Abstract
Through a highly divergent efferent projection system, the locus coeruleus-noradrenergic system supplies norepinephrine throughout the central nervous system. State-dependent neuronal discharge activity of locus coeruleus neurons has long-suggested a role of this system in the induction of an alert waking state. More recent work supports this hypothesis, demonstrating robust wake-promoting actions of the locus coeruleus-noradrenergic system. Norepinephrine enhances arousal, in part, via actions of β- and α1-receptors located within multiple subcortical structures, including the general regions of the medial septal area and the medial preoptic areas. Recent anatomical studies suggest that arousal-enhancing actions of norepinephrine are not limited to the locus coeruleus system and likely include the A1 and A2 noradrenergic cell groups. Thus, noradrenergic modulation of arousal state involves multiple noradrenergic systems acting with multiple subcortical regions. Pharmacological studies indicate that the combined actions of these systems are necessary for the sustained maintenance of arousal levels associated with spontaneous waking. Enhanced arousal state is a prominent aspect of both stress and psychostimulant drug action and evidence indicates that noradrenergic systems likely play an important role in both stress-related and psychostimulant-induced arousal. These and other observations suggest that the dysregulation of noradrenergic neurotransmission could well contribute to the dysregulation of arousal associated with a variety of behavioral disorders including insomnia and stress-related disorders.
Keywords: A1, A2, cognition, locus coeruleus, norepinephrine, sleep, psychostimulants, stress, waking
1. Introduction
The ability to detect, attend to, and respond to events is an essential aspect of normal behavior and, ultimately, survival. Conversely, sleep and the accompanying relative insensitivity to environmental events is similarly important for optimal functioning and survival. As such, the regulation of arousal is critical, with both too little and too much proving detrimental. Though many definitions are possible, for the purposes of this review, the term arousal refers to a continuum of sensitivity to environmental stimuli. Following this definition, arousal fluctuates greatly between sleeping and waking states as well as within waking (i.e. drowsy vs. hyperaroused) [9,45]. Importantly, affective, cognitive and perceptual processes display a pronounced dependency on arousal state. For example, the inverted-U relationships between arousal and learning, attention, and working memory have been well-documented (i.e. the Yerkes-Dodson law, [4,8,156]. Through this, fluctuations in arousal level can have a substantial impact on a variety of cognitive and perceptual processes.
Alterations in arousal state are accompanied by alterations in forebrain neuronal activity, reflected in electroencephalographic (EEG) signals [88,95,142,149]. The formal examination of the neural mechanisms underlying arousal dates back to the work of von Economo [153], Bremer [30] and Moruzzi and Magoun [107], which identified a critical role of the brainstem in the induction and maintenance of arousal. Given the pivotal role that arousal plays in behavior, it is not surprising that a large array of brainstem and basal forebrain neural systems participate in the regulation of behavioral state. Included among these is the locus coeruleus (LC)-noradrenergic system.
The LC is the major source of brain NE [58]. NE acts at three major receptor families, α1, α2, and β, each comprised of multiple subtypes. α1- and β-receptors are thought to exist primarily postsynaptically whereas, α2-receptors are present both pre- and postsynaptically. Early electrophysiological observations indicated that the LC-noradrenergic system may play an important role in the regulation of arousal/behavioral state (reviewed in [58,59]). For example, LC neurons display highest discharge rates during waking and lower rates during sleep [6,57,68]. Importantly, alterations in LC discharge rate precede changes in behavioral state [6,57,68]. Combined, these observations suggest a potential causal role of the LC in the regulation of arousal. As reviewed below, this question has been the focus of a large number of studies, utilizing a variety of experimental approaches. Results obtained from studies conducted over the past two decades provide relatively unambiguous evidence for a prominent arousal-promoting role of the LC and other brain noradrenergic systems. These observations are the focus of this review.
2. Choice of experimental approach
Prior to a review of experimental observations regarding arousal-enhancing actions of the LC-noradrenergic system, it is useful to review the general approaches available for studying arousal-promoting actions of a neurotransmitter system. In the examination of a causal role of a neurotransmitter system in the modulation of arousal, two general approaches are available. The first examines the effects of enhancing the activity of that neurotransmitter, while the second examines the effects of blocking transmitter action. Importantly, each approach addresses fundamentally distinct questions. The first examines the extent to which a neurotransmitter is sufficient to induce a change in arousal state; the second examines the extent to which the transmitter is necessary for the maintenance of an aroused state. The neural regulation of arousal appears to involve substantial redundancy, with multiple systems exerting wake-promoting actions [26,115,149]. Presumably because of this redundancy, inactivation of any single neurotransmitter system often has minimal effects on sleep-wake state. As such, negative effects of neurotransmitter-selective antagonists and/or lesions on sleep and waking need to be interpreted cautiously. Given this, the approach most likely to yield unambiguous evidence is the examination of whether a transmitter’s action is sufficient to increase arousal. Of course, with this approach it is important to use baseline conditions characterized by relatively low arousal levels and experimental procedures that do not non-specifically elevate arousal levels (i.e. handling-related waking/stress).
An early-used approach to examine the involvement of the LC in the regulation of sleep-wake state was the use of electrolytic or neurotoxic (6-OHDA/DSP-4) lesions of the LC-noradrenergic system. Though a reasonable sounding approach, work since then demonstrates a robust ability of noradrenergic and dopaminergic systems to mount compensatory responses that reduce the functional impact of a lesion. Most of these compensatory responses occur within 7–10 days of the lesion, a commonly used time point for testing the behavioral effects of the lesion. Importantly, microdialysis studies demonstrate that extracellular levels of NE and DA are not reduced substantially unless tissue levels have been decreased by more than 90% [1,36,117–119]. Moreover, even when tissue levels have been reduced by at least 90%, the decrease in extracellular levels of these transmitters (reflected in microdialysis measures) is substantially less than that observed in tissue measures (i.e. >90% depletion of tissue levels vs 60% reduction in extracellular levels). Given lesions also increase postsynaptic receptor number and second messenger levels [61,66,67,86,126,128], the functional impairment associated with these larger lesions (>90% tissue depletion) is likely to be less than predicted by the decrease in extracellular NE levels. Given the robust nature of these compensatory responses, it is not surprising that following substantial (though incomplete) destruction of the LC efferent system, hyperactive, rather than hypoactive, noradrenergic function has been observed [16,48,106]. These latter observations suggest that even in the presence of a statistically-significant behavioral/physiological effect of a noradrenergic lesion, care needs to be exercised in the interpretation of this effect.
3. Noradrenergic modulation of arousal
3.1. Evidence for an arousal-promoting action of the LC
A variety of lesion and pharmacological studies were initially conducted to assess whether there exists a causal relationship between LC neuronal activity and arousal (for review, [149]). In general, lesions of noradrenergic systems have had an inconsistent impact on EEG and behavioral indices of arousal [73,74,82,83]; for review, see [149], likely reflecting the occurrence of lesion-induced compensation within the LC-NE system. Consistent with this conclusion, Lidbrink [82] reported that 6-OHDA lesions of the dorsal noradrenergic bundle increased slow-wave EEG activity initially, but that this effect disappeared approximately 7 days following the lesion.
In contrast to that observed with noradrenergic lesions, acute suppression of LC-NE neurotransmission by systemic, ICV, or intra-brainstem administration of α2-agonists results in profound sedation [46,63,72,81,155]. Conversely, infusion of NE intracerebroventricularly or into forebrain sites is behaviorally activating [56,123]. Despite the consistency of these observations, due to the small size of the LC and the close proximity of this nucleus to other brainstem arousal-related nuclei, it is difficult to unambiguously identify the LC as the site of action involved in these drug-induced changes in behavioral state.
To more selectively alter LC-discharge activity, Adams and Foote [2] developed a combined recording/infusion probe that uses electrophysiological recordings to locate the LC, place small infusions (35–150 nl) of pharmacological agents within close proximity to this nucleus, and monitor the effects of these infusions on LC neuronal discharge rate. Using this probe the effects of selective alterations in LC discharge rate on forebrain EEG activity were examined in halothane-anesthetized rats [19,23]. In these studies, unilateral LC activation (via infusion of the cholinergic agonist, bethanechol) produced a bilateral and relatively rapid (within 5–10 seconds) activation of forebrain EEG in the halothane-anesthetized rat (see Figures 1, 2). A number of observations indicate that the infusion-induced alterations in LC discharge were responsible for the activation of the forebrain, rather than actions distant to the LC. These include: 1) the fact that EEG activation was prevented by pretreatment with drugs that impair NE neurotransmission (an α2-agonist and a β-antagonist); 2) infusions only activated forebrain EEG if they were placed within a radius of approximately 500 μm of the center of the LC (Figure 2); 3) a similar latency between LC activation and EEG activation occurred regardless of whether infusions were placed lateral or medial to the LC; and, 4) infusions placed anterior or posterior to the LC did not alter forebrain EEG activity state.
Figure 1.
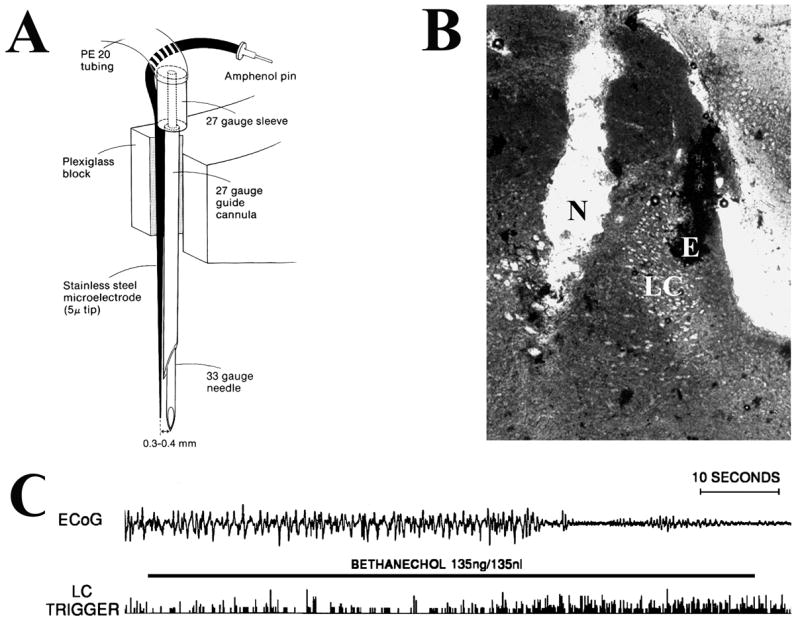
Effects of LC activation on cortical EEG (ECoG) activity state. In this experiment, the LC was first located electrophysiologically using a combined recording-infusion probe in the halothane-anesthetized rat. Once located, a small infusion of the cholinergic agonist, bethanechol was made adjacent to the LC. The effect of this infusion-induced activation of the LC on ECoG was monitored. A: Schematic of the infusion/recording probe used to activate LC. Bethanechol was infused 300–400 μm lateral or medial to the recording electrode that was used to locate the main body of the LC. B: Photomicrograph of a peri-LC infusion site. N indicates position within the track created by the infusion needle where the infusate exited the needle. E indicates position of the recording electrode within the LC. C: Effects of bethanechol infusion on LC activity and ECoG activity (recorded from the prefrontal cortex). ECoG activity is displayed in the top trace and the raw trigger output from LC activity in the bottom trace. Bethanechol infusion (indicated by horizontal bar) increased LC discharge rate approximately two-thirds of the way through the 60-second infusion. Several seconds following LC activation, an abrupt onset of EEG desynchronization is observed (see [19]).
