disease at the time restaging was to occur. Therefore, assessment of PFS was not consistent among all patients.
There are limitations to the interpretation of this study to the overall ovarian cancer patient population. This was a retrospective analysis of data collected for a large phase III clinical trial. Although there are strengths in the use of clinical trial data (e.g. control of potential confounding variables), data were not collected prospectively for the purpose of this analysis. Furthermore, body weight data were not collected indefinitely on these patients following the completion of six cycles of protocol chemotherapy. Therefore, it is unclear what changes in body weight or future treatment regimens may have occurred following the sixth course of therapy that could have impacted survival. Nevertheless, this exploratory study provides initial evidence of the potential impact of change in body weight during primary chemotherapy on OS.
There is an estimated mean body weight loss of 3.1 kg pre- to post-surgery.14 This study was limited to changes during the chemotherapy administration period, specifically to exclude the effect of surgery on body weight changes. Therefore, it is not possible to differentiate between weight gain as a result of recovery of pre-surgery body weight or as a result of increased food intake or other reasons. However, body weight change has been reported in other cancers that do not involve major surgery.9 In these cases, body weight change has been shown to be related to changes in physical activity and diet that take place during cancer treatment. 9,22 Future trials should take into account the relationship between body weight loss at the time of surgery and the amount of weight gain during treatment, as patients undergoing extensive resection may have a different pattern of weight change than those with less extensive disease. Data related to bowel resection from GOG-158 were unavailable for analysis, as these data were not routinely collected as part of the study record. Therefore, it is unclear what role bowel resection may have with body weight changes and survival in this study. However, a study of advanced ovarian cancer patients optimally debulked at primary cytoreduction surgery did not find survival differences between those who did or did not undergo bowel resection, although bowel resection was associated with improved PFS.23 Performance status data were also not available throughout the treatment period and could not be compared with changes in body weight. Similarly, serial CA-125 values, used to determine CA-125 nadir, an established prognostic indicator for recurrence and survival, were not available for comparison.24
Interventions to prevent or reduce weight loss during primary chemotherapy should be examined in a prospective trial to assess their ability to improve survival among ovarian cancer patients. Future research should include prospective assessments of body weight, gastrointestinal toxicity, physical activity, and measures of frailty and dietary intake, as well as social support and quality of life, to understand the variety of lifestyle and concomitant factors that may be associated with body weight changes during primary therapy.9,22,24 These assessments may certainly be combined with efforts to improve patient nutrition, lifestyle and eating habits, as well as to manage toxicity, in an effort to reduce weight loss during primary chemotherapy. Although only through prospective randomized trials can definitive statements be made, this exploratory trial suggests that minimizing weight loss during chemotherapy may improve patient survival.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Emory University Clinic, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, SUNY Downstate Medical Center, University of Kentucky, Community Clinical Oncology Program, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, Gynecologic Oncology Network, Oregon Health Sciences University, University of Southern California at Los Angeles, University of Miami School of Medicine, Stanford University Medical Center, Eastern Virginia Medical School, University of Arizona Health Science Center, Mayo Clinic, and Long Island Jewish Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003 Aug 20;95(16):1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 3.Modesitt SC, van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005 Oct;60(10):683–92. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 4.Hoyo C, Berchuck A, Halabi S, Bentley RC, Moorman P, Calingaert B, et al. Anthropometric Measurements and Epithelial Ovarian Cancer Risk in African-American and White women. Cancer Causes Control. 2005 Oct;16(8):955–63. doi: 10.1007/s10552-005-3205-y. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Xie X, Lee AH, Binns CW, Holman CD. Body mass index in relation to ovarian cancer survival. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1307–10. doi: 10.1158/1055-9965.EPI-04-0519. [DOI] [PubMed] [Google Scholar]
- 6.Gadducci A, Cosio S, Fanucchi A, Genazzani AR. Malnutrition and cachexia in ovarian cancer patients: pathophysiology and management. Anticancer Res. 2001 Jul-Aug;21(4B):2941–7. [PubMed] [Google Scholar]
- 7.Dell DD. Cachexia in patients with advanced cancer. Clin J Oncol Nurs. 2002 Jul-Aug;6(4):235–8. doi: 10.1188/02.CJON.235-238. [DOI] [PubMed] [Google Scholar]
- 8.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003 Apr 1;97(7):1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cance Epidemiol Biomarkers Prev. 2007 Mar;16(3):430–8. doi: 10.1158/1055-9965.EPI-06-0735. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Takahashi M, Taguchi K, Sasaki F, Todo S. Weekly paclitaxel administration in the adjuvant therapy of primary breast cancer. Gan To Kagaku Ryoho. 2003 May;30(5):653–9. [PubMed] [Google Scholar]
- 11.Hainsworth JD, Urba WJ, Hon JK, Thompson KA, Stagg MP, Hopkins LG, et al. One-hour paclitaxel plus carboplatin in the treatment of advanced non-small cell lung cancer: results of a multicentre, phase II trial. Eur J Cancer. 1998 Apr;34(5):654–8. doi: 10.1016/s0959-8049(97)10103-4. [DOI] [PubMed] [Google Scholar]
- 12.Fitch M, Deane K, Howell D, Gray RE. Women's experiences with ovarian cancer: reflections on being diagnosed. Can Oncol Nurs J. 2002 Summer;12(3):152–68. doi: 10.5737/1181912x123152159. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RL, Bagnell KG, Townley PM. Carboplatin and short-infusion paclitaxel in high-risk and advanced-stage ovarian carcinoma. Cancer J Sci Am. 1997 Jul-Aug;3(4):246–53. [PubMed] [Google Scholar]
- 14.Gil KM, Frasure HE, Hopkins MP, Jenison EL, von Gruenigen VE. Body weight and composition changes in ovarian cancer patients during adjuvant chemotherapy. Gynecol Oncol. 2006 Oct;103(1):247–52. doi: 10.1016/j.ygyno.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003 Sep 1;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 16.Homer D, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley and Sons; New York: 1999. [Google Scholar]
- 17.Pavelka JC, Brown RS, Karlan BY, Cass I, Leuchter RS, Lagasse LD, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006 Oct 1;107(7):1520–4. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 18.Kjaerbye-Thygesen A, Frederiksen K, Hogdall EV, Glud E, Christensen L, Hogdall CK, et al. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):798–803. doi: 10.1158/1055-9965.EPI-05-0897. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002 Sep;11(9):822–8. [PubMed] [Google Scholar]
- 20.Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tüchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993 July;218(1):13–21. doi: 10.1097/00000658-199307000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatch KD, Beecham JB, Blessing JA, Creasman WT. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen: A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68:269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Doyle C, Kushi LH, Byers T, Courneya KS, Denmark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2006 Nov/Dec;56(6):323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 23.Estes JM, Leath CA, 3rd, Straughn JM, Jr, Rocconi RP, Kirby TO, Huh WK, et al. Bowel resection at the time of primary debulking for epithelial ovarian carcinoma: outcomes in patients treated with platinum and taxane-based chemotherapy. J Am Coll Surg. 2006 Oct;203(4):527–32. doi: 10.1016/j.jamcollsurg.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006 Aug;17(8):1234–8. doi: 10.1093/annonc/mdl120. [DOI] [PubMed] [Google Scholar]
- 25.Rieck G, Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract Res Clin Obstet Gynaecol. 2006 Apr;20(2):227–51. doi: 10.1016/j.bpobgyn.2005.10.010. [DOI] [PubMed] [Google Scholar]