Figure 2.
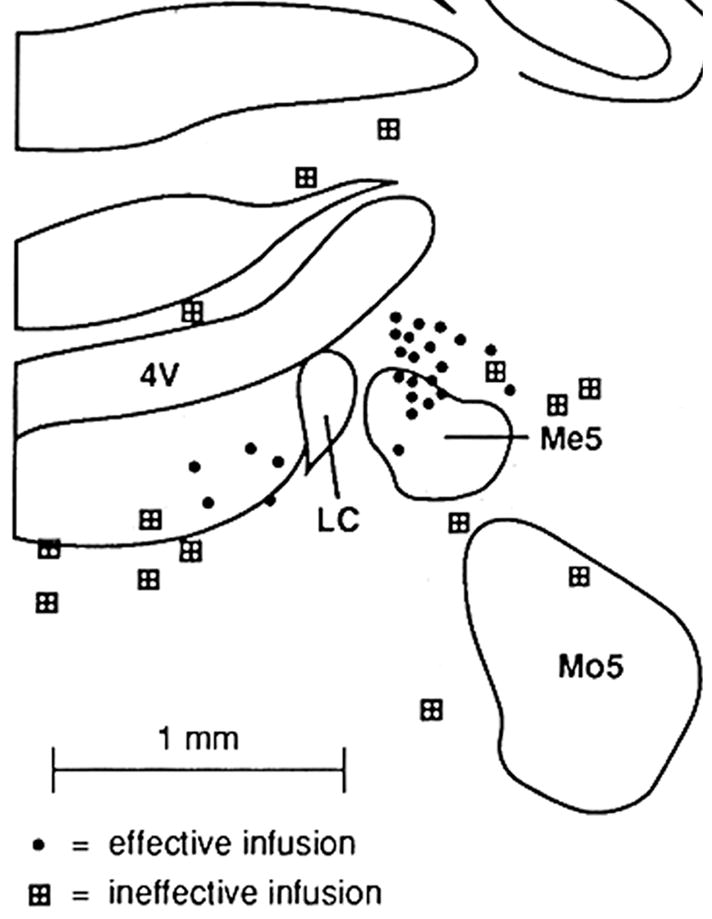
Schematic diagram depicting bethanechol infusion sites that were effective or ineffective for activating forebrain EEG. Solid circles indicate sites at which bethanechol infusion activated the EEG. Shaded boxes indicate sites at which bethanechol infusion had no obvious EEG effects. The center of the infusion site is indicated by these symbols. There is a radius of approximately 500 μm around LC within which infusions, placed either medially or laterally, activated forebrain EEG. Infusions placed immediately anterior to the LC were also ineffective (not shown). Abbreviations: Me5, mesencephalic nucleus of the trigeminal nerve; Mo5, motor nucleus of the trigeminal nerve; Pr5, principle sensory nucleus of the trigeminal nerve; 4V, fourth ventricle (see [19]).
Additional studies in the lightly-anesthetized rat, characterized by a spontaneously activated (desynchronized) forebrain EEG, demonstrated that bilateral suppression of LC neuronal discharge (via small infusions of an α2-agonist) produced a robust increase in slow-wave EEG activity (Figure 3; [23]). Importantly, in these studies even minimal LC neuronal discharge activity within a single hemisphere (i.e. 5–10% of basal levels) was sufficient to maintain bilateral forebrain activation. This is likely an important factor in why LC/noradrenergic lesions, which almost certainly never reduce completely extracellular NE levels (see above), do not result in large alterations in time spent awake.
Figure 3.
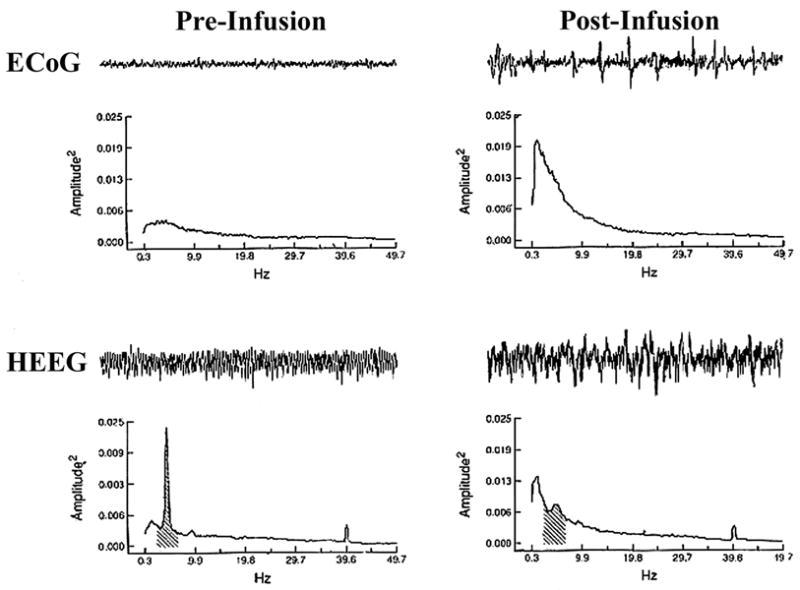
Effects of bilateral suppression of LC discharge in the lightly-anesthetized rat. Shown are the effects of bilateral clonidine infusions (100 ng in 100 nl) that resulted in the complete bilateral suppression of LC discharge activity. Shown are 25-sec raw EEG traces for cortical EEG (ECoG; top row) and hippocampal EEG (HEEG; bottom row) along with the results of power spectral analysis (PSA; graphs below the raw traces) calculated on an 8-min epoch from which the 25-sec EEG trace was taken. Data from pre-infusion (left column) and post-infusion (right column) periods are displayed. The most striking post-infusion changes in the ECoG are the increase in the slowest frequencies, and in the HEEG, the dramatic reduction in theta activity and the appearance of mixed-frequency activity. Shading in the PSA plots indicates the theta frequency band (2.3–6.9 Hz) in the HEEG power spectra (see [23]).
These observations indicate that, under these experimental conditions (the halothane-anesthetized rat), LC neuronal activity is both sufficient and necessary for the maintenance of an activated forebrain.
3.2. Site of action: NE acts at β- and α1-receptors within the medial septal and medial preoptic areas, but not the substantia innominata, to promote alert waking
LC efferents could act at a variety of cortical and subcortical sites to modulate forebrain EEG. Subcortically, the general regions of the medial septal area (MSA), the medial preoptic area (MPOA), and the substantia innominata (SI) participate in the regulation of forebrain EEG activity state [31,33,76,101,121,140]. Each of these regions receives LC-noradrenergic input [158–160]. In a series of intratissue infusion studies, the EEG and behavioral state modulatory actions of NE within these regions were examined in anesthetized and unanesthetized rats [15,18,20,22]. In these studies, small infusions (150–250 nl) of NE, an α1-agonist (phenylephrine) or a β-agonist (isoproterenol). Remote-controlled infusions were made into sleeping animals implanted with EEG/EMG electrodes to permit examination of the behavioral state-modulatory actions of intratissue infusions from a stably low-arousal state (i.e. sleeping) while avoiding the need to handle the animal to perform an infusion (see [18]).
3.2.1. Wake-promoting actions of NE β- and α1-receptors within the MSA and MPOA
Using this approach, intratissue infusion of β- or α1-receptor agonists into the MSA (Figures 4, 6, 7) or MPOA (situated immediately caudal to the MSA; Figures 4, 5, 7) produce robust and dose-dependent increases in EEG activation in anesthetized animals and increase EEG/EMG and behavioral indices of waking in unanesthetized animals [15,18,20,22,76,127]. Infusions immediately outside these regions are devoid of wake-promoting actions [15,18,20,22]. Importantly, the wake-promoting actions of β- and α1-receptors within both the MSA and MPOA are additive (Figure 7; [20]).
Figure 4.
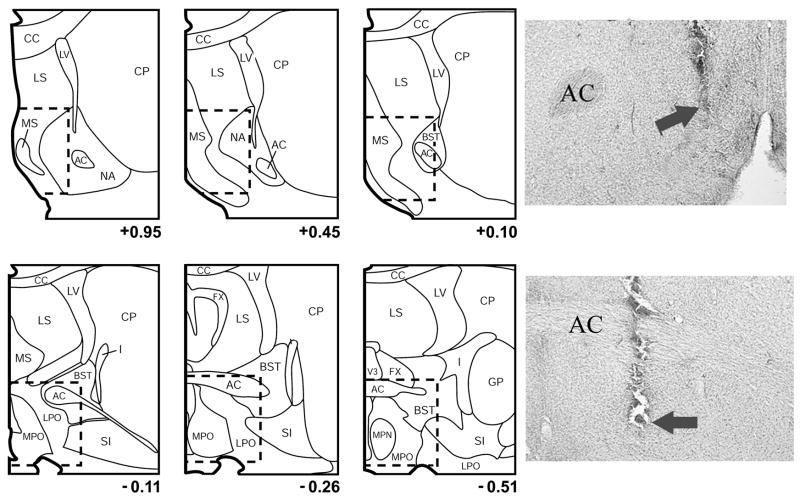
Boundaries defining the general regions of the MSA (top row) and MPOA (middle row) within which NE acts to promote waking. Previous intratissue infusion mapping studies with NE and various direct and indirect NE agonists indicate that NE acts within a nearly continuous portion of the medial basal forebrain the spans the anterior-posterior extent of the MSA and MPOA, indicated by the dotted line. The region termed the MSA encompasses the medial septum, the vertical limb of the diagonal band of Broca, the posterior portions of the shell region of the nucleus accumbens. The region termed the MPOA encompasses preoptic area of the hypothalamus and portions of the bed nucleus of the stria terminalis (BST). Previous mapping studies suggest that the shell accumbens or BST are not prominently involved in NE-induced waking. Infusions outside the MSA and MPOA are generally ineffective at increasing waking. Panels are arranged anterior-posterior with the anterior-most panel shown in the upper left and the posterior-most panel shown in the bottom right position. Photomicrographs (bottom row) are of infusion sites from experiments involving NE agonist infusions into the MSA (left image) and MPOA (right image). In these photomicrographs, the arrows indicate the most ventral extent of the infusion needle. AC, anterior commissure; CC, corpus callosum; CP, caudate-putamen; GP, globus pallidus; I, internal capsule; LS, lateral septum, LC, lateral ventricle; MS, medial septum; NA nucleus accumbens; SI, substantia innominata.
Figure 6.
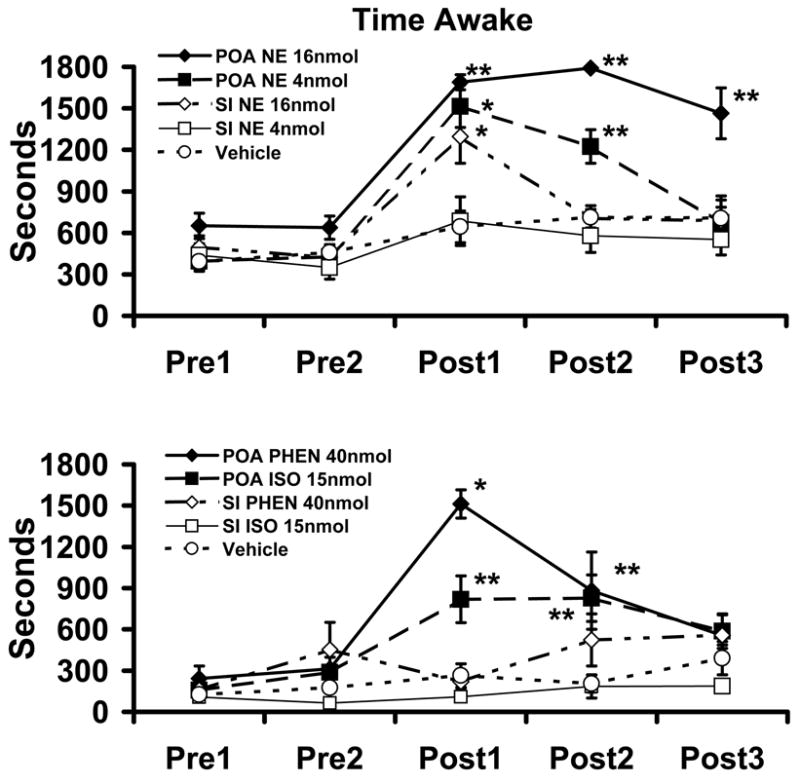
Wake-promoting effects of intra-MPOA infusion of NE, α1-agonist and β-agonist. Top Panel displays the effects of vehicle, 4 nmol NE, or 16 nmol NE, infused into either the MPOA or SI on total time spent awake. Bottom Panel displays the effects of the α1-agonist, phenylephrine (PHEN; 40 nmol), and the β-agonist, isoproterenol (ISO; 15 nmol), infused into the MPOA or SI on total time spent awake. Symbols represent mean (±SEM) of time (secs) spent awake in 30-minutes epochs. PRE1 and PRE2 represent 30-min pre-infusion epochs occurring immediately prior to the infusion. POST1-POST3 represent 30-min post-infusions epochs, beginning immediately following the infusions. NE, phenylephrine and isoproterenol increased waking when infused to the MPOA. In contrast, these treatments had little impact on time awake when infused into the SI. The only exception to this being observed with the high dose of NE. In this case the latency to waking was longer and the magnitude of waking smaller than that observed with infusions into the MPOA. Lack of visible error bars indicates the magnitude of the SEM fell within the range corresponding to the dimensions of the symbol. There were no significant differences between any of the groups during the pre-infusion epochs. *P<0.05, **P<0.01 compared to vehicle-treated controls (see [22]).
Figure 7.
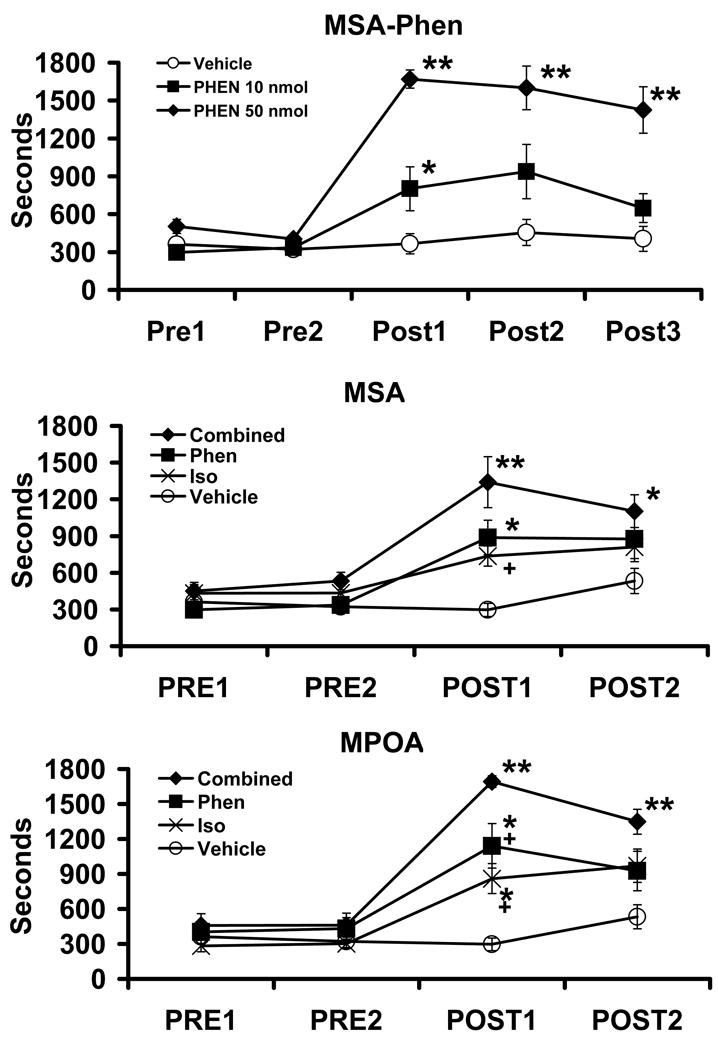
Additive wake-promoting effects of α1- and β-agonist receptor stimulation within the MSA and MPOA. Top Panel depicts dose-dependent wake-promoting actions of infusion of the α1-agonist, phenylephrine (PHEN; 10 nmol, 50 nmol in 150 nl), into the MSA. Intra-MSA infusion produced dose-dependent increases in waking. Middle and bottom panels depict the effects of infusion of vehicle, 10 nmol phenylephrine (Phen), 4 nmol of the β-agonist, isoproterenol (Iso; 4 nmol) and combined phenylephrine and isoproterenol (Combined). For both regions, when administered separately at these doses each drug had a mild wake-promoting action. In the combined treatment group, the wake-promoting effects of isoproterenol and phenylephrine appeared additive and not supra-additive. Symbols represent means (± SEM) of time (secs) spent awake per 30-min testing epoch. PRE1 and PRE2 represent pre-infusion portions of the experiment. POST1-POST3 represent post-infusions epochs. *P<0.05, **P<0.01 compared to PRE1; +P<0.05 compared to Combined (see [20]).
Figure 5.
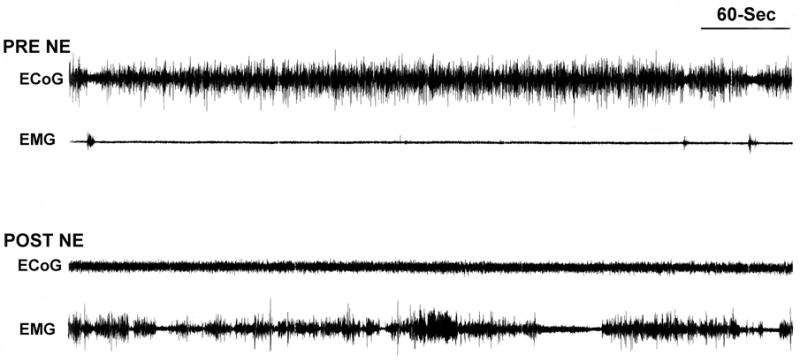
Wake-promoting effects of NE infusion into the MPOA. Shown are the effects of NE (1.2 μg (4 nmol)/150nl) infusions into the MPOA on ECoG and electromyographic activity (EMG) from a typical experiment. Shown are 10-min traces of ECoG and EMG recorded immediately prior to (top traces, PRE NE) and 10-min following (bottom traces, POST NE) NE infusion into MPOA. Prior to the infusion, the animal spent the majority of time in slow-wave sleep (resting with large amplitude, slow-wave activity present in ECoG and low-amplitude activity present in EMG). The most striking post-infusion changes are the decrease in large-amplitude, slow-wave ECoG activity and the increase in EMG amplitude, indicative of alert, active waking (see [22]).
In contrast to the above-reviewed studies that document potent wake-promoting effects of α1-agonist infusion into the MPOA, minimal sleep-wake effects of intra-MPOA infusion of the α1-agonist, methoxamine, have also been observed [150,151]. Although the basis for this apparent discrepancy is unclear, possible contributing factors include an elevated baseline level of waking and/or greater tissue damage within the MPOA around the infusion site in these more recent studies [150].
3.2.2. Neurocircuitry within the MSA and MPOA associated with NE-induced waking
The terms MSA and MPOA refer to relatively large and heterogeneous structures, each containing multiple subnuclei. The anatomical resolution of previous mapping studies (approximately 500 μm) precludes identification of the specific subnuclei involved in the wake-promoting actions of NE within these general regions. Given this, currently, the neurocircuitry within the MSA and MPOA involved in the wake-promoting actions of NE is not known.
Within the MPOA and MSA (as well as SI) evidence indicates the presences of both wake-promoting and sleep-promoting neuronal populations. For example, electrophysiological studies indicate that within these regions there are neurons that are active primarily during waking whereas other neurons are primarily active during sleep [121,140]. Within the MPOA, sleep-active neurons are found within both the median preoptic nucleus (MnPN) and the ventrolateral preoptic nucleus (VLPO) possesses sleep-active neurons. The majority of sleep-active neurons synthesize GABA or galanin, or both [121,140]. Moreover, within the SI and MPOA, sleep-active neurons have been demonstrated to express α2-receptors [90,105]. Further, α2-receptor stimulation inhibits sleep-active neurons with the general region of the MSA and MPOA [112]. Similarly, in vitro studies indicate an inhibitory action of NE on GABAergic neurons contained with the VLPO [62]. Thus, the wake-promoting actions of NE may involve the α2-receptor-dependent inhibition of sleep-active neurons. However, this mechanism does not explain the wake-promoting effects of intra-MSA and intra-MPOA infusions of α1- and β-agonists.
Results from previous noradrenergic agonist mapping studies suggest that the VLPO is not a primary site of action in the wake-promoting effects of intra-MPOA α1-agonist infusions [22]. Nonetheless, α1- and β-receptors located within the VLPO may well modulate sleep-wake state, an issue that remains to be addressed. In contrast, to the VLPO, the MnPN is situated well within the general area targeted in the previous intra-MPOA infusion studies. Nonetheless, neither an α1- or β-agonist affected on sleep-active neurons within the general region of the MPOA and MSA [112]. In contrast, α1-agonist administration, but not β-agonist, increased the activity of a subpopulation of waking-active neurons [112].
Within the MSA, evidence indicates that the activity of projections from both GABAergic and cholinergic neurons combine to influence hippocampal EEG activity [41]. Given the MSA also projects to the neocortex [92,120,131], NE could modulate hippocampal and cortical EEG activity state via actions on either of these ascending pathways. Alternatively, NE could influence EEG and behavioral activity state via alterations in efferent pathways that project to subcortical arousal-related regions, such as the hypothalamus, thalamus, or midbrain. Such actions could involve direct projections from the MSA to these regions [43,43,100,138,138] or indirect via hippocampal efferents [70,139,154].
To date, the extent to which α1- and β-receptors are differentially distributed across neurochemically-defined (i.e. cholinergic vs. GABAergic) or behaviorally-defined (i.e. sleep-active vs. waking-active) neuronal subpopulations within both the MSA and MPOA remains to be determined. If the wake-promoting effects of α1- and β-receptors involve actions on the same cell, at least two mechanisms exist through which these receptors could interact to exert additive arousal-modulating actions. First, these receptors could exert independent and additive effects on membrane potential via parallel actions on second messenger systems (e.g. phosphoinositol vs. cAMP). Additionally, evidence indicates that α1-receptors can potentiate β-receptor-mediated cAMP production [132].
Finally, the above-reviewed observations indicate that NE promotes alert waking via actions within anatomically distinct subregions (MSA vs. MPOA) that have traditionally been associated with dissimilar behavioral functions. The wake-promoting actions of NE within the MSA and MPOA may result from direct actions on arousal-modulating circuits. Alternatively, these regions may have evolved to support distinct, and possibly highly divergent behavioral processes, each requiring an alert waking state. In this case, each region may send efferents to arousal-controlling circuits to ensure coordination of the appropriate behavioral state with the state-dependent behavioral and/or physiological functions subserved by each region.
Of relevance to this discussion, the MPOA has been linked to temperature regulation, including temperature fluctuations associated with sleep and waking [75,99]. Moreover, intra-MPOA infusion of the α1-agonist, methoxamine, decreases body temperature while, intra-MPOA infusion of the α1-antagonist, prazosin, increases body temperature [152,152]. Interestingly, animals treated with intra-MPOA infusion of the α1-agonist, methoxamine, choose a warmer environment if that option is provided [150]. Under these latter conditions methoxamine was observed to no longer increase waking and instead appeared to suppress time spent awake (see 3.7 below for further discussion). Though there are a number of issues that need to be considered regarding these latter observations (see 3.7 below), they nonetheless suggest the possibility that the sleep-wake modulating effects of MPOA NE may, in part, be related to actions of NE on body temperature. This hypothesis however contradicts previous studies that described a dissociation of temperature-modulating and behavioral state-modulating actions of various noradrenergic agonists and antagonists [89]. Based on a series of pharmacological manipulations involved in these latter studies, it was concluded that α1, β- and α2-receptors play a differential role in the regulation of body temperature (α1) and sleep-wake state (α2 and β). However, this hypothesis conflicts with the prominent wake-promoting actions of MPOA α1-receptor stimulation described above. Clearly, additional work is needed to clarify the role of different MPOA NE receptor subtypes in the regulation of body temperature vs. waking and the relationship between these processes.
3.2.3. Involvement of β2-receptors in NE-induced waking
Three subtypes of β-receptors have been identified: β1-β3 [53,103,111]. Intra-tissue infusions of the β2-agonist, clenbuterol, into either the MSA or the MPOA produces dose-dependent increases in time spent awake [25]. Although clenbuterol possesses weak β1-antagonistic properties [42], β1-antagonist infusion into the MSA or MPOA had no impact on sleep-wake state [25]. Thus, it is concluded that clenbuterol-induced waking observed in these studies results from the stimulation of β2-receptors.
3.2.4. NE does not act within the SI to promote waking
The SI, situated immediately lateral to both the MSA and MPOA (see Figure 4), provides a potent activating influence on EEG, in part through the actions of cholinergic projections to the neocortex [31,101]. These projections act in parallel with projections from the MSA to regulate the activity state of the neocortex and hippocampus. Thus, it was posited that NE-driven activation of hippocampal and cortical EEG involved the simultaneous actions of NE within the MSA and SI, respectively. In contrast to this hypothesis, infusion of NE, phenylephrine (α1-agonist), isoproterenol (β-agonist), or the indirect noradrenergic agonist, amphetamine has no significant impact on time spent awake when infused into the SI [15,18,21,22]. The only exception to this is observed with the highest concentration of NE examined (15 μg/250 nl) which produced a moderate increase in waking (Figure 5; [22,35]). However, in this case, the latency to waking was substantially longer and the time spent awake substantially reduced, relative to infusion into the MPOA [22]. The general lack of EEG altering effects of NE agonist infusions into SI is in contrast to the robust EEG-activating effects of glutamate infusions into SI [15,101]. The most parsimonious explanation for these observations is that the SI is relatively insensitive to the arousal-promoting actions of NE and that at high concentrations NE diffuses from the SI to the MPOA where it acts to increase waking.
3.3. Contrasting actions of MSA/MPOA β-receptor blockade in anesthetized vs. unanesthetized animals
The above-described studies demonstrate that MSA β-receptors exert an arousal-enhancing action. Moreover, in the halothane-anesthetized animal, bilateral blockade of MSA β-receptors decreases EEG activation observed following either pharmacologically-induced LC activation or in the lightly-anesthetized preparation [27]. These observations indicate that in the anesthetized animal MSA β-receptors are necessary for forebrain EEG activation. Whether this is true of MPOA β-receptors remains to be determined. In contrast to that observed in the anesthetized animal, in the unanesthetized rat, bilateral blockade of MSA β-receptors [27] or MPOA β-receptors [76] does not alter EEG or behavioral indices of arousal. As mentioned, the suppression of LC-NE neurotransmission globally, using ICV or intrabrainstem-administered α2-agonists, decreases EEG and behavioral indices of arousal [44,47]. The lack of sedative effects of bilateral MSA β-receptor blockade likely reflects the wake-promoting actions of: 1) α1-receptors within the MSA; and 2) α1- and β-receptors located outside the MSA (i.e. MPOA). Thus, in contrast to that observed in the presence of anesthesia, the wake-promoting actions of MSA β-receptors are redundant in the unanesthetized state. It is important to emphasize that redundancy does not imply irrelevance: under normal conditions, stimulation of MSA β-receptors will promote alert waking, a behaviorally important action.
3.4. Origin and organization of the noradrenergic innervation of the MSA, MPOA and SI
The above-described observations indicate that LC neurons exert a robust excitatory influence on forebrain activity state through actions of NE within the MSA and MPOA. Nonetheless, there are a number of noradrenergic nuclei that could provide input to these regions and, thus, participate in the modulation of behavioral state. Recent retrograde tracing studies indicate that the LC provides the majority of noradrenergic input to the MSA, MPOA and SI (approx. 50%). Nonetheless, these studies further demonstrate that substantial noradrenergic projections to these three regions also arise from the A1/C1 (approx. 25%) and A2/C2 (approx. 25–40%) cell groups (Table I). These observations suggest a likely arousal-promoting role of the A1/C1 and the A2/C2 noradrenergic/adrenergic nuclei.
Table I.
Distribution of retrogradely-labeled dopamine-β-hydroxylase-immunoreactive (DBH-ir) neurons across select noradrenergic nuclei following retrograde tracer infusion into select basal forebrain regions (MSA, MPOA and SI).
| Region | Number of DBH-ir neurons per region | % DBH-ir neurons retrogradely-labeled per region | % of all DBH-ir neurons retrogradely- labeled |
|---|---|---|---|
| MSA | |||
| LC | 52.3 ± 7.4 | 23.6 ± 2.7 | 56.8 ± 5.8 |
| SubC | 7.7 ± 10.8 | 1.1 ± 0.7 | 0.5 ± 0.3 |
| A1/C1 | 12.7 ± 0.8 | 14.3 ± 2.4 | 23.6 ± 4.9 |
| A2/C2 | 14.5 ± 1.7 | 9.6 ± 5.3 | 16.0 ± 2.5 |
| A4 | 3.9 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A5 | 6.2 ± 0.7 | 4.9 ± 1.3 | 1.8 ± 1.0 |
| A7 | 9.5 ± 1.7 | 1.1 ± 1.1 | 0.3 ± 0.3 |
| C3 | 5.3 ± 0.7 | 1.5 ± 1.5 | 1.0 ± 1.0 |
|
| |||
| MPOR | |||
| LC | 56.4 ± 8.0 | 15.5 ± 2.0 | 41.7 ± 2.2 |
| SubC | 6.7 ± 0.8 | 0.0 ± 0.0 | 0 ± 0.0 |
| A1/C1 | 10.9 ± 0.7 | 25.8 ± 2.9 | 39.5 ± 1.8 |
| A2/C2 | 16.9 ± 2.1 | 5.8 ± 1.1 | 14.9 ± 1.8 |
| A4 | 2.6 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A5 | 6.4 ± 0.7 | 8.3 ± 2.8 | 2.9 ± 0.6 |
| A7 | 6.6 ± 1.7 | 2.0 ± 2.0 | 0.3 ± 0.3 |
| C3 | 4.0 ± 0.5 | 6.2 ± 4.5 | 0.7 ± 0.4 |
|
| |||
| SI | |||
| LC | 57.5 ± 9.0 | 16.1 ± 2.4 | 46.8 ± 2.5 |
| SubC | 7.5 ± 1.2 | 0.0 ± 0.0 | 0 ± 0.0 |
| A1/C1 | 11.5 ± 0.9 | 12.9 ± 2.1 | 24.8 ± 2.9 |
| A2/C2 | 15.3 ± 2.0 | 9.58 ± 1.7 | 21.1 ± 3.3 |
| A4 | 5.5 ± 0.8 | 6.7 ± 2.9 | 1.0 ± 0.1 |
| A5 | 9.2 ± 0.7 | 5.4 ± 1.2 | 6.0 ± 2.4 |
| A7 | 6.8 ± 2.6 | 1.4 ± 1.4 | 0.4 ± 0.4 |
| C3 | 4.5 ± 0.5 | 0.0 ± 0.0 | 0 ± 0.0 |
Animals received an iontophoretic infusion of the retrograde tracer fluorogold (FG) into one three regions: MSA, MPOA, and SI (Region). Tissue was processed for simultaneous immunohistochemical visualization of DBH-ir (the NE synthetic enzyme) and FG. The number and percentage of retrogradely-labeled DBH-ir neurons within noradrenergic/adrenergic cell groups (LC(A6), subcoeruleus, A1/C1, A2/C2, A4, A5, A7, C3) were then measured (double-labeled neurons containing DBH-ir + FG). Shown are: 1) Mean number (±SEM) of DBH-ir neurons per noradrenergic/adrenergic nucleus; 2) percentage (± SEM) of DBH-ir neurons from each noradrenergic nucleus that were retrogradely-labeled from the respective basal forebrain region; and 3) percentage of the total number of DBH-ir neurons across all noradrenergic nuclei that were retrogradely-labeled within a given noradrenergic nucleus. For each basal forebrain region the majority of DBH-ir retrogradely labeled neurons was observed within the LC and a lesser percentage within A1/C1 and A2/C2 (See [55]).
In terms of LC projections, basal forebrain-projecting noradrenergic neurons are largely distributed uniformly within the LC [55]. This contrasts with a rough topographic distribution of LC neurons projecting to neocortex (except the prefrontal cortex), hippocampus, cerebellum, and spinal cord [87]. Moreover, additional observations indicate that individual LC neurons display a high degree of collateralization across these three arousal-related basal forebrain regions, ensuring that alterations in LC discharge are relayed simultaneously to these regions [55]. Combined, these observations suggest that LC efferents are organized to permit coordinated actions across multiple anatomically distinct, yet functionally-related (i.e. arousal-related), basal forebrain fields.
As mentioned, a prominent difference between SI and both MSA and MPOA is that the SI is relatively insensitive to the wake-promoting actions of NE and NE agonists [22]. Nonetheless, in vitro, NE depolarizes cholinergic basal forebrain neurons, indicating a neuromodulatory role of NE within SI [60]. To date, the behavioral/cognitive functions of NE within the SI remain to be fully elucidated. However, evidence indicates a role of the SI in state-dependent attentional processes (for review, see [122]). Moreover, noradrenergic α1-receptors within the SI appear to participate in the priming effect of systemic epinephrine administration on the cerebral auditory evoked potential [11]. Thus, NE likely acts within the SI to modulate state-dependent behavioral/cognitive processes, such as attention (for review, see [122]). The high degree of collateralization across the MSA, MPOA and SI permits the LC to simultaneously modulate behavioral state while modulating SI-dependent behavioral and physiological processes.
3.5. Additional regions involved in NE-dependent waking
The above-reviewed observations suggest a prominent role of the MSA and MPOA in stimulant-induced arousal. The actions of NE within these subcortical regions occur in conjunction with the activating actions of NE directly within cortical and thalamic neurons [96]. Moreover, a variety of additional brainstem-originating systems are associated with the regulation of behavioral state including serotonergic, dopaminergic, cholinergic, and histaminergic systems [33,71,85,104,145,148,149]. These systems are highly interconnected and in many cases have been demonstrated to exert reciprocally activating effects [5,50,51,129,130,143]. To date, the extent to which NE acts on these other neurotransmitter systems to modulate behavioral state has not been examined systematically. However, via actions within the dorsal pontine tegmentum β-receptors have been demonstrated to inhibit REM sleep [143,144]
Additionally, the lateral hypothalamus (LH) has been implicated in the regulation of sleep-wake state. In particular, substantial evidence suggests prominent arousal-promoting actions of the neuropeptide family, hypocretin (orexin) and HCRT-synthesizing neurons are located solely in the perifornical region of the LH [54,137]. The LH receives a moderately dense noradrenergic innervation [10], although most of this arises from outside the LC [157]. In recently initiated studies, wake-promoting actions of the α1-agonist, phenylephrine, were observed when infused into the LH, but not when infused immediately outside this region [13]. A variety of additional regions beyond those mentioned above participate in the modulation of arousal, including the central nucleus of the amygdala [125]. Thus, the current list of known sites involved in NE-dependent modulation of waking is likely to be expanded in the future. Combined, available information indicates that the noradrenergic modulation of arousal involves coordinated actions of NE, arising from the LC and other noradrenergic nuclei (i.e. A1, A2), within a distributed network of subcortical structures.
3.6. Synergistic actions of β- and α1-receptors in the maintenance of alert waking
The above-reviewed observations indicate that stimulation of either α1- or β-receptors within any one of a number of brain regions is sufficient to induce the alert waking state. This then raises the question of whether combined actions of these receptors are necessary for the maintenance of normal alert waking. To initially address this issue, the EEG effects of β-receptor blockade (timolol infused intracerebroventricularly), α1-receptor blockade (prazosin, administered intraperitoneally), or combined β- and α1-receptor blockade were examined in rats under conditions associated with high arousal levels (a brightly-lit novel environment). These studies demonstrated profound increases in large-amplitude, slow-wave activity in cortical EEG in animals treated with combined β- and α1-antagonists (Figure 8; [17]). This increase in slow-wave activity is in contrast to the minimal EEG effects of β-antagonist treatment alone or the α1-antagonist-induced increase in sleep-spindles (Figure 8; see also [32]). Therefore, under these conditions, combined blockade of β- and α1-receptors produces synergistic sedative effects.
Figure 8.
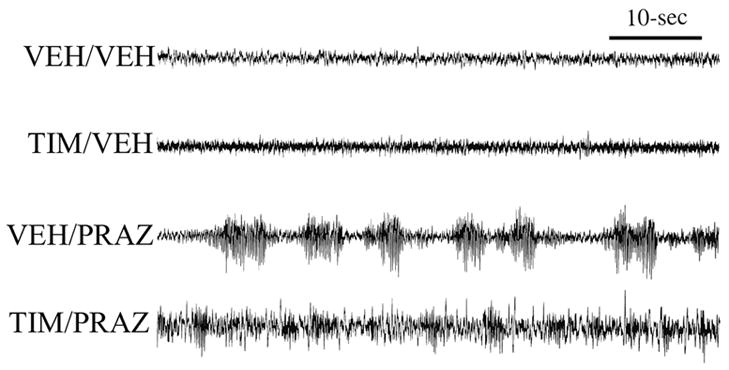
Synergistic sedative effects of α1- and β-receptor blockade. Shown are the effects of the β-antagonist, timolol (ICV), the α1-antagonist, prazosin (IP) and combined antagonist treatment on cortical EEG in animals exposed to an arousing brightly-lit novel environment. Animals were treated 30-min prior to testing with: 1) ICV vehicle + IP saline (VEH/VEH); 2) 150 μg ICV timolol + IP saline (TIM/VEH); 3) ICV vehicle + 500 μg/kg prazosin (VEH/PRAZ,), and; 4) combined timolol + prazosin (TIM/PRAZ). In this figure, EEG traces are from the second 5-min epoch of exposure to the novel environment. Vehicle-treated controls displayed behavioral and EEG indices of alert waking throughout most of the recording session. This is reflected in sustained EEG desynchronization (low-amplitude, high-frequency). β-receptor blockade alone (TIM/VEH) had no effects on EEG activity. α1-receptor blockade alone (VEH/PRAZ) increased the frequency and duration of sleep spindles (high-voltage spindles). In contrast to that observed with β-receptor blockade alone, in the presence of α1-receptor blockade, β-receptor blockade produced substantial increases in large-amplitude, slow-wave activity. Power spectral analyses provide quantification of these qualitative observations (data not shown, see [17]).
Importantly, this sedative effect was not observed during the first 5-minutes of testing. Thus, under certain conditions of elevated arousal, combined actions of α1- and β-receptors are not necessary for the maintenance of EEG indices of arousal. These observations could suggest that the combined blockade of α1- and β-receptors increases the rate of habituation to an arousing, novel environment. Alternatively, initial exposure to the novel environment may involve actions of additional systems that are capable of supporting an activated forebrain in the absence of α1 and β-receptor stimulation. It is important to note that behaviorally, although these animals were profoundly sedated following combined α1- and β-receptor blockade (i.e. nearly continuous slow-wave EEG activity), behaviorally they did not appear to be asleep. Specifically, the animals maintained sufficient postural tone to remain in an upright position, albeit with minimal movement. Thus, in the absence of α1-/β-receptor action, either other NE receptors (i.e. α2) or other neurotransmitter systems maintain a certain degree of arousal. The sedating effect of α1-receptor blockade may have relevance to the therapeutic effects of α1-antagonist in the treatment of post-traumatic stress sleep-related symptoms [116,141]. Moreover, decreased stimulation of α1- and β-receptors may participate in the well-documented anesthetic effects of α2-agonists [110].
3.7. Potential sleep-promoting actions of α1-receptors within the MPOA
In contrast to the robust wake-promoting effects of NE agonists infused into the MPOA and MSA, 6-OHDA lesions of the ventral noradrenergic bundle have been reported to increase time spent awake (VNAB; [77]). This has been interpreted as suggesting a potential sleep-promoting action of NE, particularly within the MPOA (for review see [79]). However, given the above-reviewed information regarding multiple sources of the noradrenergic innervation of the MPOA (LC, A1, A2) and lesion-induced compensation, these effects of a VNAB lesion may well reflect lesion-induced upregulation of noradrenergic neurotransmission (and/or other transmitter systems) within the MPOA and/or other arousal-related structures.
Lesions of the VNAB also disrupted the wake-promoting actions of NE when infused into the MPOA during the day and resulted in an NE-induced decrease in time spent awake when infused into the MPOA during the night [78]. Consistent with this latter observation, infusion of an α1-antagonist into the MPOA increased time spent awake [151]. Combined, these observations suggest possible arousal-attenuating actions of MPOA α1-receptors. Currently, it is not clear how to reconcile the robust wake-promoting effects of intra-MPOA NE, α1- and β-agonist infusions reported by others [20,22,76] with these more recent observations. However, there are a number of issues to consider. First, these effects appear relatively modest compared to the wake-promoting effects of NE agonist infusions. Second, baseline arousal level in these latter studies appears higher and more labile than in previous studies utilizing NE agonists. Alterations in baseline arousal level could influence the arousal-modulating actions of NE. Third, when performing intra-tissue infusions, it is critical to minimize tissue damage. Available evidence, though limited, indicates substantial tissue damage may have occurred within the MPOA in these more recent studies (see [151]). This could well alter sensitivity to NE-selective drugs. Fourth, given variability in baseline waking between groups observed in these studies, it would be useful to assess drug effects relative to within-subject baseline measures of sleep and waking (i.e. relative to the 30-minutes immediately preceding infusion). Such an analysis could yield qualitatively different results. Finally, as reviewed above, extensive evidence indicates: 1) robust arousal-enhancing actions of LC activation and intra-MPOA infusions of NE/NE agonists; 2) in intact animals, profound sedative actions of: 1) LC inhibition, specifically; 2) noradrenergic neurotransmission, globally via systemic or ICV α2-agonist administration; 3) α1-receptor and combined α1/β-receptor blockade globally. Thus, the functional significance of modest arousal-attenuating action of NE within the MPOA is unclear.
4. Noradrenergic modulation of cortical and thalamic neuronal activity state, in vitro
Cortical and thalamic neurons display distinct activity modes during sleeping and waking behavioral states. Thus, during slow-wave sleep, these neurons are hyperpolarized relative to that observed in waking and display a burst-type activity mode associated with a relative insensitivity to incoming signals. In contrast, waking is associated with a single-spike mode which permits efficient and accurate signal processing [49,93,95,108,109]. The above-described electrophysiological studies demonstrate increased rates of NE release during conditions associated with the single-spike mode (i.e. waking), suggesting that LC efferents contribute to the induction of this activity state [6,57]. Consistent with this hypothesis, in vitro, NE induces a shift in the firing pattern of cortical and thalamic neurons from a burst mode to a single-spike mode via actions at α1-receptors and β-receptors [94,97,115].
5. Noradrenergic modulation of circadian-dependent activity
LC neurons of anesthetized rats display a circadian-dependent activity (i.e. arousal-independent), with highest rates associated with the activity phase of the circadian cycle [7]. Trans-synaptic retrograde tracing studies indicate that the suprachiasmatic nucleus can influence the LC via the dorsomedial hypothalamus [7]. Moreover, in these studies lesions of the dorsomedial hypothalamus eliminated circadian-dependent LC discharge activity. Finally, noradrenergic lesions altered circadian-dependent behavioral activity in the absence of anesthesia [64]. Combined, these observations indicate a role for the LC-noradrenergic system in circadian-dependent fluctuations in arousal/activity.
6. The LC-NE system modulates state-dependent gene expression
Waking is associated with an upregulation of certain genes within the neocortex [37,38,40]. Earlier studies indicated that unilateral lesions of the LC decrease expression of certain of these genes in the ipsilateral neocortex [38]. Subsequent studies demonstrated that DSP-4-induced lesion of the LC-NE system reduced transcription levels of approximately 20% of 95 identified waking-related genes in the neocortex [39]. The majority of lesion-affected genes are associated with synaptic plasticity and/or stress responsivity. In contrast to that observed with gene transcription, and consistent with previous NE-lesion studies, DSP-4 treatment did not affect time spent awake, despite a reduction of tissue NE levels of at least 80%. These observations indicate there exist different activity-response curves for LC regulation of arousal vs. neocortical gene transcription. As reviewed above, the minimal effects on time spent awake of the DSP-4 lesions likely reflects two distinct, yet related mechanisms. First, in the anesthetized rat, sedative effects of LC inhibition are not observed until LC neuronal discharge is suppressed greater than 95% [23]. Second, DSP-4 lesions spare non-LC noradrenergic neurons, which provide a significant noradrenergic innervation to basal forebrain arousal-related regions [55].
These observations are consistent with additional evidence demonstrating stimulatory actions of NE on a variety of immediate-early genes (IEGs). For example, systemic administration of an α2-antagonist, which increases NE efflux, increases mRNA and protein levels for a variety of IEGs in rat cerebral cortex [29,65]. Additionally, direct infusion of NE into the cortex [136] or amygdala [134] also increases expression of the IEG, c-fos. Similar effects on IEG expression are observed with exposure of animals to stress, which increases LC discharge and NE release [28,29,65]. The activating effects of pharmacologically- or stressor-induced increases in NE neurotransmission on IEG expression are attenuated with pretreatment of either β- or α1-antagonists [28,29,65,134,136] as well as (in the case of stress) LC lesions [135]. Additional studies implicate the β1-receptor subtype in β-dependent activation of c-fos [133].
These observations indicate a significant impact of the LC on state-dependent expression of plasticity-related genes. Such actions likely play an important role in optimizing learning, memory and behavioral plasticity during the waking state.
7. Involvement of NE in stress-related arousal
The view of stress as a behavioral and physiological state elicited by challenging or threatening events arises from nearly a century of research starting with the seminal work of Cannon [34] and Selye [124]. This early work identified the activation of peripheral catecholamine systems and the pituitary-adrenal axis as defining features of stress. Combined, the activation of these systems facilitates contending with a challenging situation. More recent work implicates central catecholamines in stress, including the LC-noradrenergic system [12,146]. Although the affective and cognitive features of stress are less-well defined, a heightened level of readiness for action appears to be paramount to the state of stress. A prominent component of this preparatory state is an elevated level of arousal.
The LC-noradrenergic system displays a pronounced sensitivity to stressors [12,52,146]. The above-reviewed information indicates a prominent role of central noradrenergic systems in the regulation of arousal. Thus, it is posited that an activation of these systems plays a prominent role in the induction of elevated arousal levels in stress. Consistent with this hypothesis, bilateral suppression of LC discharge blocks stressor-induced EEG activation in the halothane-anesthetized rat [113,114,147]. As discussed above, the stress-related modulation of arousal by noradrenergic systems can indirectly influence a variety of cognitive and affective processes. Additional actions of NE within specific affect- and cognition-related circuits are likely to more directly impact cognitive and affective components of stress as well.
Substantial evidence indicates that activation of the prototypical stress systems (hypothalamo-pituitary-adrenal axis as well as peripheral and central catecholaminergic systems) occurs across both aversive and appetitive conditions (for review, [91]). This suggests the working hypothesis that at least a subset of the physiological indices of stress may be independent of affective valence (pleasant vs. unpleasant) and more closely aligned with arousal level, motivational state, and/or the need for action. In this context, noradrenergic modulation of behavioral state (and state-dependent cognition) may serve a critical function in stress-related behavior while not being unique to stress.
8. Involvement of NE in psychostimulant-induced arousal
Psychostimulants, exert profound arousal-enhancing actions. Neurochemically, these drugs increase rates of NE and DA neurotransmission. Surprisingly, the neural mechanisms involved in psychostimulant-induced arousal have not been well-characterized. The above-reviewed observations indicate robust wake-promoting effects of NE via actions within multiple subcortical sites. Combined, these observations suggest the involvement of NE in the arousal-promoting actions of psychostimulants. Substantial evidence supports this hypothesis (for review, [14]). This includes the fact that, similar to that of NE, amphetamine acts within the MSA and MPOA, but not the SI, to promote alert waking [21]. In general, amphetamine-induced waking observed in these studies resembles normal waking, and is not associated with increases in locomotor activity and/or stereotypy. Additionally, microdialysis studies demonstrate that a close association between amphetamine-induced waking and amphetamine-induced increases in extracellular levels of NE and DA [24]. Recent observations suggest that the wake-promoting actions of psychostimulants involve increases in NE (and possibly/probably DA) outside the prefrontal cortex (i.e. MSA, MPOA) that are equal to or greater than 100% above levels associated with quiet-resting [14,24]. Finally, evidence for a causal role of NE in psychostimulant-induced arousal, pretreatment with the β-antagonist, timolol, dose-dependently blocked amphetamine-induced EEG activation in halothane-anesthetized rats. Consistent with the above-reviewed observations, β-antagonist pretreatment had no effect on amphetamine-induced arousal in unanesthetized animals [84].
9. Broader Behavioral Actions of Norepinephrine
This review has focused on one critical aspect of noradrenergic function: the induction of an appropriate behavioral state for the detection of environmental events (i.e. waking). Nonetheless, it is important to note that NE-induced arousal occurs in tandem with a large variety of additional modulatory actions of NE on physiological and behavioral processes, including endocrine regulation, perception, motor function, attention and memory, and decision and action [3,8,26,69,80,98,102]. These actions involve a variety of cortical and subcortical noradrenergic terminal fields and noradrenergic receptors. A unifying theme to the diversity of these actions is the facilitation of the collection, processing, and responding to salient information arising from an ever-changing environment.
10. Summary
The regulation of arousal is a critical aspect of normal behavior. As such, it is not surprising that multiple systems have evolved to regulate arousal state. The above-described observations indicate a prominent role of the LC and other noradrenergic systems in the regulation of arousal state. Specifically, these systems promote alert waking via actions of α1-and β-receptors located within multiple subcortical regions. Based on available experimental evidence, it appears that under normal physiological conditions even moderate activity of the LC-NE system is incompatible with the state of sleep. Given this, excessive activity of this system may contribute to certain forms of insomnia or other conditions associated with elevated arousal levels, including stress-related disorders, such as PTSD. There is extensive evidence that NE modulates directly neural circuitry associated with cognition and affect (for review, [26]). However, affective and cognitive processes also display a pronounced sensitivity to arousal state. Thus, in addition to direct modulatory actions on cognition- and affect-related neurocircuitry, the LC-noradrenergic system may influence cognitive and affective processes indirectly via the modulation of arousal.
Acknowledgments
This work was supported by PHS grants MH62359, DA10681, DA00389 and the University of Wisconsin Graduate School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abercrombie ED, Zigmond MJ. Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci. 1989;9:4062–4067. doi: 10.1523/JNEUROSCI.09-11-04062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams LM, Foote SL. Effects of locally infused pharmacological agents on spontaneous and sensory-evoked activity of locus coeruleus neurons. Brain Res Bull. 1988;21:395–400. doi: 10.1016/0361-9230(88)90151-7. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Akaoka H, Charlety P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991;11:760–769. doi: 10.1523/JNEUROSCI.11-03-00760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 8.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 9.Aston-Jones G, Rajkowski J, Ivanova S, Usher M, Cohen J. Neuromodulation and cognitive performance: recent studies of noradrenergic locus ceruleus neurons in behaving monkeys. Adv Pharmacol. 1998;42:755–759. doi: 10.1016/s1054-3589(08)60857-1. [DOI] [PubMed] [Google Scholar]
- 10.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 11.Berntson GG, Shafi R, Knox D, Sarter M. Blockade of epinephrine priming of the cerebral auditory evoked response by cortical cholinergic deafferentation. Neuroscience. 2003;116:179–186. doi: 10.1016/s0306-4522(02)00702-9. [DOI] [PubMed] [Google Scholar]
- 12.Berridge CW. The locus coeruleus-noradrenergic system and stress: modulation of arousal state and state-dependent behavioral processes. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Part I: The Neurobiology of Stress. Elsevier B.V; Amsterdam: 2005. pp. 437–464. [Google Scholar]
- 13.Berridge CW, Schmeichel BE. Norepinephrine Acts Within the Lateral Hypothalamus to Promote Waking: Relevance to Psychostimulant-Induced Arousal. Soc Neurosci Abst. 2007 in press. [Google Scholar]
- 14.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 15.Berridge CW, Bolen SJ, Manley MS, Foote SL. Modulation of forebrain electroencephalographic activity in halothane- anesthetized rat via actions of noradrenergic beta-receptors within the medial septal region. J Neurosci. 1996;16:7010–7020. doi: 10.1523/JNEUROSCI.16-21-07010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge CW, Dunn AJ. DSP-4-induced depletion of brain norepinephrine produces opposite effects on exploratory behavior 3 and 14 days after treatment. Psychopharmacology (Berl) 1990;100:504–508. doi: 10.1007/BF02244003. [DOI] [PubMed] [Google Scholar]
- 17.Berridge CW, España RA. Synergistic sedative effects of noradrenergic alpha(1)-and beta- receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- 18.Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge CW, Isaac SO, Espana RA. Additive wake-promoting actions of medial basal forebrain noradrenergic alpha1- and beta-receptor stimulation. Behav Neurosci. 2003;117:350–359. doi: 10.1037/0735-7044.117.2.350. [DOI] [PubMed] [Google Scholar]
- 21.Berridge CW, O’Neil J, Wifler K. Amphetamine acts within the medial basal forebrain to initiate and maintain alert waking. Neuroscience. 1999;93:885–896. doi: 10.1016/s0306-4522(99)00271-7. [DOI] [PubMed] [Google Scholar]
- 22.Berridge CW, O’Neill J. Differential sensitivity to the wake-promoting actions of norepinephrine within the medial preoptic area and the substantia innominata. Behav Neurosci. 2001;115:165–174. doi: 10.1037/0735-7044.115.1.165. [DOI] [PubMed] [Google Scholar]
- 23.Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- 24.Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- 25.Berridge CW, Stellick RL, Schmeichel BE. Wake-promoting actions of medial basal forebrain beta2 receptor stimulation. Behav Neurosci. 2005;119:743–751. doi: 10.1037/0735-7044.119.3.743. [DOI] [PubMed] [Google Scholar]
- 26.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 27.Berridge CW, Wifler K. Contrasting effects of noradrenergic beta-receptor blockade within the medial septal area on forebrain electroencephalographic and behavioral activity state in anesthetized and unanesthetized rat. Neuroscience. 2000;97:543–552. doi: 10.1016/s0306-4522(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 28.Bing G, Stone EA, Zhang Y, Filer D. Immunohistochemical studies of noradrenergic-induced expression of c- fos in the rat CNS. Brain Res. 1992;592:57–62. doi: 10.1016/0006-8993(92)91658-2. [DOI] [PubMed] [Google Scholar]
- 29.Bing GY, Filer D, Miller JC, Stone EA. Noradrenergic activation of immediate early genes in rat cerebral cortex. Brain Res Mol Brain Res. 1991;11:43–46. doi: 10.1016/0169-328x(91)90019-t. [DOI] [PubMed] [Google Scholar]
- 30.Bremer F. Cerebral activity during sleep and narcosis: Contribution to the study of the mechanisms of sleep. Bulletin de l’Academie Royale Medicale Belgique. 1937;4:240–275. [Google Scholar]
- 31.Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buzsaki G, Kennedy B, Solt BV, Ziegler M. Noradrenergic control of thalamic oscillation: the role of α-2 receptors. Eur J Neurosci. 1991;3:222–229. doi: 10.1111/j.1460-9568.1991.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 33.Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 34.Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol. 1914;33:356–372. [Google Scholar]
- 35.Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castaneda E, Whishaw IQ, Robinson TE. Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci. 1990;10:1847–1854. doi: 10.1523/JNEUROSCI.10-06-01847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 38.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 39.Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirelli C, Tononi G. Differences in gene expression between sleep and waking as revealed by mRNA differential display. Brain Res Mol Brain Res. 1998;56:293–305. doi: 10.1016/s0169-328x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 41.Colom LV, Nassif-Caudarella S, Dickson CT, Smythe JW, Bland BH. In vivo intrahippocampal microinfusion of carbachol and bicuculline induces theta-like oscillations in the septally deafferented hippocampus. Hippocampus. 1991;1:381–390. doi: 10.1002/hipo.450010406. [DOI] [PubMed] [Google Scholar]
- 42.Conway PG, Tejani-Butt S, Brunswick DJ. Interaction of beta adrenergic agonists and antagonists with brain beta adrenergic receptors in vivo. J Pharmacol Exp Ther. 1987;241:755–762. [PubMed] [Google Scholar]
- 43.Cunningham JT, Nissen R, Renaud LP. Norepinephrine injections in diagonal band of Broca selectively reduced the activity of vasopressin supraoptic neurons in the rat. Brain Res. 1993;610:152–155. doi: 10.1016/0006-8993(93)91229-l. [DOI] [PubMed] [Google Scholar]
- 44.Danysz W, Dyr W, Plaznik A, Kostowski W. The effect of microinjections of clonidine into the locus coeruleus on cortical EEG in rats. Pol J Pharmacol Pharm. 1989;41:45–50. [PubMed] [Google Scholar]
- 45.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 46.De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Sarro GB, Bagetta G, Ascioti C, Libri V, Nistico G. Microinfusion of clonidine and yohimbine into locus coeruleus alters EEG power spectrum: effects of aging and reversal by phosphatidylserine. Br J Pharmacol. 1988;95:1278–1286. doi: 10.1111/j.1476-5381.1988.tb11765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz J, Ellison G, Masouka D. Stages of recovery from central norepinephrine lesions in enriched and impoverished environments: a behavioral and biochemical study. Exp Brain Res. 1978;31:117–130. doi: 10.1007/BF00235809. [DOI] [PubMed] [Google Scholar]
- 49.Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol. 1986;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dringenberg HC, Souza-Silva MA, Rossmuller J, Huston JP, Schwarting RK. Histamine H1 receptor antagonists produce increases in extracellular acetylcholine in rat frontal cortex and hippocampus. J Neurochem. 1998;70:1750–1758. doi: 10.1046/j.1471-4159.1998.70041750.x. [DOI] [PubMed] [Google Scholar]
- 51.Dringenberg HC, Souza-Silva MA, Schwarting RK, Huston JP. Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:423–429. doi: 10.1007/pl00005274. [DOI] [PubMed] [Google Scholar]
- 52.Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51:406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 53.Engel G, Maurer R, Perrot K, Richardson BP. Beta-Adrenoceptor Subtypes in Sections of Rat and Guinea-Pig Kidney. Naunyn-Schmiedebergs Archives of Pharmacology. 1985;328:354–357. doi: 10.1007/BF00515567. [DOI] [PubMed] [Google Scholar]
- 54.España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 55.España RA, Berridge CW. Organization of noradrenergic efferents to arousal-related basal forebrain structures. J Comp Neurol. 2006;496:668–683. doi: 10.1002/cne.20946. [DOI] [PubMed] [Google Scholar]
- 56.Flicker C, Geyer MA. The hippocampus as a possible site of action for increased locomotion during intracerebral infusions of norepinephrine. Behav Neural Biol. 1982;34:421–426. doi: 10.1016/s0163-1047(82)91843-x. [DOI] [PubMed] [Google Scholar]
- 57.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 59.Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- 60.Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci. 1995;7:1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 61.Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–441. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- 62.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 63.Gatti PJ, Hill KJ, Da Silva AM, Norman WP, Gillis RA. Central nervous system site of action for the hypotensive effect of clonidine in the cat. J Pharmacol Exp Ther. 1988;245:373–380. [PubMed] [Google Scholar]
- 64.Gonzalez MM, Aston-Jones G. Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep. 2006;29:1327–1336. doi: 10.1093/sleep/29.10.1327. [DOI] [PubMed] [Google Scholar]
- 65.Gubits RM, Smith TM, Fairhurst JL, Yu H. Adrenergic receptors mediate changes in c-fos mRNA levels in brain. Brain Res Mol Brain Res. 1989;6:39–45. doi: 10.1016/0169-328x(89)90026-0. [DOI] [PubMed] [Google Scholar]
- 66.Hallman H, Jonsson G. Pharmacological modifications of the neurotoxic action of the noradrenaline neurotoxin DSP4 on central noradrenaline neurons. Eur J Pharmacol. 1984;103:269–278. doi: 10.1016/0014-2999(84)90487-4. [DOI] [PubMed] [Google Scholar]
- 67.Harik SI, Duckrow RB, LaManna JC, Rosenthal M, Sharma VK, Banerjee SP. Cerebral compensation for chronic noradrenergic denervation induced by locus ceruleus lesion: recovery of receptor binding, isoproterenol- induced adenylate cyclase activity, and oxidative metabolism. J Neurosci. 1981;1:641–649. doi: 10.1523/JNEUROSCI.01-06-00641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 69.Hurlemann R. Noradrenergic control of emotion-induced amnesia and hypermnesia. Rev Neurosci. 2006;17:525–532. doi: 10.1515/revneuro.2006.17.5.525. [DOI] [PubMed] [Google Scholar]
- 70.Ino T, Itoh K, Kamiya H, Shigemoto R, Akiguchi I, Mizuno N. Direct projections of non-pyramidal neurons of Ammon’s horn to the supramammillary region in the cat. Brain Res. 1988;460:173–177. doi: 10.1016/0006-8993(88)91219-x. [DOI] [PubMed] [Google Scholar]
- 71.Isaac SO, Berridge CW. Wake-promoting actions of dopamine D1 and D2 receptor stimulation. J Pharmacol Exp Ther. 2003;307:386–394. doi: 10.1124/jpet.103.053918. [DOI] [PubMed] [Google Scholar]
- 72.Itil TM, Itil KZ. Central mechanisms of clonidine and propranolol in man. Quantitative Pharmaco-EEG with antihypertensive compounds. Chest. 1983;83:411–416. [PubMed] [Google Scholar]
- 73.Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58:157–177. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]
- 74.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 75.Krilowicz BL, Szymusiak R, McGinty D. Regulation of posterior lateral hypothalamic arousal related neuronal discharge by preoptic anterior hypothalamic warming. Brain Res. 1994;668:30–38. doi: 10.1016/0006-8993(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 76.Kumar V, Datta S, Chhina GS, Gandhi N, Singh B. Sleep-awake responses elicited from medial preoptic area on application of norepinephrine and phenoxybenzamine in free moving rats. Brain Res. 1984;322:322–325. doi: 10.1016/0006-8993(84)90125-2. [DOI] [PubMed] [Google Scholar]
- 77.Kumar VM, Sharma R, Wadhwa S, Manchanda SK. Sleep-inducing function of noradrenergic fibers in the medial preoptic area. Brain Res Bull. 1993;32:153–158. doi: 10.1016/0361-9230(93)90069-n. [DOI] [PubMed] [Google Scholar]
- 78.Kumar VM, Vetrivelan R, Mallick HN. Alpha-1 adrenergic receptors in the medial preoptic area are involved in the induction of sleep. Neurochem Res. 2006;31:1095–1102. doi: 10.1007/s11064-006-9109-8. [DOI] [PubMed] [Google Scholar]
- 79.Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: Neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochem Int. 2007;50:783–790. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- 81.Laverty R, Taylor KM. Behavioural and biochemical effects of 2-(2,6-dichlorophenylamino)-2-imidazoline hydrochloride (St 155) on the central nervous system. Br J Pharmacol. 1969;35:253–264. doi: 10.1111/j.1476-5381.1969.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lidbrink P. The effect of lesions of ascending noradrenaline pathways on sleep and waking in the rat. Brain Res. 1974;74:19–40. doi: 10.1016/0006-8993(74)90109-7. [DOI] [PubMed] [Google Scholar]
- 83.Lidbrink P, Fuxe K. Effects of intracerebral injections of 6-hydroxydopamine on sleep and waking in the rat. J Pharm Pharmacol. 1973;25:84–87. doi: 10.1111/j.2042-7158.1973.tb09125.x. [DOI] [PubMed] [Google Scholar]
- 84.Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–326. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- 85.Lin JS, Sakai K, Jouvet M. Role of hypothalamic histaminergic systems in the regulation of vigilance states in cats. C R Acad Sci III. 1986;303:469–474. [PubMed] [Google Scholar]
- 86.Logue MP, Growdon JH, Coviella IL, Wurtman RJ. Differential effects of DSP-4 administration on regional brain norepinephrine turnover in rats. Life Sci. 1985;37:403–409. doi: 10.1016/0024-3205(85)90401-1. [DOI] [PubMed] [Google Scholar]
- 87.Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986;18:307–319. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- 88.Makeig S, Inlow M. Lapses in alertness: coherence of fluctuations in performance and EEG spectrum. Electroencephalogr Clin Neurophysiol. 1993;86:23–35. doi: 10.1016/0013-4694(93)90064-3. [DOI] [PubMed] [Google Scholar]
- 89.Mallick BN, Alam MN. Different types of norepinephrinergic receptors are involved in preoptic area mediated independent modulation of sleep-wakefulness and body temperature. Brain Res. 1992;591:8–19. doi: 10.1016/0006-8993(92)90972-c. [DOI] [PubMed] [Google Scholar]
- 90.Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18:723–727. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- 91.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 92.Marston HM, West HL, Wilkinson LS, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the septum and vertical limb nucleus of the diagonal band of Broca on conditional visual discrimination: relationship between performance and choline acetyltransferase activity in the cingulate cortex. J Neurosci. 1994;14:2009–2019. doi: 10.1523/JNEUROSCI.14-04-02009.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCarley RW, Benoit O, Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983;50:798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- 94.McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 95.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 96.McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- 97.McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- 98.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 99.McGinty D, Metes A, Alam MN, Megirian D, Stewart D, Szymusiak R. Preoptic hypothalamic warming suppresses laryngeal dilator activity during sleep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1129–R1137. doi: 10.1152/ajpregu.00296.2003. [DOI] [PubMed] [Google Scholar]
- 100.Meibach RC, Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977;119:1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- 101.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milstein JA, Lehmann O, Theobald DE, Dalley JW, Robbins TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl) 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minneman KP, Hegstrand LR, Molinoff PB. Pharmacological Specificity of Beta-1 and Beta-2 Adrenergic-Receptors in Rat-Heart and Lung In vitro. Molecular Pharmacology. 1979;16:21–33. [PubMed] [Google Scholar]
- 104.Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–394. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 105.Modirrousta M, Mainville L, Jones BE. Gabaergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 106.Mogilnicka E. Increase in beta- and alpha 1-adrenoceptor binding sites in the rat brain and in the alpha 1-adrenoceptor functional sensitivity after the DSP-4-induced noradrenergic denervation. Pharmacol Biochem Behav. 1986;25:743–746. doi: 10.1016/0091-3057(86)90380-1. [DOI] [PubMed] [Google Scholar]
- 107.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 108.Mukhametov LM, Rizzolatti G, Seitun A. An analysis of the spontaneous activity of lateral geniculate neurons and of optic tract fibers in free moving cats. Arch Ital Biol. 1970;108:325–347. [PubMed] [Google Scholar]
- 109.Mukhametov LM, Rizzolatti G, Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970;210:651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 111.Nisoli E, Carruba MO. Pharmacological properties of beta(3)-adrenoceptors. Trends in Pharmacological Sciences. 1997;18:257–258. [PubMed] [Google Scholar]
- 112.Osaka T, Matsumura H. Noradrenaline inhibits preoptic sleep-active neurons through alpha 2-receptors in the rat. Neurosci Res. 1995;21:323–330. doi: 10.1016/0168-0102(94)00871-c. [DOI] [PubMed] [Google Scholar]
- 113.Page ME, Akaoka H, Aston-Jones G, Valentino RJ. Bladder distention activates noradrenergic locus coeruleus neurons by an excitatory amino acid mechanism. Neuroscience. 1992;51:555–563. doi: 10.1016/0306-4522(92)90295-d. [DOI] [PubMed] [Google Scholar]
- 114.Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- 115.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 116.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O’Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 117.Robinson TE, Castaneda E, Whishaw IQ. Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol. 1990;44:253–275. doi: 10.1037/h0084241. [DOI] [PubMed] [Google Scholar]
- 118.Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- 120.Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- 121.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 122.Sarter M, Bruno JP. Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends Neurosci. 1999;22:67–74. doi: 10.1016/s0166-2236(98)01289-2. [DOI] [PubMed] [Google Scholar]
- 123.Segal DS, Mandell AJ. Behavioral activation of rats during intraventricular infusion of norepinephrine. Proc Natl Acad Sci U S A. 1970;66:289–293. doi: 10.1073/pnas.66.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 125.Silvestri AJ, Kapp BS. Amygdaloid modulation of mesopontine peribrachial neuronal activity: implications for arousal. Behav Neurosci. 1998;112:571–588. doi: 10.1037//0735-7044.112.3.571. [DOI] [PubMed] [Google Scholar]
- 126.Skolnick P, Stalvey LP, Daly JW, Hoyler E, Davis JN. Binding of alpha-and beta-adrenergic ligands to cerebral cortical membranes: effect of 6-hydroxydopamine treatment and relationship to the responsiveness of cyclic amp-generating systems in two rat strains. Eur J Pharmacol. 1978;47:201–210. doi: 10.1016/0014-2999(78)90391-6. [DOI] [PubMed] [Google Scholar]
- 127.Sood S, Dhawan JK, Ramesh V, John J, Gopinath G, Kumar VM. Role of medial preoptic area beta adrenoceptors in the regulation of sleep-wakefulness. Pharmacol Biochem Behav. 1997;57:1–5. doi: 10.1016/s0091-3057(96)00384-x. [DOI] [PubMed] [Google Scholar]
- 128.Sporn JR, Wolfe BB, Harden TK, Molinoff PB. Supersensitivity in rat cerebral cortex: pre- and postsynaptic effects of 6-hydroxydopamine at noradrenergic synapses. Mol Pharmacol. 1977;13:1170–1180. [PubMed] [Google Scholar]
- 129.Stevens DR, Birnstiel S, Gerber U, McCarley RW, Greene RW. Nicotinic depolarizations of rat medial pontine reticular formation neurons studied in vitro. Neuroscience. 1993;57:419–424. doi: 10.1016/0306-4522(93)90073-o. [DOI] [PubMed] [Google Scholar]
- 130.Stevens DR, McCarley RW, Greene RW. The mechanism of noradrenergic alpha 1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994;14:6481–6487. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stewart DJ, MacFabe DF, Leung LW. Topographical projection of cholinergic neurons in the basal forebrain to the cingulate cortex in the rat. Brain Res. 1985;358:404–407. doi: 10.1016/0006-8993(85)90994-1. [DOI] [PubMed] [Google Scholar]
- 132.Stone EA, McEwen BS, Herrera AS, Carr KD. Regulation of alpha and beta components of noradrenergic cyclic AMP response in cortical slices. Eur J Pharmacol. 1987;141:347–356. doi: 10.1016/0014-2999(87)90551-6. [DOI] [PubMed] [Google Scholar]
- 133.Stone EA, Zhang Y. Adrenoceptor antagonists block c-fos response to stress in the mouse brain. Brain Res. 1995;694:279–286. doi: 10.1016/0006-8993(95)00882-q. [DOI] [PubMed] [Google Scholar]
- 134.Stone EA, Zhang Y, Hiller JM, Simon EJ, Hillman DE. Activation of fos in mouse amygdala by local infusion of norepinephrine or atipamezole. Brain Res. 1997;778:1–5. doi: 10.1016/s0006-8993(97)00667-7. [DOI] [PubMed] [Google Scholar]
- 135.Stone EA, Zhang Y, John S, Filer D, Bing G. Effect of locus coeruleus lesion on c-fos expression in the cerebral cortex caused by yohimbine injection or stress. Brain Res. 1993;603:181–185. doi: 10.1016/0006-8993(93)91236-l. [DOI] [PubMed] [Google Scholar]
- 136.Stone EA, Zhang Y, John SM, Bing G. c-Fos response to administration of catecholamines into brain by microdialysis. Neurosci Lett. 1991;133:33–35. doi: 10.1016/0304-3940(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 137.Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 138.Swanson LW, Cowan WM. The connections of the septal region in the rat. Comp Neur. 1979;186:621–656. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- 139.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 140.Szymusiak R, Gvilia I, McGinty D. Hypothalamic control of sleep. Sleep Med. 2007;8:291–301. doi: 10.1016/j.sleep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 141.Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 142.Timo-Iaria C, Negrao N, Schmidek WR, Hoshino K, Lobato de Menezes CE, Leme dR. Phases and states of sleep in the rat. Physiol Behav. 1970;5:1057–1062. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- 143.Tononi G, Pompeiano M, Pompeiano O. Desynchronized sleep suppression after microinjection of the beta- adrenergic agonist isoproterenol in the dorsal pontine tegmentum. Arch Ital Biol. 1988;126:125–128. [PubMed] [Google Scholar]
- 144.Tononi G, Pompeiano M, Pompeiano O. Modulation of desynchronized sleep through microinjection of beta- adrenergic agonists and antagonists in the dorsal pontine tegmentum of the cat. Pflugers Arch. 1989;415:142–149. doi: 10.1007/BF00370584. [DOI] [PubMed] [Google Scholar]
- 145.Trampus M, Ongini E. The D1 dopamine receptor antagonist SCH 23390 enhances REM sleep in the rat. Neuropharmacology. 1990;29:889–893. doi: 10.1016/0028-3908(90)90138-h. [DOI] [PubMed] [Google Scholar]
- 146.Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- 147.Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- 148.Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- 149.Vanderwolf CH, Robinson TE. Reticulo-cortical activity and behavior: A critique of the arousal theory and a new synthesis. Behavioral Brain Science. 1981;4:459–514. [Google Scholar]
- 150.Vetrivelan R, Mallick HN, Kumar VM. Unmasking of alpha1 adrenoceptor induced hypnogenic response from medial preoptic area. Physiol Behav. 2005;84:641–650. doi: 10.1016/j.physbeh.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 151.Vetrivelan R, Mallick HN, Kumar VM. Sleep induction and temperature lowering by medial preoptic alpha(1) adrenergic receptors. Physiol Behav. 2006;87:707–713. doi: 10.1016/j.physbeh.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 152.Vetrivelan R, Mallick HN, Kumar VM. Tonic activity of alpha1 adrenergic receptors of the medial preoptic area contributes towards increased sleep in rats. Neuroscience. 2006;139:1141–1151. doi: 10.1016/j.neuroscience.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 153.Von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- 154.Walaas I, Fonnum F. Biochemical evidence for glutamate as a transmitter in hippocampal efferents to the basal forebrain and hypothalamus in the rat brain. Neuroscience. 1980;5:1691–1698. doi: 10.1016/0306-4522(80)90088-3. [DOI] [PubMed] [Google Scholar]
- 155.Waterman A, Livingston A, Bouchenafa O. Analgesic effects of intrathecally-applied alpha 2-adrenoceptor agonists in conscious, unrestrained sheep. Neuropharmacology. 1988;27:213–216. doi: 10.1016/0028-3908(88)90173-6. [DOI] [PubMed] [Google Scholar]
- 156.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- 157.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2005;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zaborszky L. Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. In: Frotscher M, Misgel U, editors. Central cholinergic synaptic transmission. Birkhauser; Basel: 1989. pp. 12–32. [DOI] [PubMed] [Google Scholar]
- 159.Zaborszky L, Cullinan WE. Direct catecholaminergic-cholinergic interactions in the basal forebrain. I. Dopamine-beta-hydroxylase- and tyrosine hydroxylase input to cholinergic neurons. J Comp Neurol. 1996;374:535–554. doi: 10.1002/(SICI)1096-9861(19961028)374:4<535::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 160.Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic projection neurons: an update. Adv Exp Med Biol. 1991;295:43–100. doi: 10.1007/978-1-4757-0145-6_2. [DOI] [PubMed] [Google Scholar]